Mechanistic Insights into the Successful Development of Combination Therapy of Enfortumab Vedotin and Pembrolizumab for the Treatment of Locally Advanced or Metastatic Urothelial Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Development of Enfortumab Vedotin for the Treatment of la/mUC
3. Development of Enfortumab Vedotin and Pembrolizumab as a Combination Therapy for Patients with la/mUC
4. Mechanisms of Resistance to Enfortumab Vedotin Treatment for Urothelial Carcinoma
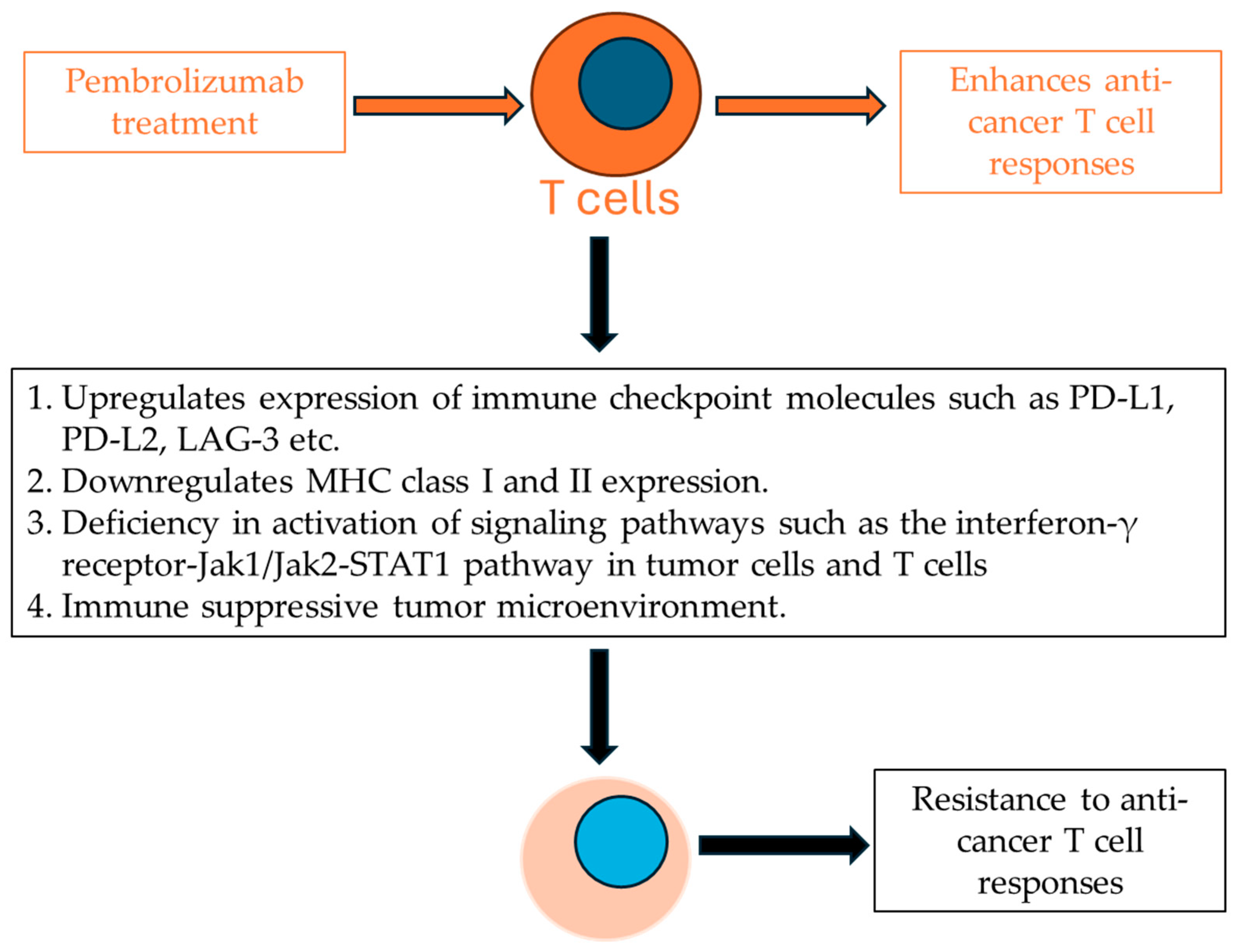
5. Mechanisms of Resistance to Pembrolizumab Treatment in Urothelial Carcinoma
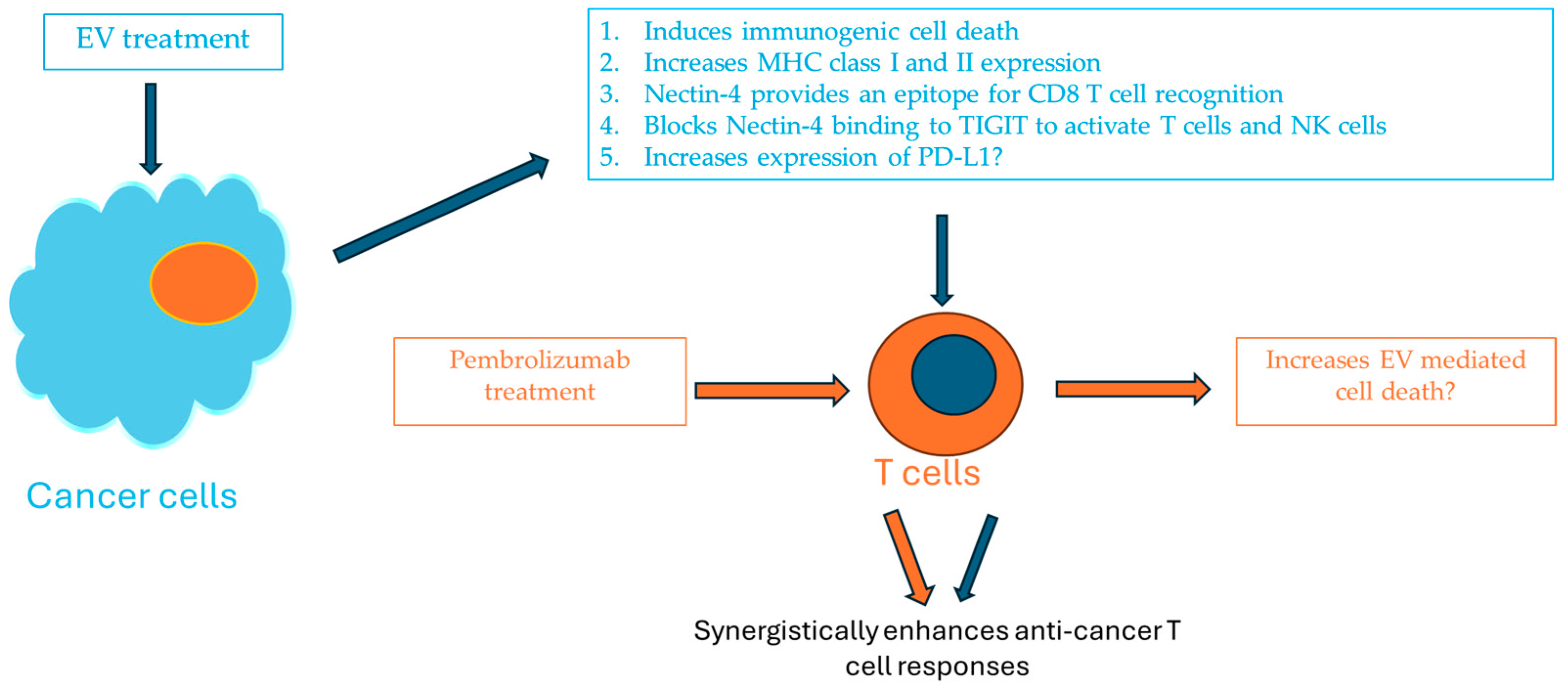
6. The Successful Development of Combined Enfortumab Vedotin with Pembrolizumab for the Treatment of Urothelial Carcinoma
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Disclaimer
References
- Baron, J.; Wang, E.S. Gemtuzumab ozogamicin for the treatment of acute myeloid leukemia. Expert Rev. Clin. Pharmacol. 2018, 11, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Castaigne, S.; Pautas, C.; Terré, C.; Raffoux, E.; Bordessoule, D.; Bastie, J.-N.; Legrand, O.; Thomas, X.; Turlure, P.; Reman, O.; et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): A randomised, open-label, phase 3 study. Lancet 2012, 379, 1508–1516. [Google Scholar] [CrossRef]
- Liu, K.; Li, M.; Li, Y.; Li, Y.; Chen, Z.; Tang, Y.; Yang, M.; Deng, G.; Liu, H. A review of the clinical efficacy of FDA-Approved antibody–drug conjugates in human cancers. Mol. Cancer 2024, 23, 62. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, P.; Carmagnani Pestana, R.; Corti, C.; Modi, S.; Bardia, A.; Tolaney, S.M.; Cortes, J.; Soria, J.-C.; Curigliano, G. Antibody–drug conjugates: Smart chemotherapy delivery across tumor histologies. CA Cancer J. Clin. 2022, 72, 165–182. [Google Scholar] [CrossRef]
- Drago, J.Z.; Modi, S.; Chandarlapaty, S. Unlocking the potential of antibody–drug conjugates for cancer therapy. Nat. Rev. Clin. Oncol. 2021, 18, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Dumontet, C.; Reichert, J.M.; Senter, P.D.; Lambert, J.M.; Beck, A. Antibody–drug conjugates come of age in oncology. Nat. Rev. Drug Discov. 2023, 22, 641–661. [Google Scholar] [CrossRef]
- Khongorzul, P.; Ling, C.J.; Khan, F.U.; Ihsan, A.U.; Zhang, J. Antibody–Drug Conjugates: A Comprehensive Review. Mol. Cancer Res. 2020, 18, 3–19. [Google Scholar] [CrossRef]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef]
- Narayan, P.; Dilawari, A.; Osgood, C.; Feng, Z.; Bloomquist, E.; Pierce, W.F.; Jafri, S.; Kalavar, S.; Kondratovich, M.; Jha, P.; et al. US Food and Drug Administration Approval Summary: Fam-Trastuzumab Deruxtecan-nxki for Human Epidermal Growth Factor Receptor 2-Low Unresectable or Metastatic Breast Cancer. J. Clin. Oncol. 2023, 41, 2108–2116. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- D’Arienzo, A.; Verrazzo, A.; Pagliuca, M.; Napolitano, F.; Parola, S.; Viggiani, M.; Caputo, R.; Puglisi, F.; Giuliano, M.; Del Mastro, L.; et al. Toxicity profile of antibody-drug conjugates in breast cancer: Practical considerations. EClinicalMedicine 2023, 62, 102113. [Google Scholar] [CrossRef]
- Abelman, R.O.; Wu, B.; Spring, L.M.; Ellisen, L.W.; Bardia, A. Mechanisms of Resistance to Antibody-Drug Conjugates. Cancers 2023, 15, 1278. [Google Scholar] [CrossRef]
- Chang, H.L.; Schwettmann, B.; McArthur, H.L.; Chan, I.S. Antibody-Drug conjugates in breast cancer: Overcoming resistance and boosting immune response. J. Clin. Investig. 2023, 133, e172156. [Google Scholar] [CrossRef]
- García-Alonso, S.; Ocaña, A.; Pandiella, A. Resistance to Antibody–Drug Conjugates. Cancer Res. 2018, 78, 2159–2165. [Google Scholar] [CrossRef]
- Loganzo, F.; Sung, M.; Gerber, H.-P. Mechanisms of Resistance to Antibody–Drug Conjugates. Mol. Cancer Ther. 2016, 15, 2825–2834. [Google Scholar] [CrossRef]
- Challita-Eid, P.M.; Satpayev, D.; Yang, P.; An, Z.; Morrison, K.; Shostak, Y.; Raitano, A.; Nadell, R.; Liu, W.; Lortie, D.R.; et al. Enfortumab Vedotin Antibody–Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Res. 2016, 76, 3003–3013. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.-L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef]
- Chang, E.; Weinstock, C.; Zhang, L.; Charlab, R.; Dorff, S.E.; Gong, Y.; Hsu, V.; Li, F.; Ricks, T.K.; Song, P.; et al. FDA Approval Summary: Enfortumab Vedotin for Locally Advanced or Metastatic Urothelial Carcinoma. Clin. Cancer Res. 2021, 27, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Strategies and challenges for the next generation of antibody–drug conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Maecker, H.; Jonnalagadda, V.; Bhakta, S.; Jammalamadaka, V.; Junutula, J.R. Exploration of the antibody-drug conjugate clinical landscape. MAbs 2023, 15, 2229101. [Google Scholar] [CrossRef]
- Zhou, W.; Fang, P.; Yu, D.; Ren, H.; You, M.; Yin, L.; Mei, F.; Zhu, H.; Wang, Z.; Xu, H.; et al. Preclinical Evaluation of 9MW2821, a Site-Specific Monomethyl Auristatin E–Based Antibody–Drug Conjugate for Treatment of Nectin-4–Expressing Cancers. Mol. Cancer Ther. 2023, 22, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Rigby, M.; Bennett, G.; Chen, L.; Mudd, G.E.; Harrison, H.; Beswick, P.J.; Van Rietschoten, K.; Watcham, S.M.; Scott, H.S.; Brown, A.N. BT8009; a nectin-4 targeting Bicycle toxin conjugate for treatment of solid tumors. Mol. Cancer Ther. 2022, 21, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, H.; Takao, S.; Furusawa, A.; Valera Romero, V.; Gurram, S.; Kato, T.; Okuyama, S.; Kano, M.; Choyke, P.L.; Kobayashi, H. Near-Infrared photoimmunotherapy targeting Nectin-4 in a preclinical model of bladder cancer. Cancer Lett. 2024, 585, 216606. [Google Scholar] [CrossRef] [PubMed]
- Tsuchikama, K.; Anami, Y.; Ha, S.Y.Y.; Yamazaki, C.M. Exploring the next generation of antibody–drug conjugates. Nat. Rev. Clin. Oncol. 2024, 21, 203–223. [Google Scholar] [CrossRef]
- Fuentes-Antras, J.; Genta, S.; Vijenthira, A.; Siu, L.L. Antibody-drug conjugates: In search of partners of choice. Trends Cancer 2023, 9, 339–354. [Google Scholar] [CrossRef]
- McGregor, B.A.; Sonpavde, G.P.; Kwak, L.; Regan, M.M.; Gao, X.; Hvidsten, H.; Mantia, C.M.; Wei, X.X.; Berchuck, J.E.; Berg, S.A.; et al. The Double Antibody Drug Conjugate (DAD) phase I trial: Sacituzumab govitecan plus enfortumab vedotin for metastatic urothelial carcinoma. Ann. Oncol. 2024, 35, 91–97. [Google Scholar] [CrossRef]
- Powles, T.; Valderrama, B.P.; Gupta, S.; Bedke, J.; Kikuchi, E.; Hoffman-Censits, J.; Iyer, G.; Vulsteke, C.; Park, S.H.; Shin, S.J.; et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N. Engl. J. Med. 2024, 390, 875–888. [Google Scholar] [CrossRef]
- Reymond, N.; Fabre, S.; Lecocq, E.; Adelaıde, J.; Dubreuil, P.; Lopez, M. Nectin4/PRR4, a New Afadin-associated Member of the Nectin Family That Trans-interacts with Nectin1/PRR1 through V Domain Interaction. J. Biol. Chem. 2001, 276, 43205–43215. [Google Scholar] [CrossRef]
- Takai, Y.; Ikeda, W.; Ogita, H.; Rikitake, Y. The Immunoglobulin-Like Cell Adhesion Molecule Nectin and Its Associated Protein Afadin. Annu. Rev. Cell Dev. Biol. 2008, 24, 309–342. [Google Scholar] [CrossRef]
- Heath, E.I.; Rosenberg, J.E. The biology and rationale of targeting nectin-4 in urothelial carcinoma. Nat. Rev. Urol. 2021, 18, 93–103. [Google Scholar] [CrossRef]
- Buchanan, P.C.; Boylan, K.L.M.; Walcheck, B.; Heinze, R.; Geller, M.A.; Argenta, P.A.; Skubitz, A.P.N. Ectodomain shedding of the cell adhesion molecule Nectin-4 in ovarian cancer is mediated by ADAM10 and ADAM17. J. Biol. Chem. 2017, 292, 6339–6351. [Google Scholar] [CrossRef] [PubMed]
- Fabre-Lafay, S.; Garrido-Urbani, S.; Reymond, N.; Goncalves, A.; Dubreuil, P.; Lopez, M. Nectin-4, a new serological breast cancer marker, is a substrate for tumor necrosis factor-alpha-converting enzyme (TACE)/ADAM-17. J. Biol. Chem. 2005, 280, 19543–19550. [Google Scholar] [CrossRef]
- Fabre-Lafay, S.; Monville, F.; Garrido-Urbani, S.; Berruyer-Pouyet, C.; Ginestier, C.; Reymond, N.; Finetti, P.; Sauvan, R.; Adelaide, J.; Geneix, J.; et al. Nectin-4 is a new histological and serological tumor associated marker for breast cancer. BMC Cancer 2007, 7, 73. [Google Scholar] [CrossRef]
- Noyce, R.S.; Richardson, C.D. Nectin 4 is the epithelial cell receptor for measles virus. Trends Microbiol. 2012, 20, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Rikitake, Y.; Mandai, K.; Takai, Y. The role of nectins in different types of cell–cell adhesion. J. Cell Sci. 2012, 125, 3713–3722. [Google Scholar] [CrossRef]
- Takahashi, S.; Uemura, M.; Kimura, T.; Kawasaki, Y.; Takamoto, A.; Yamaguchi, A.; Melhem-Bertrandt, A.; Gartner, E.M.; Inoue, T.; Akazawa, R.; et al. A phase I study of enfortumab vedotin in Japanese patients with locally advanced or metastatic urothelial carcinoma. Investig. New Drugs 2020, 38, 1056–1066. [Google Scholar] [CrossRef]
- Yu, E.Y.; Petrylak, D.P.; O’Donnell, P.H.; Lee, J.-L.; van der Heijden, M.S.; Loriot, Y.; Stein, M.N.; Necchi, A.; Kojima, T.; Harrison, M.R.; et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV-201): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2021, 22, 872–882. [Google Scholar] [CrossRef]
- DeRycke, M.S.; Pambuccian, S.E.; Gilks, C.B.; Kalloger, S.E.; Ghidouche, A.; Lopez, M.; Bliss, R.L.; Geller, M.A.; Argenta, P.A.; Harrington, K.M.; et al. Nectin 4 Overexpression in Ovarian Cancer Tissues and Serum: Potential Role as a Serum Biomarker. Am. J. Clin. Pathol. 2010, 134, 835–845. [Google Scholar] [CrossRef]
- Takano, A.; Ishikawa, N.; Nishino, R.; Masuda, K.; Yasui, W.; Inai, K.; Nishimura, H.; Ito, H.; Nakayama, H.; Miyagi, Y.; et al. Identification of Nectin-4 Oncoprotein as a Diagnostic and Therapeutic Target for Lung Cancer. Cancer Res. 2009, 69, 6694–6703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, G.; Qi, J.; Li, Y.; He, Y.; Xu, X.; Shi, J.; Zhang, C.W.H.; Yan, J.; Gao, G.F. Structure of measles virus hemagglutinin bound to its epithelial receptor nectin-4. Nat. Struct. Mol. Biol. 2013, 20, 67–72. [Google Scholar] [CrossRef]
- Mühlebach, M.D.; Mateo, M.; Sinn, P.L.; Prüfer, S.; Uhlig, K.M.; Leonard, V.H.J.; Navaratnarajah, C.K.; Frenzke, M.; Wong, X.X.; Sawatsky, B.; et al. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature 2011, 480, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Bouleftour, W.; Guillot, A.; Magne, N. The Anti-Nectin 4: A Promising Tumor Cells Target. A Systematic Review. Mol. Cancer Ther. 2022, 21, 493–501. [Google Scholar] [CrossRef]
- Ogita, H.; Takai, Y. Nectins and nectin-like molecules: Roles in cell adhesion, polarization, movement, and proliferation. IUBMB Life 2006, 58, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Harrison, O.J.; Vendome, J.; Brasch, J.; Jin, X.; Hong, S.; Katsamba, P.S.; Ahlsen, G.; Troyanovsky, R.B.; Troyanovsky, S.M.; Honig, B.; et al. Nectin ectodomain structures reveal a canonical adhesive interface. Nat. Struct. Mol. Biol. 2012, 19, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Brancati, F.; Fortugno, P.; Bottillo, I.; Lopez, M.; Josselin, E.; Boudghene-Stambouli, O.; Agolini, E.; Bernardini, L.; Bellacchio, E.; Iannicelli, M. Mutations in PVRL4, encoding cell adhesion molecule nectin-4, cause ectodermal dysplasia-syndactyly syndrome. Am. J. Hum. Genet. 2010, 87, 265–273. [Google Scholar] [CrossRef]
- Chatterjee, S.; Sinha, S.; Kundu, C.N. Nectin cell adhesion molecule-4 (NECTIN-4): A potential target for cancer therapy. Eur. J. Pharmacol. 2021, 911, 174516. [Google Scholar] [CrossRef]
- Liu, Y.; Han, X.; Li, L.; Zhang, Y.; Huang, X.; Li, G.; Xu, C.; Yin, M.; Zhou, P.; Shi, F.; et al. Role of Nectin-4 protein in cancer (Review). Int. J. Oncol. 2021, 59, 93. [Google Scholar] [CrossRef]
- Boylan, K.L.; Buchanan, P.C.; Manion, R.D.; Shukla, D.M.; Braumberger, K.; Bruggemeyer, C.; Skubitz, A.P. The expression of Nectin-4 on the surface of ovarian cancer cells alters their ability to adhere, migrate, aggregate, and proliferate. Oncotarget 2017, 8, 9717–9738. [Google Scholar] [CrossRef]
- Martin, T.A.; Lane, J.; Harrison, G.M.; Jiang, W.G. The expression of the Nectin complex in human breast cancer and the role of Nectin-3 in the control of tight junctions during metastasis. PLoS ONE 2013, 8, e82696. [Google Scholar] [CrossRef]
- Deng, H.; Shi, H.; Chen, L.; Zhou, Y.; Jiang, J. Over-Expression of Nectin-4 promotes progression of esophageal cancer and correlates with poor prognosis of the patients. Cancer Cell Int. 2019, 19, 106. [Google Scholar] [CrossRef]
- Nishiwada, S.; Sho, M.; Yasuda, S.; Shimada, K.; Yamato, I.; Akahori, T.; Kinoshita, S.; Nagai, M.; Konishi, N.; Nakajima, Y. Nectin-4 expression contributes to tumor proliferation, angiogenesis and patient prognosis in human pancreatic cancer. J. Exp. Clin. Cancer Res. 2015, 34, 30. [Google Scholar] [CrossRef] [PubMed]
- Klümper, N.; Ralser, D.J.; Ellinger, J.; Roghmann, F.; Albrecht, J.; Below, E.; Alajati, A.; Sikic, D.; Breyer, J.; Bolenz, C.; et al. Membranous NECTIN-4 Expression Frequently Decreases during Metastatic Spread of Urothelial Carcinoma and Is Associated with Enfortumab Vedotin Resistance. Clin. Cancer Res. 2023, 29, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Satapathy, S.R.; Siddharth, S.; Nayak, A.; Kundu, C.N. NECTIN-4 increased the 5-FU resistance in colon cancer cells by inducing the PI3K–AKT cascade. Cancer Chemother. Pharmacol. 2015, 76, 471–479. [Google Scholar] [CrossRef]
- Liu, B.A.; Olson, D.; Snead, K.; Gosink, J.; Tenn, E.-M.; Zaval, M.; Cao, A.; Sahetya, D.; Nesterova, A.; Hensley, K.; et al. Abstract 5581: Enfortumab vedotin, an anti-Nectin-4 ADC demonstrates bystander cell killing and immunogenic cell death anti-tumor activity mechanisms of action in urothelial cancers. Cancer Res. 2020, 80, 5581. [Google Scholar] [CrossRef]
- Rosenberg, J.; Sridhar, S.S.; Zhang, J.; Smith, D.; Ruether, D.; Flaig, T.W.; Baranda, J.; Lang, J.; Plimack, E.R.; Sangha, R.; et al. EV-101: A Phase I Study of Single-Agent Enfortumab Vedotin in Patients with Nectin-4-Positive Solid Tumors, including Metastatic Urothelial Carcinoma. J. Clin. Oncol. 2020, 38, 1041–1049. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Powles, T.; Sonpavde, G.P.; Loriot, Y.; Duran, I.; Lee, J.L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Mamtani, R.; et al. EV-301 long-term outcomes: 24-month findings from the phase III trial of enfortumab vedotin versus chemotherapy in patients with previously treated advanced urothelial carcinoma. Ann. Oncol. 2023, 34, 1047–1054. [Google Scholar] [CrossRef]
- Swami, U.; Maughan, B.L.; Boucher, K.M.; Nordblad, B.; Lloyd, J.; Batten, J.; Phunrab, T.K.; Quertinmont, J.; Kohli, M.; Gupta, S.; et al. Enfortumab vedotin (EV) as monotherapy in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC): Results of stage I of a phase 2 trial. J. Clin. Oncol. 2024, 42, 152. [Google Scholar] [CrossRef]
- Bruce, J.Y.; Pusztai, L.; Braiteh, F.S.; Gorla, S.R.; Wu, C.; Baranda, J. EV-202: A phase II study of enfortumab vedotin in patients with select previously treated locally advanced or metastatic solid tumors. J. Clin. Oncol. 2020, 38, TPS3647. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Milowsky, M.; Ramamurthy, C.; Mar, N.; McKay, R.R.; Friedlander, T.; Ferrario, C.; Bracarda, S.; George, S.; Moon, H.; et al. LBA73 Study EV-103 Cohort K: Antitumor activity of enfortumab vedotin (EV) monotherapy or in combination with pembrolizumab (P) in previously untreated cisplatin-ineligible patients (pts) with locally advanced or metastatic urothelial cancer (la/mUC). Ann. Oncol. 2022, 33, S1441. [Google Scholar] [CrossRef]
- O’Donnell, P.H.; Rosenberg, J.E.; Hoimes, C.J.; Petrylak, D.P.; Milowsky, M.I.; McKay, R.R.; Srinivas, S.; Friedlander, T.W.; Ramamurthy, C.; Bilen, M.A.; et al. Enfortumab vedotin (EV) alone or in combination with pembrolizumab (P) in previously untreated cisplatin-ineligible patients with locally advanced or metastatic urothelial cancer (la/mUC): Subgroup analyses of confirmed objective response rate (cORR) from EV-103 cohort K. J. Clin. Oncol. 2023, 41, 499. [Google Scholar] [CrossRef]
- Msaouel, P.; Sweis, R.F.; Bupathi, M.; Heath, E.; Goodman, O.B.; Hoimes, C.J.; Milowsky, M.I.; Davis, N.; Kalebasty, A.R.; Picus, J.; et al. A Phase 2 Study of Sitravatinib in Combination with Nivolumab in Patients with Advanced or Metastatic Urothelial Carcinoma. Eur. Urol. Oncol. 2023, 7, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.; Xiao, J.-F.; Agarwal, N.; Duex, J.E.; Theodorescu, D. Advances in bladder cancer biology and therapy. Nat. Rev. Cancer 2021, 21, 104–121. [Google Scholar] [CrossRef]
- Konala, V.M.; Adapa, S.; Aronow, W.S. Immunotherapy in Bladder Cancer. Am. J. Ther. 2022, 29, e334–e337. [Google Scholar] [CrossRef]
- Roviello, G.; Catalano, M.; Santi, R.; Palmieri, V.E.; Vannini, G.; Galli, I.C.; Buttitta, E.; Villari, D.; Rossi, V.; Nesi, G. Immune Checkpoint Inhibitors in Urothelial Bladder Cancer: State of the Art and Future Perspectives. Cancers 2021, 13, 4411. [Google Scholar] [CrossRef]
- Powles, T.; van der Heijden, M.S.; Castellano, D.; Galsky, M.D.; Loriot, Y.; Petrylak, D.P.; Ogawa, O.; Park, S.H.; Lee, J.-L.; De Giorgi, U.; et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020, 21, 1574–1588. [Google Scholar] [CrossRef]
- Vlachostergios, P.J.; Faltas, B.M. Treatment resistance in urothelial carcinoma: An evolutionary perspective. Nat. Rev. Clin. Oncol. 2018, 15, 495–509. [Google Scholar] [CrossRef]
- Bellmunt, J.; de Wit, R.; Vaughn David, J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang Nicholas, J.; Climent Miguel, A.; Petrylak Daniel, P.; Choueiri Toni, K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Balar, A.V.; Kamat, A.M.; Kulkarni, G.S.; Uchio, E.M.; Boormans, J.L.; Roumiguié, M.; Krieger, L.E.M.; Singer, E.A.; Bajorin, D.F.; Grivas, P.; et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): An open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 2021, 22, 919–930. [Google Scholar] [CrossRef]
- Powles, T.; Csőszi, T.; Özgüroğlu, M.; Matsubara, N.; Géczi, L.; Cheng, S.Y.S.; Fradet, Y.; Oudard, S.; Vulsteke, C.; Morales Barrera, R.; et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Zengin, Z.B.; Meza, L.; Pal, S.K.; Grivas, P. Chemoimmunotherapy in urothelial cancer: Concurrent or sequential? Lancet Oncol. 2021, 22, 894–896. [Google Scholar] [CrossRef] [PubMed]
- Apolo, A.B.; Ballman, K.V.; Sonpavde, G.P.; Berg, S.A.; Kim, W.Y.; Parikh, R.A.; Teo, M.Y.; Sweis, R.F.; Geynisman, D.M.; Grivas, P.; et al. AMBASSADOR Alliance A031501: Phase III randomized adjuvant study of pembrolizumab in muscle-invasive and locally advanced urothelial carcinoma (MIUC) vs observation. J. Clin. Oncol. 2024, 42, LBA531. [Google Scholar] [CrossRef]
- Powles, T.; Park Se, H.; Voog, E.; Caserta, C.; Valderrama Begoña, P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.M.; Mateen, R.; Qaddour, N.; Carrillo, A.; Verschraegen, C.; Yang, Y.; Li, Z.; Sundi, D.; Mortazavi, A.; Collier, K.A. A Comprehensive Review of Immunotherapy Clinical Trials for Metastatic Urothelial Carcinoma: Immune Checkpoint Inhibitors Alone or in Combination, Novel Antibodies, Cellular Therapies, and Vaccines. Cancers 2024, 16, 335. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, M.S.; Sonpavde, G.; Powles, T.; Necchi, A.; Burotto, M.; Schenker, M.; Sade, J.P.; Bamias, A.; Beuzeboc, P.; Bedke, J.; et al. Nivolumab plus Gemcitabine–Cisplatin in Advanced Urothelial Carcinoma. N. Engl. J. Med. 2023, 389, 1778–1789. [Google Scholar] [CrossRef] [PubMed]
- Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Grivas, P.; Vuky, J.; Powles, T.; Plimack, E.R.; Hahn, N.M.; de Wit, R.; Pang, L.; et al. First-Line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 1483–1492. [Google Scholar] [CrossRef]
- Vuky, J.; Balar, A.V.; Castellano, D.E.; O’Donnell, P.H.; Grivas, P.; Bellmunt, J.; Powles, T.; Bajorin, D.F.; Hahn, N.M.; De Wit, R.; et al. Updated efficacy and safety of KEYNOTE-052: A single-arm phase 2 study investigating first-line pembrolizumab (pembro) in cisplatin-ineligible advanced urothelial cancer (UC). J. Clin. Oncol. 2018, 36, 4524. [Google Scholar] [CrossRef]
- Fradet, Y.; Bellmunt, J.; Vaughn, D.J.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; Necchi, A.; et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: Results of >2 years of follow-up. Ann. Oncol. 2019, 30, 970–976. [Google Scholar] [CrossRef]
- Parikh, M.; Pan, C.-X.; Beckett, L.; Li, Y.; Robles, D.; DeVisser, C.; Lara, P. Combination checkpoint immunotherapy and cytotoxic chemotherapy: Further results from a phase Ib/II trial of pembrolizumab and docetaxel or gemcitabine in patients with advanced or metastatic urothelial cancer. J. Clin. Oncol. 2018, 36, 525. [Google Scholar] [CrossRef]
- Giannatempo, P.; Raggi, D.; Marandino, L.; Bandini, M.; Farè, E.; Calareso, G.; Colecchia, M.; Gallina, A.; Ross, J.S.; Alessi, A.; et al. Pembrolizumab and nab-paclitaxel as salvage therapy for platinum-treated, locally advanced or metastatic urothelial carcinoma: Interim results of the open-label, single-arm, phase II PEANUT study. Ann. Oncol. 2020, 31, 1764–1772. [Google Scholar] [CrossRef]
- Bitting, R.L.; Vile, D.C.; Tooze, J.A.; Thomas, C.Y.; Neve, M.; Kooshki, M.; Brown, J.; Dubey, P.; Triozzi, P.; Goodman, M.M. Single-Arm phase II study of low-dose paclitaxel and pembrolizumab in platinum-refractory metastatic urothelial carcinoma (UC). J. Clin. Oncol. 2021, 39, 433. [Google Scholar] [CrossRef]
- Grivas, P.; Pouessel, D.; Park, C.H.; Barthelemy, P.; Bupathi, M.; Petrylak, D.P.; Agarwal, N.; Gupta, S.; Fléchon, A.; Ramamurthy, C.; et al. Sacituzumab Govitecan in Combination with Pembrolizumab for Patients with Metastatic Urothelial Cancer That Progressed after Platinum-Based Chemotherapy: TROPHY-U-01 Cohort 3. J. Clin. Oncol. 2024, 42, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, P.H.; Milowsky, M.I.; Petrylak, D.P.; Hoimes, C.J.; Flaig, T.W.; Mar, N.; Moon, H.H.; Friedlander, T.W.; McKay, R.R.; Bilen, M.A.; et al. Enfortumab Vedotin with or without Pembrolizumab in Cisplatin-Ineligible Patients with Previously Untreated Locally Advanced or Metastatic Urothelial Cancer. J. Clin. Oncol. 2023, 41, 4107–4117. [Google Scholar] [CrossRef]
- Jiang, M.; Li, Q.; Xu, B. Spotlight on ideal target antigens and resistance in antibody-drug conjugates: Strategies for competitive advancement. Drug Resist. Updates 2024, 75, 101086. [Google Scholar] [CrossRef]
- Hoffman-Censits, J.; Lombardo, K.; McConkey, D.; Hahn, N.M.; Bashir, B.; Kelly, W.K.; Johnson, B.; Matoso, A. New and topics: Enfortumab vedotin mechanisms of response and resistance in urothelial cancer—What do we understand so far? Urol. Oncol. 2021, 39, 619–622. [Google Scholar] [CrossRef]
- Maas, M.; Stühler, V.; Walz, S.; Stenzl, A.; Bedke, J. Enfortumab vedotin—Next game-changer in urothelial cancer. Expert Opin. Biol. Ther. 2021, 21, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Cabaud, O.; Berger, L.; Crompot, E.; Adélaide, J.; Finetti, P.; Garnier, S.; Guille, A.; Carbuccia, N.; Farina, A.; Agavnian, E.; et al. Overcoming Resistance to Anti–Nectin-4 Antibody-Drug Conjugate. Mol. Cancer Ther. 2022, 21, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Aggen, D.H.; Chu, C.E.; Rosenberg, J.E. Scratching the Surface: NECTIN-4 as a Surrogate for Enfortumab Vedotin Resistance. Clin. Cancer Res. 2023, 29, 1377–1380. [Google Scholar] [CrossRef]
- Klümper, N.; Eckstein, M. Biomarkers of Response to Anti-NECTIN4 Antibody-Drug Conjugate Enfortumab Vedotin in Urothelial Cancer. Eur. Urol. Focus 2024, 10, 224–226. [Google Scholar] [CrossRef]
- Klümper, N.; Tran, N.K.; Zschäbitz, S.; Hahn, O.; Büttner, T.; Roghmann, F.; Bolenz, C.; Zengerling, F.; Schwab, C.; Nagy, D.; et al. NECTIN4 Amplification Is Frequent in Solid Tumors and Predicts Enfortumab Vedotin Response in Metastatic Urothelial Cancer. J. Clin. Oncol. 2024, 42, JCO-23. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.E.; Sjöström, M.; Egusa, E.A.; Gibb, E.A.; Badura, M.L.; Zhu, J.; Koshkin, V.S.; Stohr, B.A.; Meng, M.V.; Pruthi, R.S.; et al. Heterogeneity in NECTIN4 Expression Across Molecular Subtypes of Urothelial Cancer Mediates Sensitivity to Enfortumab Vedotin. Clin. Cancer Res. 2021, 27, 5123–5130. [Google Scholar] [CrossRef]
- Deininger, S.; Törzsök, P.; Oswald, D.; Lusuardi, L. Current Systemic Treatment Options in Metastatic Urothelial Carcinoma after Progression on Checkpoint Inhibition Therapy—A Systemic Review Combined with Single-Group Meta-Analysis of Three Studies Testing Enfortumab Vedotin. Cancers 2021, 13, 3206. [Google Scholar] [CrossRef] [PubMed]
- Garczyk, S.; Degener, S.; Bischoff, F.; Schnitzler, T.; Salz, A.; Golz, R.; Buchner, A.; Schulz, G.B.; Schneider, U.; Gaisa, N.T.; et al. Heterogenous NECTIN4 expression in urothelial high-risk non-muscle-invasive bladder cancer. Virchows Arch. 2022, 481, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Hoffman-Censits, J.; Maldonado, L. Targeted Treatment of Locally Advanced and Metastatic Urothelial Cancer: Enfortumab Vedotin in Context. Onco Targets Ther. 2022, 15, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Lodha, R.; Delavan, H.M.; Winebaum, J.; Porten, S.P.; Feng, F.Y.; Chu, C.E.; Chou, J. Mechanisms and strategies to overcome resistance to enfortumab vedotin in bladder cancer. J. Clin. Oncol. 2024, 42, 690. [Google Scholar] [CrossRef]
- Kotono, M.; Kijima, T.; Takada-Owada, A.; Okubo, N.; Kurashina, R.; Kokubun, H.; Uematsu, T.; Takei, K.; Ishida, K.; Kamai, T. Increased expression of ATP-binding cassette transporters in enfortumab vedotin-resistant urothelial cancer. IJU Case Rep. 2024, 7, 173–176. [Google Scholar] [CrossRef]
- Shimoda, H.; Takada-Owada, A.; Kijima, T.; Kokubun, H.; Uematsu, T.; Takei, K.; Nakazato, Y.; Yashi, M.; Ishida, K.; Kamai, T. ABC transporter expression to predict therapeutic effect of enfortumab vedotin in urothelial cancer. J. Clin. Oncol. 2024, 42, 693. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Assaraf, Y.G.; Zhao, K.; Xu, X.; Xie, J.; Yang, D.-H.; Chen, Z.-S. Overcoming ABC transporter-mediated multidrug resistance: Molecular mechanisms and novel therapeutic drug strategies. Drug Resist. Updates 2016, 27, 14–29. [Google Scholar] [CrossRef]
- Loganzo, F.; Tan, X.; Sung, M.; Jin, G.; Myers, J.S.; Melamud, E.; Wang, F.; Diesl, V.; Follettie, M.T.; Musto, S.; et al. Tumor Cells Chronically Treated with a Trastuzumab–Maytansinoid Antibody–Drug Conjugate Develop Varied Resistance Mechanisms but Respond to Alternate Treatments. Mol. Cancer Ther. 2015, 14, 952–963. [Google Scholar] [CrossRef]
- Jindal, T.; Zhu, X.; Bose, R.; Kumar, V.; Maldonado, E.; Deshmukh, P.; Shipp, C.; Feng, S.; Johnson, M.S.; Angelidakis, A.; et al. Somatic alterations of TP53 and MDM2 associated with response to enfortumab vedotin in patients with advanced urothelial cancer. Front. Oncol. 2023, 13, 1161089. [Google Scholar] [CrossRef]
- Yamazaki, C.M.; Yamaguchi, A.; Anami, Y.; Xiong, W.; Otani, Y.; Lee, J.; Ueno, N.T.; Zhang, N.; An, Z.; Tsuchikama, K. Antibody-Drug conjugates with dual payloads for combating breast tumor heterogeneity and drug resistance. Nat. Commun. 2021, 12, 3528. [Google Scholar] [CrossRef]
- Journeaux, T.; Bernardes, G.J.L. Homogeneous multi-payload antibody–drug conjugates. Nat. Chem. 2024, 16, 854–870. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. Mech. Dis. 2021, 16, 223–249. [Google Scholar] [CrossRef]
- Fares, C.M.; Van Allen, E.M.; Drake, C.G.; Allison, J.P.; Hu-Lieskovan, S. Mechanisms of Resistance to Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immunotherapy Not Work for All Patients? Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Kalbasi, A.; Ribas, A. Tumour-Intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 2020, 20, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Karasarides, M.; Cogdill, A.P.; Robbins, P.B.; Bowden, M.; Burton, E.M.; Butterfield, L.H.; Cesano, A.; Hammer, C.; Haymaker, C.L.; Horak, C.E.; et al. Hallmarks of Resistance to Immune-Checkpoint Inhibitors. Cancer Immunol. Res. 2022, 10, 372–383. [Google Scholar] [CrossRef]
- Morad, G.; Helmink, B.A.; Sharma, P.; Wargo, J.A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 2021, 184, 5309–5337. [Google Scholar] [CrossRef]
- Syn, N.L.; Teng, M.W.L.; Mok, T.S.K.; Soo, R.A. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017, 18, e731–e741. [Google Scholar] [CrossRef]
- Paschen, A.; Melero, I.; Ribas, A. Central Role of the Antigen-Presentation and Interferon-γ Pathways in Resistance to Immune Checkpoint Blockade. Annu. Rev. Cancer Biol. 2022, 6, 85–102. [Google Scholar] [CrossRef]
- van Dorp, J.; van der Heijden, M.S. The bladder cancer immune micro-environment in the context of response to immune checkpoint inhibition. Front. Immunol. 2023, 14, 1235884. [Google Scholar] [CrossRef]
- Schneider, A.K.; Chevalier, M.F.; Derré, L. The multifaceted immune regulation of bladder cancer. Nat. Rev. Urol. 2019, 16, 613–630. [Google Scholar] [CrossRef]
- Koyama, S.; Akbay, E.A.; Li, Y.Y.; Herter-Sprie, G.S.; Buczkowski, K.A.; Richards, W.G.; Gandhi, L.; Redig, A.J.; Rodig, S.J.; Asahina, H.; et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 2016, 7, 10501. [Google Scholar] [CrossRef]
- Pérez-Ruiz, E.; Melero, I.; Kopecka, J.; Sarmento-Ribeiro, A.B.; García-Aranda, M.; De Las Rivas, J. Cancer immunotherapy resistance based on immune checkpoints inhibitors: Targets, biomarkers, and remedies. Drug Resist. Updates 2020, 53, 100718. [Google Scholar] [CrossRef]
- Huang, R.-Y.; Francois, A.; McGray, A.J.R.; Miliotto, A.; Odunsi, K. Compensatory upregulation of PD-1, LAG-3, and CTLA-4 limits the efficacy of single-agent checkpoint blockade in metastatic ovarian cancer. OncoImmunology 2017, 6, e1249561. [Google Scholar] [CrossRef] [PubMed]
- Boorjian, S.A.; Sheinin, Y.; Crispen, P.L.; Farmer, S.A.; Lohse, C.M.; Kuntz, S.M.; Leibovich, B.C.; Kwon, E.D.; Frank, I. T-Cell Coregulatory Molecule Expression in Urothelial Cell Carcinoma: Clinicopathologic Correlations and Association with Survival. Clin. Cancer Res. 2008, 14, 4800–4808. [Google Scholar] [CrossRef]
- Miao, Y.R.; Thakkar, K.N.; Qian, J.; Kariolis, M.S.; Huang, W.; Nandagopal, S.; Yang, T.T.C.; Diep, A.N.; Cherf, G.M.; Xu, Y.; et al. Neutralization of PD-L2 is Essential for Overcoming Immune Checkpoint Blockade Resistance in Ovarian Cancer. Clin. Cancer Res. 2021, 27, 4435–4448. [Google Scholar] [CrossRef] [PubMed]
- Amaria, R.N.; Postow, M.; Burton, E.M.; Tetzlaff, M.T.; Ross, M.I.; Torres-Cabala, C.; Glitza, I.C.; Duan, F.; Milton, D.R.; Busam, K.; et al. Neoadjuvant relatlimab and nivolumab in resectable melanoma. Nature 2022, 611, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Tawbi Hussein, A.; Schadendorf, D.; Lipson Evan, J.; Ascierto Paolo, A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas Helen, J.; Lao Christopher, D.; De Menezes Juliana, J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Ngiow, S.F.; Manne, S.; Huang, Y.J.; Azar, T.; Chen, Z.; Mathew, D.; Chen, Q.; Khan, O.; Wu, J.E.; Alcalde, V.; et al. LAG-3 sustains TOX expression and regulates the CD94/NKG2-Qa-1b axis to govern exhausted CD8 T cell NK receptor expression and cytotoxicity. Cell 2024, 187, 4336–4354.e19. [Google Scholar] [CrossRef]
- Cillo, A.R.; Cardello, C.; Shan, F.; Karapetyan, L.; Kunning, S.; Sander, C.; Rush, E.; Karunamurthy, A.; Massa, R.C.; Rohatgi, A.; et al. Blockade of LAG-3 and PD-1 leads to co-expression of cytotoxic and exhaustion gene modules in CD8 + T cells to promote antitumor immunity. Cell 2024, 187, 4373–4388.e15. [Google Scholar] [CrossRef]
- Andrews, L.P.; Butler, S.C.; Cui, J.; Cillo, A.R.; Cardello, C.; Liu, C.; Brunazzi, E.A.; Baessler, A.; Xie, B.; Kunning, S.R.; et al. LAG-3 and PD-1 synergize on CD8 + T cells to drive T cell exhaustion and hinder autocrine IFN-γ-dependent anti-tumor immunity. Cell 2024, 187, 4355–4372.e22. [Google Scholar] [CrossRef]
- McGranahan, N.; Rosenthal, R.; Hiley, C.T.; Rowan, A.J.; Watkins, T.B.K.; Wilson, G.A.; Birkbak, N.J.; Veeriah, S.; Van Loo, P.; Herrero, J.; et al. Allele-Specific HLA Loss and Immune Escape in Lung Cancer Evolution. Cell 2017, 171, 1259–1271.e11. [Google Scholar] [CrossRef]
- Romero, J.M.; Jiménez, P.; Cabrera, T.; Cózar, J.M.; Pedrinaci, S.; Tallada, M.; Garrido, F.; Ruiz-Cabello, F. Coordinated downregulation of the antigen presentation machinery and HLA class I/β2-microglobulin complex is responsible for HLA-ABC loss in bladder cancer. Int. J. Cancer 2005, 113, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.S.; Zhang, W.; Wang, X.; Jiang, P.; Traugh, N.; Li, Z.; Meyer, C.; Stewig, B.; Xie, Y.; Bu, X.; et al. Therapeutically Increasing MHC-I Expression Potentiates Immune Checkpoint Blockade. Cancer Discov. 2021, 11, 1524–1541. [Google Scholar] [CrossRef]
- Mpakali, A.; Stratikos, E. The Role of Antigen Processing and Presentation in Cancer and the Efficacy of Immune Checkpoint Inhibitor Immunotherapy. Cancers 2021, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Benci, J.L.; Xu, B.; Qiu, Y.; Wu, T.J.; Dada, H.; Twyman-Saint Victor, C.; Cucolo, L.; Lee, D.S.M.; Pauken, K.E.; Huang, A.C.; et al. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell 2016, 167, 1540–1554.e12. [Google Scholar] [CrossRef]
- Cassetta, L.; Kitamura, T. Targeting Tumor-Associated Macrophages as a Potential Strategy to Enhance the Response to Immune Checkpoint Inhibitors. Front. Cell Dev. Biol. 2018, 6, 38. [Google Scholar] [CrossRef]
- Arlauckas, S.P.; Garris, C.S.; Kohler, R.H.; Kitaoka, M.; Cuccarese, M.F.; Yang, K.S.; Miller, M.A.; Carlson, J.C.; Freeman, G.J.; Anthony, R.M.; et al. In vivo imaging reveals a tumor-associated macrophage–mediated resistance pathway in anti–PD-1 therapy. Sci. Transl. Med. 2017, 9, eaal3604. [Google Scholar] [CrossRef]
- Pathria, P.; Louis, T.L.; Varner, J.A. Targeting Tumor-Associated Macrophages in Cancer. Trends Immunol. 2019, 40, 310–327. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Skora, A.D.; Li, Z.; Liu, Q.; Tam, A.J.; Blosser, R.L.; Diaz, L.A.; Papadopoulos, N.; Kinzler, K.W.; Vogelstein, B.; et al. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc. Natl. Acad. Sci. USA 2014, 111, 11774–11779. [Google Scholar] [CrossRef]
- Gabrilovich, D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef]
- Saleh, R.; Elkord, E. Treg-Mediated acquired resistance to immune checkpoint inhibitors. Cancer Lett. 2019, 457, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Jin, J.Q.; Xia, L.; Xiao, T.; Mei, S.; Wang, X.; Huang, X.; Chen, J.; Liu, M.; Chen, C.; et al. Pharmacological inhibition of β-catenin/BCL9 interaction overcomes resistance to immune checkpoint blockades by modulating Treg cells. Sci. Adv. 2019, 5, eaau5240. [Google Scholar] [CrossRef]
- Wang, L.; Sfakianos, J.P.; Beaumont, K.G.; Akturk, G.; Horowitz, A.; Sebra, R.P.; Farkas, A.M.; Gnjatic, S.; Hake, A.; Izadmehr, S.; et al. Myeloid Cell–Associated Resistance to PD-1/PD-L1 Blockade in Urothelial Cancer Revealed through Bulk and Single-cell RNA Sequencing. Clin. Cancer Res. 2021, 27, 4287–4300. [Google Scholar] [CrossRef] [PubMed]
- Takeyama, Y.; Kato, M.; Tamada, S.; Azuma, Y.; Shimizu, Y.; Iguchi, T.; Yamasaki, T.; Gi, M.; Wanibuchi, H.; Nakatani, T. Myeloid-Derived suppressor cells are essential partners for immune checkpoint inhibitors in the treatment of cisplatin-resistant bladder cancer. Cancer Lett. 2020, 479, 89–99. [Google Scholar] [CrossRef]
- Li, T.; Liu, T.; Zhu, W.; Xie, S.; Zhao, Z.; Feng, B.; Guo, H.; Yang, R. Targeting MDSC for Immune-Checkpoint Blockade in Cancer Immunotherapy: Current Progress and New Prospects. Clin. Med. Insights Oncol. 2021, 15, 11795549211035540. [Google Scholar] [CrossRef]
- Anami, T.; Komohara, Y.; Miura, Y.; Yamanaka, K.; Kurahashi, R.; Segawa, T.; Motoshima, T.; Murakami, Y.; Yatsuda, J.; Yamaguchi, T.; et al. High T-Cell infiltration in tumor tissue and younger age predict the response to pembrolizumab in recurrent urothelial cancer. Med. Mol. Morphol. 2021, 54, 316–323. [Google Scholar] [CrossRef]
- Audisio, A.; Buttigliero, C.; Delcuratolo, M.D.; Parlagreco, E.; Audisio, M.; Ungaro, A.; Di Stefano, R.F.; Di Prima, L.; Turco, F.; Tucci, M. New Perspectives in the Medical Treatment of Non-Muscle-Invasive Bladder Cancer: Immune Checkpoint Inhibitors and Beyond. Cells 2022, 11, 357. [Google Scholar] [CrossRef]
- Rebuzzi, S.E.; Banna, G.L.; Murianni, V.; Damassi, A.; Giunta, E.F.; Fraggetta, F.; De Giorgi, U.; Cathomas, R.; Rescigno, P.; Brunelli, M.; et al. Prognostic and Predictive Factors in Advanced Urothelial Carcinoma Treated with Immune Checkpoint Inhibitors: A Review of the Current Evidence. Cancers 2021, 13, 5517. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.G.; Meghani, K.; Cooley, L.F.; McLaughlin, K.A.; Fall, L.A.; Yu, Y.; Castro, M.A.A.; Groeneveld, C.S.; de Reyniès, A.; Nazarov, V.I.; et al. Expression-Based subtypes define pathologic response to neoadjuvant immune-checkpoint inhibitors in muscle-invasive bladder cancer. Nat. Commun. 2023, 14, 2126. [Google Scholar] [CrossRef] [PubMed]
- van Wilpe, S.; Gerretsen, E.C.F.; van der Heijden, A.G.; de Vries, I.J.M.; Gerritsen, W.R.; Mehra, N. Prognostic and Predictive Value of Tumor-Infiltrating Immune Cells in Urothelial Cancer of the Bladder. Cancers 2020, 12, 2692. [Google Scholar] [CrossRef]
- Boll, L.M.; Vazquez Montes de Oca, S.; Camarena, M.E.; Castelo, R.; Bellmunt, J.; Perera-Bel, J.; Alba, M.M. Predicting immunotherapy response in advanced bladder cancer: A meta-analysis of six independent cohorts. bioRxiv 2024. [Google Scholar]
- Sha, D.; Jin, Z.; Budczies, J.; Kluck, K.; Stenzinger, A.; Sinicrope, F.A. Tumor Mutational Burden as a Predictive Biomarker in Solid Tumors. Cancer Discov. 2020, 10, 1808–1825. [Google Scholar] [CrossRef]
- Jardim, D.L.; Goodman, A.; de Melo Gagliato, D.; Kurzrock, R. The Challenges of Tumor Mutational Burden as an Immunotherapy Biomarker. Cancer Cell 2021, 39, 154–173. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Marcus, L.; Fashoyin-Aje, L.A.; Donoghue, M.; Yuan, M.; Rodriguez, L.; Gallagher, P.S.; Philip, R.; Ghosh, S.; Theoret, M.R.; Beaver, J.A.; et al. FDA Approval Summary: Pembrolizumab for the Treatment of Tumor Mutational Burden–High Solid Tumors. Clin. Cancer Res. 2021, 27, 4685–4689. [Google Scholar] [CrossRef]
- Bakaloudi, D.R.; Talukder, R.; Makrakis, D.; Diamantopoulos, L.N.; Patgunarajah, U.; Thomas, V.M.; Jindal, T.; Brown, J.R.; Miletic, M.; Johnson, J.; et al. Role of tumor mutational burden (TMB) and microsatellite instability (MSI) in patients (pts) with advanced urothelial carcinoma (aUC) treated with immune checkpoint inhibitor (ICI). J. Clin. Oncol. 2024, 42, 654. [Google Scholar] [CrossRef]
- Samstein, R.M.; Lee, C.-H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Gooden, M.J.M.; de Bock, G.H.; Leffers, N.; Daemen, T.; Nijman, H.W. The prognostic influence of tumour-infiltrating lymphocytes in cancer: A systematic review with meta-analysis. Br. J. Cancer 2011, 105, 93–103. [Google Scholar] [CrossRef]
- Lobo, N.; Mount, C.; Omar, K.; Nair, R.; Thurairaja, R.; Khan, M.S. Landmarks in the treatment of muscle-invasive bladder cancer. Nat. Rev. Urol. 2017, 14, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.-M.; Black, P.C.; Eigl, B.J. Predictive Biomarkers for Checkpoint Blockade in Urothelial Cancer: A Systematic Review. J. Urol. 2019, 202, 49–56. [Google Scholar] [CrossRef]
- Mandrekar, S.J.; Sargent, D.J. Clinical Trial Designs for Predictive Biomarker Validation: Theoretical Considerations and Practical Challenges. J. Clin. Oncol. 2009, 27, 4027–4034. [Google Scholar] [CrossRef]
- Pignon, J.-C.; Jegede, O.; Shukla, S.A.; Braun, D.A.; Horak, C.E.; Wind-Rotolo, M.; Ishii, Y.; Catalano, P.J.; Grosha, J.; Flaifel, A.; et al. irRECIST for the Evaluation of Candidate Biomarkers of Response to Nivolumab in Metastatic Clear Cell Renal Cell Carcinoma: Analysis of a Phase II Prospective Clinical Trial. Clin. Cancer Res. 2019, 25, 2174–2184. [Google Scholar] [CrossRef]
- Powles, T.; Sridhar, S.S.; Loriot, Y.; Bellmunt, J.; Mu, X.J.; Ching, K.A.; Pu, J.; Sternberg, C.N.; Petrylak, D.P.; Tambaro, R.; et al. Avelumab maintenance in advanced urothelial carcinoma: Biomarker analysis of the phase 3 JAVELIN Bladder 100 trial. Nat. Med. 2021, 27, 2200–2211. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, B.A.; Di Maio, M.; Cerbone, L.; Maiello, E.; Procopio, G.; Roviello, G.; Meet, U.R.O.G. Significance of PD-L1 in Metastatic Urothelial Carcinoma Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2024, 7, e241215. [Google Scholar] [CrossRef] [PubMed]
- Schepisi, G.; Santoni, M.; Massari, F.; Gurioli, G.; Salvi, S.; Conteduca, V.; Montironi, R.; De Giorgi, U. Urothelial Cancer: Inflammatory Mediators and Implications for Immunotherapy. BioDrugs 2016, 30, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Havel, J.J.; Chowell, D.; Chan, T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef]
- Holder, A.M.; Dedeilia, A.; Sierra-Davidson, K.; Cohen, S.; Liu, D.; Parikh, A.; Boland, G.M. Defining clinically useful biomarkers of immune checkpoint inhibitors in solid tumours. Nat. Rev. Cancer 2024, 24, 498–512. [Google Scholar] [CrossRef]
- Gibney, G.T.; Weiner, L.M.; Atkins, M.B. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016, 17, e542–e551. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017, 17, 97–111. [Google Scholar] [CrossRef]
- Kroemer, G.; Galassi, C.; Zitvogel, L.; Galluzzi, L. Immunogenic cell stress and death. Nat. Immunol. 2022, 23, 487–500. [Google Scholar] [CrossRef]
- Olson, D.; Younan, P.; Liu, B.; Blahnik-Fagan, G.; Gosink, J.; Snead, K.; Tenn, E.; Hensley, K.; Sahetya, D.; Nesterova, A.; et al. 1187 Enfortumab vedotin induces immunogenic cell death, elicits antitumor immune memory, and shows enhanced preclinical activity in combination with immune checkpoint inhibitors. J. ImmunoTher. Cancer 2022, 10, A1231. [Google Scholar] [CrossRef]
- Hoimes, C.J.; Flaig, T.W.; Milowsky, M.I.; Friedlander, T.W.; Bilen, M.A.; Gupta, S.; Srinivas, S.; Merchan, J.R.; McKay, R.R.; Petrylak, D.P. Enfortumab vedotin plus pembrolizumab in previously untreated advanced urothelial cancer. J. Clin. Oncol. 2023, 41, 22–31. [Google Scholar] [CrossRef]
- Ghatalia, P.; Plimack, E.R. Harnessing potent therapies with care: Enfortumab vedotin plus pembrolizumab for advanced-stage urothelial carcinoma. Nat. Rev. Clin. Oncol. 2023, 20, 818–819. [Google Scholar] [CrossRef]
- Jhunjhunwala, S.; Hammer, C.; Delamarre, L. Antigen presentation in cancer: Insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer 2021, 21, 298–312. [Google Scholar] [CrossRef]
- Lopez, M.; Ghidouche, A.; Rochas, C.; Godelaine, D.; Carrasco, J.; Colau, D.; Hames, G.; Montero-Julian, F.A.; Coulie, P.G.; Olive, D. Identification of a naturally processed HLA-A* 02: 01-restricted CTL epitope from the human tumor-associated antigen Nectin-4. Cancer Immunol. Immunother. 2016, 65, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Ueki, H.; Hinata, N.; Kitagawa, K.; Hara, T.; Terakawa, T.; Furukawa, J.; Harada, K.; Nakano, Y.; Komatsu, M.; Fujisawa, M.; et al. Expressions of PD-L1 and Nectin-4 in urothelial cancer patients treated with pembrolizumab. Clin. Transl. Oncol. 2022, 24, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; Sasaki, M.; Scharer, C.D.; Kissick, H.T.; Patterson, D.G.; Magliocca, K.R.; Seykora, J.T.; Sapkota, B.; Gutman, D.A.; Cooper, L.A.; et al. Phosphoinositide 3-Kinase Signaling Can Modulate MHC Class I and II Expression. Mol. Cancer Res. 2019, 17, 2395–2409. [Google Scholar] [CrossRef] [PubMed]
- Nicolò, E.; Giugliano, F.; Ascione, L.; Tarantino, P.; Corti, C.; Tolaney, S.M.; Cristofanilli, M.; Curigliano, G. Combining antibody-drug conjugates with immunotherapy in solid tumors: Current landscape and future perspectives. Cancer Treat. Rev. 2022, 106, 102395. [Google Scholar] [CrossRef]
- Iwata, T.N.; Ishii, C.; Ishida, S.; Ogitani, Y.; Wada, T.; Agatsuma, T. A HER2-Targeting Antibody–Drug Conjugate, Trastuzumab Deruxtecan (DS-8201a), Enhances Antitumor Immunity in a Mouse Model. Mol. Cancer Ther. 2018, 17, 1494–1503. [Google Scholar] [CrossRef]
- Jindal, T.; Zhang, L.; Jiang, C.; Kilari, D.; Alhalabi, O.; Nizam, A.; Basu, A.; Bilen, M.A.; Zakharia, Y.; Milowsky, M.I.; et al. Independent biomarkers predictive of outcomes with enfortumab vedotin (EV) in patients (pts) with advanced urothelial carcinoma (aUC): Analysis of the UNITE study. J. Clin. Oncol. 2023, 41, 4573. [Google Scholar] [CrossRef]
- Vannini, A.; Leoni, V.; Barboni, C.; Sanapo, M.; Zaghini, A.; Malatesta, P.; Campadelli-Fiume, G.; Gianni, T. αvβ3-integrin regulates PD-L1 expression and is involved in cancer immune evasion. Proc. Natl. Acad. Sci. USA 2019, 116, 20141–20150. [Google Scholar] [CrossRef] [PubMed]
- Manieri, N.A.; Chiang, E.Y.; Grogan, J.L. TIGIT: A Key Inhibitor of the Cancer Immunity Cycle. Trends Immunol. 2017, 38, 20–28. [Google Scholar] [CrossRef]
- Cho, B.C.; Abreu, D.R.; Hussein, M.; Cobo, M.; Patel, A.J.; Secen, N.; Lee, K.H.; Massuti, B.; Hiret, S.; Yang, J.C.H.; et al. Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): Primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol. 2022, 23, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Banta, K.L.; Xu, X.; Chitre, A.S.; Au-Yeung, A.; Takahashi, C.; O’Gorman, W.E.; Wu, T.D.; Mittman, S.; Cubas, R.; Comps-Agrar, L.; et al. Mechanistic convergence of the TIGIT and PD-1 inhibitory pathways necessitates co-blockade to optimize anti-tumor CD8+ T cell responses. Immunity 2022, 55, 512–526.e9. [Google Scholar] [CrossRef]
- Kraehenbuehl, L.; Weng, C.-H.; Eghbali, S.; Wolchok, J.D.; Merghoub, T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat. Rev. Clin. Oncol. 2022, 19, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Harden, K.; Gonzalez, L.C.; Francesco, M.; Chiang, E.; Irving, B.; Tom, I.; Ivelja, S.; Refino, C.J.; Clark, H.; et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2009, 10, 48–57. [Google Scholar] [CrossRef]
- Ge, Z.; Peppelenbosch, M.P.; Sprengers, D.; Kwekkeboom, J. TIGIT, the Next Step Towards Successful Combination Immune Checkpoint Therapy in Cancer. Front. Immunol. 2021, 12, 699895. [Google Scholar] [CrossRef]
- Stanietsky, N.; Simic, H.; Arapovic, J.; Toporik, A.; Levy, O.; Novik, A.; Levine, Z.; Beiman, M.; Dassa, L.; Achdout, H.; et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 17858–17863. [Google Scholar] [CrossRef]
- Reches, A.; Ophir, Y.; Stein, N.; Kol, I.; Isaacson, B.; Charpak Amikam, Y.; Elnekave, A.; Tsukerman, P.; Kucan Brlic, P.; Lenac, T.; et al. Nectin4 is a novel TIGIT ligand which combines checkpoint inhibition and tumor specificity. J. Immunother. Cancer 2020, 8, e000266. [Google Scholar] [CrossRef]
- Ganguli, N.; Kumari, P.; Dash, S.; Samanta, D. Molecular and structural basis of TIGIT: Nectin-4 interaction, a recently discovered pathway crucial for cancer immunotherapy. Biochem. Biophys. Res. Commun. 2023, 677, 31–37. [Google Scholar] [CrossRef]
- Tomiyama, E.; Fujita, K.; Rodriguez Pena, M.D.; Taheri, D.; Banno, E.; Kato, T.; Hatano, K.; Kawashima, A.; Ujike, T.; Uemura, M.; et al. Expression of Nectin-4 and PD-L1 in Upper Tract Urothelial Carcinoma. Int. J. Mol. Sci. 2020, 21, 5390. [Google Scholar] [CrossRef] [PubMed]
- Bahlinger, V.; Branz, A.; Strissel, P.L.; Strick, R.; Lange, F.; Geppert, C.I.; Klümper, N.; Hölzel, M.; Wach, S.; Taubert, H.; et al. Associations of TACSTD2/TROP2 and NECTIN-4/NECTIN-4 with molecular subtypes, PD-L1 expression, and FGFR3 mutational status in two advanced urothelial bladder cancer cohorts. Histopathology 2024, 84, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Esteva, F.J.; Beresford, M.; Saura, C.; De Laurentiis, M.; Kim, S.-B.; Im, S.-A.; Wang, Y.; Salgado, R.; Mani, A.; et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): A phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. 2020, 21, 1283–1295. [Google Scholar] [CrossRef]
- Nakajima, S.; Mimura, K.; Matsumoto, T.; Thar Min, A.K.; Ito, M.; Nakano, H.; Neupane, P.; Kanke, Y.; Okayama, H.; Saito, M. The effects of T-DXd on the expression of HLA class I and chemokines CXCL9/10/11 in HER2-overexpressing gastric cancer cells. Sci. Rep. 2021, 11, 16891. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, L.; Wilson, M.; Mettetal, J.; Proia, T. 1115 T-DXd increases immune cell infiltration and enhances activity of immune checkpoint blockade in murine tumor models. J. ImmunoTher. Cancer 2022, 10, A1158. [Google Scholar] [CrossRef]
- Herbst Roy, S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios Carlos, H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1–Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef]
- Bellmunt, J.; Nadal, R. Enfortumab vedotin and pembrolizumab combination as a relevant game changer in urothelial carcinoma: What is left behind? Med 2024, 5, 490–492. [Google Scholar] [CrossRef]
- Santoni, M.; Takeshita, H.; Massari, F.; Bamias, A.; Cerbone, L.; Fiala, O.; Mollica, V.; Buti, S.; Santoni, A.; Bellmunt, J. Pembrolizumab plus enfortumab vedotin in urothelial cancer. Nat. Rev. Urol. 2024, 21, 387–388. [Google Scholar] [CrossRef]
- Brown, J.R.; Koshkin, V.S. Therapies After Progression on Enfortumab Vedotin and Pembrolizumab: Navigating Second-line Options for Metastatic Urothelial Carcinoma in the New Treatment Landscape. Eur. Urol. Focus 2024, 10, 231–233. [Google Scholar] [CrossRef]
| Study | Regimen | Inclusion Criteria | HR (OS or PFS) | ORR (%) | DoR (months) | mOS (months) | mPFS (months) | Citation |
|---|---|---|---|---|---|---|---|---|
| EV101 Phase I NCT02091999 | EV | ≥1 L chemo. and Nectin-4-positive solid tumors, including mUC * | N/A | 43 | 7.4 | 12.3 | 5.4 | [55] |
| NCT03070990 | EV | 2 L la/mUC Dose escalation in Japanese patients with la/mUC | N/A | 35.3 | N/A | N/A | N/A | [36] |
| EV-201 Phase II NCT03219333 | EV | 2 L UC | N/A | 52 (95% CI 41, 62) | 10.9 (95% CI 5.78, NR) | 14.7 (95% CI 10.5, 18.2) | 5.8 (95% CI 5.0, 8.3) | [37] |
| EV-301 Phase III NCT03474107 | EV vs. Chemo. | 2 L la/mUC | 0.70 (95% CI 0.56, 0.89) | 40.6 vs. 17.9 | 7.39 vs. 8.11 | 12.88 vs. 8.97 | 5.55 vs. 3.71 | [17] |
| EV-103/KEYNOTE-869 Cohort K Phase Ib/II NCT03288545 | EV +/− pembro. | 1 L la/mUC | N/A | 64.5 vs. 45.2 | Not reached vs. 13.2 | 22.3 vs. 21.7 | Not reached vs. 8 | [59,60] |
| 516-003 trial Phase II NCT03606174 | EV + sitravatinib + pembro.? | 2 L la/mUC | N/A | 25 | 11.8 | 10.8 | 4.0 | [61] |
| EV-302 Phase III NCT04223856 | EV + pembro. vs. gemcitabine + platinum | 1 L la/mUC | 0.45 (95% CI 0.38, 0.54) | 67.7 vs. 44.4 | Not reached vs. 7.0 | 31.5 vs. 16.1 | 12.5 vs. 6.3 | [27] |
| Phase IINCT06356155 | EV +/− pembro. as neoadjuvant | Not yet recruiting | N/A | N/A | N/A | N/A | N/A | ClinicalTrials.com |
| Phase I/II NCT05845814 | EV + pembro. plus investigational agents (anti-LAG-3 favezelimab or anti-Tight vibostolimab) Vs. EV + pembro. | Active, not recruiting | N/A | N/A | N/A | N/A | N/A | ClinicalTrials.com |
| Study | Regimen | Inclusion Criteria | HR (mPFS) | ORR (months) | DoR (months) | mOS (months) | mPFS (months) | Citation |
|---|---|---|---|---|---|---|---|---|
| KEYNOTE-052 Phase II NCT02335424 | Pembrolizumab | 1 L la/mUC | N/A | 24 | NR (95% CI 9, NR) | N/A | N/A | [75] |
| KEYNOTE-052 Phase II NCT02335424 (long-term follow up) | Pembrolizumab | 1 L la/mUC | N/A | 28.6 (95% CI 24.1, 33.5) | 30.1 (95% CI 18.1, NR) | 11.3 (95% CI 9.7, 13.1) | 2.2 | [76] |
| KEYNOTE-045 Phase III NCT02256436 (two years follow-up) | Pembrolizumab vs. chemotherapy | 2 L mUC | N/A | 21.1 vs. 11.0% | Not reached vs. 4.4 | 10.3 vs. 7.4 | 2.1 vs. 3.3 | [77] |
| KEYNOTE-057 | Pembrolizumab | High-risk, non-muscle invasive UC | N/A | 41 CR | N/A | N/A | N/A | [68] |
| KEYNOTE-361 Phase III NCT02853305 | Pembrolizumab + chemo vs. pembrolizumab vs. chemo | 1 L mUC | 0.78 (95% CI 0.65, 0.93) (excludes monotherapy) | 54.7 vs. 30.3 vs. 44.9 | 8.5 vs. 28.2 vs. 6.2 | 17.0 vs. 15.6 vs. 14.3 | 8.3 vs. 7.1 (excludes monotherapy) | [69] |
| Phase I/II NCT02437370 | Pembrolizumab + either docetaxel or gemcitabine | 2 L mUC | N/A | 44 vs. 45 | N/A | N/A | 13.3 vs. 5.9 | [78] |
| PEANUT Phase II NCT03464734 | Pembrolizumab + nab-paclitaxel | ≥2 L mUC | N/A | 38.6 (95% CI 27, 51) | Not reached | Not reached (media follow-up 8.9) | 5.9 (95% CI 3.1, 11.5) | [79] |
| Phase II NCT02581982 | Pembrolizumab + paclitaxel | 2 L mUC | N/A | 33 | N/A | 11.7 months (95% CI 8.7, NR) | 6 PFS 46.8 | [80] |
| TROPHY U-01 cohort 3 Phase II NCT03547973 | Pembrolizumab + sacituzumab govitecan | mUC after platinum | N/A | 41 (95% CI, 26.3 to 57.9; 20% complete response rate) | 11.1 (95% CI 4.8, NE) | 12.7 (10.7, NE) | 5.3 (95% CI 3.4–10.2) | [81] |
| RC48G001 Phase I/II NCT04879329 | Disitamab vedotin +/− pembrolizumab | HER2+ 1 L mUC | Not recruiting | N/A | N/A | N/A | N/A | ClinicalTrials.gov |
| Phase III NCT05911295 | Disitamab vedotin + pembrolizumab vs. chemotherapy | HER2+ 1 L la/mUC | Not recruiting | N/A | N/A | N/A | N/A | ClinicalTrials.gov |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, C.; Patterson, K.M.; Friedman, D.; Bacot, S.M.; Feldman, G.M.; Wang, T. Mechanistic Insights into the Successful Development of Combination Therapy of Enfortumab Vedotin and Pembrolizumab for the Treatment of Locally Advanced or Metastatic Urothelial Cancer. Cancers 2024, 16, 3071. https://doi.org/10.3390/cancers16173071
Taylor C, Patterson KM, Friedman D, Bacot SM, Feldman GM, Wang T. Mechanistic Insights into the Successful Development of Combination Therapy of Enfortumab Vedotin and Pembrolizumab for the Treatment of Locally Advanced or Metastatic Urothelial Cancer. Cancers. 2024; 16(17):3071. https://doi.org/10.3390/cancers16173071
Chicago/Turabian StyleTaylor, Caroline, Kamai M. Patterson, Devira Friedman, Silvia M. Bacot, Gerald M. Feldman, and Tao Wang. 2024. "Mechanistic Insights into the Successful Development of Combination Therapy of Enfortumab Vedotin and Pembrolizumab for the Treatment of Locally Advanced or Metastatic Urothelial Cancer" Cancers 16, no. 17: 3071. https://doi.org/10.3390/cancers16173071
APA StyleTaylor, C., Patterson, K. M., Friedman, D., Bacot, S. M., Feldman, G. M., & Wang, T. (2024). Mechanistic Insights into the Successful Development of Combination Therapy of Enfortumab Vedotin and Pembrolizumab for the Treatment of Locally Advanced or Metastatic Urothelial Cancer. Cancers, 16(17), 3071. https://doi.org/10.3390/cancers16173071





