Cabozantinib Plus Nivolumab in Adult Patients with Advanced or Metastatic Renal Cell Carcinoma: A Retrospective, Non-Interventional Study in a Real-World Cohort/GUARDIANS Project
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort and Treatment
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
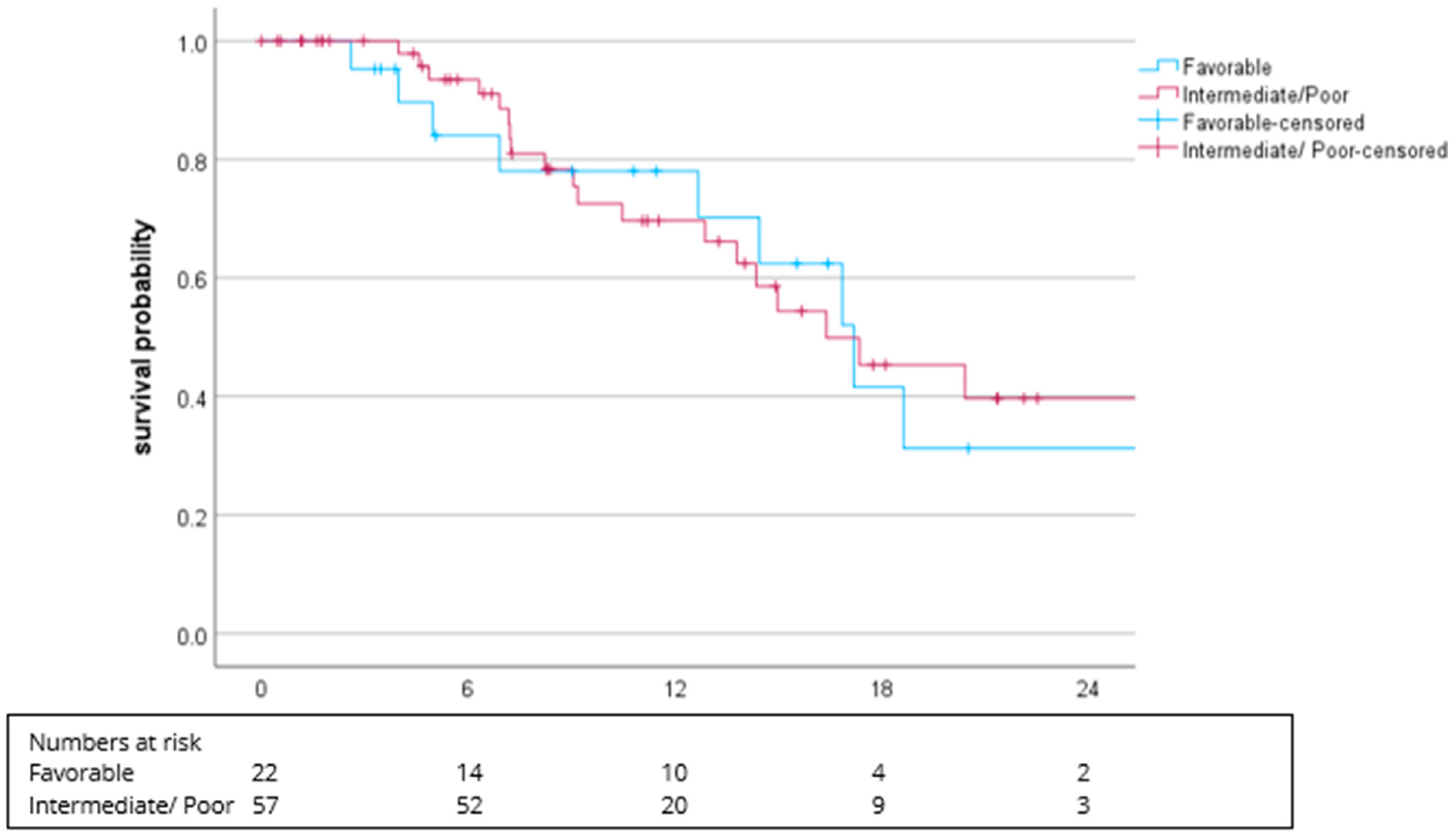
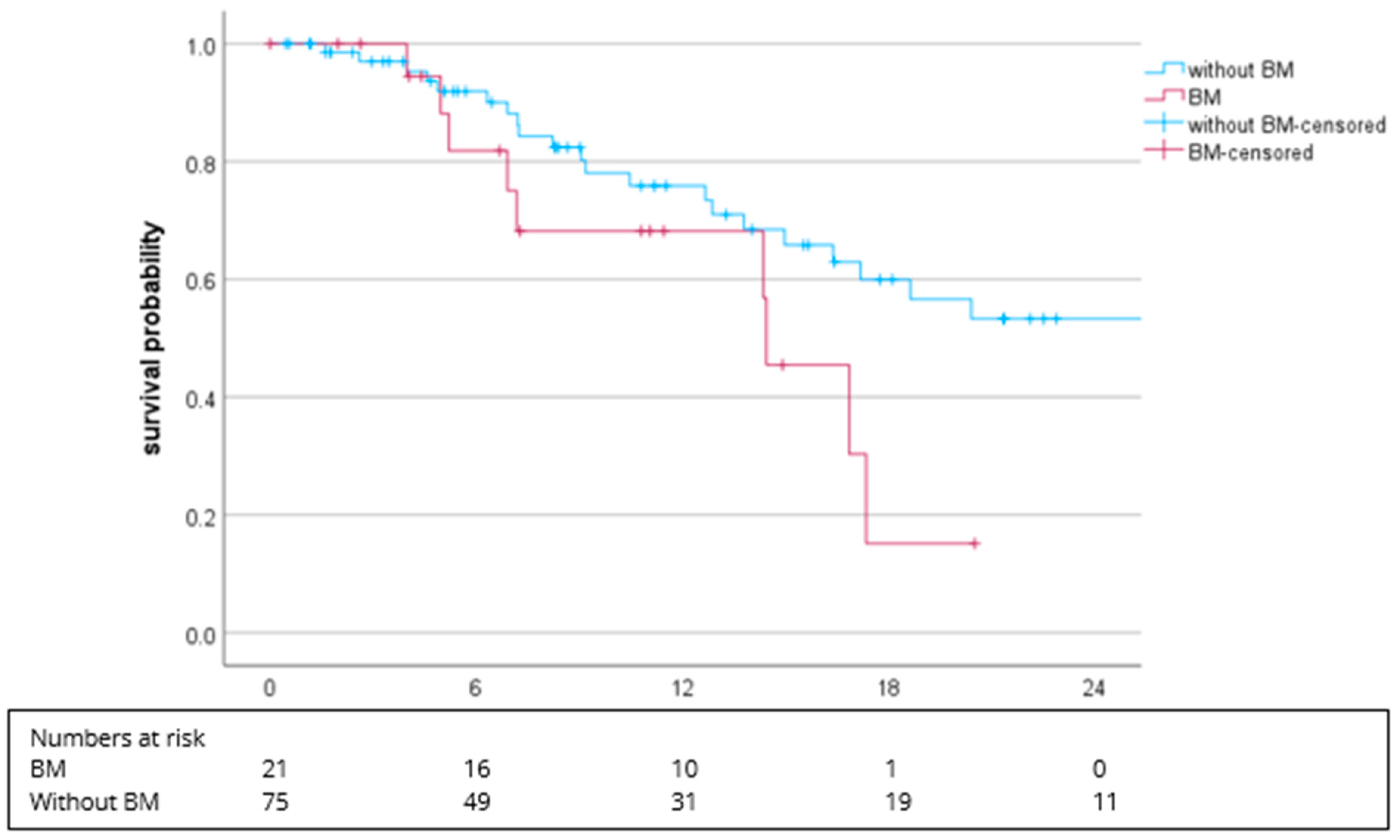
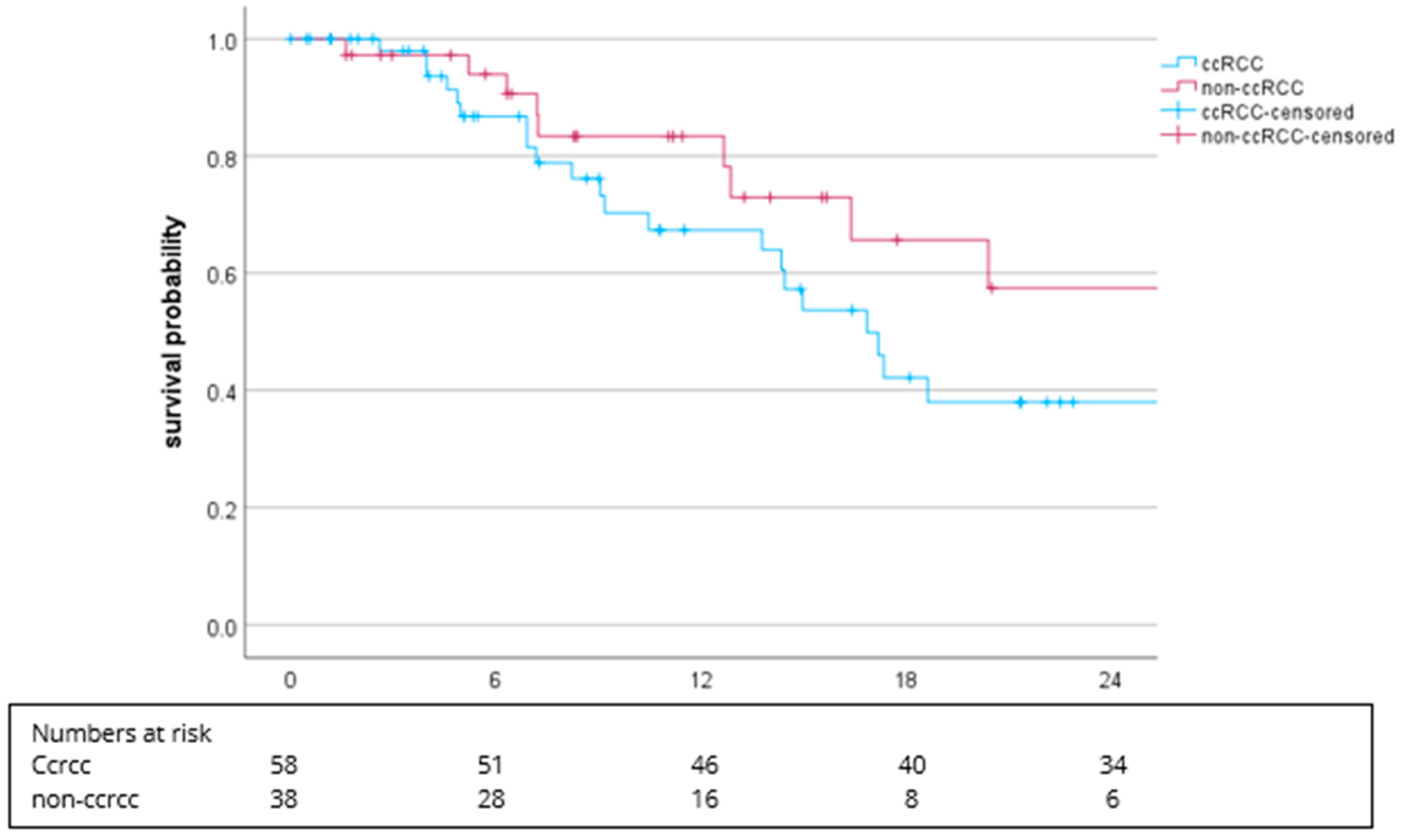
3.2. Efficacy
3.3. Safety/Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of Renal Cell Carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef]
- Humphrey, P.A.; Moch, H.; Cubilla, A.L.; Ulbright, T.M.; Reuter, V.E. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur. Urol. 2016, 70, 106–119. [Google Scholar] [CrossRef]
- Amato, R.J. Chemotherapy for renal cell carcinoma. Semin. Oncol. 2000, 27, 177–186. [Google Scholar]
- Kim, H.; Shim, B.Y.; Lee, S.J.; Lee, J.Y.; Lee, H.J.; Kim, I.H. Loss of Von Hippel-Lindau (VHL) Tumor Suppressor Gene Function: VHL-HIF Pathway and Advances in Treatments for Metastatic Renal Cell Carcinoma (RCC). Int. J. Mol. Sci. 2021, 22, 9795. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.; Lister, J.; Neumann, M.; Wiecek, W.; Fu, S.; Vataire, A.L.; Sostar, J.; Huang, S.; Marteau, F. Cabozantinib Versus Standard-of-Care Comparators in the Treatment of Advanced/Metastatic Renal Cell Carcinoma in Treatment-naïve Patients: A Systematic Review and Network Meta-Analysis. Target. Oncol. 2018, 13, 205–216. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Halabi, S.; Sanford, B.L.; Hahn, O.; Michaelson, M.D.; Walsh, M.K.; Feldman, D.R.; Olencki, T.; Picus, J.; Small, E.J.; et al. Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial. J. Clin. Oncol. 2017, 35, 591–597. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Escudier, B.; Powles, T.; Tannir, N.M.; Mainwaring, P.N.; Rini, B.I.; Hammers, H.J.; Donskov, F.; Roth, B.J.; Peltola, K.; et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): Final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016, 17, 917–927. [Google Scholar] [CrossRef]
- Saeed, A.; Phadnis, M.; Park, R.; Sun, W.; Al-Rajabi, R.M.d.T.; Baranda, J.C.; Williamson, S.K.; Collins, Z.; Firth-Braun, J.; Saeed, A.; et al. Cabozantinib (cabo) combined with durvalumab (durva) in gastroesophageal (GE) cancer and other gastrointestinal (GI) malignancies: Preliminary phase Ib CAMILLA study results. J. Clin. Oncol. 2020, 38, 4563. [Google Scholar] [CrossRef]
- Bergerot, P.; Lamb, P.; Wang, E.; Pal, S.K. Cabozantinib in Combination with Immunotherapy for Advanced Renal Cell Carcinoma and Urothelial Carcinoma: Rationale and Clinical Evidence. Mol. Cancer Ther. 2019, 18, 2185–2193. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Horner, J.W.; Paul, E.; Shang, X.; Troncoso, P.; Deng, P.; Jiang, S.; Chang, Q.; Spring, D.J.; Sharma, P.; et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 2017, 543, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Apolo, A.B.; Nadal, R.; Tomita, Y.; Davarpanah, N.N.; Cordes, L.M.; Steinberg, S.M.; Cao, L.; Parnes, H.L.; Costello, R.; Merino, M.J.; et al. Cabozantinib in patients with platinum-refractory metastatic urothelial carcinoma: An open-label, single-centre, phase 2 trial. Lancet Oncol. 2020, 21, 1099–1109. [Google Scholar] [CrossRef]
- Grimm, M.O.; Leucht, K.; Grünwald, V.; Foller, S. New First Line Treatment Options of Clear Cell Renal Cell Cancer Patients with PD-1 or PD-L1 Immune-Checkpoint Inhibitor-Based Combination Therapies. J. Clin. Med. 2020, 9, 565. [Google Scholar] [CrossRef]
- Gulati, S.; Labaki, C.; Karachaliou, G.S.; Choueiri, T.K.; Zhang, T. First-Line Treatments for Metastatic Clear Cell Renal Cell Carcinoma: An Ever-Enlarging Landscape. Oncologist 2022, 27, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Motzer, R.J. Systemic Therapy for Metastatic Renal-Cell Carcinoma. N. Engl. J. Med. 2017, 376, 354–366. [Google Scholar] [CrossRef]
- Motzer, R.; Alekseev, B.; Rha, S.Y.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Hutson, T.E.; Kopyltsov, E.; Méndez-Vidal, M.J.; et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Waddell, T.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulieres, D.; Melichar, B.; et al. Pembrolizumab plus axitinib versus sunitinib as first-line therapy for advanced clear cell renal cell carcinoma: 5-year analysis of KEYNOTE-426. J. Clin. Oncol. 2023, 41, LBA4501. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Motzer, R.J.; Rini, B.I.; Haanen, J.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Gravis-Mescam, G.; Uemura, M.; Lee, J.L.; et al. Updated efficacy results from the JAVELIN Renal 101 trial: First-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann. Oncol. 2020, 31, 1030–1039. [Google Scholar] [CrossRef]
- Motzer, R.J.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Hutson, T.E.; Alekseev, B.; Rha, S.Y.; Merchan, J.; Goh, J.C.; et al. Lenvatinib Plus Pembrolizumab Versus Sunitinib in First-Line Treatment of Advanced Renal Cell Carcinoma: Final Prespecified Overall Survival Analysis of CLEAR, a Phase III Study. J. Clin. Oncol. 2024, 42, 1222–1228. [Google Scholar] [CrossRef]
- Motzer, R.J.; Penkov, K.; Uemura, H.; Campbell, M.T.; Kollmannsberger, C.K.; Lee, J.-L.; Venugopal, B.; Eertwegh, A.V.D.; Negrier, S.; Gurney, H.; et al. Avelumab + axitinib vs sunitinib in patients (pts) with advanced renal cell carcinoma (aRCC): Final overall survival (OS) analysis from the JAVELIN Renal 101 phase 3 trial. J. Clin. Oncol. 2024, 42, 4508. [Google Scholar] [CrossRef]
- Motzer, R.J.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Shah, A.Y.; Suárez, C.; Hamzaj, A.; Porta, C.; Hocking, C.M.; et al. Nivolumab plus cabozantinib versus sunitinib in first-line treatment for advanced renal cell carcinoma (CheckMate 9ER): Long-term follow-up results from an open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Bourlon, M.T.; Escudier, B.; Burotto, M.; Powles, T.; Apolo, A.B.; Shah, A.Y.; Porta, C.; Suárez, C.; Barrios, C.H.; Richardet, M.; et al. Nivolumab plus cabozantinib (N+C) vs sunitinib (S) for previously untreated advanced renal cell carcinoma (aRCC): Results from 55-month follow-up of the CheckMate 9ER trial. J. Clin. Oncol. 2024, 42, 362. [Google Scholar] [CrossRef]
- Lee, C.H.; Voss, M.H.; Carlo, M.I.; Chen, Y.B.; Zucker, M.; Knezevic, A.; Lefkowitz, R.A.; Shapnik, N.; Dadoun, C.; Reznik, E.; et al. Phase II Trial of Cabozantinib Plus Nivolumab in Patients With Non-Clear-Cell Renal Cell Carcinoma and Genomic Correlates. J. Clin. Oncol. 2022, 40, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Apolo, A.B.; Nadal, R.; Girardi, D.M.; Niglio, S.A.; Ley, L.; Cordes, L.M.; Steinberg, S.M.; Sierra Ortiz, O.; Cadena, J.; Diaz, C.; et al. Phase I Study of Cabozantinib and Nivolumab Alone or With Ipilimumab for Advanced or Metastatic Urothelial Carcinoma and Other Genitourinary Tumors. J. Clin. Oncol. 2020, 38, 3672–3684. [Google Scholar] [CrossRef]
- Powles, T.; Burotto, M.; Escudier, B.; Apolo, A.B.; Bourlon, M.T.; Shah, A.Y.; Suárez, C.; Porta, C.; Barrios, C.H.; Richardet, M.; et al. Nivolumab plus cabozantinib versus sunitinib for first-line treatment of advanced renal cell carcinoma: Extended follow-up from the phase III randomised CheckMate 9ER trial. ESMO Open 2024, 9, 102994. [Google Scholar] [CrossRef]
- Yuasa, T.; Urakami, S.; Yamamoto, S.; Yonese, J.; Saito, K.; Takahashi, S.; Hatake, K.; Fukui, I. Treatment outcome and prognostic factors in renal cell cancer patients with bone metastasis. Clin. Exp. Metastasis 2011, 28, 405–411. [Google Scholar] [CrossRef]
- Toyoda, Y.; Shinohara, N.; Harabayashi, T.; Abe, T.; Akino, T.; Sazawa, A.; Nonomura, K. Survival and prognostic classification of patients with metastatic renal cell carcinoma of bone. Eur. Urol. 2007, 52, 163–168. [Google Scholar] [CrossRef]
- Grünwald, V.; Eberhardt, B.; Bex, A.; Flörcken, A.; Gauler, T.; Derlin, T.; Panzica, M.; Dürr, H.R.; Grötz, K.A.; Giles, R.H.; et al. An interdisciplinary consensus on the management of bone metastases from renal cell carcinoma. Nat. Rev. Urol. 2018, 15, 511–521. [Google Scholar] [CrossRef]

| Characteristic, No. (%) | All Patients (N = 96) | Patients with ccRCC (N = 61) | Patients with Non-ccRCC (N = 35) |
|---|---|---|---|
| Median age at start of nivolumab/cabozantinib in years (range) | 66.0 (22–85) | 67.1 (35–85) | 65.4 (22–78) |
| Age < 75 years | 80 (83.3) | 53 (86.9) | 27 (77.1) |
| Age ≥ 75 years | 11 (11.5) | 5 (8.2) | 6 (17.1) |
| Unknown | 5 (5.2) | 3 (4.9) | 2 (5.7) |
| Gender | |||
| Male | 64 (66.6) | 39 (63.9) | 25 (71.4) |
| Female | 32 (33.3) | 22 (36.1) | 10 (28.6) |
| Prior definitive treatment | |||
| Yes | 58 (60.4) | 37 (60.6) | 21 (60) |
| No | 38 (39.6) | 24 (39.4) | 14 (40) |
| T-status | |||
| T1 | 4 (4.2) | 0 (0) | 4 (11.4) |
| T2 | 8 (8.3) | 6 (9.8) | 2 (5.7) |
| T3 | 24 (25) | 18 (29.5) | 6 (17.1) |
| T4 | 12 (12.5) | 7 (11.5) | 5 (8.2) |
| Unknown | 48 (49.0) | 30 (49.2) | 18 (51.4) |
| N-status | |||
| N0 | 31 (32.2) | 21 (34.4) | 10 (28.6) |
| N1 (regional) | 19 (19.8) | 13 (21.3) | 6 (17.1) |
| N2 (non-regional) | 29 (30.2) | 19 (31.1) | 10 (28.6) |
| Unknown | 17 (17.7) | 8 (13.1) | 9 (25.7) |
| M-Status | |||
| M0 | 12 (12.5) | 8 (13.1) | 4 (11.4) |
| M1 | 84 (87.5) | 53 (86.9) | 31 (88.5) |
| ECOG performance status score | |||
| 0 | 40 (41.7) | 24 (39.4) | 16 (45.7) |
| 1 | 26 (27.1) | 11 (18.0) | 15 (42.9) |
| 2 | 8 (8.3) | 8 (13.1) | 0 (0) |
| 3 | 6 (6.3) | 6 (9.8) | 0 (0) |
| 4 | 0 (0) | 0 (0) | 0 (0) |
| Unknown | 16 (16.7) | 12 (19.7) | 4 (6.5) |
| IMDC | |||
| Favorable (0 points) | 22 (22.9) | 18 (29.5) | 4 (11.4) |
| Intermediate (1–2 points) | 41 (42.7) | 23 (37.7) | 18 (51.4) |
| Poor (≥3 points) | 16 (16.7) | 13 (21.3) | 3 (8.6) |
| Unknown | 17 (17.7) | 7 (11.4) | 10 (28.6) |
| Site of metastases | |||
| Lymph nodes | 48 (50) | 32 (52.4) | 16 (45.7) |
| Lung | 53 (55.2) | 40 (65.6) | 13 (37.1) |
| Bone | 39 (40.6) | 25 (41.0) | 14 (40) |
| Liver | 19 (19.8) | 8 (13.1) | 11(31.4) |
| Adrenal | 14 (14.6) | 12 (19.7) | 2 (5.7) |
| Brain | 13 (13.5) | 8 (13.1) | 5 (14.2) |
| Other | 15 (15.6) | 12 (19.7) | 3 (8.6) |
| No. (%) | All Patients (N = 96) |
|---|---|
| ORR | 44 (45.8) |
| CR | 2 (2.0) |
| PR | 42 (43.8) |
| SD | 31 (32.3) |
| PD | 8 (8.3) |
| Not evaluable | 13 (13.5) |
| DCR | 75 (78.1) |
| No. (%) | All Patients (N = 96) |
|---|---|
| Treatment-related adverse events, any grade | 79 (82.3) |
| Grade ≥ 3 TRAEs | 40 (41.7) |
| Treatment-related adverse events resulting in any treatment discontinuation | 24 (25) |
| Treatment-related adverse events leading to death | 1 (1.0) |
| No. (%) | All Grades | Grades 3–5 |
|---|---|---|
| Increased liver enzymes | 33 (34.4) | 13 (13.5) |
| Diarrhea | 30 (31.3) | 8 (8.3) |
| Palmar–plantar erythrodysesthesia | 28 (29.2) | 9 (9.4) |
| Fatigue | 18 (18.8) | 3 (3.1) |
| Hypothyroidism | 12 (12.5) | 8 (8.3) |
| Nausea | 11 (11.5) | 1 (1.0) |
| Hypertension | 11 (11.5) | 8 (8.3) |
| Mucosal inflammation | 10 (10.4) | 3 (3,1) |
| Loss of weight | 8 (8.3) | 2 (2.1) |
| Thrombopenia | 7 (7.3) | 1 (1.0) |
| Leukopenia | 5 (5.2) | 1 (1.0) |
| Anemia | 5 (5.2) | 1 (1.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilser, T.; Darr, C.; Niegisch, G.; Schnabel, M.J.; Foller, S.; Häuser, L.; Zschäbitz, S.; Lewerich, J.; Ivanyi, P.; Schlack, K.; et al. Cabozantinib Plus Nivolumab in Adult Patients with Advanced or Metastatic Renal Cell Carcinoma: A Retrospective, Non-Interventional Study in a Real-World Cohort/GUARDIANS Project. Cancers 2024, 16, 2998. https://doi.org/10.3390/cancers16172998
Hilser T, Darr C, Niegisch G, Schnabel MJ, Foller S, Häuser L, Zschäbitz S, Lewerich J, Ivanyi P, Schlack K, et al. Cabozantinib Plus Nivolumab in Adult Patients with Advanced or Metastatic Renal Cell Carcinoma: A Retrospective, Non-Interventional Study in a Real-World Cohort/GUARDIANS Project. Cancers. 2024; 16(17):2998. https://doi.org/10.3390/cancers16172998
Chicago/Turabian StyleHilser, Thomas, Christopher Darr, Günter Niegisch, Marco Julius Schnabel, Susan Foller, Lorine Häuser, Stefanie Zschäbitz, Jonas Lewerich, Philipp Ivanyi, Katrin Schlack, and et al. 2024. "Cabozantinib Plus Nivolumab in Adult Patients with Advanced or Metastatic Renal Cell Carcinoma: A Retrospective, Non-Interventional Study in a Real-World Cohort/GUARDIANS Project" Cancers 16, no. 17: 2998. https://doi.org/10.3390/cancers16172998
APA StyleHilser, T., Darr, C., Niegisch, G., Schnabel, M. J., Foller, S., Häuser, L., Zschäbitz, S., Lewerich, J., Ivanyi, P., Schlack, K., Paffenholz, P., Daetwyler, E., Niedersüß-Beke, D., & Grünwald, V. (2024). Cabozantinib Plus Nivolumab in Adult Patients with Advanced or Metastatic Renal Cell Carcinoma: A Retrospective, Non-Interventional Study in a Real-World Cohort/GUARDIANS Project. Cancers, 16(17), 2998. https://doi.org/10.3390/cancers16172998








