Dual-Action Therapeutics: DNA Alkylation and Antimicrobial Peptides for Cancer Therapy
Abstract
Simple Summary
Abstract
1. Introduction
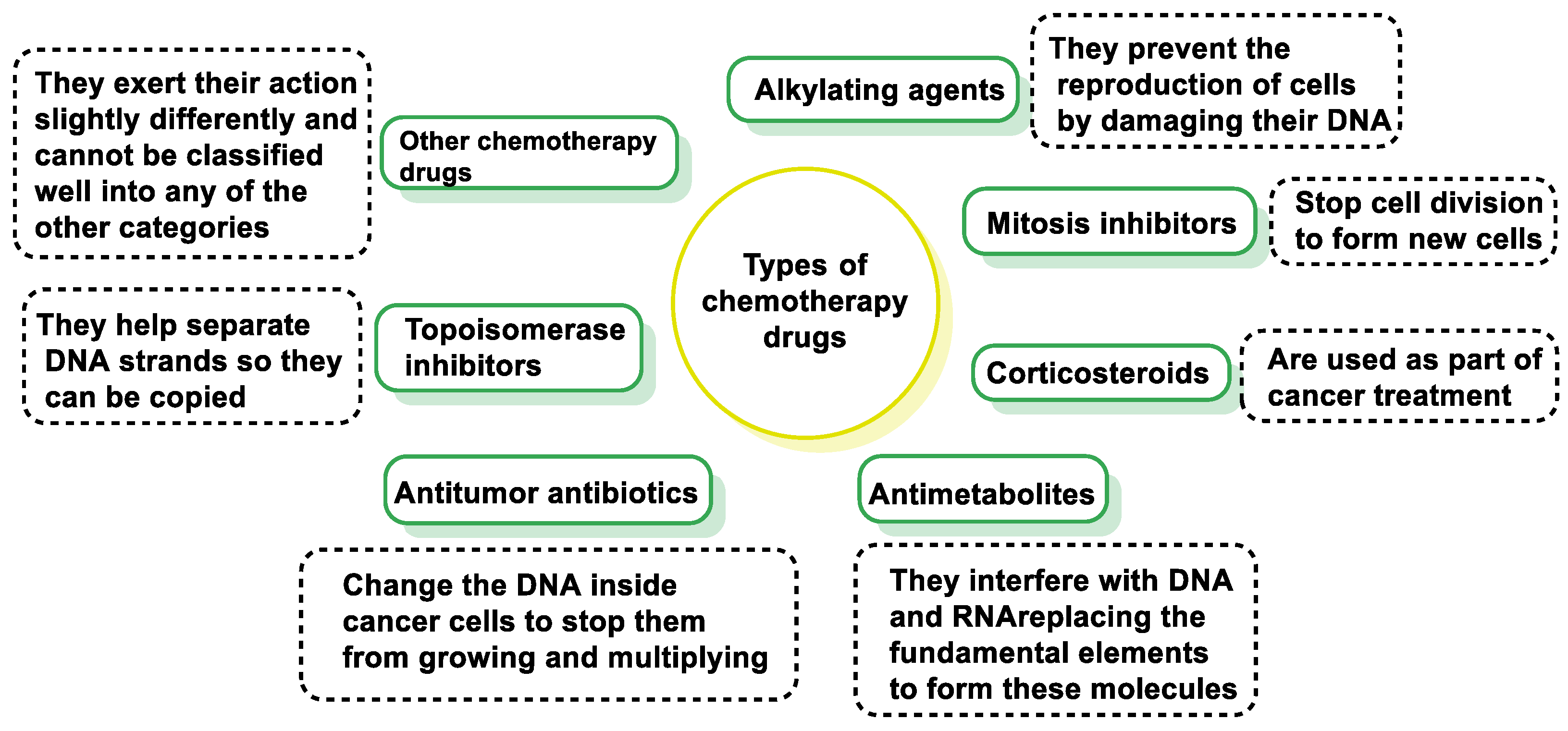
2. Types of Chemotherapy Drugs
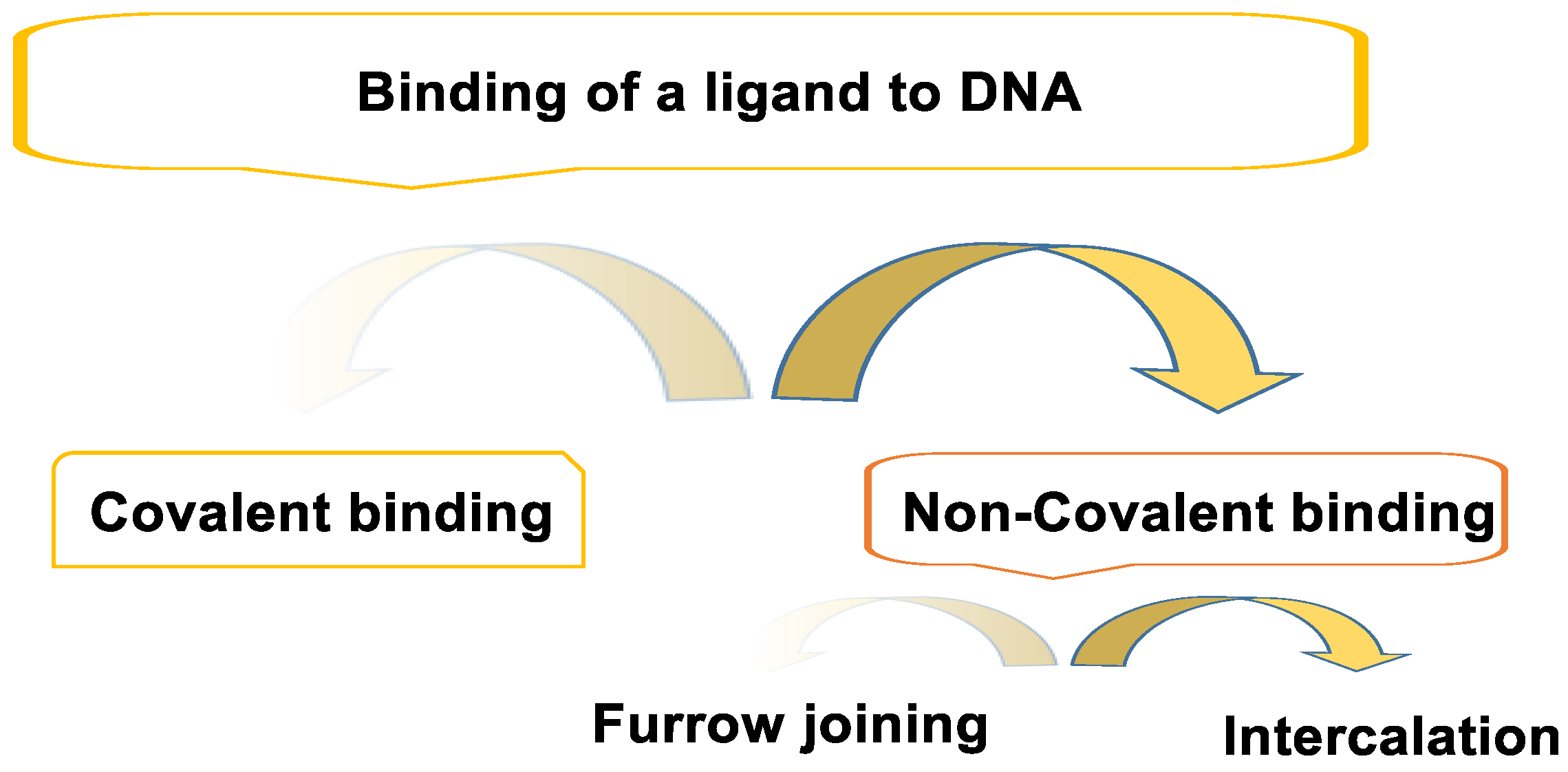
3. Binding of a Ligand to DNA
3.1. Non-Covalent Bond
3.2. Covalent Bonding
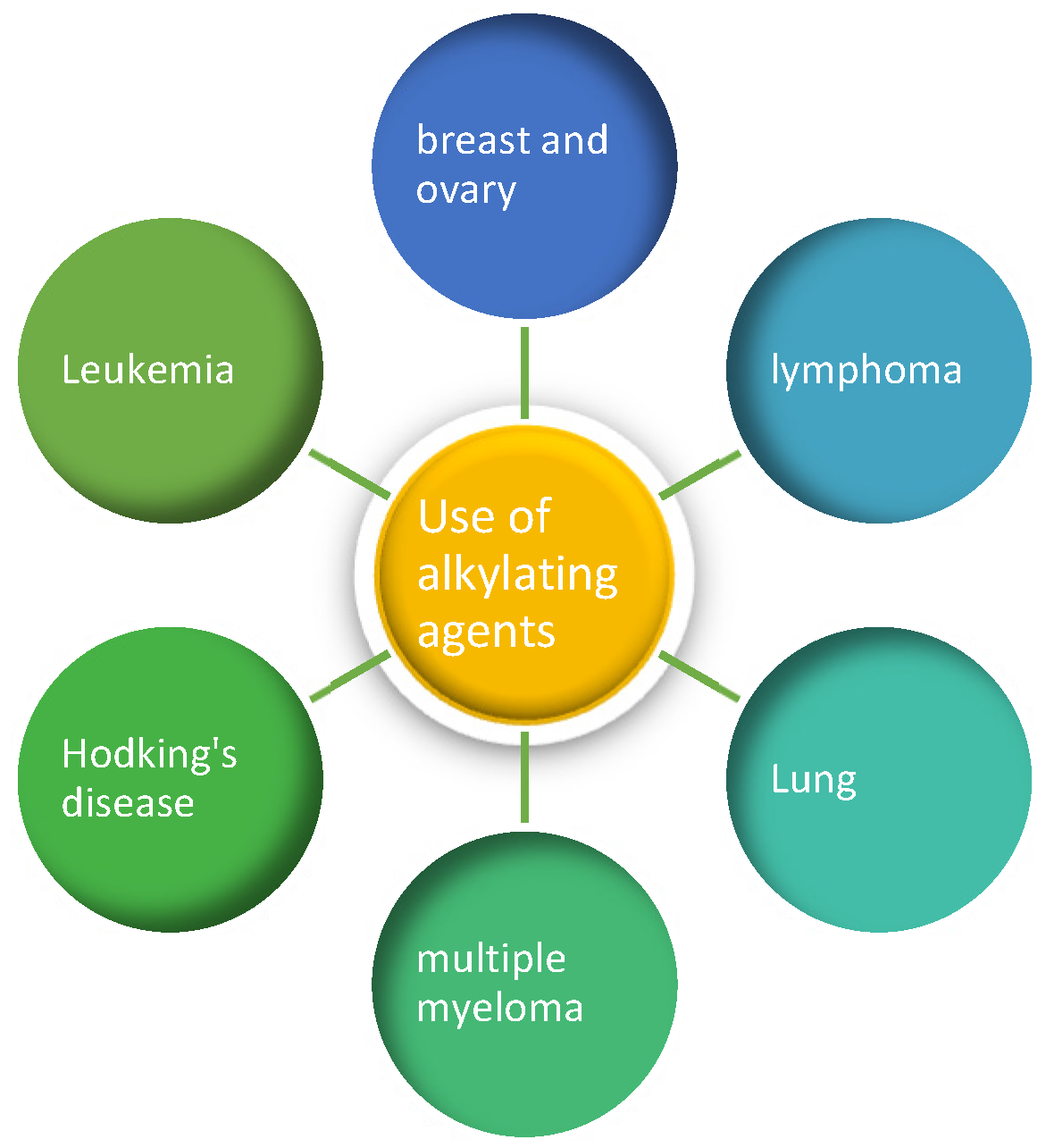
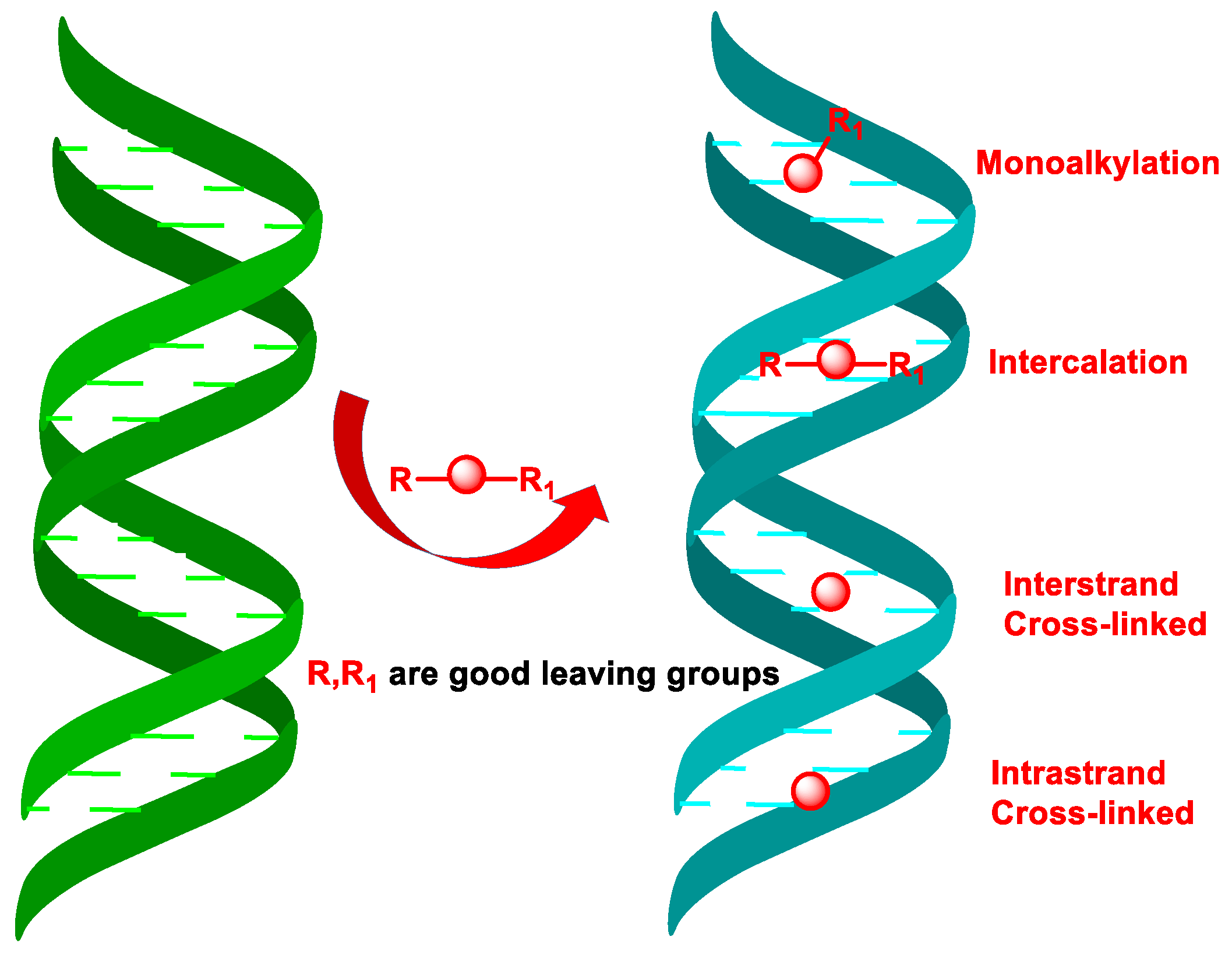
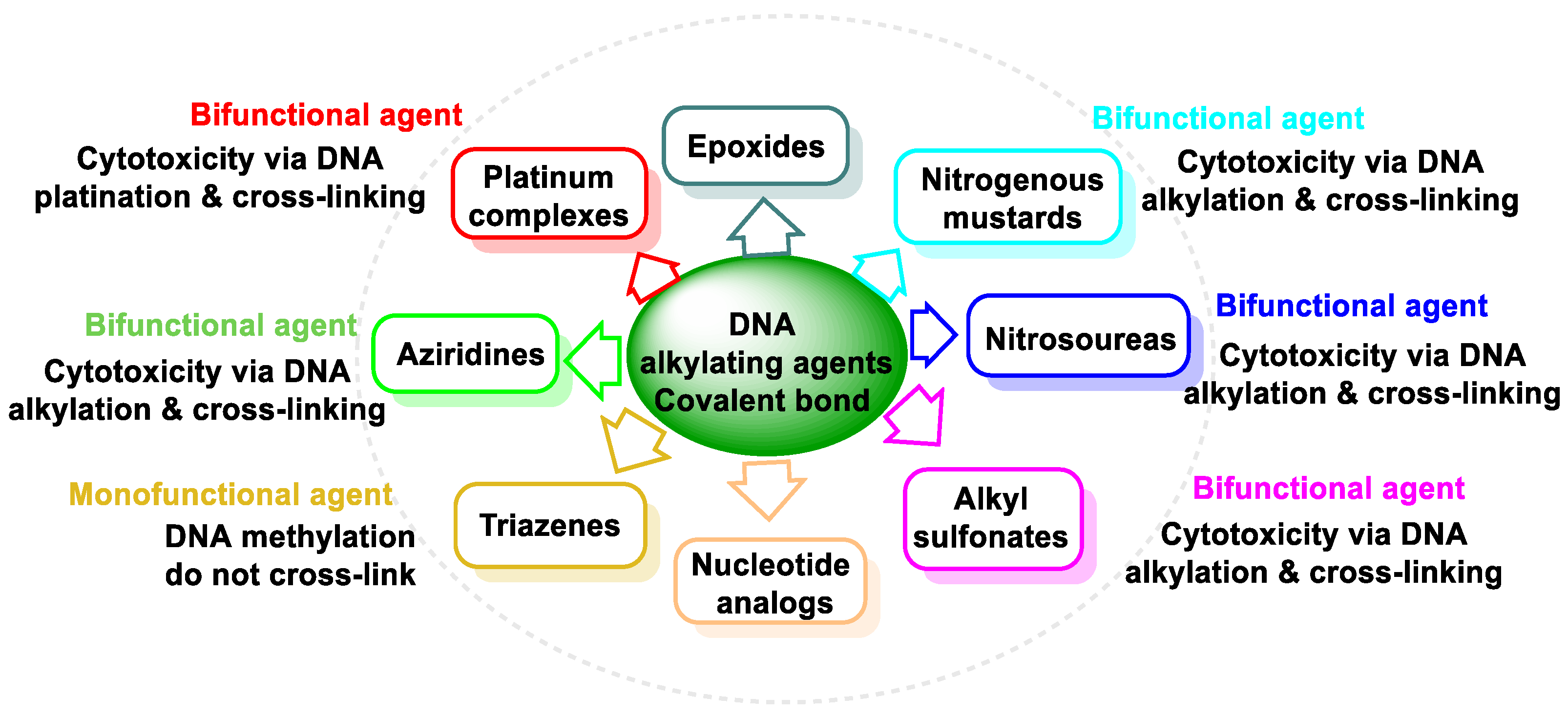
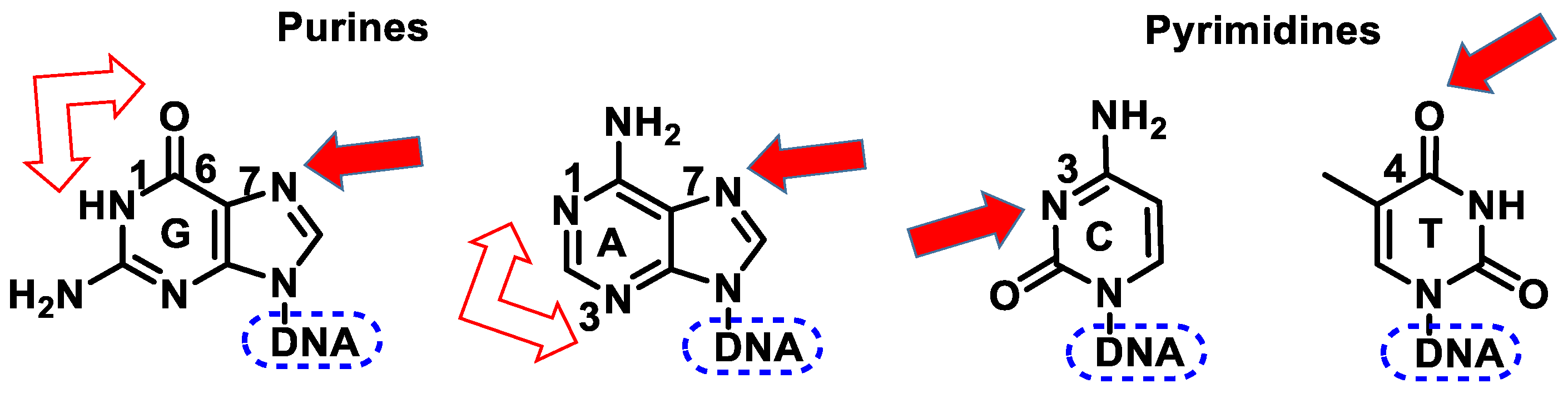
4. DNA Alkylating Agents
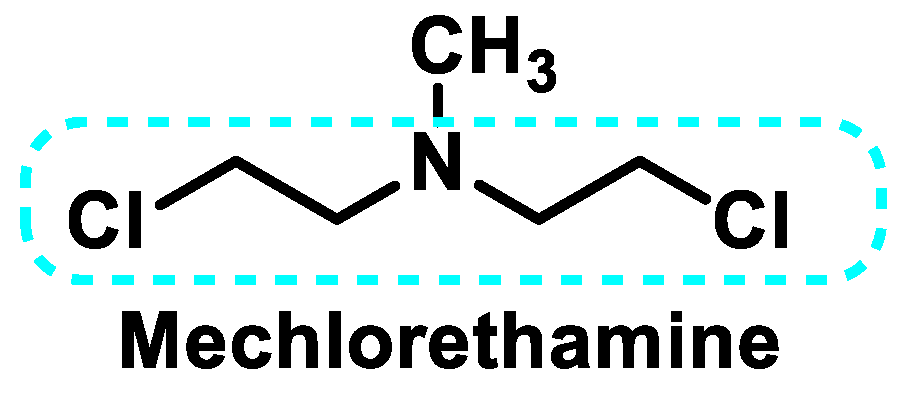
5. Nitrogen Mustards
5.1. Aliphatic Mustard
5.2. Phosphamide Nitrogen Mustard
5.3. Aromatic Nitrogen Mustards
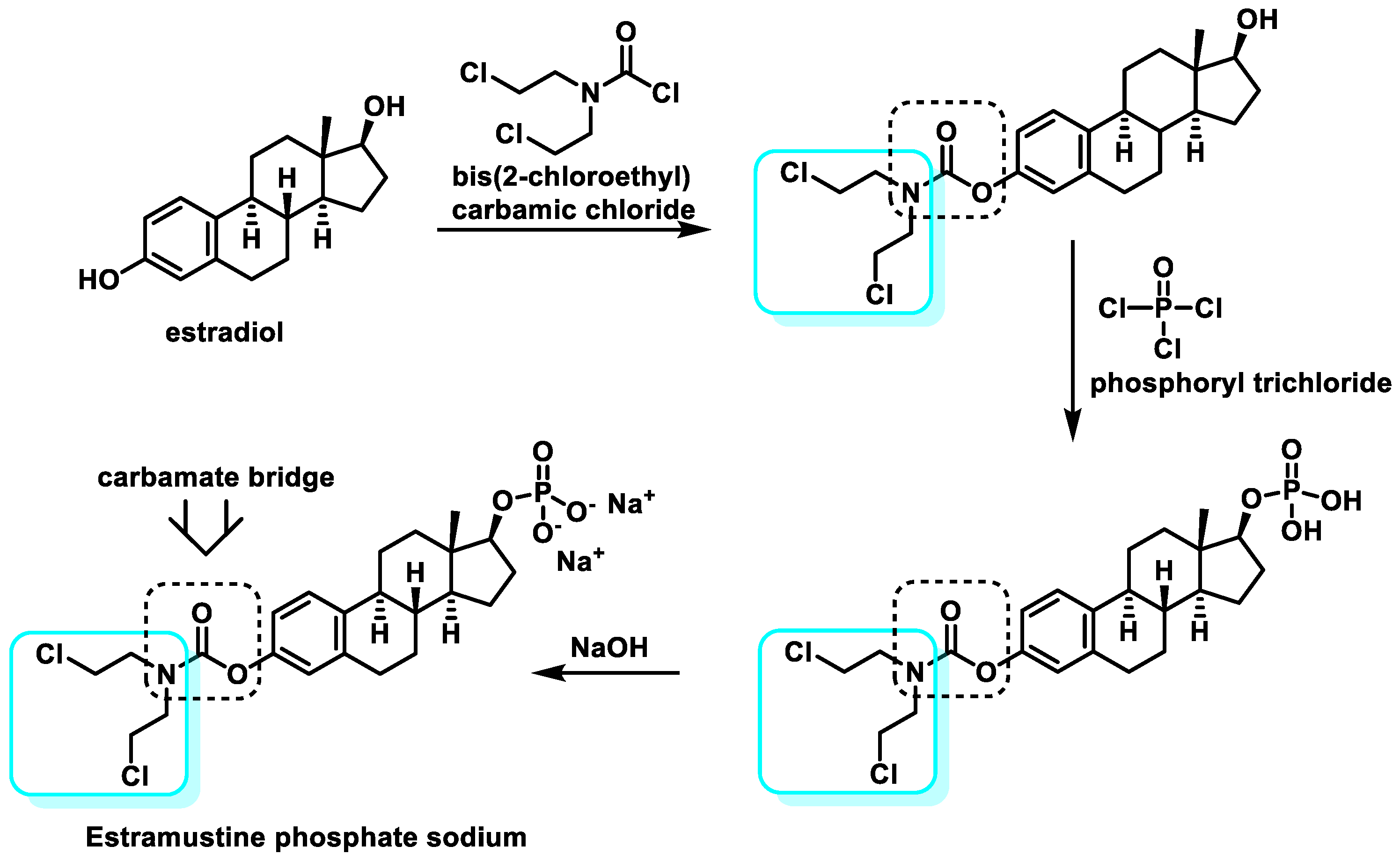
5.4. Steroid-Coupled Nitrogen Mustards
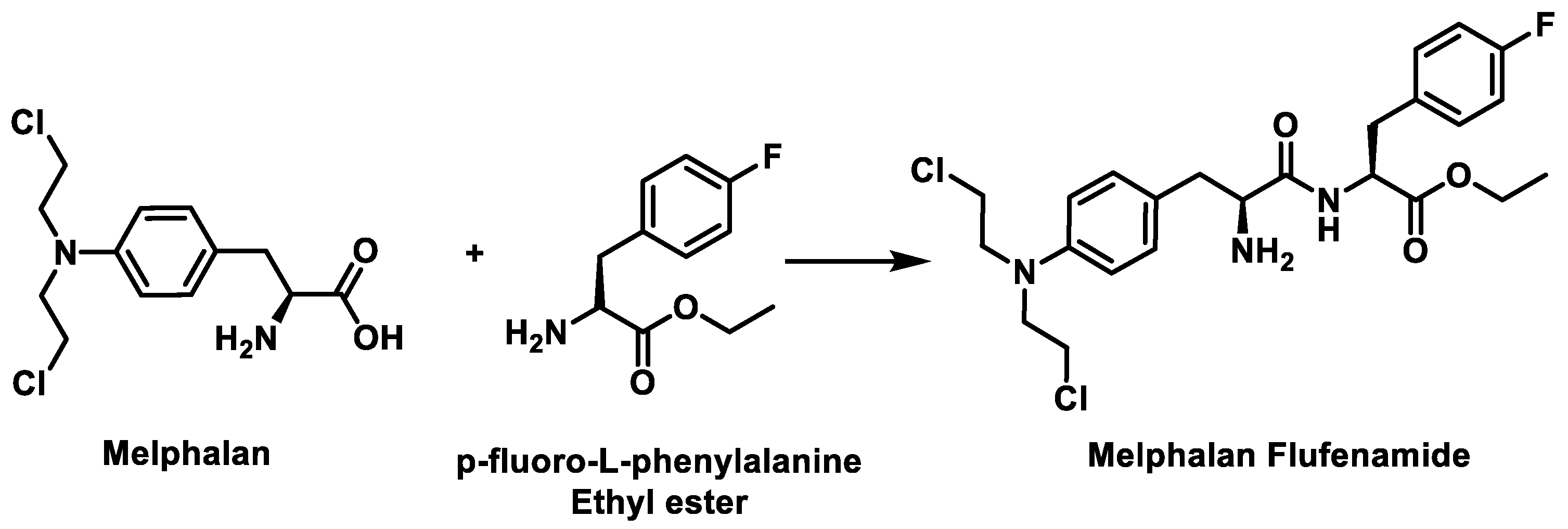
5.5. Peptide-Coupled Nitrogen Mustards
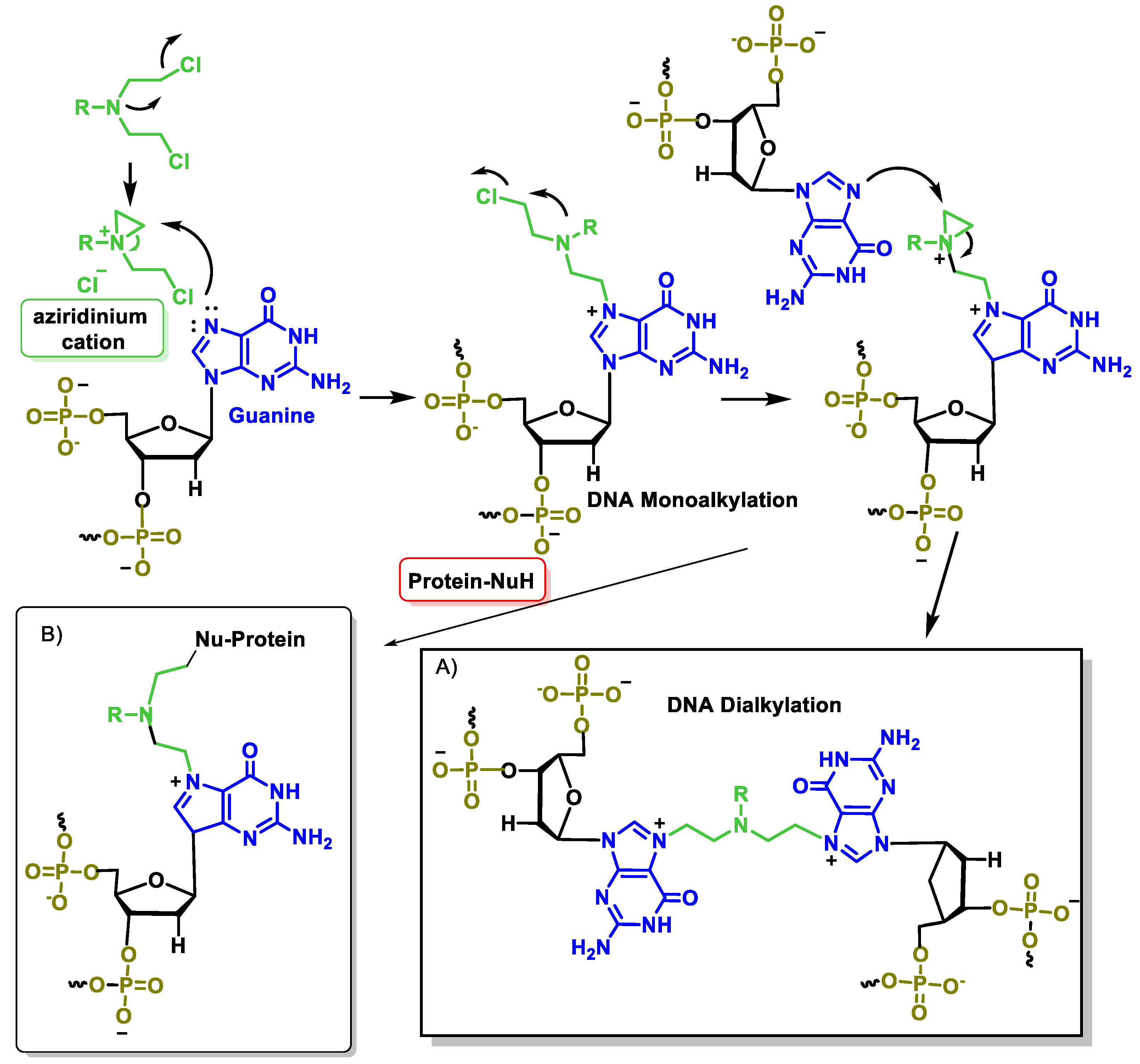
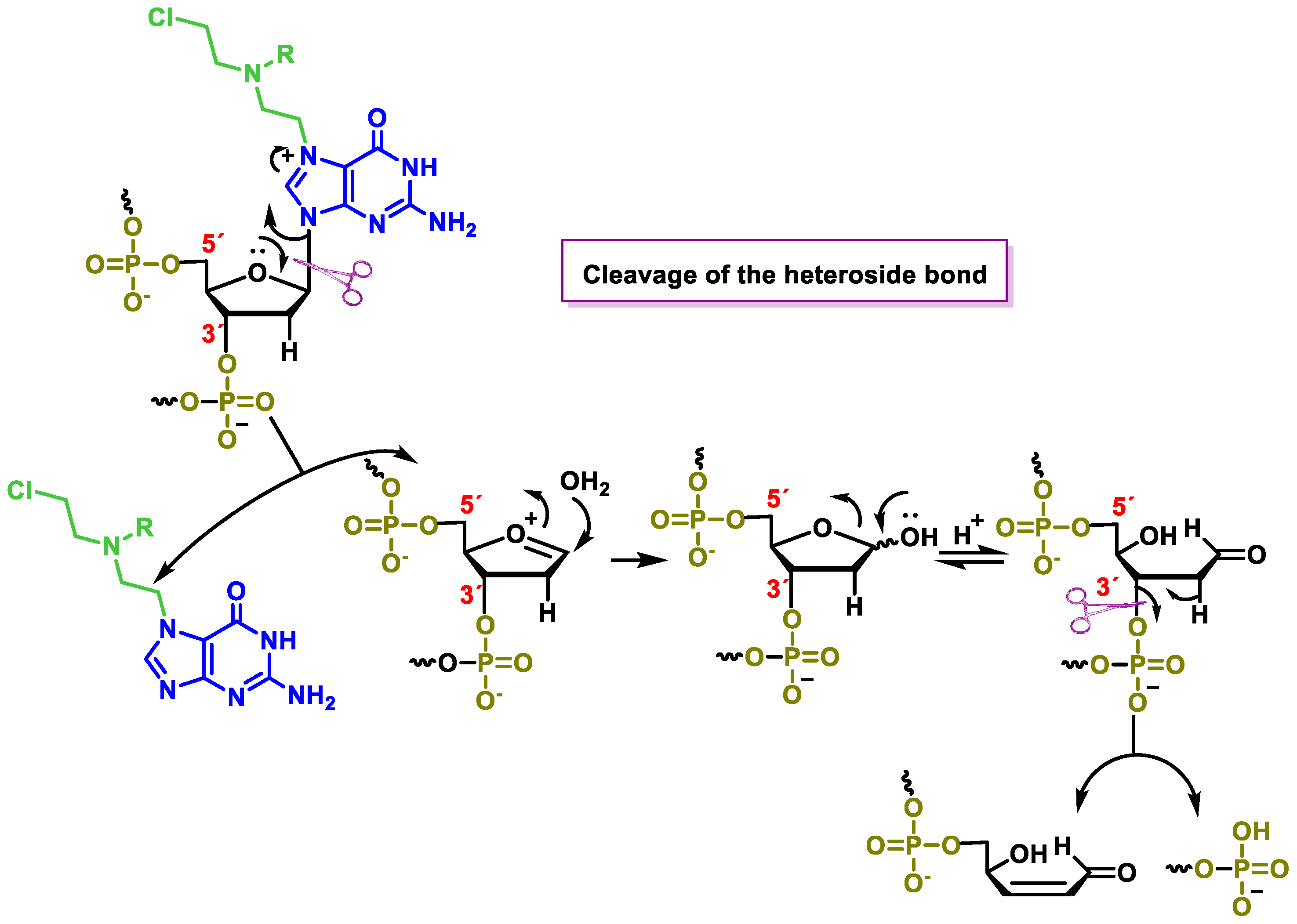
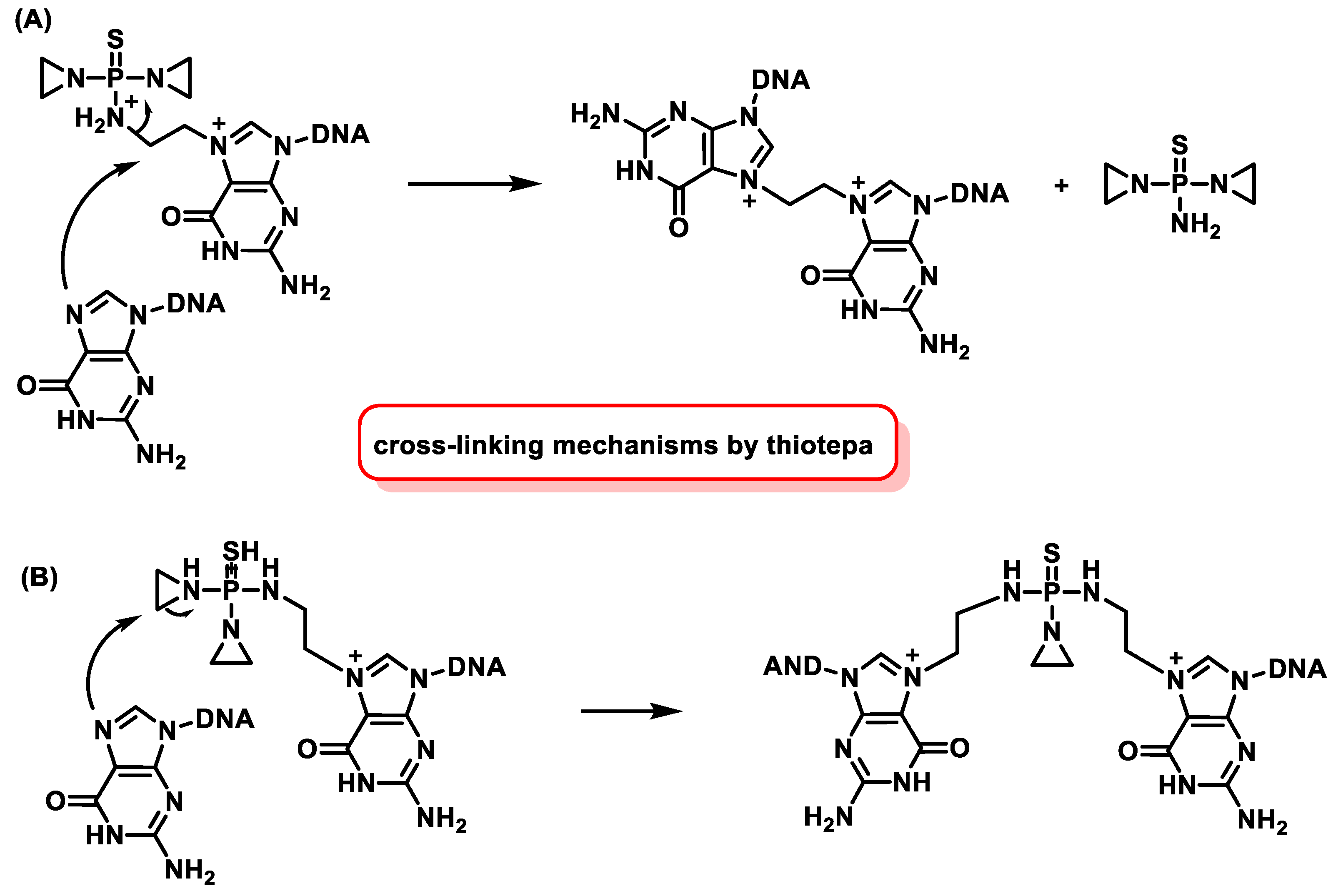
5.6. Mode of Action of Nitrogen Mustards
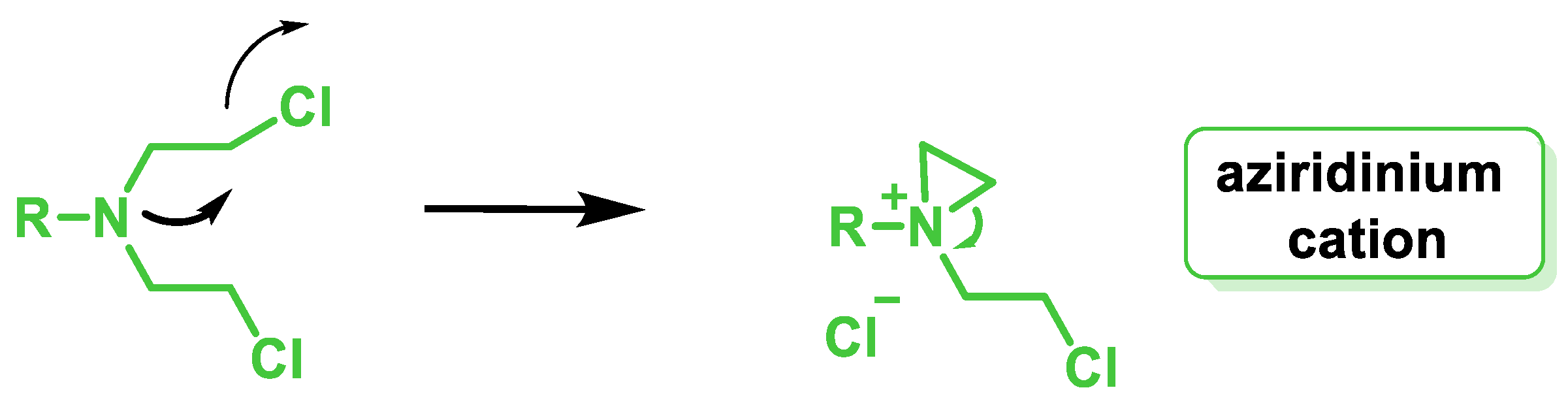
6. Aziridines
6.1. Aziridines Linked to a Benzoquinone System
6.1.1. Natural Aziridinyylbenzoquinone
6.1.2. Synthetic Aziridinyylbenzoquinone
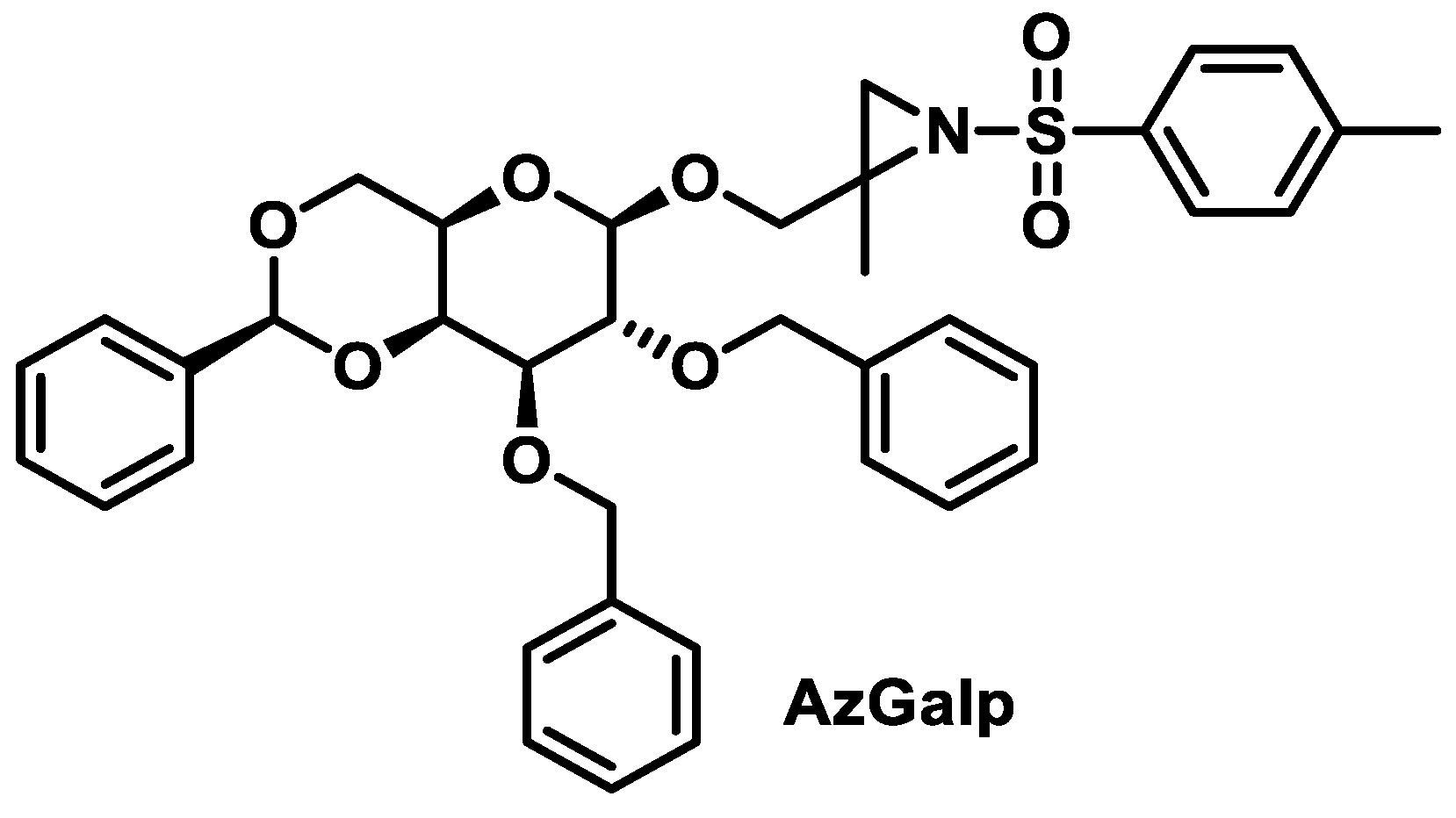
6.1.3. Aziridines β-D-Galactopyranosides
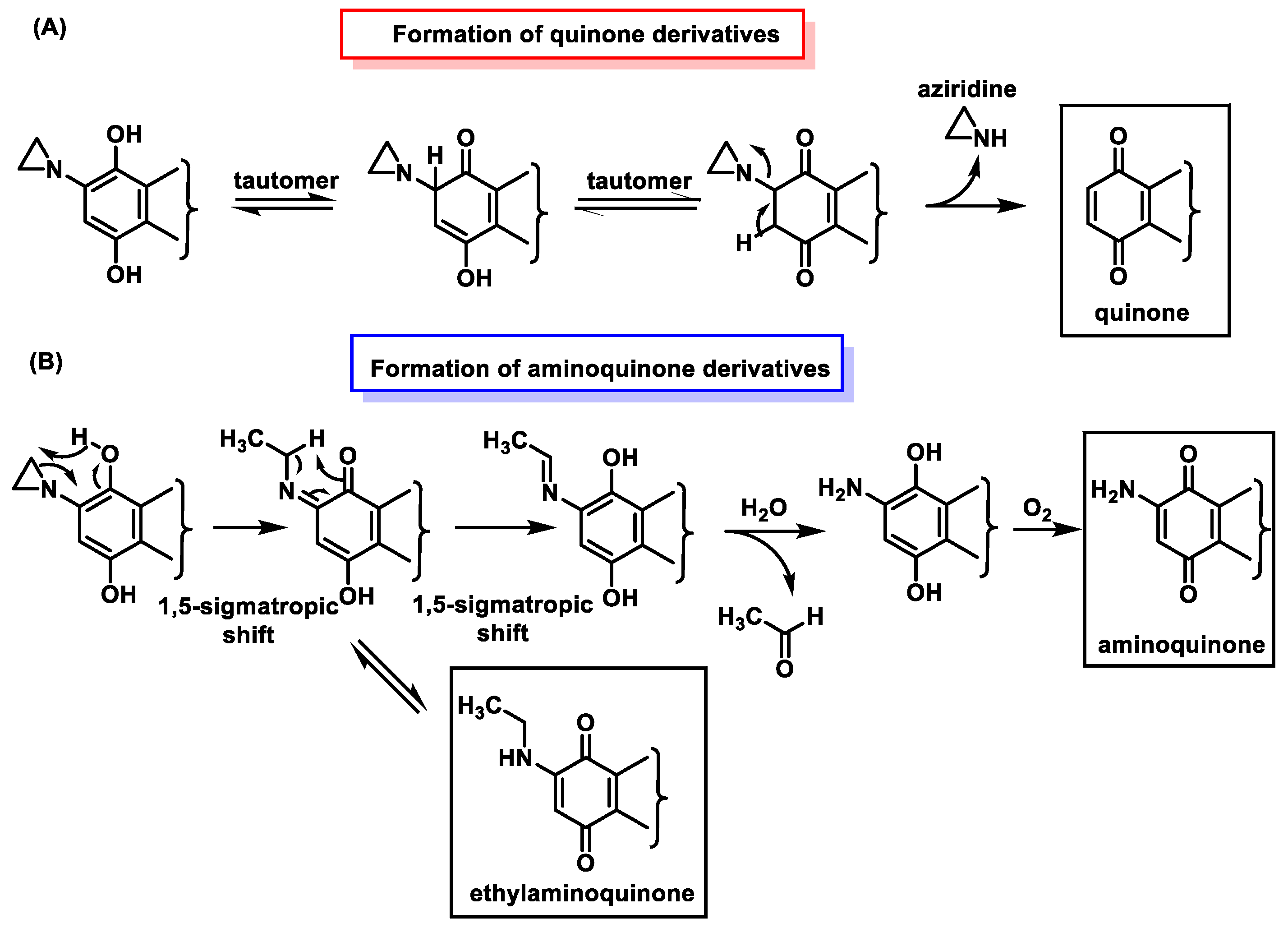
6.2. Mechanism of DNA Alkylation by Aziridinylbenzoquinones
6.3. Inactivation of Aziridinylbenzoquinones
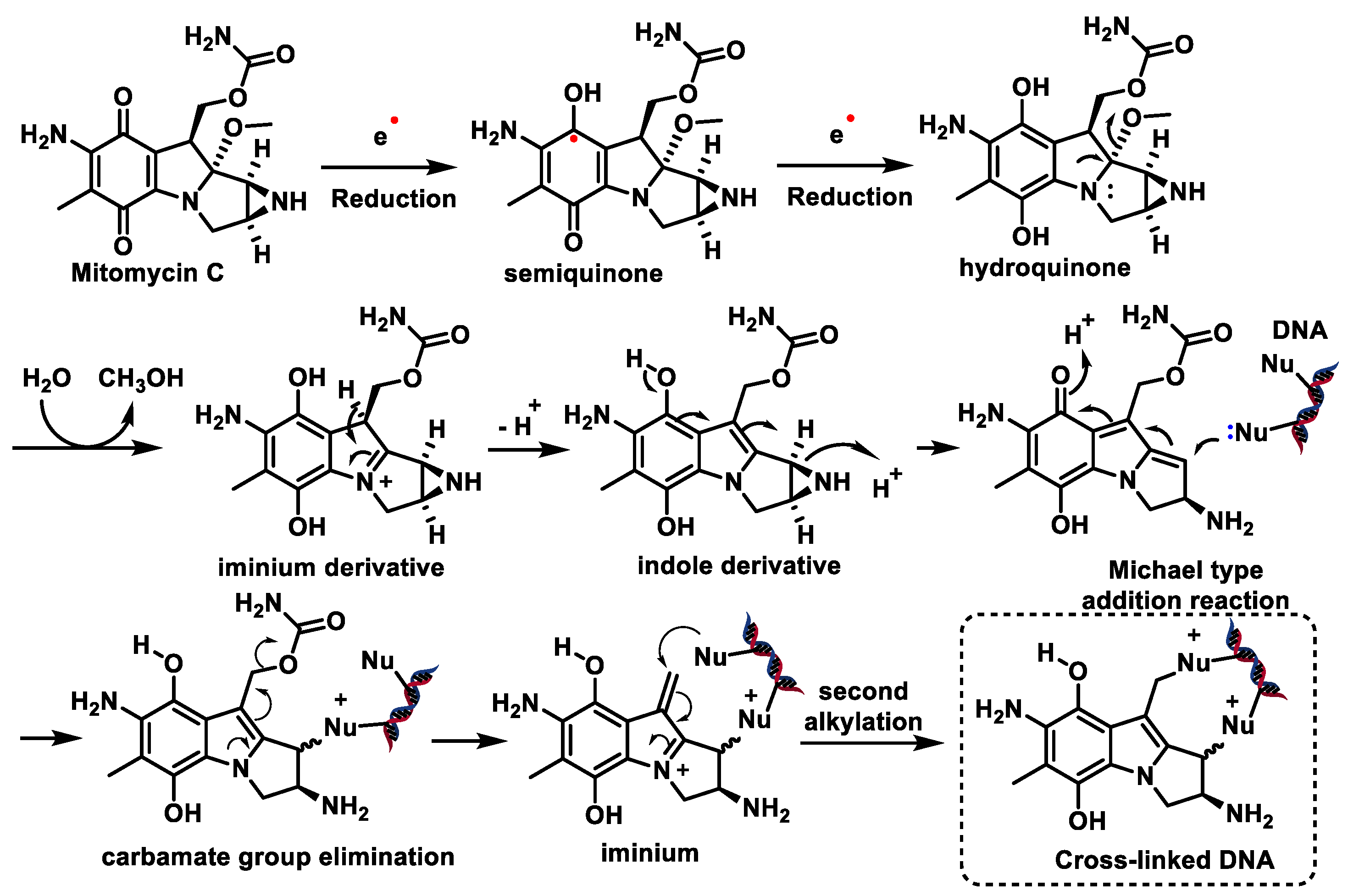
7. Epoxides
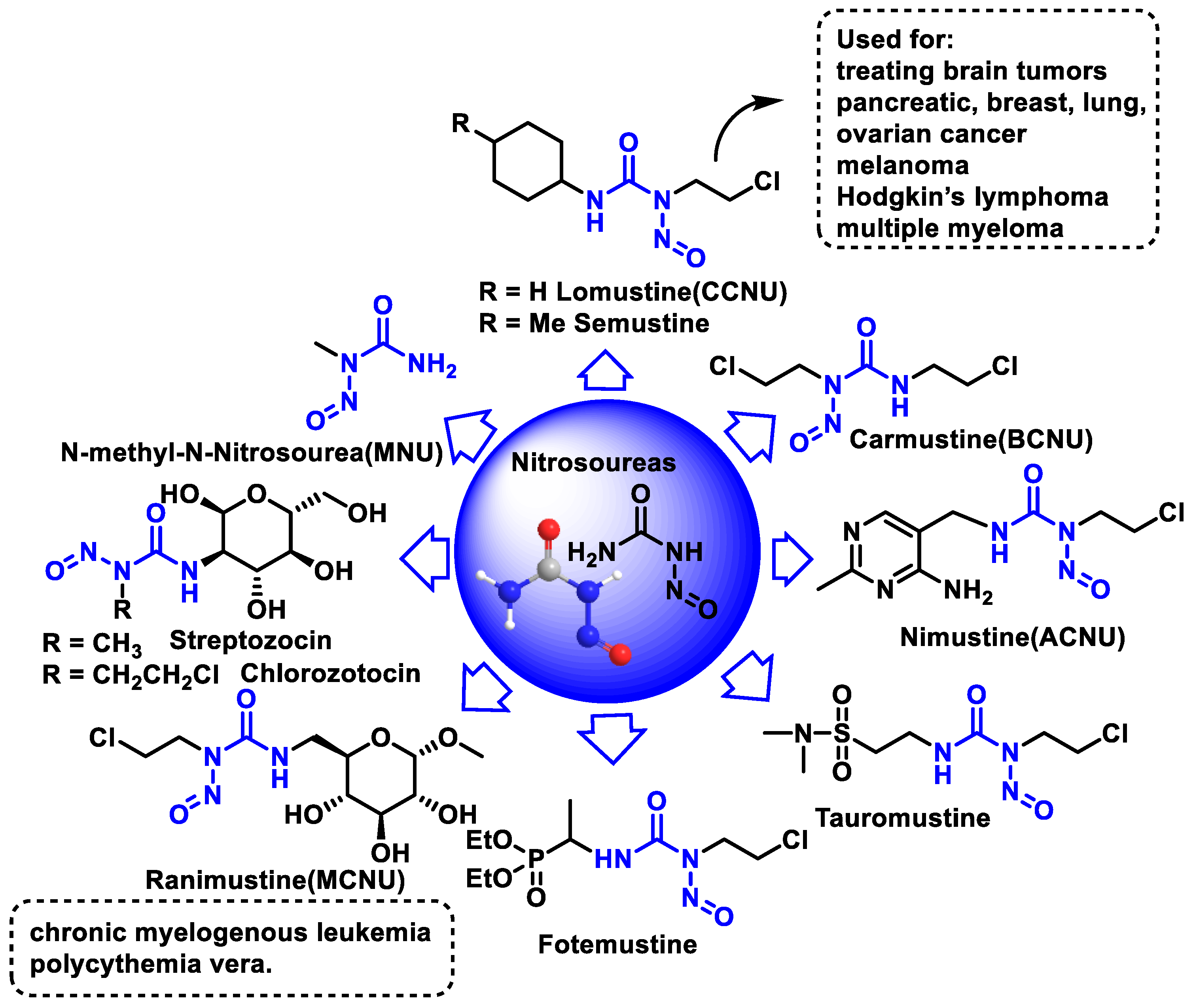
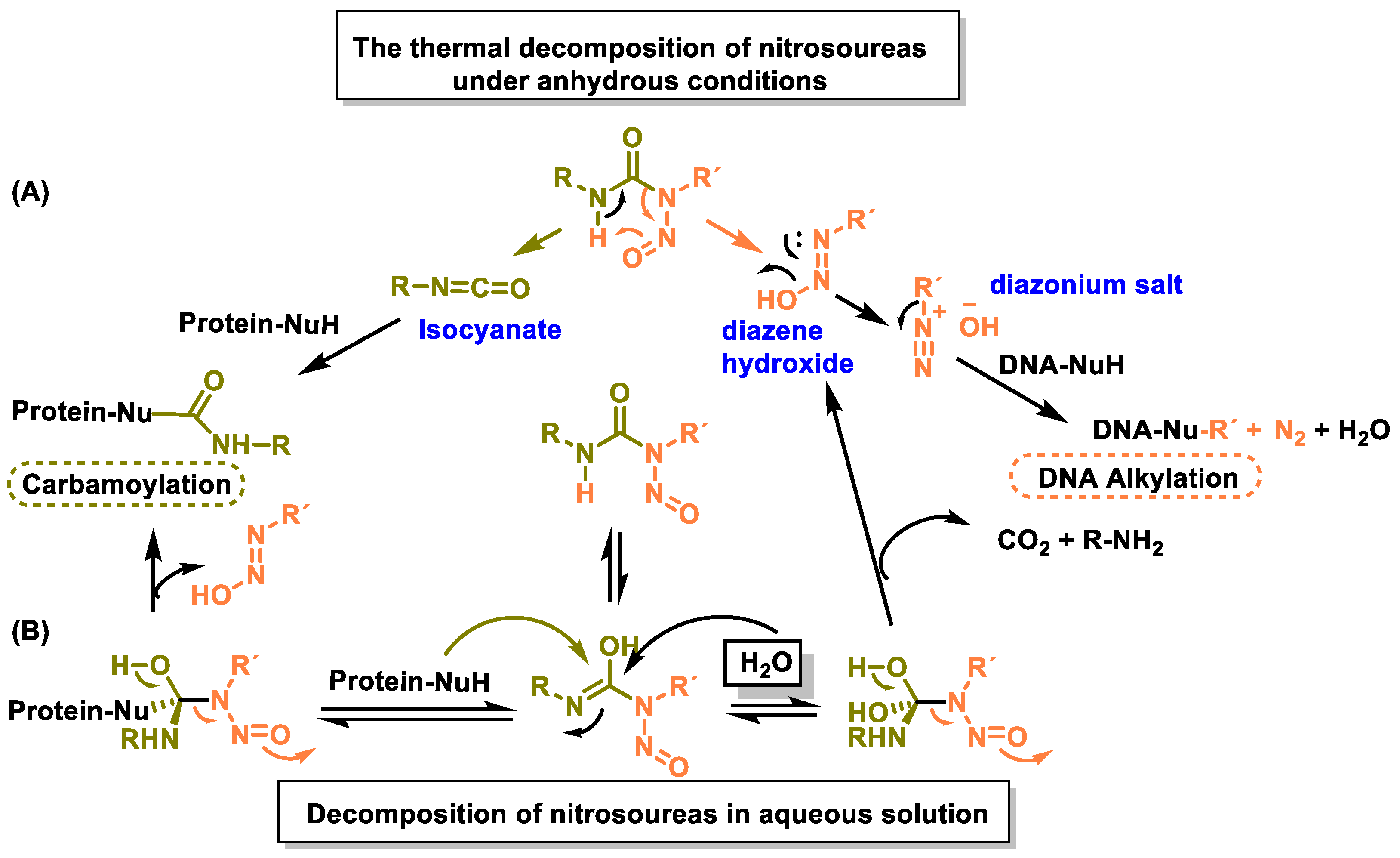
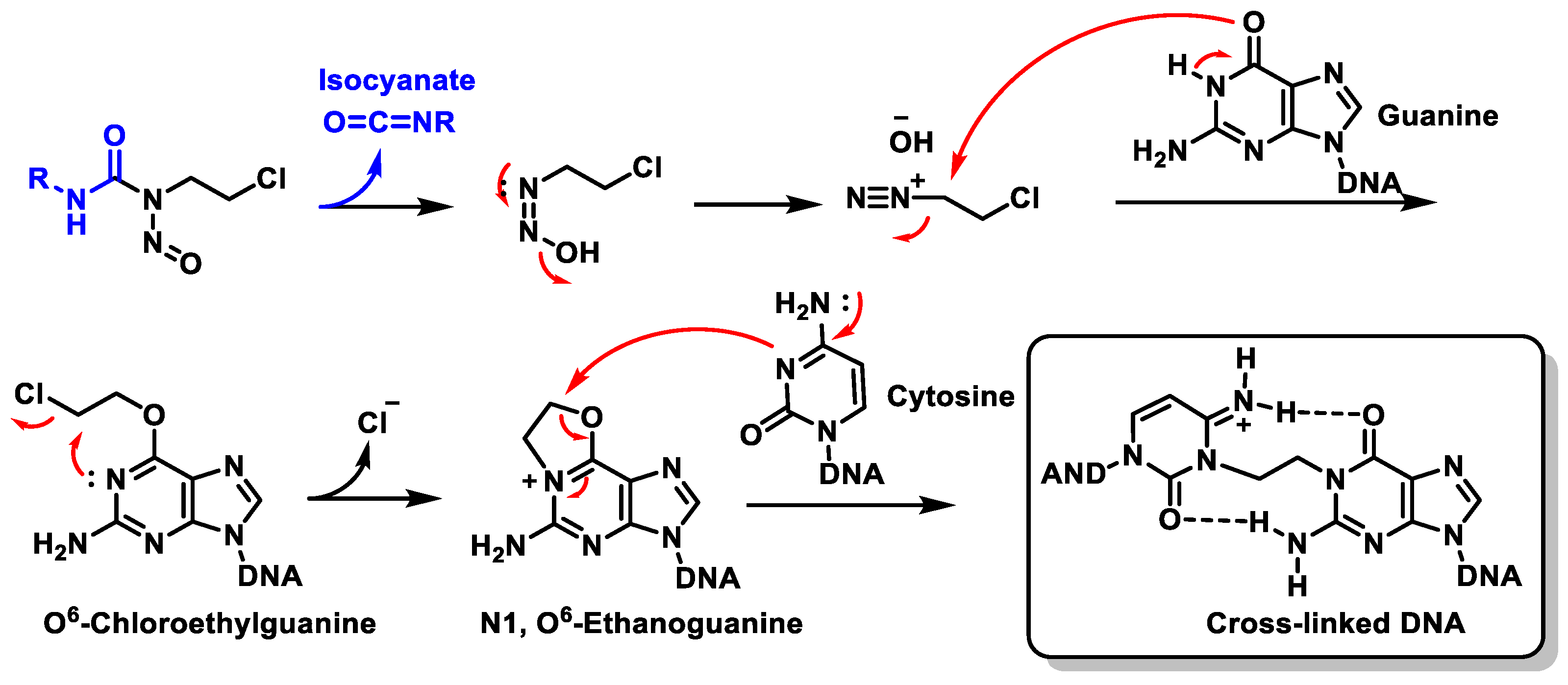
8. Nitrosoureas
Mechanism of Action of Nitrosoureas
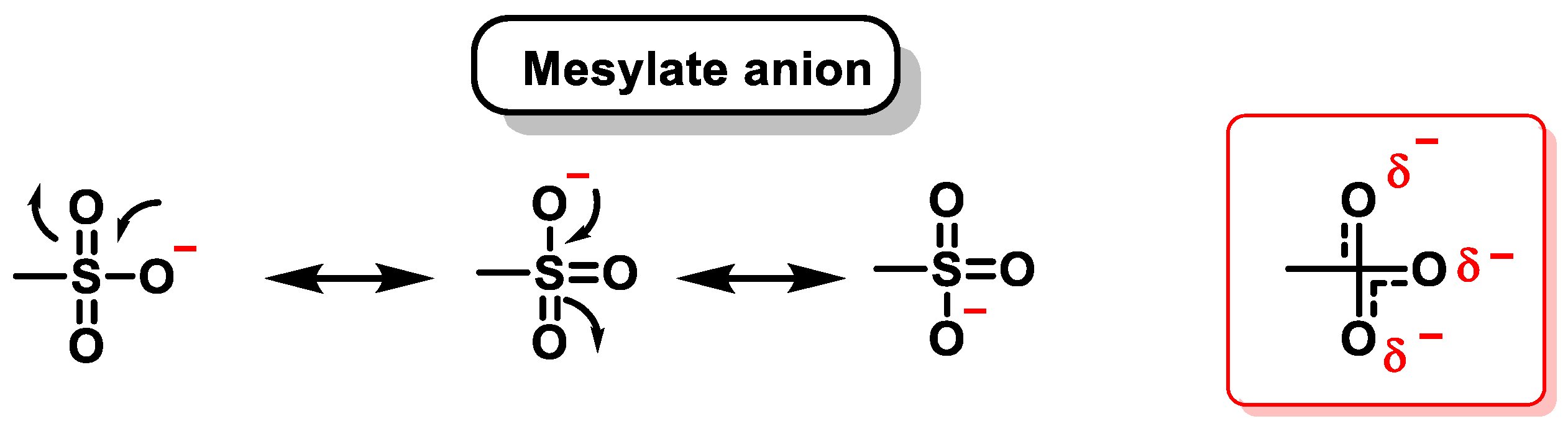
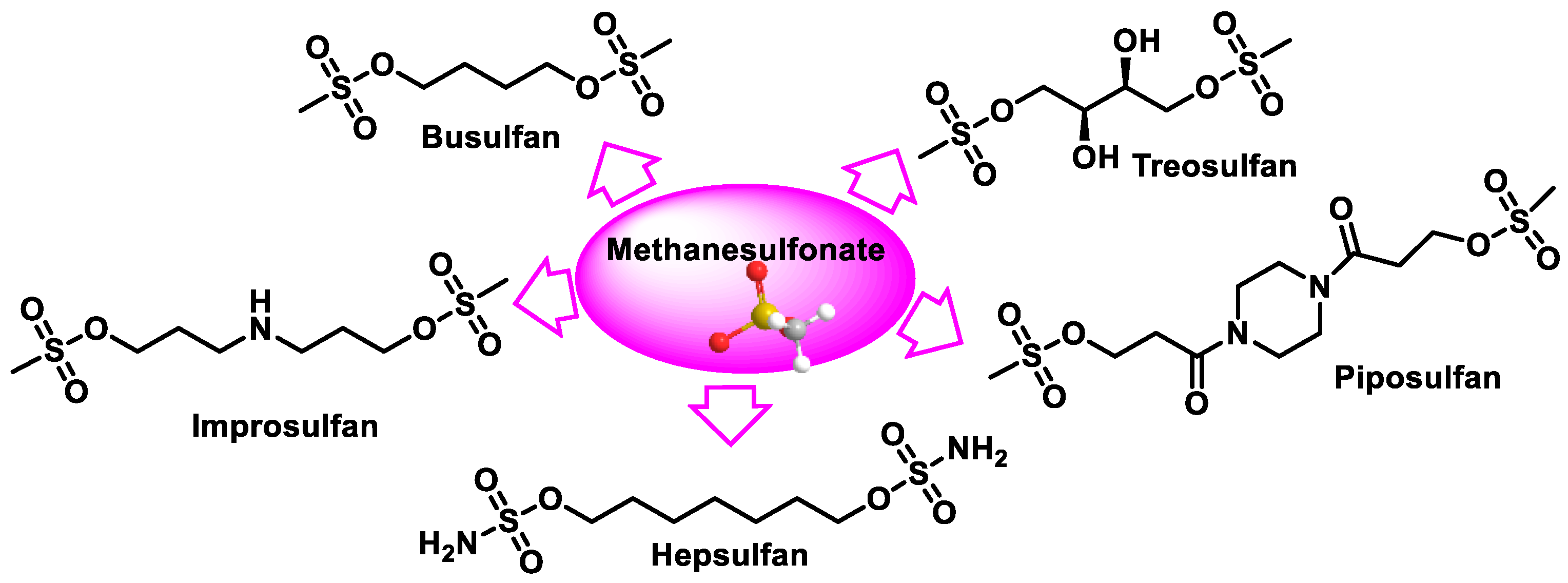
9. Methanesulphonates
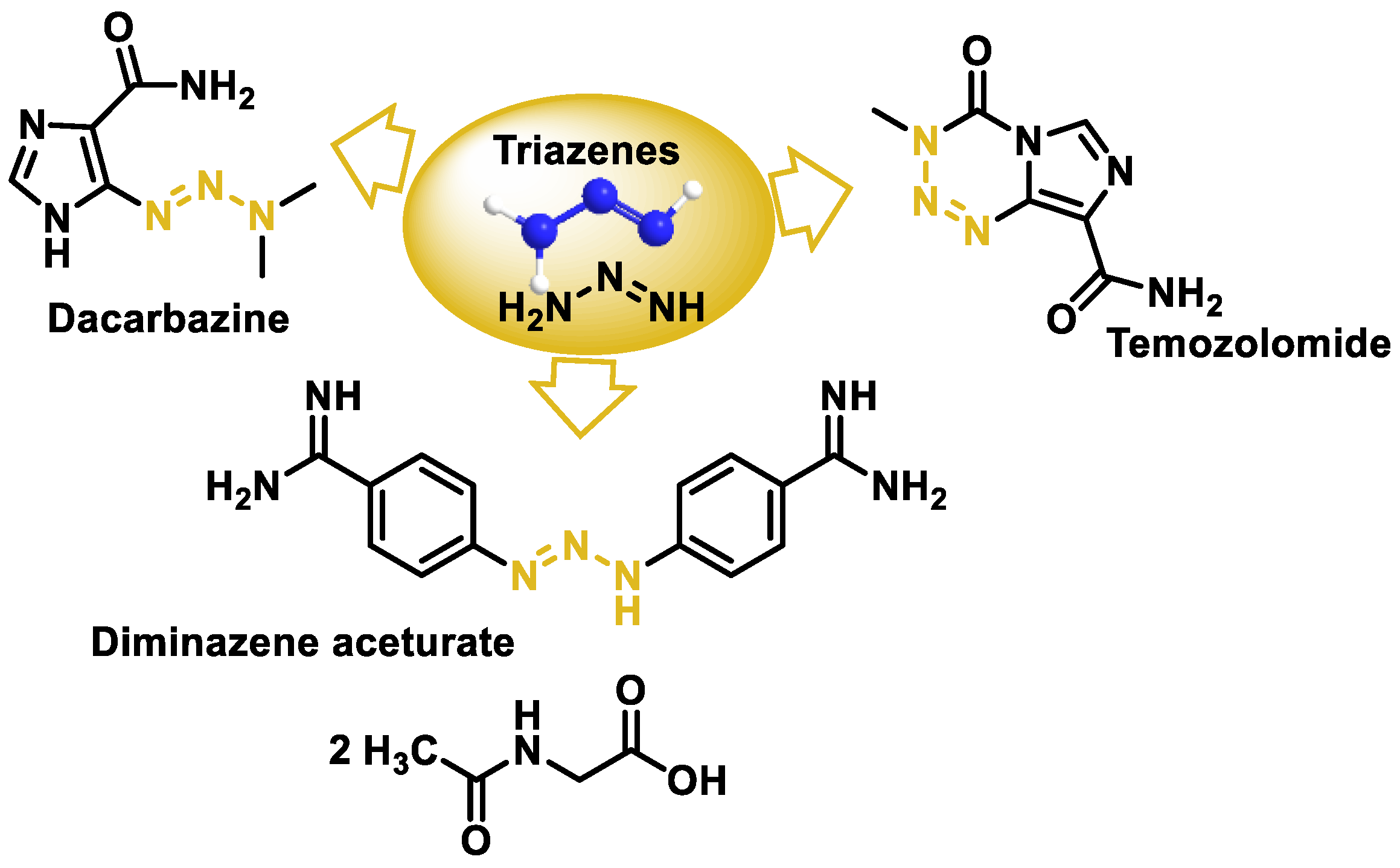
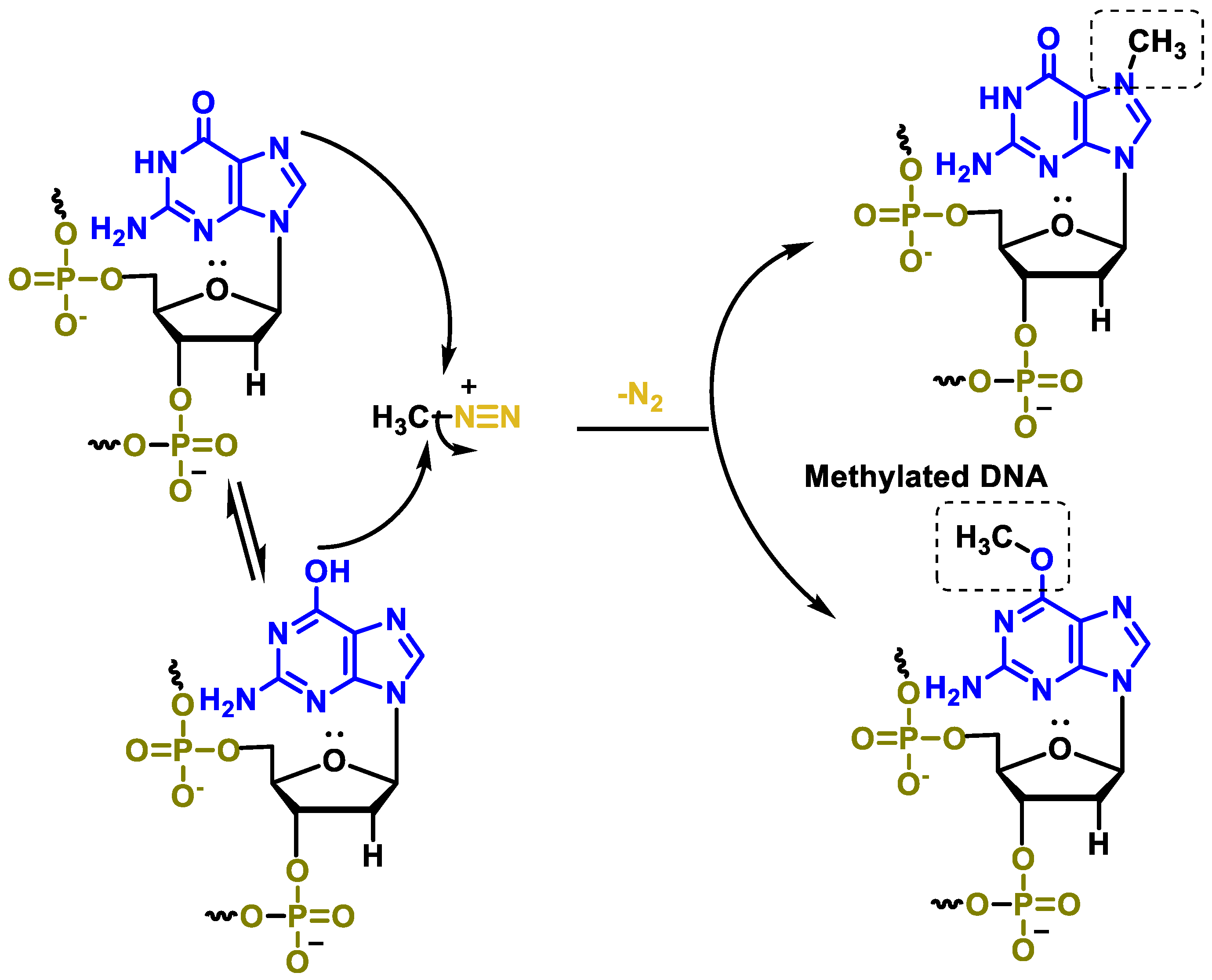
10. Triazenes
11. Fate and Effects of Anticancer Drugs in the Environment
12. Antimicrobial Peptides
13. Mechanisms of Action of AMPs in Cancer Cells
14. Advantages of AMPs and Their Synergy with Alkylating Agents
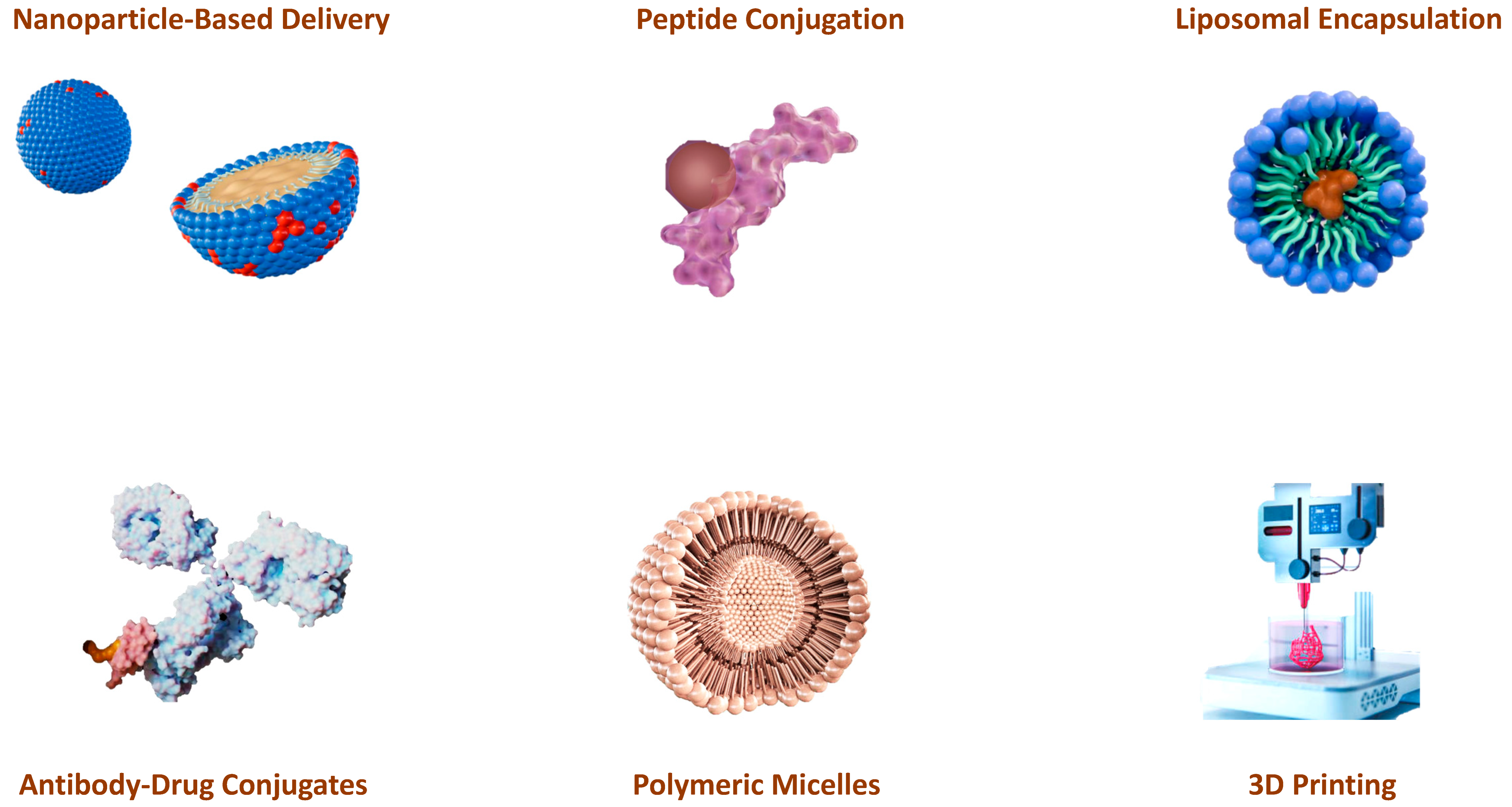
15. Technological Advances in the Administration of AMPs and Alkylating Drugs
16. Can AI Tools Accelerate Current Research into Alkylating Agents?
17. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Amjad, M.T.; Chidharla, A.; Kasi, A. Cancer Chemotherapy; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef] [PubMed]
- Wiltse, J.; Dellarco, V.L. US Environmental Protection Agency guidelines for carcinogen risk assessment: Past and future. Mutat. Res./Rev. Genet. Toxicol. 1996, 365, 3–15. [Google Scholar] [CrossRef] [PubMed]
- De, Surya Kanta. An Overview of Cancer. In Fundamentals of Cancer Detection, Treatment, and Prevention; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 1–20. [Google Scholar]
- Passaro, A.; Al Bakir, M.; Hamilton, E.G.; Diehn, M.; André, F.; Roy-Chowdhuri, S.; Mountzios, G.; Wistuba, I.I.; Swanton, C.; Peters, S. Cancer biomarkers: Emerging trends and clinical implications for personalized treatment. Cell 2024, 187, 1617–1635. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Misiak, M.; Mantegazza, F.; Beretta, L.G. Methods for Elucidation of DNA-Anticancer Drug Interactions and their Applications in the Development of New Drugs. Curr. Pharm. Des. 2016, 22, 6596–6611. [Google Scholar] [CrossRef]
- Thurston, D.E.; Pysz, I. Chemistry and Pharmacology of Anticancer Drugs; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Lagunas-Rangel, F.A.; Liu, W.; Schiöth, H.B. Can Exposure to Environmental Pollutants Be Associated with Less Effective Chemotherapy in Cancer Patients? Int. J. Environ. Res. Public Health 2022, 19, 2064. [Google Scholar] [CrossRef] [PubMed]
- Hoofnagle, J.H.; Serrano, J.; Knoben, J.E.; Navarro, V.J. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Holland, J.F. Holland-Frei Cancer Medicine 8; PMPH-USA: Shelton, CT, USA, 2010; Volume 8. [Google Scholar]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Kołat, D.; Kałuzińska-Kołat, Ż.; Celik, I.; Kontek, R. Doxorubicin—An Agent with Multiple Mechanisms of Anticancer Activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef]
- van der Zanden, S.Y.; Qiao, X.; Neefjes, J. New insights into the activities and toxicities of the old anticancer drug doxorubicin. FEBS J. 2021, 288, 6095–6111. [Google Scholar] [CrossRef]
- Carvalho, C.; Santos, R.X.; Cardoso, S.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The good, the bad and the ugly effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Lipscomb, L.A.; Peek, M.E.; Zhou, F.X.; Bertrand, J.A.; VanDerveer, D.; Williams, L.D. Water ring structure at DNA interfaces: Hydration and dynamics of DNA-anthracycline complexes. Biochemistry 1994, 33, 3649–3659. [Google Scholar] [CrossRef] [PubMed]
- Howerton, S.B.; Nagpal, A.; Dean Williams, L. Surprising roles of electrostatic interactions in DNA–ligand complexes. Biopolymers 2003, 69, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Zlatanova, J.; Victor, J.M. How are nucleosomes disrupted during transcription elongation? HFSP J. 2009, 3, 373–378. [Google Scholar] [CrossRef]
- Yang, F.; Kemp, C.J.; Henikoff, S. Doxorubicin enhances nucleosome turnover around promoters. Curr. Biol. 2013, 23, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Salerno, D.; Brogioli, D.; Cassina, V.; Turchi, D.; Beretta, G.L.; Seruggia, D.; Ziano, R.; Zunino, F.; Mantegazza, F. Magnetic tweezers measurements of the nanomechanical properties of DNA in the presence of drugs. Nucleic Acids Res. 2010, 38, 7089–7099. [Google Scholar] [CrossRef][Green Version]
- Yang, F.; Teves, S.S.; Kemp, C.J.; Henikoff, S. Doxorubicin, DNA torsion, and chromatin dynamics. Biochim. Biophys. Acta 2014, 1845, 84–89. [Google Scholar] [CrossRef]
- Hande, K.R. Clinical applications of anticancer drugs targeted to topoisomerase II. Biochim. Biophys. Acta 1998, 1400, 173–184. [Google Scholar] [CrossRef]
- Lemke, K.; Wojciechowski, M.; Laine, W.; Bailly, C.; Colson, P.; Baginski, M.; Larsen, A.K.; Skladanowski, A. Induction of unique structural changes in guanine-rich DNA regions by the triazoloacridone C-1305, a topoisomerase II inhibitor with antitumor activities. Nucleic Acids Res. 2005, 33, 6034–6047. [Google Scholar] [CrossRef]
- Króliczewski, J.; Bartoszewska, S.; Dudkowska, M.; Janiszewska, D.; Biernatowska, A.; Crossman, D.K.; Krzymiński, K.; Wysocka, M.; Romanowska, A.; Baginski, M.; et al. Utilizing Genome-Wide mRNA Profiling to Identify the Cytotoxic Chemotherapeutic Mechanism of Triazoloacridone C-1305 as Direct Microtubule Stabilization. Cancers 2020, 12, 864. [Google Scholar] [CrossRef]
- Kellett, A.; Molphy, Z.; Slator, C.; McKee, V.; Farrell, N.P. Molecular methods for assessment of non-covalent metallodrug-DNA interactions. Chem. Soc. Rev. 2019, 48, 971–988. [Google Scholar] [CrossRef]
- Bauer, G.B.; Povirk, L.F. Specificity and kinetics of interstrand and intrastrand bifunctional alkylation by nitrogen mustards at a G-G-C sequence. Nucleic Acids Res. 1997, 25, 1211–1218. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Lastra, J.M.P.d.l.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Chemical Insights into Oxidative and Nitrative Modifications of DNA. Int. J. Mol. Sci. 2023, 24, 15240. [Google Scholar] [CrossRef] [PubMed]
- David, E.G.; Luminita, M.V. Intercalative Binding of Small Molecules to Nucleic Acids. Curr. Org. Chem. 2000, 4, 915–929. [Google Scholar] [CrossRef]
- Ralhan, R.; Kaur, J. Alkylating agents and cancer therapy. Expert Opin. Ther. Pat. 2007, 17, 1061–1075. [Google Scholar] [CrossRef]
- Guerra, C.F.; Bickelhaupt, F.M. CHAPTER 4-Watson–Crick hydrogen bonds: Nature and role in DNA replication. In Modern Methods for Theoretical Physical Chemistry of Biopolymers; Starikov, E.B., Lewis, J.P., Tanaka, S., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2006; pp. 79–97. [Google Scholar]
- Langkjær, N.; Wengel, J.; Pasternak, A. Watson-Crick hydrogen bonding of unlocked nucleic acids. Bioorg. Med. Chem. Lett. 2015, 25, 5064–5066. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.M.; Ferguson, L.R.; Denny, W.A. DNA and the chromosome-varied targets for chemotherapy. Cell Chromosome 2004, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Bhattacharya, S. Chemistry and biology of DNA-binding small molecules. Curr. Sci. 2012, 102, 212–231. [Google Scholar]
- Highley, M.S.; Landuyt, B.; Prenen, H.; Harper, P.G.; De Bruijn, E.A. The Nitrogen Mustards. Pharmacol. Rev. 2022, 74, 552–599. [Google Scholar] [CrossRef]
- Chen, Y.; Jia, Y.; Song, W.; Zhang, L. Therapeutic Potential of Nitrogen Mustard Based Hybrid Molecules. Front. Pharmacol. 2018, 9, 1453. [Google Scholar] [CrossRef]
- Li, J.J.; Li, J.J. Cancer Drugs: From Nitrogen Mustards to Gleevec. In Laughing Gas, Viagra, and Lipitor: The Human Stories Behind the Drugs We Use; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Ogino, M.H.; Tadi, P. Cyclophosphamide. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
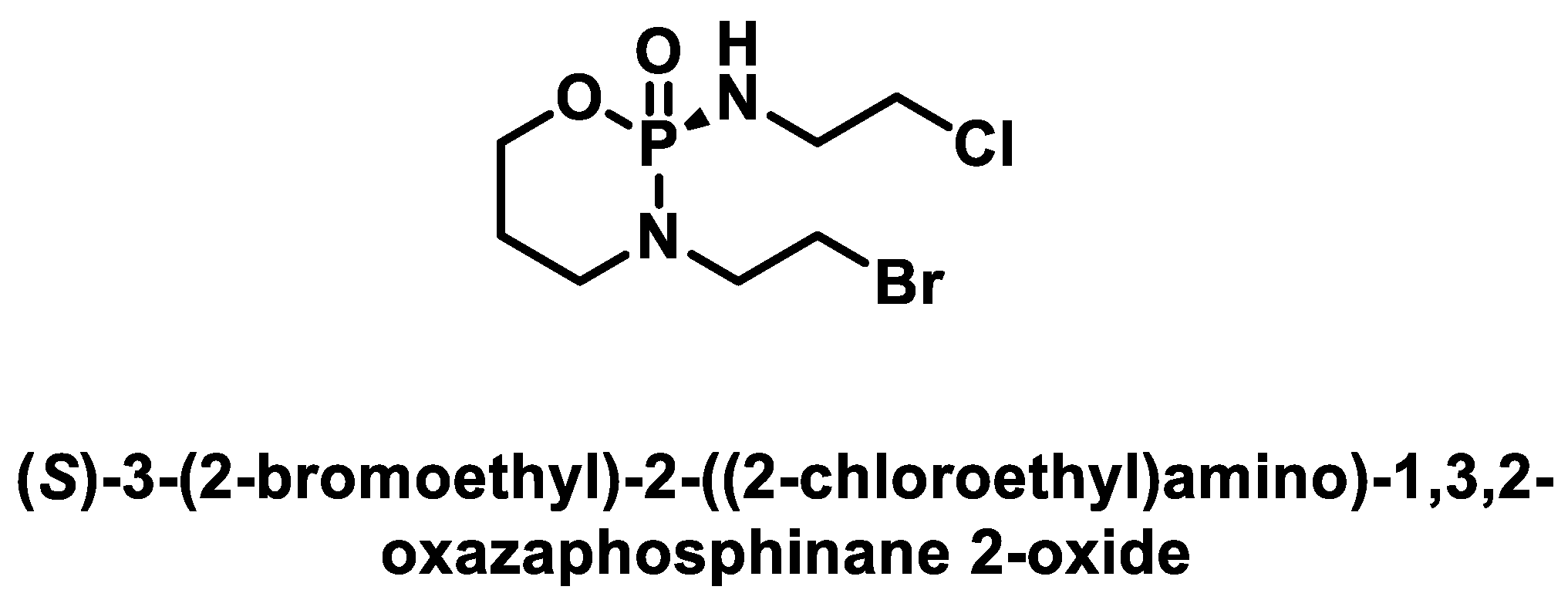
- Idle, J.R.; Beyoğlu, D. Ifosfamide-History, efficacy, toxicity and encephalopathy. Pharmacol. Ther. 2023, 243, 108366. [Google Scholar] [CrossRef]
- Scripture, C.D.; Sparreboom, A.; Figg, W.D. Modulation of cytochrome P450 activity: Implications for cancer therapy. Lancet Oncol. 2005, 6, 780–789. [Google Scholar] [CrossRef]
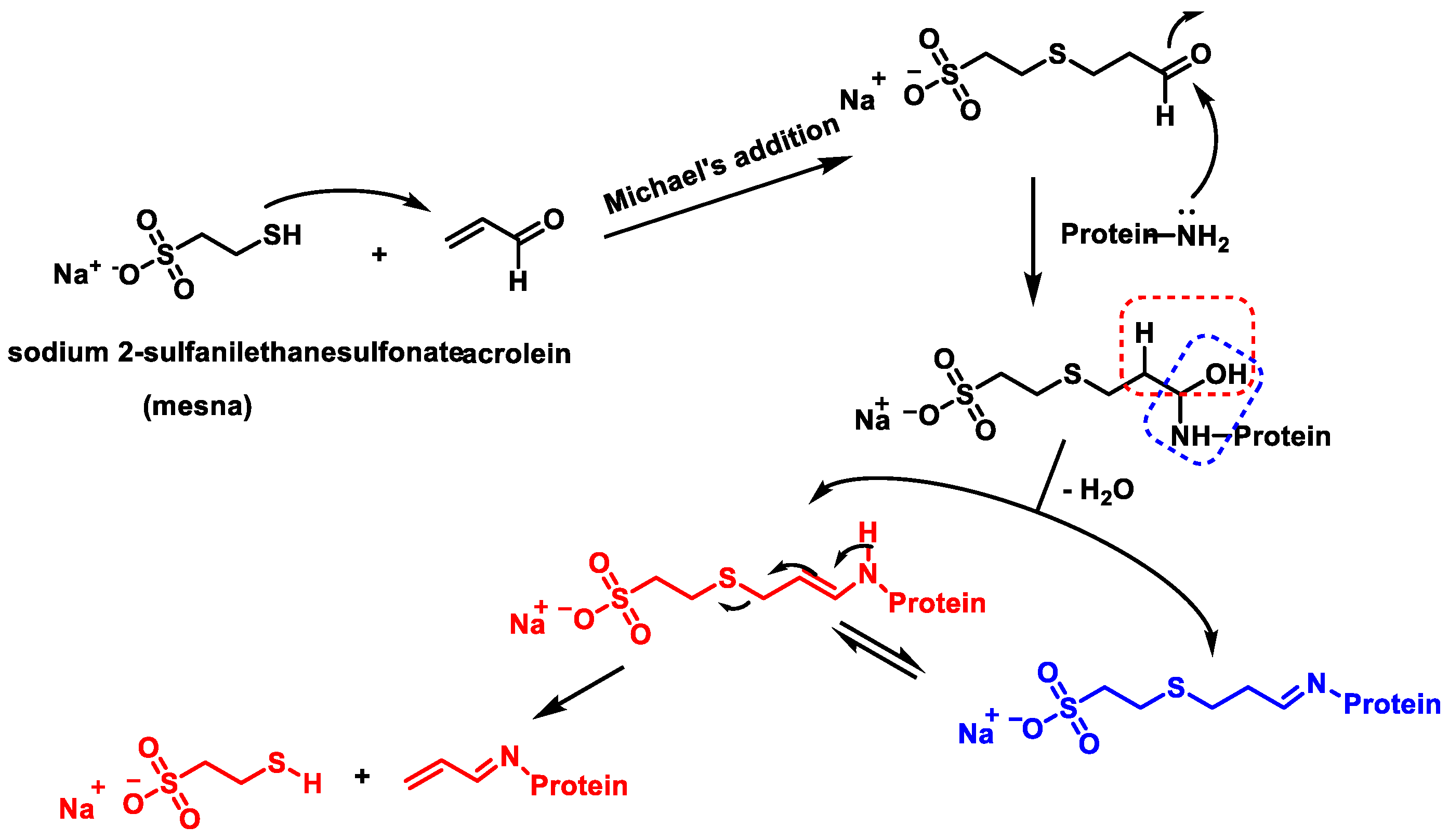
- Burcham, P.C.; Fontaine, F.R.; Kaminskas, L.M.; Petersen, D.R.; Pyke, S.M. Protein adduct-trapping by hydrazinophthalazine drugs: Mechanisms of cytoprotection against acrolein-mediated toxicity. Mol. Pharmacol. 2004, 65, 655–664. [Google Scholar] [CrossRef]
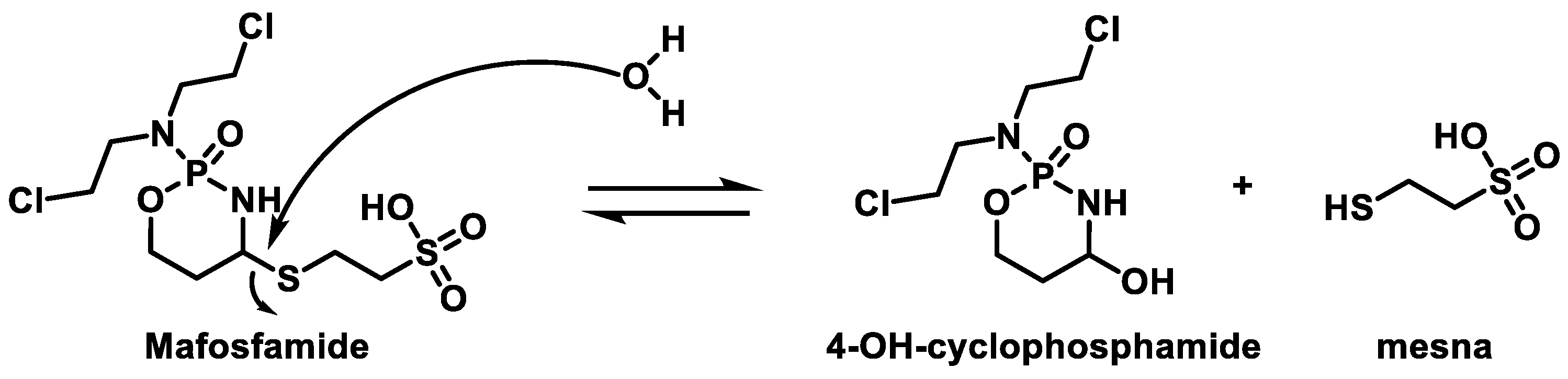
- Martinez-Sanchez, J.; Pascual-Diaz, R.; Palomo, M.; Moreno-Castaño, A.B.; Ventosa, H.; Salas, M.Q.; Rovira, M.; Escolar, G.; Carreras, E.; Diaz-Ricart, M. Mafosfamide, a cyclophosphamide analog, causes a proinflammatory response and increased permeability on endothelial cells in vitro. Bone Marrow Transplant. 2023, 58, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.-Y.; Kwon, C.-H. N3-methyl-mafosfamide as a chemically stable, alternative prodrug of mafosfamide. Bioorganic Med. Chem. Lett. 1998, 8, 1673–1678. [Google Scholar] [CrossRef] [PubMed]
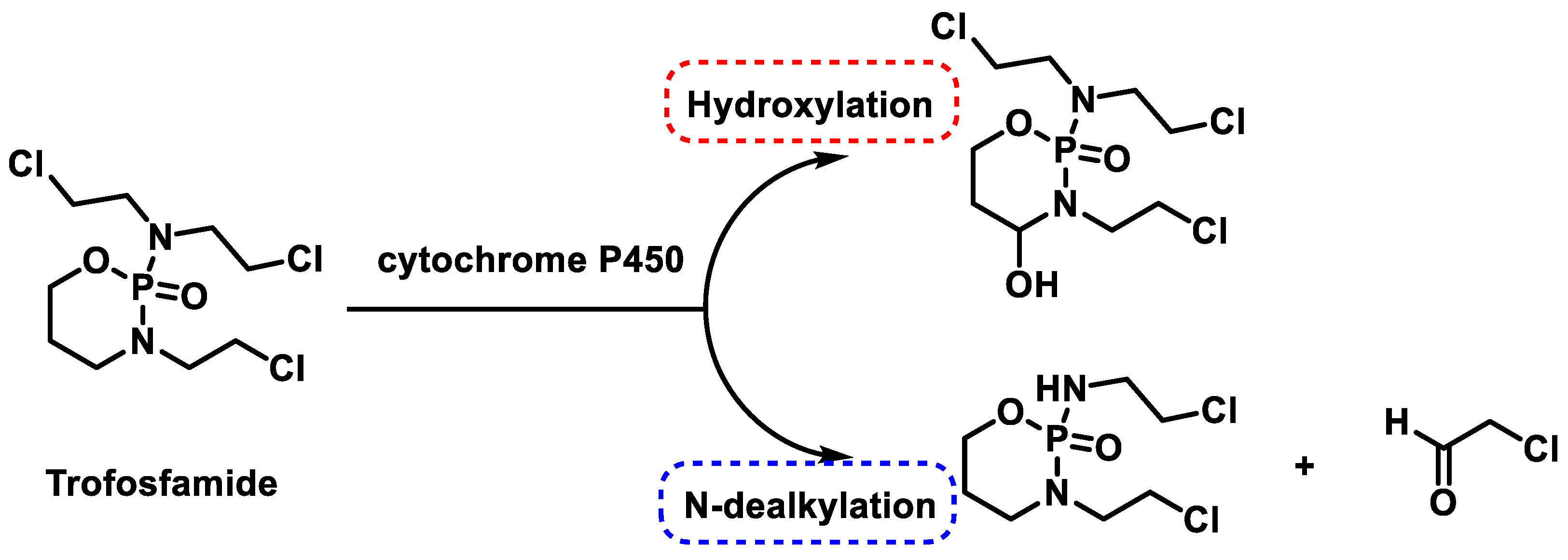
- May-Manke, A.; Kroemer, H.; Hempel, G.; Bohnenstengel, F.; Hohenlöchter, B.; Blaschke, G.; Boos, J. Investigation of the major human hepatic cytochrome P450 involved in 4-hydroxylation and N-dechloroethylation of trofosfamide. Cancer Chemother. Pharmacol. 1999, 44, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Huang, M.; Duan, W.; Yu, X.Q.; Zhou, S. Design of new oxazaphosphorine anticancer drugs. Curr. Pharm. Des. 2007, 13, 963–978. [Google Scholar] [CrossRef]
- O’Byrne, K.; Steward, W.P. The role of chemotherapy in the treatment of adult soft tissue sarcomas. Oncology 1999, 56, 13–23. [Google Scholar] [CrossRef]
- Advani, S.H. The role of ifosfamide in paediatric cancer. Aust. N. Z. J. Med. 1998, 28, 410–413. [Google Scholar] [CrossRef]
- Dayyani, F.; Macarulla, T.; Johnson, A.; Wainberg, Z.A. Second-line treatment options for patients with metastatic pancreatic ductal adenocarcinoma: A systematic literature review. Cancer Treat. Rev. 2023, 113, 102502. [Google Scholar] [CrossRef]
- Diethelm-Varela, B.; Ai, Y.; Liang, D.; Xue, F. Nitrogen Mustards as Anticancer Chemotherapies: Historic Perspective, Current Developments and Future Trends. Curr. Top. Med. Chem. 2019, 19, 691–712. [Google Scholar] [CrossRef]
- Summerfield, G.P.; Taylor, P.R.; Mounter, P.J.; Proctor, S.J. High-dose chlorambucil for the treatment of chronic lymphocytic leukaemia and low-grade non-Hodgkin’s lymphoma. Br. J. Haematol. 2002, 116, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Lalic, H.; Aurer, I.; Batinic, D.; Visnjic, D.; Smoljo, T.; Babic, A. Bendamustine: A review of pharmacology, clinical use and immunological effects (Review). Oncol. Rep. 2022, 47, 114. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Kumar, S.; Prasad, D.N.; Bhardwaj, T.R. Therapeutic journery of nitrogen mustard as alkylating anticancer agents: Historic to future perspectives. Eur. J. Med. Chem. 2018, 151, 401–433. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Debnath, C.; Bérubé, G. Steroid-linked nitrogen mustards as potential anticancer therapeutics: A review. J. Steroid Biochem. Mol. Biol. 2013, 137, 271–300. [Google Scholar] [CrossRef] [PubMed]
- Scholar, E. Estramustine. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2007; pp. 1–5. [Google Scholar]
- Bastholt, L.; Johansson, C.J.; Pfeiffer, P.; Svensson, L.; Johansson, S.A.; Gunnarsson, P.O.; Mouridsen, H. A pharmacokinetic study of prednimustine as compared with prednisolone plus chlorambucil in cancer patients. Cancer Chemother. Pharmacol. 1991, 28, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Zolottsev, V.A.; Latysheva, A.S.; Pokrovsky, V.S.; Khan, I.I.; Misharin, A.Y. Promising applications of steroid conjugates for cancer research and treatment. Eur. J. Med. Chem. 2021, 210, 113089. [Google Scholar] [CrossRef]
- Trafalis, D.T.P. Hybrid aza-steroid alkylators in the treatment of colon cancer. Cancer Lett. 2006, 243, 202–210. [Google Scholar] [CrossRef]
- Trafalis, D.; Geromichalou, E.; Dalezis, P.; Nikoleousakos, N.; Sarli, V. Synthesis and evaluation of new steroidal lactam conjugates with aniline mustards as potential antileukemic therapeutics. Steroids 2016, 115, 1–8. [Google Scholar] [CrossRef]
- Wickström, M.; Nygren, P.; Larsson, R.; Harmenberg, J.; Lindberg, J.; Sjöberg, P.; Jerling, M.; Lehmann, F.; Richardson, P.; Anderson, K.; et al. Melflufen—A peptidase-potentiated alkylating agent in clinical trials. Oncotarget 2017, 8, 66641–66655. [Google Scholar] [CrossRef]
- Mateos, M.V.; Bladé, J.; Bringhen, S.; Ocio, E.M.; Efebera, Y.; Pour, L.; Gay, F.; Sonneveld, P.; Gullbo, J.; Richardson, P.G. Melflufen: A Peptide-Drug Conjugate for the Treatment of Multiple Myeloma. J. Clin. Med. 2020, 9, 3120. [Google Scholar] [CrossRef]
- Lehmann, F.; Wennerberg, J. Evolution of Nitrogen-Based Alkylating Anticancer Agents. Processes 2021, 9, 377. [Google Scholar] [CrossRef]
- Olivier, T.; Prasad, V. The approval and withdrawal of melphalan flufenamide (melflufen): Implications for the state of the FDA. Transl. Oncol. 2022, 18, 101374. [Google Scholar] [CrossRef] [PubMed]
- Persmark, M.; Guengerich, F.P. Spectroscopic and thermodynamic characterization of the interaction of N7-guanyl thioether derivatives of d(TGCTG*CAAG) with potential complements. Biochemistry 1994, 33, 14368. [Google Scholar] [CrossRef]
- Ryan, B.J.; Yang, H.; Bacurio, J.H.T.; Smith, M.R.; Basu, A.K.; Greenberg, M.M.; Freudenthal, B.D. Structural Dynamics of a Common Mutagenic Oxidative DNA Lesion in Duplex DNA and during DNA Replication. J. Am. Chem. Soc. 2022, 144, 8054–8065. [Google Scholar] [CrossRef]
- Burgos-Morón, E.; Pastor, N.; Orta, M.L.; Jiménez-Alonso, J.J.; Palo-Nieto, C.; Vega-Holm, M.; Vega-Pérez, J.M.; Iglesias-Guerra, F.; Mateos, S.; López-Lázaro, M.; et al. In Vitro Anticancer Activity and Mechanism of Action of an Aziridinyl Galactopyranoside. Biomedicines 2022, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Eder, J.P.; Elias, A.; Shea, T.C.; Schryber, S.M.; Teicher, B.A.; Hunt, M.; Burke, J.; Siegel, R.; Schnipper, L.; Frei, E., 3rd. A phase I-II study of cyclophosphamide, thiotepa, and carboplatin with autologous bone marrow transplantation in solid tumor patients. J. Clin. Oncol. 1990, 8, 1239–1245. [Google Scholar] [CrossRef]
- Langer, C.J.; Nash, S.; Catalano, R.; Rosenblum, N.G.; Hogan, W.M.; Comis, R.L.; O’Dwyer, P.J. Phase II trial of thio-TEPA in relapsed and refractory ovarian carcinoma. Gynecol. Oncol. 1991, 43, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Teicher, B.A.; Herman, T.S.; Holden, S.A.; Epelbaum, R.; Liu, S.-d.; Frei, E., III. Lonidamine as a modulator of alkylating agent activity in vitro and in vivo. Cancer Res. 1991, 51, 780–784. [Google Scholar]
- Miller, B.; Tenenholz, T.; Egorin, M.J.; Sosnovsky, G.; Rao, N.U.M.; Gutierrez, P.L. Cellular pharmacology of N, N′, N ″-triethylene thiophosphoramide. Cancer Lett. 1988, 41, 157–168. [Google Scholar] [CrossRef]
- MILLER, D.G. ALKYLATING AGENTS AND HUMAN SPERMATOGENESIS. Obstet. Gynecol. Surv. 1972, 27, 150–151. [Google Scholar] [CrossRef]
- Van Maanen, M.; Smeets, C.; Beijnen, J. Chemistry, pharmacology and pharmacokinetics of N, N′, N′′-triethylenethiophosphoramide (ThioTEPA). Cancer Treat. Rev. 2000, 26, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Beall, H.D.; Winski, S. Mechanisms of action of quinone-containing alkylating agents. I: NQO1-directed drug development. Front. Biosci. 2000, 5, D639–D648. [Google Scholar] [PubMed]
- Begleiter, A. Clinical applications of quinone-containing alkylating agents. Front. Biosci. 2000, 5, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Bass, P.D.; Gubler, D.A.; Judd, T.C.; Williams, R.M. Mitomycinoid alkaloids: Mechanism of action, biosynthesis, total syntheses, and synthetic approaches. Chem. Rev. 2013, 113, 6816–6863. [Google Scholar] [CrossRef] [PubMed]
- Puyo, S.; Montaudon, D.; Pourquier, P. From old alkylating agents to new minor groove binders. Crit. Rev. Oncol./Hematol. 2014, 89, 43–61. [Google Scholar] [CrossRef] [PubMed]
- DeVita, V.T.; Lawrence, T.S.; Rosenberg, S.A. DeVita, Hellman, and Rosenberg’s Cancer: Principles & Practice of Oncology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; Volume 2. [Google Scholar]
- Haffty, B.G.; Son, Y.H.; Wilson, L.D.; Papac, R.; Fischer, D.; Rockwell, S.; Sartorelli, A.C.; Ross, D.; Sasaki, C.T.; Fischer, J.J. Bioreductive alkylating agent porfiromycin in combination with radiation therapy for the management of squamous cell carcinoma of the head and neck. Radiat. Oncol. Investig. Clin. Basic Res. 1997, 5, 235–245. [Google Scholar] [CrossRef]
- Wolkenberg, S.E.; Boger, D.L. Mechanisms of in situ activation for DNA-targeting antitumor agents. Chem. Rev. 2002, 102, 2477–2496. [Google Scholar] [CrossRef]
- Paz, M.M.; Pritsos, C.A. Chapter Seven-The Molecular Toxicology of Mitomycin C. In Advances in Molecular Toxicology; Fishbein, J.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 6, pp. 243–299. [Google Scholar]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Bustamante Munguira, E.; Andrés Juan, C.; Pérez-Lebeña, E. Michael Acceptors as Anti-Cancer Compounds: Coincidence or Causality? Int. J. Mol. Sci. 2024, 25, 6099. [Google Scholar] [CrossRef]
- Tomasz, M.; Palom, Y. The mitomycin bioreductive antitumor agents: Cross-linking and alkylation of DNA as the molecular basis of their activity. Pharmacol. Ther. 1997, 76, 73–87. [Google Scholar] [CrossRef]
- Tomasz, M.; Chawla, A.K.; Lipman, R. Mechanism of monofunctional and bifunctional alkylation of DNA by mitomycin C. Biochemistry 1988, 27, 3182–3187. [Google Scholar] [CrossRef]
- Eagan, R.T.; Dinapoli, R.P.; Cascino, T.L.; Scheithauer, B.; O’Neill, B.P.; O’Fallon, J.R. Comprehensive phase II evaluation of aziridinylbenzoquinone (AZQ, diaziquone) in recurrent human primary brain tumors. J. Neuro-Oncol. 1987, 5, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Obe, G.; Beek, B. Trenimon: Biochemical, physiological and genetic effects on cells and organisms. Mutat. Res. 1979, 65, 21–70. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.M.; Hendriks, H.R.; Peters, G.J. EO9 (Apaziquone): From the clinic to the laboratory and back again. Br. J. Pharmacol. 2013, 168, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Pierce, S.E.; Guziec, L.J.; Guziec, F.S., Jr.; Brodbelt, J.S. Characterization of Aziridinylbenzoquinone DNA Cross-Links by Liquid Chromatography− Infrared Multiphoton Dissociation− Mass Spectrometry. Chem. Res. Toxicol. 2010, 23, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, R.; Hartley, J.A.; Butler, J. Mechanisms of action of quinone-containing alkylating agents: DNA alkylation by aziridinylquinones. Front. Biosci. 2000, 5, E172–E180. [Google Scholar] [CrossRef]
- Naylor, M.; Thomson, P. Recent advances in bioreductive drug targeting. Mini Rev. Med. Chem. 2001, 1, 17–29. [Google Scholar] [CrossRef]
- Fourie, J.; Guziec, F., Jr.; Guziec, L.; Monterrosa, C.; Fiterman, D.J.; Begleiter, A. Structure-activity study with bioreductive benzoquinone alkylating agents: Effects on DT-diaphorase-mediated DNA crosslink and strand break formation in relation to mechanisms of cytotoxicity. Cancer Chemother. Pharmacol. 2004, 53, 191–203. [Google Scholar] [CrossRef]
- Danson, S.; Ward, T.H.; Butler, J.; Ranson, M. DT-diaphorase: A target for new anticancer drugs. Cancer Treat. Rev. 2004, 30, 437–449. [Google Scholar] [CrossRef]
- Denny, W.A. Prodrug strategies in cancer therapy. Eur. J. Med. Chem. 2001, 36, 577–595. [Google Scholar] [CrossRef]
- Zhou, R.; Skibo, E.B. Chemistry of the pyrrolo [1, 2-a] benzimidazole antitumor agents: Influence of the 7-substituent on the ability to alkylate DNA and inhibit topoisomerase II. J. Med. Chem. 1996, 39, 4321–4331. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gutierrez, P.L.; Amstad, P.; Blough, N.V. Hydroxyl radical production by mouse epidermal cell lines in the presence of quinone anti-cancer compounds. Chem. Res. Toxicol. 1999, 12, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Hartley, J.; O’hare, C.; Baumgart, J. DNA alkylation and interstrand cross-linking by treosulfan. Br. J. Cancer 1999, 79, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, J.; Barna, K.; Uher, F.; Milosevits, J.; Pálóczi, K.; Gaál, D.; Petrányi, G.; Kelemen, E. Comparison of the lymphoid toxicities of mitobronitol and busulphan in mice: Reduced B cell toxicity and improved thymic recovery as possible contributors to the reduced risk for complications following BMT with mitobronitol preconditioning. Leukemia 1997, 11, 1769–1774. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schepartz, S.A. Early history and development of the nitrosoureas. Cancer Treat. Rep. 1976, 60, 647. [Google Scholar] [PubMed]
- Kohn, K.W. Interstrand cross-linking of DNA by 1, 3-bis (2-chloroethyl)-1-nitrosourea and other 1-(2-haloethyl)-1-nitrosoureas. Cancer Res. 1977, 37, 1450–1454. [Google Scholar] [PubMed]
- Panasci, L.C.; Green, D.; Nagourney, R.; Fox, P.; Schein, P.S. A structure-activity analysis of chemical and biological parameters of chloroethylnitrosoureas in mice. Cancer Res. 1977, 37, 2615–2618. [Google Scholar] [PubMed]
- Samson, M.; Baker, L.; Cummings, G.; Talley, R.; McDonald, B.; Bhathena, D. Clinical trial of chlorozotocin, DTIC, and dactinomycin in metastatic malignant melanoma. Cancer Treat. Rep. 1982, 66, 371–373. [Google Scholar]
- Montgomery, J.A.; James, R.; McCaleb, G.S.; Johnston, T.P. The modes of decomposition of 1, 3-bis (2-chloroethyl)-1-nitrosourea and related compounds. J. Med. Chem. 1967, 10, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Buggia, I.; Locatelli, F.; Regazzi, M.B.; Zecca, M. Busulfan. Ann. Pharmacother. 1994, 28, 1055–1062. [Google Scholar] [CrossRef]
- Krivoy, N.; Hoffer, E.; Lurie, Y.; Bentur, Y.; Rowe, J.M. Busulfan use in hematopoietic stem cell transplantation: Pharmacology, dose adjustment, safety and efficacy in adults and children. Curr. Drug Saf. 2008, 3, 60–66. [Google Scholar] [CrossRef]
- Iwamoto, T.; Hiraku, Y.; Oikawa, S.; Mizutani, H.; Kojima, M.; Kawanishi, S. DNA intrastrand cross-link at the 5′-GA-3′ sequence formed by busulfan and its role in the cytotoxic effect. Cancer Sci. 2004, 95, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.L.; Kawedia, J.D.; Champlin, R.E.; Kramer, M.A.; Nieto, Y.; Ghose, R.; Andersson, B.S. Clarifying busulfan metabolism and drug interactions to support new therapeutic drug monitoring strategies: A comprehensive review. Expert Opin. Drug Metab. Toxicol. 2017, 13, 901–923. [Google Scholar] [CrossRef] [PubMed]
- Farmer, P. Metabolism and reactions of alkylating agents. Pharmacol. Ther. 1987, 35, 301–358. [Google Scholar] [CrossRef] [PubMed]
- Bedford, P.; Fox, B.W. DNA-DNA interstrand crosslinking by dimethanesulphonic acid esters: Correlation with cytotoxicity and antitumour activity in the yoshida lymphosarcoma model and relationship to chain length. Biochem. Pharmacol. 1983, 32, 2297–2301. [Google Scholar] [CrossRef]
- Serrone, L.; Zeuli, M.; Sega, F.M.; Cognetti, F. Dacarbazine-based chemotherapy for metastatic melanoma: Thirty-year experience overview. J. Exp. Clin. Cancer Res. 2000, 19, 21–34. [Google Scholar]
- Reid, J.M.; Kuffel, M.J.; Miller, J.K.; Rios, R.; Ames, M.M. Metabolic activation of dacarbazine by human cytochromes P450: The role of CYP1A1, CYP1A2, and CYP2E1. Clin. Cancer Res. 1999, 5, 2192–2197. [Google Scholar]
- Nussbaumer, S.; Bonnabry, P.; Veuthey, J.L.; Fleury-Souverain, S. Analysis of anticancer drugs: A review. Talanta 2011, 85, 2265–2289. [Google Scholar] [CrossRef]
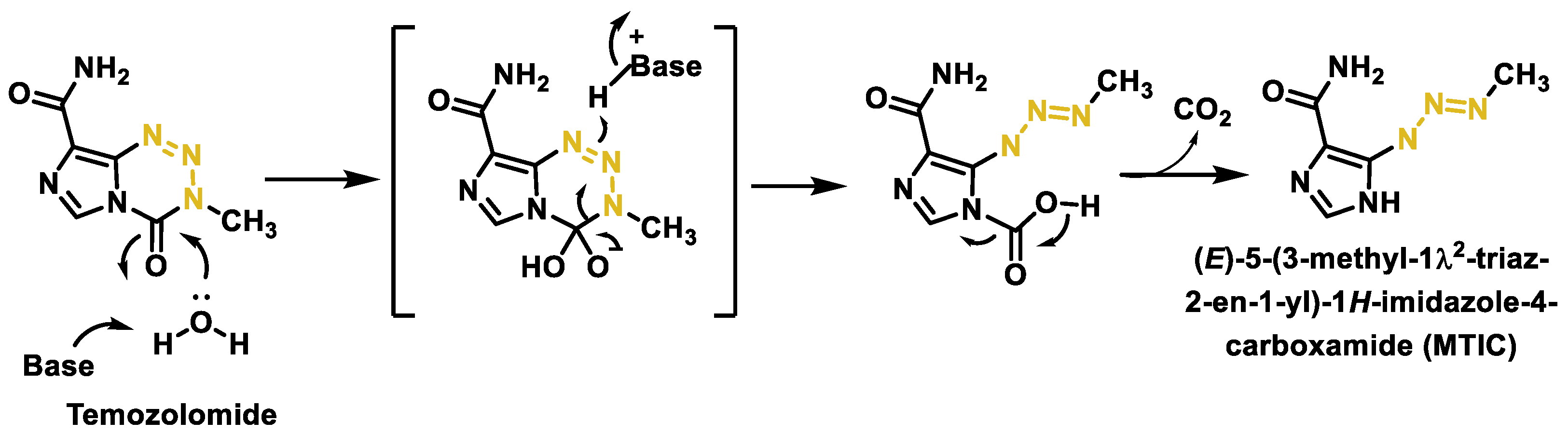
- Moody, C.L.; Wheelhouse, R.T. The medicinal chemistry of imidazotetrazine prodrugs. Pharmaceuticals 2014, 7, 797–838. [Google Scholar] [CrossRef] [PubMed]
- García-del-Muro, X.; López-Pousa, A.; Maurel, J.; Martín, J.; Martínez-Trufero, J.; Casado, A.; Gómez-España, A.; Fra, J.; Cruz, J.; Poveda, A. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: A Spanish Group for Research on Sarcomas study. J. Clin. Oncol. 2011, 29, 2528–2533. [Google Scholar] [CrossRef]
- Bouraoui, S.; Brahem, A.; Tabka, F.; Mrizek, N.; Saad, A.; Elghezal, H. Assessment of chromosomal aberrations, micronuclei and proliferation rate index in peripheral lymphocytes from Tunisian nurses handling cytotoxic drugs. Environ. Toxicol. Pharmacol. 2011, 31, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Meer, L.; Janzer, R.C.; Kleihues, P.; Kolar, G.F. In vivo metabolism and reaction with DNA of the cytostatic agent, 5-(3,3-dimethyl-1-triazeno)imidazole-4-carboxamide (DTIC). Biochem. Pharmacol. 1986, 35, 3243–3247. [Google Scholar] [CrossRef] [PubMed]
- Kyrtopoulos, S.A.; Souliotis, V.L.; Valavanis, C.; Boussiotis, V.A.; Pangalis, G.A. Accumulation of O6-methylguanine in human DNA after therapeutic exposure to methylating agents and its relationship with biological effects. Environ. Health Perspect. 1993, 99, 143–147. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Horton, J.K.; Stevens, M.F. A new light on the photo-decomposition of the antitumour drug DTIC. J. Pharm. Pharmacol. 1981, 33, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T.; Hiraku, Y.; Okuda, M.; Kawanishi, S. Mechanism of UVA-dependent DNA damage induced by an antitumor drug dacarbazine in relation to its photogenotoxicity. Pharm. Res. 2008, 25, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Newlands, E.S.; Stevens, M.F.; Wedge, S.R.; Wheelhouse, R.T.; Brock, C. Temozolomide: A review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat. Rev. 1997, 23, 35–61. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.D.; Wirth, M.; Statkevich, P.; Reidenberg, P.; Alton, K.; Sartorius, S.E.; Dugan, M.; Cutler, D.; Batra, V.; Grochow, L.B.; et al. Absorption, metabolism, and excretion of 14C-temozolomide following oral administration to patients with advanced cancer. Clin. Cancer Res. 1999, 5, 309–317. [Google Scholar] [PubMed]
- Turci, R.; Sottani, C.; Spagnoli, G.; Minoia, C. Biological and environmental monitoring of hospital personnel exposed to antineoplastic agents: A review of analytical methods. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003, 789, 169–209. [Google Scholar] [CrossRef]
- Ioannou-Ttofa, L.; Fatta-Kassinos, D. Cytostatic drug residues in wastewater treatment plants: Sources, removal efficiencies and current challenges. In Fate and Effects of Anticancer Drugs in the Environment; Springer: Cham, Switzerland, 2020; pp. 103–138. [Google Scholar]
- Li, D.; Chen, H.; Liu, H.; Schlenk, D.; Mu, J.; Lacorte, S.; Ying, G.-G.; Xie, L. Anticancer drugs in the aquatic ecosystem: Environmental occurrence, ecotoxicological effect and risk assessment. Environ. Int. 2021, 153, 106543. [Google Scholar] [CrossRef]
- Tripathi, A.K.; David, A.; Govil, T.; Rauniyar, S.; Rathinam, N.K.; Goh, K.M.; Sani, R.K. Environmental Remediation of Antineoplastic Drugs: Present Status, Challenges, and Future Directions. Processes 2020, 8, 747. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Borrelli, A.; Buonaguro, L.; Buonaguro, F.M.; Tornesello, M.L. Antimicrobial peptides as anticancer agents: Functional properties and biological activities. Molecules 2020, 25, 2850. [Google Scholar] [CrossRef]
- Mader, J.S.; Hoskin, D.W. Cationic antimicrobial peptides as novel cytotoxic agents for cancer treatment. Expert Opin. Investig. Drugs 2006, 15, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Roudi, R.; Syn, N.L.; Roudbary, M. Antimicrobial peptides as biologic and immunotherapeutic agents against cancer: A comprehensive overview. Front. Immunol. 2017, 8, 1320. [Google Scholar] [CrossRef] [PubMed]
- Deslouches, B.; Di, Y.P. Antimicrobial peptides with selective antitumor mechanisms: Prospect for anticancer applications. Oncotarget 2017, 8, 46635. [Google Scholar] [CrossRef] [PubMed]
- Erdem Büyükkiraz, M.; Kesmen, Z. Antimicrobial peptides (AMPs): A promising class of antimicrobial compounds. J. Appl. Microbiol. 2022, 132, 1573–1596. [Google Scholar]
- Wang, G.S. Antimicrobial Peptides: Discovery, Design and Novel Therapeutic Strategies; Cabi: Wallingford, UK, 2017. [Google Scholar]
- Narayana, J.L.; Chen, J.-Y. Antimicrobial peptides: Possible anti-infective agents. Peptides 2015, 72, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Y.; Xue, Z.; Jia, Y.; Li, R.; He, C.; Chen, H. The structure-mechanism relationship and mode of actions of antimicrobial peptides: A review. Trends Food Sci. Technol. 2021, 109, 103–115. [Google Scholar]
- Decker, A.P.; Mechesso, A.F.; Wang, G. Expanding the landscape of amino acid-rich antimicrobial peptides: Definition, deployment in nature, implications for peptide design and therapeutic potential. Int. J. Mol. Sci. 2022, 23, 12874. [Google Scholar] [CrossRef] [PubMed]
- Luong, H.X.; Thanh, T.T.; Tran, T.H. Antimicrobial peptides–Advances in development of therapeutic applications. Life Sci. 2020, 260, 118407. [Google Scholar]
- Ramos-Martín, F.; Herrera-León, C.; d’Amelio, N. Molecular basis of the anticancer, apoptotic and antibacterial activities of Bombyx mori Cecropin A. Arch. Biochem. Biophys. 2022, 715, 109095. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Gomes, I.; da Lima, A.B.; da Silva Brito, D.M.; Almeida Lima, A.; de Oliveira, F.L.; Espino Zelaya, E.A.; Magalhães Rebello Alencar, L.; Castelo Branco de Souza Collares Maia, D.; Amaral de Moraes, M.E.; Pantoja Mesquita, F. Recalculating the Route: Repositioning Antimicrobial Peptides for Cancer Treatment. Chem. Biodivers. 2024, 21, e202301840. [Google Scholar] [CrossRef]
- Tolos, A.M.; Moisa, C.; Dochia, M.; Popa, C.; Copolovici, L.; Copolovici, D.M. Anticancer potential of antimicrobial peptides: Focus on buforins. Polymers 2024, 16, 728. [Google Scholar] [CrossRef] [PubMed]
- Kanaujia, K.A.; Mishra, N.; Rajinikanth, P.; Saraf, S.A. Antimicrobial peptides as antimicrobials for wound care management: A comprehensive review. J. Drug Deliv. Sci. Technol. 2024, 95, 105570. [Google Scholar] [CrossRef]
- van den Boogaard, W.M.; Komninos, D.S.; Vermeij, W.P. Chemotherapy side-effects: Not all DNA damage is equal. Cancers 2022, 14, 627. [Google Scholar] [CrossRef] [PubMed]
- Toribio, M.; Campos, E.L.; Monteiro, G.J.; Henriques, J.C.G.; Gonzaga, W.J.C.; Lorenzon, E.N.; Miranda, C.S.S. The combination of peptides and cisplatin for the treatment of oral cancer. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2024, 137, e308. [Google Scholar] [CrossRef]
- Khine, H.E.E.; Ecoy, G.A.U.; Roytrakul, S.; Phaonakrop, N.; Pornputtapong, N.; Prompetchara, E.; Chanvorachote, P.; Chaotham, C. Chemosensitizing activity of peptide from Lentinus squarrosulus (Mont.) on cisplatin-induced apoptosis in human lung cancer cells. Sci. Rep. 2021, 11, 4060. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, R.; Dey, T.; Kumar, L.; Kar, S.; Sarkar, R.; Ghorai, M.; Malik, S.; Jha, N.K.; Vellingiri, B.; Kesari, K.K. Cellular landscaping of cisplatin resistance in cervical cancer. Biomed. Pharmacother. 2022, 153, 113345. [Google Scholar] [CrossRef]
- Aghamiri, S.; Zandsalimi, F.; Raee, P.; Abdollahifar, M.-A.; Tan, S.C.; Low, T.Y.; Najafi, S.; Ashrafizadeh, M.; Zarrabi, A.; Ghanbarian, H. Antimicrobial peptides as potential therapeutics for breast cancer. Pharmacol. Res. 2021, 171, 105777. [Google Scholar] [CrossRef]
- Teng, Q.-X.; Luo, X.; Lei, Z.-N.; Wang, J.-Q.; Wurpel, J.; Qin, Z.; Yang, D.-H. The multidrug resistance-reversing activity of a novel antimicrobial peptide. Cancers 2020, 12, 1963. [Google Scholar] [CrossRef]
- Jafari, A.; Babajani, A.; Sarrami Forooshani, R.; Yazdani, M.; Rezaei-Tavirani, M. Clinical applications and anticancer effects of antimicrobial peptides: From bench to bedside. Front. Oncol. 2022, 12, 819563. [Google Scholar] [CrossRef]
- Seyfi, R.; Kahaki, F.A.; Ebrahimi, T.; Montazersaheb, S.; Eyvazi, S.; Babaeipour, V.; Tarhriz, V. Antimicrobial peptides (AMPs): Roles, functions and mechanism of action. Int. J. Pept. Res. Ther. 2020, 26, 1451–1463. [Google Scholar] [CrossRef]
- Aria, H.; Rezaei, M. Immunogenic cell death inducer peptides: A new approach for cancer therapy, current status and future perspectives. Biomed. Pharmacother. 2023, 161, 114503. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Vishwanatha, J.K. Role of anti-cancer peptides as immunomodulatory agents: Potential and design strategy. Pharmaceutics 2022, 14, 2686. [Google Scholar] [CrossRef] [PubMed]
- Kardani, K.; Bolhassani, A. Antimicrobial/anticancer peptides: Bioactive molecules and therapeutic agents. Immunotherapy 2021, 13, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Al-Ostoot, F.H.; Salah, S.; Khamees, H.A.; Khanum, S.A. Tumor angiogenesis: Current challenges and therapeutic opportunities. Cancer Treat. Res. Commun. 2021, 28, 100422. [Google Scholar] [CrossRef]
- Hu, Q.; Chen, C.; Lin, Z.; Zhang, L.; Guan, S.; Zhuang, X.; Dong, G.; Shen, J. The antimicrobial peptide Esculentin-1a (1–21) NH2 stimulates wound healing by promoting angiogenesis through the PI3K/AKT pathway. Biol. Pharm. Bull. 2023, 46, 382–393. [Google Scholar] [CrossRef]
- Kang, M.J.; Roh, K.-H.; Lee, J.S.; Lee, J.H.; Park, S.; Lim, D.W. Vascular Endothelial Growth Factor Receptor 1 Targeting Fusion Polypeptides with Stimuli-Responsiveness for Anti-angiogenesis. ACS Appl. Mater. Interfaces 2023, 15, 32201–32214. [Google Scholar] [CrossRef]
- Del Genio, V.; Bellavita, R.; Falanga, A.; Hervé-Aubert, K.; Chourpa, I.; Galdiero, S. Peptides to overcome the limitations of current anticancer and antimicrobial nanotherapies. Pharmaceutics 2022, 14, 1235. [Google Scholar] [CrossRef]
- Basak, D.; Arrighi, S.; Darwiche, Y.; Deb, S. Comparison of anticancer drug toxicities: Paradigm shift in adverse effect profile. Life 2021, 12, 48. [Google Scholar] [CrossRef]
- Szlasa, W.; Zendran, I.; Zalesińska, A.; Tarek, M.; Kulbacka, J. Lipid composition of the cancer cell membrane. J. Bioenerg. Biomembr. 2020, 52, 321–342. [Google Scholar] [CrossRef]
- Vedadghavami, A.; Zhang, C.; Bajpayee, A.G. Overcoming negatively charged tissue barriers: Drug delivery using cationic peptides and proteins. Nano Today 2020, 34, 100898. [Google Scholar] [CrossRef]
- Divyashree, M.; Mani, M.K.; Reddy, D.; Kumavath, R.; Ghosh, P.; Azevedo, V.; Barh, D. Clinical applications of antimicrobial peptides (AMPs): Where do we stand now? Protein Pept. Lett. 2020, 27, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Chernov, A.N.; Kim, A.V.; Skliar, S.S.; Fedorov, E.V.; Tsapieva, A.N.; Filatenkova, T.A.; Chutko, A.L.; Matsko, M.V.; Galimova, E.S.; Shamova, O.V. Expression of molecular markers and synergistic anticancer effects of chemotherapy with antimicrobial peptides on glioblastoma cells. Cancer Chemother. Pharmacol. 2024, 93, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Jana, A.; Narula, P.; Chugh, A.; Kulshreshtha, R. Efficient delivery of anti-miR-210 using Tachyplesin, a cell penetrating peptide, for glioblastoma treatment. Int. J. Pharm. 2019, 572, 118789. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, X.; Zhang, J.; Chen, J.; Wang, Y.; Wei, T.; Ma, J.; Li, Y.; Mo, T.; He, Z.; et al. Tachyplesin induces apoptosis in non-small cell lung cancer cells and enhances the chemosensitivity of A549/DDP cells to cisplatin by activating Fas and necroptosis pathway. Chem. Biol. Drug Des. 2021, 97, 809–820. [Google Scholar] [CrossRef]
- Lin, M.-C.; Lin, S.-B.; Chen, J.-C.; Hui, C.-F.; Chen, J.-Y. Shrimp anti-lipopolysaccharide factor peptide enhances the antitumor activity of cisplatin in vitro and inhibits HeLa cells growth in nude mice. Peptides 2010, 31, 1019–1025. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Q.; Jiang, Y.; Xu, L.; Wei, N.; Lu, C.; Chang, C.; Song, D.; Wang, Y.; Wu, L.; et al. A novel anti-angiogenesis peptide in combination with cisplatin self-assembling into tube-like nanomedicine for oral treatment of gastric cancer. Chem. Eng. J. 2024, 496, 154169. [Google Scholar] [CrossRef]
- Luo, X.; Teng, Q.-X.; Dong, J.-Y.; Yang, D.-H.; Wang, M.; Dessie, W.; Qin, J.-J.; Lei, Z.-N.; Wang, J.-Q.; Qin, Z. Antimicrobial peptide reverses ABCB1-mediated chemotherapeutic drug resistance. Front. Pharmacol. 2020, 11, 1208. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Carbone, C.; Sousa, M.C.; Espina, M.; Garcia, M.L.; Sanchez-Lopez, E.; Souto, E.B. Nanomedicines for the delivery of antimicrobial peptides (AMPs). Nanomaterials 2020, 10, 560. [Google Scholar] [CrossRef]
- Parchebafi, A.; Tamanaee, F.; Ehteram, H.; Ahmad, E.; Nikzad, H.; Haddad Kashani, H. The dual interaction of antimicrobial peptides on bacteria and cancer cells; mechanism of action and therapeutic strategies of nanostructures. Microb. Cell Factories 2022, 21, 118. [Google Scholar] [CrossRef]
- Gera, S.; Kankuri, E.; Kogermann, K. Antimicrobial peptides–unleashing their therapeutic potential using nanotechnology. Pharmacol. Ther. 2022, 232, 107990. [Google Scholar] [CrossRef]
- Tang, Z.; Ma, Q.; Chen, X.; Chen, T.; Ying, Y.; Xi, X.; Wang, L.; Ma, C.; Shaw, C.; Zhou, M. Recent advances and challenges in nanodelivery systems for antimicrobial peptides (AMPs). Antibiotics 2021, 10, 990. [Google Scholar] [CrossRef]
- Ghafari, M.; Haghiralsadat, F.; Khanamani Falahati-pour, S.; Zavar Reza, J. Development of a novel liposomal nanoparticle formulation of cisplatin to breast cancer therapy. J. Cell. Biochem. 2020, 121, 3584–3592. [Google Scholar] [CrossRef]
- Tsvetkova, D.; Ivanova, S. Application of approved cisplatin derivatives in combination therapy against different cancer diseases. Molecules 2022, 27, 2466. [Google Scholar] [CrossRef] [PubMed]
- Renault-Mahieux, M.; Vieillard, V.; Seguin, J.; Espeau, P.; Le, D.T.; Lai-Kuen, R.; Mignet, N.; Paul, M.; Andrieux, K. Co-encapsulation of fisetin and cisplatin into liposomes for glioma therapy: From formulation to cell evaluation. Pharmaceutics 2021, 13, 970. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Wang, Y.; Wu, Z.; Zhou, Q.; Tang, Y.; Liu, Z.; Yuan, G.; Luo, S.; Zou, Y.; Guo, S. The role of Her-2 in penile squamous cell carcinoma progression and cisplatin chemoresistance and potential for antibody-drug conjugate-based therapy. Eur. J. Cancer 2023, 194, 113360. [Google Scholar] [CrossRef]
- Fuentes-Antrás, J.; Genta, S.; Vijenthira, A.; Siu, L.L. Antibody–drug conjugates: In search of partners of choice. Trends Cancer 2023, 9, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Işık, G. Development and Characterization of PEG-B-PCL Micelles Carrying Anticancer Agents. Ph.D. Thesis, Middle East Technical University, Ankara, Turkey, 2022. [Google Scholar]
- Xia, W.; Tao, Z.; Zhu, B.; Zhang, W.; Liu, C.; Chen, S.; Song, M. Targeted delivery of drugs and genes using polymer nanocarriers for cancer therapy. Int. J. Mol. Sci. 2021, 22, 9118. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, R.; Asoodeh, A.; Mousavi, S.-D.; Firouzi, Z. The effect of a novel drug delivery system using encapsulated antimicrobial peptide protonectin (IL-12) into nano micelle PEG-PCL on A549 adenocarcinoma lung cell line. J. Polym. Res. 2021, 28, 341. [Google Scholar] [CrossRef]
- Gessner, I.; Neundorf, I. Nanoparticles modified with cell-penetrating peptides: Conjugation mechanisms, physicochemical properties, and application in cancer diagnosis and therapy. Int. J. Mol. Sci. 2020, 21, 2536. [Google Scholar] [CrossRef]
- Zhou, M.; Zou, X.; Cheng, K.; Zhong, S.; Su, Y.; Wu, T.; Tao, Y.; Cong, L.; Yan, B.; Jiang, Y. The role of cell-penetrating peptides in potential anti-cancer therapy. Clin. Transl. Med. 2022, 12, e822. [Google Scholar] [CrossRef]
- Singh, T.; Kang, D.H.; Kim, T.W.; Kong, H.J.; Ryu, J.S.; Jeon, S.; Ahn, T.S.; Jeong, D.; Baek, M.J.; Im, J. Intracellular delivery of oxaliplatin conjugate via cell penetrating peptide for the treatment of colorectal carcinoma in vitro and in vivo. Int. J. Pharm. 2021, 606, 120904. [Google Scholar] [CrossRef]
- Izabela, R.; Jarosław, R.; Magdalena, A.; Piotr, R.; Ivan, K. Transportan 10 improves the anticancer activity of cisplatin. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2016, 389, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Śmiłowicz, D.; Metzler-Nolte, N. Bioconjugates of Co(III) complexes with Schiff base ligands and cell penetrating peptides: Solid phase synthesis, characterization and antiproliferative activity. J. Inorg. Biochem. 2020, 206, 111041. [Google Scholar] [CrossRef]
- Jiang, M.; Fang, X.; Ma, L.; Liu, M.; Chen, M.; Liu, J.; Yang, Y.; Wang, C. A nucleus-targeting peptide antagonist towards EZH2 displays therapeutic efficacy for lung cancer. Int. J. Pharm. 2022, 622, 121894. [Google Scholar] [CrossRef] [PubMed]
- Safhi, A.Y. Three-dimensional (3D) printing in cancer therapy and diagnostics: Current status and future perspectives. Pharmaceuticals 2022, 15, 678. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zheng, J.; Oh, X.Y.; Chan, C.Y.; Low, B.Q.L.; Tor, J.Q.; Jiang, W.; Ye, E.; Loh, X.J.; Li, Z. Nanoarchitecture-integrated hydrogel systems toward therapeutic applications. ACS Nano 2023, 17, 7953–7978. [Google Scholar] [CrossRef] [PubMed]
- Binaymotlagh, R.; Chronopoulou, L.; Haghighi, F.H.; Fratoddi, I.; Palocci, C. Peptide-based hydrogels: New materials for biosensing and biomedical applications. Materials 2022, 15, 5871. [Google Scholar] [CrossRef]
- Chinnaiyan, K.; Mugundhan Laakshmi, S.; Narayanasamy, D.; Mohan, M. Revolutionizing Healthcare and Drug Discovery: The Impact of Artificial Intelligence on Pharmaceutical Development. Curr. Drug Ther. 2024, 19, 1–16. [Google Scholar] [CrossRef]
- Saraf, S.; De, A.; Tripathy, B. Effective Use of Computational Biology and Artificial Intelligence in the Domain of Medical Oncology. In Computational Intelligence for Oncology and Neurological Disorders; CRC Press: Boca Raton, FL, USA, 2024; pp. 228–252. [Google Scholar]
- Dlamini, Z.; Francies, F.Z.; Hull, R.; Marima, R. Artificial intelligence (AI) and big data in cancer and precision oncology. Comput. Struct. Biotechnol. J. 2020, 18, 2300–2311. [Google Scholar] [CrossRef]
- Riaz, I.B.; Khan, M.A.; Haddad, T.C. Potential application of artificial intelligence in cancer therapy. Curr. Opin. Oncol. 2024, 36, 437–448. [Google Scholar] [CrossRef]
- Kim, J.; Kusko, R.; Zeskind, B.; Zhang, J.; Escalante-Chong, R. A primer on applying AI synergistically with domain expertise to oncology. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2021, 1876, 188548. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Roy, D.; Thakur, S. Transforming Cancer Care: The Impact of AI-Driven Strategies. Curr. Cancer Drug Targets 2024, 24, 1–4. [Google Scholar] [CrossRef] [PubMed]




















| Category | Mechanism of Action | Examples |
|---|---|---|
| Alkylating Agents | Act on the entire reproduction process; most effective during the DNA synthesis phase (S). | Derivatives of Nitrogen Mustards: cyclophosphamide, chlorambucil, ifosfamide. Tetrazenes–Triazenes: dacarbazine. Derivatives of Platinum: cisplatin, carboplatin, oxaliplatin. |
| Medications that Affect the Entire Process of Reproduction | Interfere with various stages of the cell cycle and reproduction process. | Nitrosureas: carmustine (bcnu), lomustine (ccnu), estramustine. |
| Antitumor Antibiotics | Interfere with DNA duplication and alter the membrane surrounding the cells. | Anthracyclines: doxorubicin, daunorubicin, epirrubicin, idarubicin, mitoxantrone. Others: bleomycin, mitomycin c, dactinomycin. |
| Antimetabolites | Act on the S phase (DNA synthesis), interfere with DNA and RNA. | Folic Acid Analogues: methotrexate. Pyrimidine Analogues: fluorouracil, cytarabine, gemcitabine, capecitabine. Purine Analogues: fludarabine, mercaptopurine, cladribine. |
| Campotecin Derivatives | Inhibit topoisomerase I, leading to DNA damage. | Irinotecan, topotecan. |
| Mitotic Inhibitors | Act during the M phase (mitosis), inhibit or stop mitosis, or inhibit enzymes needed for cell reproduction. | Vinca Alkaloids: vincristine, vinblastine, vindesine, vinorelbine. Taxoids: paclitaxel, docetaxel. Epipodophyllotoxins: etoposide, teniposide. |
| Other Mechanisms of Action | Affect tumors with hormone dependence or stimulate the immune system to attack cancer cells. | Hormones: diethylbestrol. Antiandrogens: flutamide, nilutamide, cyproterone. Antiestrogens: tamoxifen, anastrazole. Gonadotrophin Agonists: leuprolide, goserelin, buserelin, triptorelin. Progestagens: megestrol, medroxyprogesterone. |
| Immunotherapy | Stimulates the immune system’s natural defenses to destroy abnormal cells. | Interferons: interferon alpha 2a, alpha 2b. Monoclonal Antibodies: rituximab, cetuximab, trastuzumab. |
| Mechanism | Description | Reference |
|---|---|---|
| Membrane Disruption | AMPs disrupt cancer cell membranes, leading to cell lysis, facilitating the entry of alkylating agents. | [155] |
| DNA Damage | Alkylating agents cause DNA strand breaks, facilitated by AMPs. | [156] |
| Immune Modulation–Induction of Apoptosis | AMPs trigger apoptosis, which can be amplified by alkylating agents. | [157,158] |
| Anti-Angiogenesis | AMPs inhibit angiogenesis, which complements the cytotoxic effects of alkylating agents. | [159] |
| Overcoming Drug Resistance | AMPs reduce the potential for drug resistance to alkylating agents. | [160] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrés, C.M.C.; Pérez de la Lastra, J.M.; Munguira, E.B.; Andrés Juan, C.; Pérez-Lebeña, E. Dual-Action Therapeutics: DNA Alkylation and Antimicrobial Peptides for Cancer Therapy. Cancers 2024, 16, 3123. https://doi.org/10.3390/cancers16183123
Andrés CMC, Pérez de la Lastra JM, Munguira EB, Andrés Juan C, Pérez-Lebeña E. Dual-Action Therapeutics: DNA Alkylation and Antimicrobial Peptides for Cancer Therapy. Cancers. 2024; 16(18):3123. https://doi.org/10.3390/cancers16183123
Chicago/Turabian StyleAndrés, Celia María Curieses, José Manuel Pérez de la Lastra, Elena Bustamante Munguira, Celia Andrés Juan, and Eduardo Pérez-Lebeña. 2024. "Dual-Action Therapeutics: DNA Alkylation and Antimicrobial Peptides for Cancer Therapy" Cancers 16, no. 18: 3123. https://doi.org/10.3390/cancers16183123
APA StyleAndrés, C. M. C., Pérez de la Lastra, J. M., Munguira, E. B., Andrés Juan, C., & Pérez-Lebeña, E. (2024). Dual-Action Therapeutics: DNA Alkylation and Antimicrobial Peptides for Cancer Therapy. Cancers, 16(18), 3123. https://doi.org/10.3390/cancers16183123





