The Impact of Oral Microbiome Dysbiosis on the Aetiology, Pathogenesis, and Development of Oral Cancer
Abstract
Simple Summary
Abstract
1. Introduction
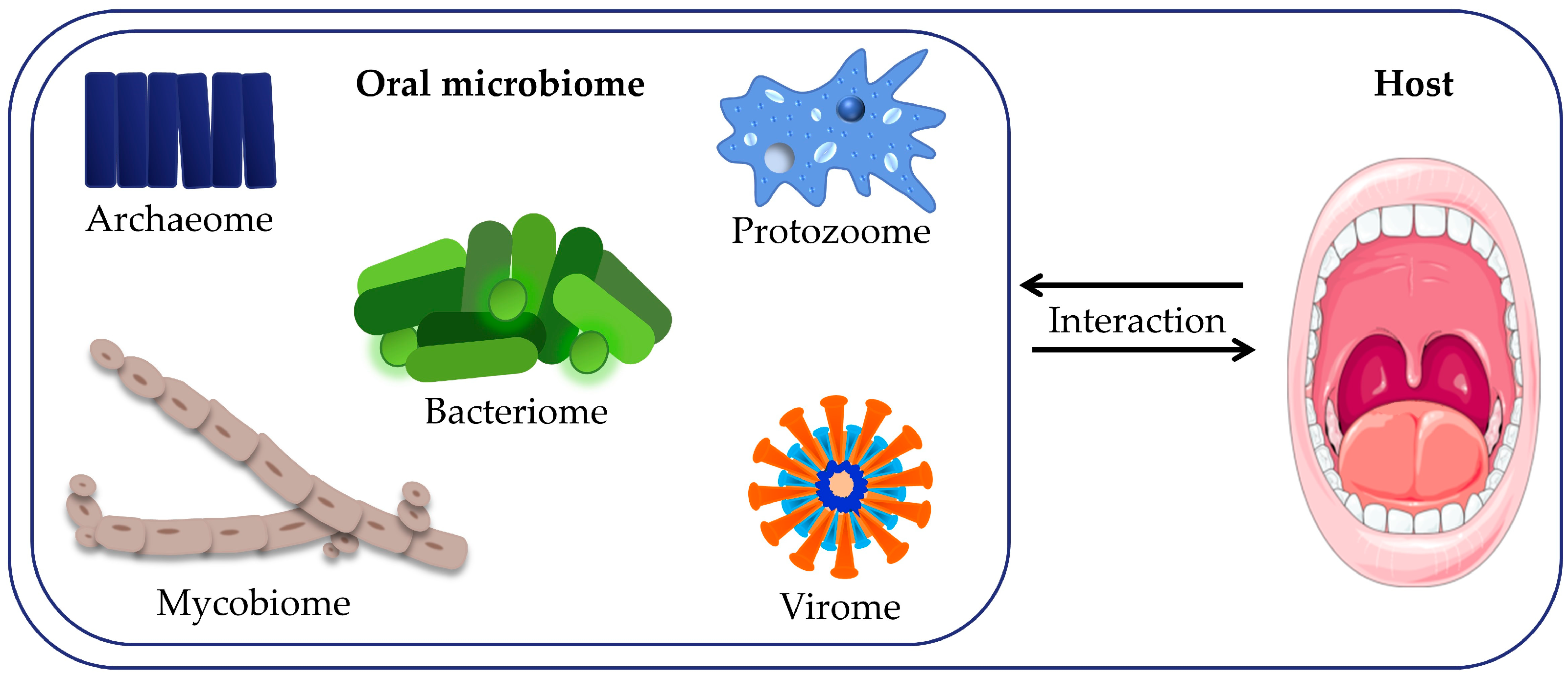
2. Microbiome of the Oral Cavity
Role of Biofilm in the Development of Oral Cavity Cancer
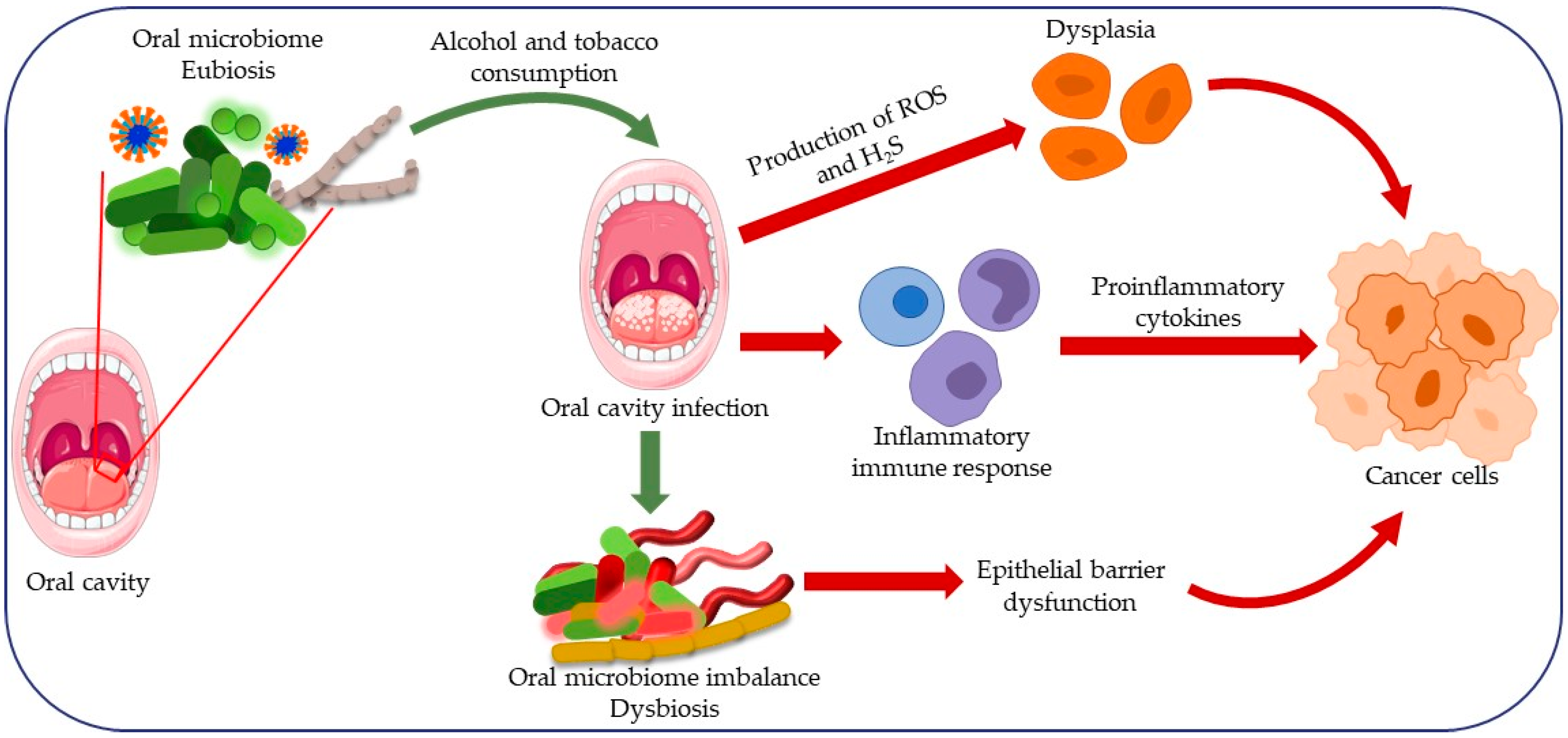
3. Dysbiosis of the Oral Microbiota
3.1. Bacteria Essential for the Development of Oral Cavity Cancer
3.2. Viral Causes of Oral Cavity Carcinoma
3.2.1. Human Papillomavirus (HPV)
3.2.2. Epstein-Barr Virus (EBV)
3.2.3. Human Herpesvirus 8 (HHV-8)
3.2.4. Immunologic Microenvironment and Therapeutic Implications
3.3. Role of Fungi in Oral Carcinoma
3.4. Role of Protozoa in Oral Carcinoma
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Badwelan, M.; Muaddi, H.; Ahmed, A.; Lee, K.T.; Tran, S.D. Oral Squamous Cell Carcinoma and Concomitant Primary Tumors, What Do We Know? A Review of the Literature. Curr. Oncol. 2023, 30, 3721–3734. [Google Scholar] [CrossRef] [PubMed]
- Montero, P.H.; Patel, S.G. Cancer of the Oral Cavity. Surg. Oncol. Clin. N. Am. 2015, 24, 491–508. [Google Scholar] [CrossRef]
- Monteiro, J.S.; Kaushik, K.; de Arruda, J.A.A.; Georgakopoulou, E.; Vieira, A.T.; Silva, T.A.; Devadiga, D.; Anyanechi, C.E.; Shetty, S. Fungal Footprints in Oral Cancer: Unveiling the Oral Mycobiome. Front. Oral Health 2024, 5, 1360340. [Google Scholar] [CrossRef]
- Farah, C.S.; Woo, S.B.; Zain, R.B.; Sklavounou, A.; McCullough, M.J.; Lingen, M. Oral Cancer and Oral Potentially Malignant Disorders. Int. J. Dent. 2014, 2014, 853479. [Google Scholar] [CrossRef]
- Kumari, P.; Debta, P.; Dixit, A. Oral Potentially Malignant Disorders: Etiology, Pathogenesis, and Transformation Into Oral Cancer. Front. Pharmacol. 2022, 13, 825266. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, K.; Kavitha, L. Oral Epithelial Dysplasia: Classifications and Clinical Relevance in Risk Assessment of Oral Potentially Malignant Disorders. J. Oral Maxillofac. Pathol. 2019, 23, 19–27. [Google Scholar] [CrossRef]
- Farooq, I.; Bugshan, A. Oral Squamous Cell Carcinoma: Metastasis, Potentially Associated Malignant Disorders, Etiology and Recent Advancements in Diagnosis. F1000Research 2020, 9, 229. [Google Scholar]
- Romano, A.; Di Stasio, D.; Petruzzi, M.; Fiori, F.; Lajolo, C.; Santarelli, A.; Lucchese, A.; Serpico, R.; Contaldo, M. Noninvasive Imaging Methods to Improve the Diagnosis of Oral Carcinoma and Its Precursors: State of the Art and Proposal of a Three-Step Diagnostic Process. Cancers 2021, 13, 2864. [Google Scholar] [CrossRef]
- Haj-Hosseini, N.; Lindblad, J.; Hasséus, B.; Kumar, V.V.; Subramaniam, N.; Hirsch, J.M. Early Detection of Oral Potentially Malignant Disorders: A Review on Prospective Screening Methods with Regard to Global Challenges. J. Maxillofac. Oral Surg. 2024, 23, 23–32. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Ferreira e Costa, R.; Leão, M.L.B.; Sant’Ana, M.S.P.; Mesquita, R.A.; Gomez, R.S.; Santos-Silva, A.R.; Khurram, S.A.; Tailor, A.; Schouwstra, C.M.; Robinson, L.; et al. Oral Squamous Cell Carcinoma Frequency in Young Patients from Referral Centers Around the World. Head Neck Pathol. 2022, 16, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Tranby, E.P.; Heaton, L.J.; Tomar, S.L.; Kelly, A.L.; Fager, G.L.; Backley, M.; Frantsve-Hawley, J. Oral Cancer Prevalence, Mortality, and Costs in Medicaid and Commercial Insurance Claims Data. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1849–1857. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Asmin, P.K.; Nusrath, F.; Divakar, D.D. Occurrence and Distribution of Cancers with Emphasis Upon Oral Cancers in Registered Oncology Institutes of South India—A Retrospective Study. Indian J. Community Med. 2024, 49, 120–130. [Google Scholar] [CrossRef]
- Cigic, L.; Martinovic, D.; Martinic, J.; Kovic, M.; Druzijanic, A.; Galic, I.; Tadin, A.; Lukanovic, B.; Duzel, M.; Poklepovic-Pericic, T. Increased Prevalence of Oral Potentially Malignant Lesions among Croatian War Invalids, a Cross-Sectional Study. J. Clin. Exp. Dent. 2023, 15, e734–e741. [Google Scholar] [CrossRef] [PubMed]
- Girisa, S.; Kumar, A.; Rana, V.; Parama, D.; Daimary, U.D.; Warnakulasuriya, S.; Kumar, A.P.; Kunnumakkara, A.B. From Simple Mouth Cavities to Complex Oral Mucosal Disorders-Curcuminoids as a Promising Therapeutic Approach. ACS Pharmacol. Transl. Sci. 2021, 4, 647–665. [Google Scholar] [CrossRef]
- Wierzbicka, M.; San Giorgi, M.R.M.; Dikkers, F.G. Transmission and Clearance of Human Papillomavirus Infection in the Oral Cavity and Its Role in Oropharyngeal Carcinoma—A Review. Rev. Med. Virol. 2023, 33, e2337. [Google Scholar] [CrossRef]
- Dellino, M.; Pinto, G.; D’Amato, A.; Barbara, F.; Di Gennaro, F.; Saracino, A.; Laganà, A.S.; Vimercati, A.; Malvasi, A.; Malvasi, V.M.; et al. Analogies between HPV Behavior in Oral and Vaginal Cavity: Narrative Review on the Current Evidence in the Literature. J. Clin. Med. 2024, 13, 1429. [Google Scholar] [CrossRef]
- Mohideen, K.; Krithika, C.; Jeddy, N.; Bharathi, R.; Thayumanavan, B.; Sankari, S.L. Meta-Analysis on Risk Factors of Squamous Cell Carcinoma of the Tongue in Young Adults. J. Oral Maxillofac. Pathol. 2019, 23, 450–457. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Q.; Wu, J.; Yuan, R.; Zhao, X.; Li, Y.; Cheng, X.; Wu, B.; Zhu, N. Trends in Cutaneous Squamous Cell Carcinoma on the Lip Incidence and Mortality in the United States, 2000–2019. Front. Oncol. 2023, 13, 1111907. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Kerr, A.R. Oral Cancer Screening: Past, Present, and Future. J. Dent. Res. 2021, 100, 1313–1320. [Google Scholar] [CrossRef]
- Neumann, F.W.; Neumann, H.; Spieth, S.; Remmerbach, T.W. Retrospective Evaluation of the Oral Brush Biopsy in Daily Dental Routine—An Effective Way of Early Cancer Detection. Clin. Oral Investig. 2022, 26, 6653–6659. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, V.; Shekar, K. Oral Squamous Cell Carcinoma: Diagnosis and Treatment Planning. In Oral and Maxillofacial Surgery for the Clinician; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1853–1867. [Google Scholar]
- Mortazavi, H.; Safi, Y.; Baharvand, M.; Rahmani, S.; Jafari, S. Peripheral Exophytic Oral Lesions: A Clinical Decision Tree. Int. J. Dent. 2017, 2017, 9193831. [Google Scholar] [CrossRef] [PubMed]
- Öhman, J.; Zlotogorski-Hurvitz, A.; Dobriyan, A.; Reiter, S.; Vered, M.; Willberg, J.; Lajolo, C.; Siponen, M. Oral Erythroplakia and Oral Erythroplakia-like Oral Squamous Cell Carcinoma—What’s the Difference? BMC Oral Health 2023, 23, 859. [Google Scholar] [CrossRef] [PubMed]
- Alwahaibi, N.; Alghallabi, A.; Alsinawi, S.; Aldairi, N. Cytological Smear and Cell Block Versus Tissue Biopsies in the Diagnosis of Malignant Tumours in Non-Gynaecologic Specimens. Ethiop. J. Health Sci. 2018, 28, 583–588. [Google Scholar] [CrossRef]
- Edirisinghe, S.T.; Devmini, T.; Pathmaperuma, S.; Weerasekera, M.; De Silva, K.; Liyanage, I.; Niluka, M.; Madushika, K.; Deegodagamage, S.; Wijesundara, C.; et al. Risk Assessment of Alcohol Consumption for Oral Cancer: A Case-Control Study in Patients Attending the National Cancer Institute (Apeksha Hospital, Maharagama) of Sri Lanka. Asian Pac. J. Cancer Prev. 2023, 24, 1181–1185. [Google Scholar] [CrossRef]
- Unlu, O.; Demirci, M.; Paksoy, T.; Eden, A.B.; Tansuker, H.D.; Dalmizrak, A.; Aktan, C.; Senel, F.; Sunter, A.V.; Yigit, O.; et al. Oral Microbial Dysbiosis in Patients with Oral Cavity Cancers. Clin. Oral Investig. 2024, 28, 377. [Google Scholar] [CrossRef]
- Irfan, M.; Delgado, R.Z.R.; Frias-Lopez, J. The Oral Microbiome and Cancer. Front. Immunol. 2020, 11, 591088. [Google Scholar] [CrossRef]
- Deo, P.N.; Deshmukh, R. Oral Microbiome: Unveiling the Fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef]
- Dong, L.; Yin, J.; Zhao, J.; Ma, S.R.; Wang, H.R.; Wang, M.; Chen, W.; Wei, W.Q. Microbial Similarity and Preference for Specific Sites in Healthy Oral Cavity and Esophagus. Front. Microbiol. 2018, 9, 1603. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, S.; Giudice, A.; Polizzi, A.; Troiano, G.; Merlo, E.M.; Sclafani, R.; Grosso, G.; Isola, G. A Cross-Talk between Diet and the Oral Microbiome: Balance of Nutrition on Inflammation and Immune System’s Response during Periodontitis. Nutrients 2022, 14, 2426. [Google Scholar] [CrossRef]
- Lee, Y.H.; Chung, S.W.; Auh, Q.S.; Hong, S.J.; Lee, Y.A.; Jung, J.; Lee, G.J.; Park, H.J.; Shin, S.; Hong, J.Y. Progress in Oral Microbiome Related to Oral and Systemic Diseases: An Update. Diagnostics 2021, 11, 1283. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Passarelli, P.C.; Azzolino, D.; Bottalico, L.; Charitos, I.A.; Cazzolla, A.P.; Colella, M.; Topi, S.; Godoy, F.G.; D’Addona, A. Oral Microbiota in Human Health and Disease: A Perspective. Exp. Biol. Med. 2023, 248, 1288–1301. [Google Scholar] [CrossRef] [PubMed]
- Radaic, A.; Kapila, Y.L. The Oralome and Its Dysbiosis: New Insights into Oral Microbiome-Host Interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef]
- Xiao, L.; Zhao, F. Microbial Transmission, Colonisation and Succession: From Pregnancy to Infancy. Gut 2023, 72, 772–786. [Google Scholar] [CrossRef]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The Oral Microbiome: Role of Key Organisms and Complex Networks in Oral Health and Disease. Periodontol. 2000 2021, 87, 107–131. [Google Scholar] [CrossRef]
- Kaan, A.M.; Kahharova, D.; Zaura, E. Acquisition and Establishment of the Oral Microbiota. Periodontol. 2000 2021, 86, 123–141. [Google Scholar] [CrossRef]
- Mombelli, A. Microbial Colonization of the Periodontal Pocket and Its Significance for Periodontal Therapy. Periodontol. 2000 2018, 76, 85–96. [Google Scholar] [CrossRef]
- Valm, A.M. The Structure of Dental Plaque Microbial Communities in the Transition from Health to Dental Caries and Periodontal Disease. J. Mol. Biol. 2019, 431, 2957–2969. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.P.; Feng, V.; Cao, G.Z.; Feng, X.P.; Chen, X. Microbiota of Preterm Infant Develops over Time along with the First Teeth Eruption. Front. Microbiol. 2022, 13, 1049021. [Google Scholar] [CrossRef] [PubMed]
- Talapko, J.; Meštrović, T.; Dmitrović, B.; Juzbašić, M.; Matijević, T.; Bekić, S.; Erić, S.; Flam, J.; Belić, D.; Petek Erić, A.; et al. A Putative Role of Candida Albicans in Promoting Cancer Development: A Current State of Evidence and Proposed Mechanisms. Microorganisms 2023, 11, 1476. [Google Scholar] [CrossRef]
- Talapko, J.; Meštrović, T.; Juzbašić, M.; Tomas, M.; Erić, S.; Horvat Aleksijević, L.; Bekić, S.; Schwarz, D.; Matić, S.; Neuberg, M.; et al. Antimicrobial Peptides—Mechanisms of Action, Antimicrobial Effects and Clinical Applications. Antibiotics 2022, 11, 1417. [Google Scholar] [CrossRef]
- Sharma, N.; Bhatia, S.; Sodhi, A.S.; Batra, N. Oral Microbiome and Health. AIMS Microbiol. 2018, 4, 42–66. [Google Scholar] [CrossRef] [PubMed]
- Zaatout, N. Presence of Non-Oral Bacteria in the Oral Cavity. Arch. Microbiol. 2021, 203, 2747–2760. [Google Scholar] [CrossRef]
- Escobar-Arregocés, F.; Eras, M.A.; Bustos, A.; Suárez-Castillo, A.; García-Robayo, D.A.; del Pilar Bernal, M. Characterization of the Oral Microbiota and the Relationship of the Oral Microbiota with the Dental and Periodontal Status in Children and Adolescents with Nonsyndromic Cleft Lip and Palate. Systematic Literature Review and Meta-Analysis. Clin. Oral Investig. 2024, 28, 245. [Google Scholar] [CrossRef]
- Morrison, A.G.; Sarkar, S.; Umar, S.; Lee, S.T.M.; Thomas, S.M. The Contribution of the Human Oral Microbiome to Oral Disease: A Review. Microorganisms 2023, 11, 318. [Google Scholar] [CrossRef]
- Upadhyay, M.; Swaroop, A.; Sinhal, V.K.; Srivastava, A.; Garg, S.K.; Singh, V.P.; Arora, P.K. Role of Human Oral Microbiome in Diseases. J. Pure Appl. Microbiol. 2024, 18, 168–176. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Yang, X.; Li, C.; Song, Z. The Oral Microbiota: Community Composition, Influencing Factors, Pathogenesis, and Interventions. Front. Microbiol. 2022, 13, 895537. [Google Scholar] [CrossRef]
- Volmer, J.G.; McRae, H.; Morrison, M. The Evolving Role of Methanogenic Archaea in Mammalian Microbiomes. Front. Microbiol. 2023, 14, 1268451. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.K.; Chan, J.Y.K.; Wong, M.C.S.; Wong, P.Y.; Lei, P.; Cai, L.; Lan, L.; Ho, W.C.S.; Yeung, A.C.M.; Chan, P.K.S.; et al. Determinants and Interactions of Oral Bacterial and Fungal Microbiota in Healthy Chinese Adults. Microbiol. Spectr. 2022, 10, e0241021. [Google Scholar] [CrossRef]
- Vallianou, N.; Kounatidis, D.; Christodoulatos, G.S.; Panagopoulos, F.; Karampela, I.; Dalamaga, M. Mycobiome and Cancer: What Is the Evidence? Cancers 2021, 13, 3149. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; Peterson, C.B.; Sahasrabhojane, P.; Ajami, N.J.; Shelburne, S.A.; Kontoyiannis, D.P.; Galloway-Peña, J.R. Observational Cohort Study of Oral Mycobiome and Interkingdom Interactions over the Course of Induction Therapy for Leukemia. mSphere 2020, 5, e00048-20. [Google Scholar] [CrossRef]
- Girija, A.S.S.; Ganesh, P.S. Functional Biomes beyond the Bacteriome in the Oral Ecosystem. Jpn. Dent. Sci. Rev. 2022, 58, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Di Spirito, F.; Di Palo, M.P.; Folliero, V.; Cannatà, D.; Franci, G.; Martina, S.; Amato, M. Oral Bacteria, Virus and Fungi in Saliva and Tissue Samples from Adult Subjects with Oral Squamous Cell Carcinoma: An Umbrella Review. Cancers 2023, 15, 5540. [Google Scholar] [CrossRef]
- Lorini, L.; Atín, C.B.; Thavaraj, S.; Müller-Richter, U.; Ferranti, M.A.; Romero, J.P.; Barba, M.S.; García-Cuenca, A.d.P.; García, I.B.; Bossi, P.; et al. Overview of Oral Potentially Malignant Disorders: From Risk Factors to Specific Therapies. Cancers 2021, 13, 3696. [Google Scholar] [CrossRef]
- Radaic, A.; Shamir, E.R.; Jones, K.; Villa, A.; Garud, N.R.; Tward, A.D.; Kamarajan, P.; Kapila, Y.L. Specific Oral Microbial Differences in Proteobacteria and Bacteroidetes Are Associated with Distinct Sites When Moving from Healthy Mucosa to Oral Dysplasia-A Microbiome and Gene Profiling Study and Focused Review. Microorganisms 2023, 11, 2250. [Google Scholar] [CrossRef]
- Pietrobon, G.; Tagliabue, M.; Stringa, L.M.; De Berardinis, R.; Chu, F.; Zocchi, J.; Carlotto, E.; Chiocca, S.; Ansarin, M. Leukoplakia in the Oral Cavity and Oral Microbiota: A Comprehensive Review. Cancers 2021, 13, 4439. [Google Scholar] [CrossRef]
- Herreros-Pomares, A.; Llorens, C.; Soriano, B.; Zhang, F.; Gallach, S.; Bagan, L.; Murillo, J.; Jantus-Lewintre, E.; Bagan, J. Oral Microbiome in Proliferative Verrucous Leukoplakia Exhibits Loss of Diversity and Enrichment of Pathogens. Oral Oncol. 2021, 120, 105404. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Verma, M.; Panda, M. Role of Oral Microbiome Signatures in Diagnosis and Prognosis of Oral Cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819867354. [Google Scholar] [CrossRef]
- Shih, Y.H.; Wang, T.H.; Shieh, T.M.; Tseng, Y.H. Oral Submucous Fibrosis: A Review on Etiopathogenesis, Diagnosis, and Therapy. Int. J. Mol. Sci. 2019, 20, 2940. [Google Scholar] [CrossRef]
- Chocolatewala, N.; Chaturvedi, P.; Desale, R. The Role of Bacteria in Oral Cancer. Indian J. Med. Paediatr. Oncol. 2010, 31, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, G.M.; Horowitz, R.A.; Johnson, R.; Prestiano, R.A.; Klein, B.I. The Systemic Oral Health Connection: Biofilms. Medicine 2022, 101, E30517. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and Its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Murray, B.; Choi, S. Biofilm and Cancer: Interactions and Future Directions for Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 12836. [Google Scholar] [CrossRef]
- Talapko, J.; Škrlec, I. The Principles, Mechanisms, and Benefits of Unconventional Agents in the Treatment of Biofilm Infection. Pharmaceuticals 2020, 13, 299. [Google Scholar] [CrossRef]
- Aleksijević, L.H.; Aleksijević, M.; Škrlec, I.; Šram, M.; Talapko, J. Porphyromonas gingivalis Virulence Factors and Clinical Significance in Periodontal Disease and Coronary Artery Diseases. Pathogens 2022, 11, 1173. [Google Scholar] [CrossRef]
- Sterzenbach, T.; Helbig, R.; Hannig, C.; Hannig, M. Bioadhesion in the Oral Cavity and Approaches for Biofilm Management by Surface Modifications. Clin. Oral Investig. 2020, 24, 4237–4260. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Sun, J.; Liu, Y. Understanding Bacterial Biofilms: From Definition to Treatment Strategies. Front. Cell. Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef]
- Pignatelli, P.; Nuccio, F.; Piattelli, A.; Curia, M.C. The Role of Fusobacterium nucleatum in Oral and Colorectal Carcinogenesis. Microorganisms 2023, 11, 2358. [Google Scholar] [CrossRef]
- Zepeda-Rivera, M.; Minot, S.S.; Bouzek, H.; Wu, H.; Blanco-Míguez, A.; Manghi, P.; Jones, D.S.; LaCourse, K.D.; Wu, Y.; McMahon, E.F.; et al. A Distinct Fusobacterium nucleatum Clade Dominates the Colorectal Cancer Niche. Nature 2024, 628, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Gerits, E.; Verstraeten, N.; Michiels, J. New Approaches to Combat Porphyromonas gingivalis Biofilms. J. Oral Microbiol. 2017, 9, 1300366. [Google Scholar] [CrossRef]
- Chenicheri, S.; Usha, R.; Ramachandran, R.; Thomas, V.; Wood, A. Insight into Oral Biofilm: Primary, Secondary and Residual Caries and Phyto-Challenged Solutions. Open Dent. J. 2017, 11, 312–333. [Google Scholar] [CrossRef]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The Biofilm Life Cycle: Expanding the Conceptual Model of Biofilm Formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, Z.; Ding, T. Quorum-Sensing Regulation of Antimicrobial Resistance in Bacteria. Microorganisms 2020, 8, 425. [Google Scholar] [CrossRef] [PubMed]
- Balducci, E.; Papi, F.; Capialbi, D.E.; Del Bino, L. Polysaccharides’ Structures and Functions in Biofilm Architecture of Antimicrobial-Resistant (AMR) Pathogens. Int. J. Mol. Sci. 2023, 24, 4030. [Google Scholar] [CrossRef]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef]
- Rumbaugh, K.P.; Sauer, K. Biofilm Dispersion. Nat. Rev. Microbiol. 2020, 18, 571. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef]
- Che, S.; Yan, Z.; Feng, Y.; Zhao, H. Unveiling the Intratumoral Microbiota within Cancer Landscapes. iScience 2024, 27, 109893. [Google Scholar] [CrossRef]
- Kay, J.; Thadhani, E.; Samson, L.; Engelward, B. Inflammation-Induced DNA Damage, Mutations and Cancer. DNA Repair 2019, 83, 102673. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Liu, S.Y.; Chua, S.L.; Khoo, B.L. The Effects of Biofilms on Tumor Progression in a 3D Cancer-Biofilm Microfluidic Model. Biosens. Bioelectron. 2021, 180, 113113. [Google Scholar] [CrossRef] [PubMed]
- Ciernikova, S.; Sevcikova, A.; Stevurkova, V.; Mego, M. Tumor Microbiome—An Integral Part of the Tumor Microenvironment. Front. Oncol. 2022, 12, 1063100. [Google Scholar] [CrossRef] [PubMed]
- Min, Z.; Yang, L.; Hu, Y.; Huang, R. Oral Microbiota Dysbiosis Accelerates the Development and Onset of Mucositis and Oral Ulcers. Front. Microbiol. 2023, 14, 1061032. [Google Scholar] [CrossRef]
- Spatafora, G.; Li, Y.; He, X.; Cowan, A.; Tanner, A.C.R. The Evolving Microbiome of Dental Caries. Microorganisms 2024, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Lavoro, A.; Cultrera, G.; Gattuso, G.; Lombardo, C.; Falzone, L.; Saverio, C.; Libra, M.; Salmeri, M. Role of Oral Microbiota Dysbiosis in the Development and Progression of Oral Lichen Planus. J. Pers. Med. 2024, 14, 386. [Google Scholar] [CrossRef]
- Siddiqui, R.; Badran, Z.; Boghossian, A.; Alharbi, A.M.; Alfahemi, H.; Khan, N.A. The Increasing Importance of the Oral Microbiome in Periodontal Health and Disease. Future Sci. OA 2023, 9, FSO856. [Google Scholar] [CrossRef]
- la Rosa, G.R.M.; Gattuso, G.; Pedullà, E.; Rapisarda, E.; Nicolosi, D.; Salmeri, M. Association of Oral Dysbiosis with Oral Cancer Development. Oncol. Lett. 2020, 19, 3045–3058. [Google Scholar] [CrossRef]
- Sarkar, P.; Malik, S.; Laha, S.; Das, S.; Bunk, S.; Ray, J.G.; Chatterjee, R.; Saha, A. Dysbiosis of Oral Microbiota During Oral Squamous Cell Carcinoma Development. Front. Oncol. 2021, 11, 614448. [Google Scholar] [CrossRef]
- Yang, J.; He, P.; Zhou, M.; Li, S.; Zhang, J.; Tao, X.; Wang, A.; Wu, X. Variations in Oral Microbiome and Its Predictive Functions between Tumorous and Healthy Individuals. J. Med. Microbiol. 2022, 71, 001568. [Google Scholar] [CrossRef]
- Delaney, C.; Veena, C.L.R.; Butcher, M.C.; McLean, W.; Shaban, S.M.A.; Nile, C.J.; Ramage, G. Limitations of Using 16S RRNA Microbiome Sequencing to Predict Oral Squamous Cell Carcinoma. APMIS 2023, 131, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Su Mun, L.; Wye Lum, S.; Kong Yuiin Sze, G.; Hock Yoong, C.; Ching Yung, K.; Kah Lok, L.; Gopinath, D. Association of Microbiome with Oral Squamous Cell Carcinoma: A Systematic Review of the Metagenomic Studies. Int. J. Environ. Res. Public Health 2021, 18, 7224. [Google Scholar] [CrossRef]
- Sukmana, B.I.; Saleh, R.O.; Najim, M.A.; AL-Ghamdi, H.S.; Achmad, H.; Al-Hamdani, M.M.; Taher, A.A.Y.; Alsalamy, A.; Khaledi, M.; Javadi, K. Oral Microbiota and Oral Squamous Cell Carcinoma: A Review of Their Relation and Carcinogenic Mechanisms. Front. Oncol. 2024, 14, 1319777. [Google Scholar] [CrossRef] [PubMed]
- Vyhnalova, T.; Danek, Z.; Gachova, D.; Linhartova, P.B. The Role of the Oral Microbiota in the Etiopathogenesis of Oral Squamous Cell Carcinoma. Microorganisms 2021, 9, 1549. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hu, Y.; Zhou, X.; Liu, S.; Han, Q.; Cheng, L. Role of Oral Bacteria in the Development of Oral Squamous Cell Carcinoma. Cancers 2020, 12, 2797. [Google Scholar] [CrossRef]
- Ye, C.; Liu, X.; Liu, Z.; Pan, C.; Zhang, X.; Zhao, Z.; Sun, H. Fusobacterium nucleatum in Tumors: From Tumorigenesis to Tumor Metastasis and Tumor Resistance. Cancer Biol. Ther. 2024, 25, 2306676. [Google Scholar] [CrossRef]
- Alon-Maimon, T.; Mandelboim, O.; Bachrach, G. Fusobacterium nucleatum and Cancer. Periodontol. 2000 2022, 89, 166–180. [Google Scholar] [CrossRef]
- McIlvanna, E.; Linden, G.J.; Craig, S.G.; Lundy, F.T.; James, J.A. Fusobacterium nucleatum and Oral Cancer: A Critical Review. BMC Cancer 2021, 21, 1212. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Z.; Tang, Z.; Huang, Y.; Huang, M.; Liu, H.; Ziebolz, D.; Schmalz, G.; Jia, B.; Zhao, J. More Than Just a Periodontal Pathogen—The Research Progress on Fusobacterium Nucleatum. Front. Cell. Infect. Microbiol. 2022, 12, 815318. [Google Scholar] [CrossRef]
- Yang, Y.L.; Yang, F.; Huang, Z.Q.; Li, Y.Y.; Shi, H.Y.; Sun, Q.; Ma, Y.; Wang, Y.; Zhang, Y.; Yang, S.; et al. T Cells, NK Cells, and Tumor-Associated Macrophages in Cancer Immunotherapy and the Current State of the Art of Drug Delivery Systems. Front. Immunol. 2023, 14, 1199173. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Mima, K.; Ishimoto, T.; Ogata, Y.; Imai, K.; Miyamoto, Y.; Akiyama, T.; Daitoku, N.; Hiyoshi, Y.; Iwatsuki, M.; et al. Relationship between Fusobacterium nucleatum and Antitumor Immunity in Colorectal Cancer Liver Metastasis. Cancer Sci. 2021, 112, 4470–4477. [Google Scholar] [CrossRef] [PubMed]
- Groeger, S.; Zhou, Y.; Ruf, S.; Meyle, J. Pathogenic Mechanisms of Fusobacterium nucleatum on Oral Epithelial Cells. Front. Oral Health 2022, 3, 831607. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Tang, P.; Li, C.; Yang, Q.; Xu, Y.; Su, C.; Li, L. Fusobacterium nucleatum and Its Associated Systemic Diseases: Epidemiologic Studies and Possible Mechanisms. J. Oral Microbiol. 2022, 15, 2145729. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Li, J.; Zhao, L.; Yan, W.; Lin, B.; Guo, X.; Wei, Y. Fusobacterium nucleatum Acts as a Pro-Carcinogenic Bacterium in Colorectal Cancer: From Association to Causality. Front. cell Dev. Biol. 2021, 9, 710165. [Google Scholar] [CrossRef]
- Liang, B.; Wu, C.; Wang, C.; Sun, W.; Chen, W.; Hu, X.; Liu, N.; Xing, D. New Insights into Bacterial Mechanisms and Potential Intestinal Epithelial Cell Therapeutic Targets of Inflammatory Bowel Disease. Front. Microbiol. 2022, 13, 1065608. [Google Scholar] [CrossRef]
- Sun, J.; Tang, Q.; Yu, S.; Xie, M.; Zheng, W.; Chen, G.; Yin, Y.; Huang, X.; Wo, K.; Lei, H.; et al. F. Nucleatum Facilitates Oral Squamous Cell Carcinoma Progression via GLUT1-Driven Lactate Production. EBioMedicine 2023, 88, 104444. [Google Scholar] [CrossRef]
- Sezgin, E.; Terlemez, G.; Bozkurt, B.; Bengi, G.; Akpinar, H.; Büyüktorun, İ. Quantitative Real-Time PCR Analysis of Bacterial Biomarkers Enable Fast and Accurate Monitoring in Inflammatory Bowel Disease. PeerJ 2022, 10, e14217. [Google Scholar] [CrossRef]
- Xue, X.; Li, R.; Chen, Z.; Li, G.; Liu, B.; Guo, S.; Yue, Q.; Yang, S.; Xie, L.; Zhang, Y.; et al. The Role of the Symbiotic Microecosystem in Cancer: Gut Microbiota, Metabolome, and Host Immunome. Front. Immunol. 2023, 14, 1235827. [Google Scholar] [CrossRef] [PubMed]
- Tuominen, H.; Rautava, J. Oral Microbiota and Cancer Development. Pathobiology 2021, 88, 116–126. [Google Scholar] [CrossRef]
- Bi, R.; Yang, Y.; Liao, H.; Ji, G.; Ma, Y.; Cai, L.; Li, J.; Yang, J.; Sun, M.; Liang, J.; et al. Porphyromonas gingivalis Induces an Inflammatory Response via the CGAS-STING Signaling Pathway in a Periodontitis Mouse Model. Front. Microbiol. 2023, 14, 1183415. [Google Scholar] [CrossRef]
- Yi, M.; Li, T.; Niu, M.; Zhang, H.; Wu, Y.; Wu, K.; Dai, Z. Targeting Cytokine and Chemokine Signaling Pathways for Cancer Therapy. Signal Transduct. Target. Ther. 2024, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.A.; Dou, Y.; Fletcher, H.M.; Boskovic, D.S. Local and Systemic Effects of Porphyromonas gingivalis Infection. Microorganisms 2023, 11, 470. [Google Scholar] [CrossRef]
- Kumar, S.; Jeong, Y.; Ashraf, M.U.; Bae, Y.S. Dendritic Cell-Mediated Th2 Immunity and Immune Disorders. Int. J. Mol. Sci. 2019, 20, 2159. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Medel, M.; Pinto, M.P.; Goralsky, L.; Cáceres, M.; Villarroel-Espíndola, F.; Manque, P.; Pinto, A.; Garcia-Bloj, B.; de Mayo, T.; Godoy, J.A.; et al. Porphyromonas Gingivalis, a Bridge between Oral Health and Immune Evasion in Gastric Cancer. Front. Oncol. 2024, 14, 1403089. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Collyer, C.A. Gingipains from Porphyromonas gingivalis—Complex Domain Structures Confer Diverse Functions. Eur. J. Microbiol. Immunol. 2011, 1, 41–58. [Google Scholar] [CrossRef]
- Mu, W.; Jia, Y.; Chen, X.; Li, H.; Wang, Z.; Cheng, B. Intracellular Porphyromonas gingivalis Promotes the Proliferation of Colorectal Cancer Cells via the MAPK/ERK Signaling Pathway. Front. Cell. Infect. Microbiol. 2020, 10, 584798. [Google Scholar] [CrossRef]
- Shahoumi, L.A.; Saleh, M.H.A.; Meghil, M.M. Virulence Factors of the Periodontal Pathogens: Tools to Evade the Host Immune Response and Promote Carcinogenesis. Microorganisms 2023, 11, 115. [Google Scholar] [CrossRef]
- Mei, F.; Xie, M.; Huang, X.; Long, Y.; Lu, X.; Wang, X.; Chen, L. Porphyromonas gingivalis and Its Systemic Impact: Current Status. Pathogens 2020, 9, 944. [Google Scholar] [CrossRef]
- Yáñez, L.; Soto, C.; Tapia, H.; Pacheco, M.; Tapia, J.; Osses, G.; Salinas, D.; Rojas-Celis, V.; Hoare, A.; Quest, A.F.G.; et al. Co-Culture of P. Gingivalis and F. Nucleatum Synergistically Elevates IL-6 Expression via TLR4 Signaling in Oral Keratinocytes. Int. J. Mol. Sci. 2024, 25, 3611. [Google Scholar] [CrossRef]
- Chopra, A.; Bhat, S.G.; Sivaraman, K. Porphyromonas gingivalis Adopts Intricate and Unique Molecular Mechanisms to Survive and Persist within the Host: A Critical Update. J. Oral Microbiol. 2020, 12, 1801090. [Google Scholar] [CrossRef]
- Chan, K.T.; Song, X.; Shen, L.; Liu, N.; Zhou, X.; Cheng, L.; Chen, J. Nisin and Its Application in Oral Diseases. J. Funct. Foods 2023, 105, 105559. [Google Scholar] [CrossRef]
- Barranca-Enríquez, A.; Romo-González, T. Your Health Is in Your Mouth: A Comprehensive View to Promote General Wellness. Front. Oral Health 2022, 3, 971223. [Google Scholar] [CrossRef]
- Brookes, Z.; McGrath, C.; McCullough, M. Antimicrobial Mouthwashes: An Overview of Mechanisms-What Do We Still Need to Know? Int. Dent. J. 2023, 73 (Suppl. S2), S64–S68. [Google Scholar] [CrossRef]
- Ciani, L.; Libonati, A.; Dri, M.; Pomella, S.; Campanella, V.; Barillari, G. About a Possible Impact of Endodontic Infections by Fusobacterium nucleatum or Porphyromonas gingivalis on Oral Carcinogenesis: A Literature Overview. Int. J. Mol. Sci. 2024, 25, 5083. [Google Scholar] [CrossRef] [PubMed]
- Talapko, J.; Juzbašić, M.; Meštrović, T.; Matijević, T.; Mesarić, D.; Katalinić, D.; Erić, S.; Milostić-Srb, A.; Flam, J.; Škrlec, I. Aggregatibacter actinomycetemcomitans: From the Oral Cavity to the Heart Valves. Microorganisms 2024, 12, 1451. [Google Scholar] [CrossRef] [PubMed]
- Baima, G.; Minoli, M.; Michaud, D.S.; Aimetti, M.; Sanz, M.; Loos, B.G.; Romandini, M. Periodontitis and Risk of Cancer: Mechanistic Evidence. Periodontol. 2000 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef]
- Nokovitch, L.; Maquet, C.; Crampon, F.; Taihi, I.; Roussel, L.M.; Obongo, R.; Virard, F.; Fervers, B.; Deneuve, S. Oral Cavity Squamous Cell Carcinoma Risk Factors: State of the Art. J. Clin. Med. 2023, 12, 3264. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Liu, T.; Yao, Y. Implications of Viral Infections and Oncogenesis in Uterine Cervical Carcinoma Etiology and Pathogenesis. Front. Microbiol. 2023, 14, 1194431. [Google Scholar] [CrossRef]
- Gupta, S.L.; Basu, S.; Soni, V.; Jaiswal, R.K. Immunotherapy: An Alternative Promising Therapeutic Approach against Cancers. Mol. Biol. Rep. 2022, 49, 9903–9913. [Google Scholar] [CrossRef]
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-Associated Oropharyngeal Cancer: Epidemiology, Molecular Biology and Clinical Management. Nat. Rev. Clin. Oncol. 2022, 19, 306–327. [Google Scholar] [CrossRef]
- Egawa, N. Papillomaviruses and Cancer: Commonalities and Differences in HPV Carcinogenesis at Different Sites of the Body. Int. J. Clin. Oncol. 2023, 28, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Akagi, K.; Xiao, W.; Jiang, B.; Pickard, R.K.L.; Li, J.; Swanson, B.J.; Agrawal, A.D.; Zucker, M.; Stache-Crain, B.; et al. Human Papillomavirus and the Landscape of Secondary Genetic Alterations in Oral Cancers. Genome Res. 2019, 29, 1–17. [Google Scholar] [CrossRef]
- Yeo-Teh, N.S.L.; Ito, Y.; Jha, S. High-Risk Human Papillomaviral Oncogenes E6 and E7 Target Key Cellular Pathways to Achieve Oncogenesis. Int. J. Mol. Sci. 2018, 19, 1706. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.V. The Human Papillomavirus Replication Cycle, and Its Links to Cancer Progression: A Comprehensive Review. Clin. Sci. 2017, 131, 2201–2221. [Google Scholar] [CrossRef]
- Berman, T.A.; Schiller, J.T. Human Papillomavirus in Cervical Cancer and Oropharyngeal Cancer: One Cause, Two Diseases. Cancer 2017, 123, 2219–2229. [Google Scholar] [CrossRef]
- Chihu-Amparan, L.; Pedroza-Saavedra, A.; Gutierrez-Xicotencatl, L. The Immune Response Generated against HPV Infection in Men and Its Implications in the Diagnosis of Cancer. Microorganisms 2023, 11, 1609. [Google Scholar] [CrossRef]
- Moody, C.A. Regulation of the Innate Immune Response during the Human Papillomavirus Life Cycle. Viruses 2022, 14, 1797. [Google Scholar] [CrossRef]
- Jiang, Y.; Tsoi, L.C.; Billi, A.C.; Ward, N.L.; Harms, P.W.; Zeng, C.; Maverakis, E.; Michelle Kahlenberg, J.; Gudjonsson, J.E. Cytokinocytes: The Diverse Contribution of Keratinocytes to Immune Responses in Skin. JCI Insight 2020, 5, e142067. [Google Scholar] [CrossRef]
- van Bockel, D.; Kelleher, A. The Crossroads: Divergent Roles of Virus-Specific CD4+ T Lymphocytes in Determining the Outcome for Human Papillomavirus Infection. Immunol. Cell Biol. 2023, 101, 525–534. [Google Scholar] [CrossRef]
- Lechien, J.R.; Seminerio, I.; Descamps, G.; Mat, Q.; Mouawad, F.; Hans, S.; Julieron, M.; Dequanter, D.; Vanderhaegen, T.; Journe, F.; et al. Impact of HPV Infection on the Immune System in Oropharyngeal and Non-Oropharyngeal Squamous Cell Carcinoma: A Systematic Review. Cells 2019, 8, 1061. [Google Scholar] [CrossRef]
- Damania, B.; Kenney, S.C.; Raab-Traub, N. Epstein-Barr Virus: Biology and Clinical Disease. Cell 2022, 185, 3652–3670. [Google Scholar] [CrossRef]
- Wang, L.; Ning, S. New Look of EBV LMP1 Signaling Landscape. Cancers 2021, 13, 5451. [Google Scholar] [CrossRef] [PubMed]
- Cereser, B.; Jansen, M.; Austin, E.; Elia, G.; McFarlane, T.; van Deurzen, C.H.M.; Sieuwerts, A.M.; Daidone, M.G.; Tadrous, P.J.; Wright, N.A.; et al. Analysis of Clonal Expansions through the Normal and Premalignant Human Breast Epithelium Reveals the Presence of Luminal Stem Cells. J. Pathol. 2018, 244, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Rex, V.; Zargari, R.; Stempel, M.; Halle, S.; Brinkmann, M.M. The Innate and T-Cell Mediated Immune Response during Acute and Chronic Gammaherpesvirus Infection. Front. Cell. Infect. Microbiol. 2023, 13, 146381. [Google Scholar] [CrossRef]
- Silva, J.d.M.; Alves, C.E.d.C.; Pontes, G.S. Epstein-Barr Virus: The Mastermind of Immune Chaos. Front. Immunol. 2024, 15, 1297994. [Google Scholar] [CrossRef]
- Chakravorty, S.; Afzali, B.; Kazemian, M. EBV-Associated Diseases: Current Therapeutics and Emerging Technologies. Front. Immunol. 2022, 13, 1059133. [Google Scholar] [CrossRef]
- Li, W.; Duan, X.; Chen, X.; Zhan, M.; Peng, H.; Meng, Y.; Li, X.; Li, X.Y.; Pang, G.; Dou, X. Immunotherapeutic Approaches in EBV-Associated Nasopharyngeal Carcinoma. Front. Immunol. 2023, 13, 1079515. [Google Scholar] [CrossRef]
- Berwanger, A.; Stein, S.C.; Kany, A.M.; Gartner, M.; Loretz, B.; Lehr, C.M.; Hirsch, A.K.H.; Schulz, T.F.; Empting, M. Disrupting Kaposi’s Sarcoma-Associated Herpesvirus (KSHV) Latent Replication with a Small Molecule Inhibitor. J. Med. Chem. 2023, 66, 10782–10790. [Google Scholar] [CrossRef]
- Reddy, N.A.; Mays, S.R.; Pacha, O. Kaposi’s Sarcoma in the Immunosuppressed. J. Immunother. Precis. Oncol. 2019, 2, 74–78. [Google Scholar] [CrossRef]
- Lopes, A.D.; Marinho, P.D.; Medeiros, L.D.; de Paula, V.S. Human Gammaherpesvirus 8 Oncogenes Associated with Kaposi’s Sarcoma. Int. J. Mol. Sci. 2022, 23, 7203. [Google Scholar] [CrossRef] [PubMed]
- Angius, F.; Ingianni, A.; Pompei, R. Human Herpesvirus 8 and Host-Cell Interaction: Long-Lasting Physiological Modifications, Inflammation and Related Chronic Diseases. Microorganisms 2020, 8, 388. [Google Scholar] [CrossRef]
- Schulz, T.F.; Cesarman, E. Kaposi Sarcoma-Associated Herpesvirus: Mechanisms of Oncogenesis. Curr. Opin. Virol. 2015, 14, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Tornesello, A.L.; Tagliamonte, M.; Buonaguro, F.M.; Tornesello, M.L.; Buonaguro, L. Virus-like Particles as Preventive and Therapeutic Cancer Vaccines. Vaccines 2022, 10, 227. [Google Scholar] [CrossRef]
- Kamel, M.S.; Munds, R.A.; Verma, M.S. The Quest for Immunity: Exploring Human Herpesviruses as Vaccine Vectors. Int. J. Mol. Sci. 2023, 24, 16112. [Google Scholar] [CrossRef]
- Mamilos, A.; Lein, A.; Winter, L.; Ettl, T.; Künzel, J.; Reichert, T.E.; Spanier, G.; Brochhausen, C. Tumor Immune Microenvironment Heterogeneity at the Invasion Front and Tumor Center in Oral Squamous Cell Carcinoma as a Perspective of Managing This Cancer Entity. J. Clin. Med. 2023, 12, 1704. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Borsetto, D.; Tomasoni, M.; Payne, K.; Polesel, J.; Deganello, A.; Bossi, P.; Tysome, J.R.; Masterson, L.; Tirelli, G.; Tofanelli, M.; et al. Prognostic Significance of CD4+ and CD8+ Tumor-Infiltrating Lymphocytes in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Cancers 2021, 13, 781. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tian, S.; Sun, J.; Zhang, J.; Lin, L.; Hu, C. The Presence of Tumour-Infiltrating Lymphocytes (TILs) and the Ratios between Different Subsets Serve as Prognostic Factors in Advanced Hypopharyngeal Squamous Cell Carcinoma. BMC Cancer 2020, 20, 731. [Google Scholar] [CrossRef]
- Brierly, G.; Celentano, A.; Breik, O.; Moslemivayeghan, E.; Patini, R.; McCullough, M.; Yap, T. Tumour Necrosis Factor Alpha (TNF-α) and Oral Squamous Cell Carcinoma. Cancers 2023, 15, 1841. [Google Scholar] [CrossRef]
- Laliberté, C.; Ng, N.; Eymael, D.; Higgins, K.; Ali, A.; Kiss, A.; Bradley, G.; Magalhaes, M.A.O. Characterization of Oral Squamous Cell Carcinoma Associated Inflammation: A Pilot Study. Front. Oral Health 2021, 2, 740469. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Joyce, J.A. The Evolving Tumor Microenvironment: From Cancer Initiation to Metastatic Outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Salemme, V.; Centonze, G.; Cavallo, F.; Defilippi, P.; Conti, L. The Crosstalk Between Tumor Cells and the Immune Microenvironment in Breast Cancer: Implications for Immunotherapy. Front. Oncol. 2021, 11, 610303. [Google Scholar] [CrossRef] [PubMed]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab Alone or with Chemotherapy versus Cetuximab with Chemotherapy for Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (KEYNOTE-048): A Randomised, Open-Label, Phase 3 Study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Wang, H.Q.; Fu, R.; Man, Q.W.; Yang, G.; Liu, B.; Bu, L.L. Advances in CAR-T Cell Therapy in Head and Neck Squamous Cell Carcinoma. J. Clin. Med. 2023, 12, 2173. [Google Scholar] [CrossRef]
- Cheng, W.; Li, F.; Gao, Y.; Yang, R. Fungi and Tumors: The Role of Fungi in Tumorigenesis (Review). Int. J. Oncol. 2024, 64, 52. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Jurevic, R.J.; Mukherjee, P.K.; Cui, F.; Sikaroodi, M.; Naqvi, A.; Gillevet, P.M. Characterization of the Oral Fungal Microbiome (Mycobiome) in Healthy Individuals. PLoS Pathog. 2010, 6, e1000713. [Google Scholar] [CrossRef] [PubMed]
- Pires, R.H.; Martinez, L.R.; Mendes-Giannini, M.J.S.; Stoianoff, M.A.R. Editorial: Pathogenesis of Fungal Biofilms in Different Environmental Conditions and Clinical Outcomes. Front. Cell. Infect. Microbiol. 2021, 11, 778458. [Google Scholar] [CrossRef]
- Cui, L.; Morris, A.; Ghedin, E. The Human Mycobiome in Health and Disease. Genome Med. 2013, 5, 63. [Google Scholar] [CrossRef]
- Wang, X.; Wu, S.; Wu, W.; Zhang, W.; Li, L.; Liu, Q.; Yan, Z. Candida Albicans Promotes Oral Cancer via IL-17A/IL-17RA-Macrophage Axis. MBio 2023, 14, e0044723. [Google Scholar] [CrossRef]
- Ladjevac, N.; Milovanovic, M.; Jevtovic, A.; Arsenijevic, D.; Stojanovic, B.; Dimitrijevic Stojanovic, M.; Stojanovic, B.; Arsenijevic, N.; Arsenijevic, A.; Milovanovic, J. The Role of IL-17 in the Pathogenesis of Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2023, 24, 9874. [Google Scholar] [CrossRef] [PubMed]
- Bilal, H.; Khan, M.N.; Khan, S.; Fang, W.; Chang, W.; Yin, B.; Song, N.J.; Liu, Z.; Zhang, D.; Yao, F.; et al. Risk of Candidiasis Associated with Interleukin-17 Inhibitors: Implications and Management. Mycology 2023, 15, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G. In It Together: Candida-Bacterial Oral Biofilms and Therapeutic Strategies. Environ. Microbiol. Rep. 2022, 14, 183–196. [Google Scholar] [CrossRef]
- Du, Q.; Ren, B.; Zhou, X.; Zhang, L.; Xu, X. Cross-Kingdom Interaction between Candida Albicans and Oral Bacteria. Front. Microbiol. 2022, 13, 911623. [Google Scholar] [CrossRef]
- de Jongh, C.A.; Bikker, F.J.; de Vries, T.J.; Werner, A.; Gibbs, S.; Krom, B.P. Porphyromonas gingivalis Interaction with Candida Albicans Allows for Aerobic Escape, Virulence and Adherence. Biofilm 2023, 7, 100172. [Google Scholar] [CrossRef]
- Martorano-Fernandes, L.; Goodwine, J.S.; Ricomini-Filho, A.P.; Nobile, C.J.; Del Bel Cury, A.A. Candida Albicans Adhesins Als1 and Hwp1 Modulate Interactions with Streptococcus Mutans. Microorganisms 2023, 11, 1391. [Google Scholar] [CrossRef]
- Ciurea, C.N.; Kosovski, I.-B.; Mare, A.D.; Toma, F.; Pintea-Simon, I.A.; Man, A. Candida and Candidiasis—Opportunism Versus Pathogenicity: A Review of the Virulence Traits. Microorganisms 2020, 8, 857. [Google Scholar] [CrossRef] [PubMed]
- Bartnicka, D.; Gonzalez-Gonzalez, M.; Sykut, J.; Koziel, J.; Ciaston, I.; Adamowicz, K.; Bras, G.; Zawrotniak, M.; Karkowska-Kuleta, J.; Satala, D.; et al. Candida Albicans Shields the Periodontal Killer Porphyromonas gingivalis from Recognition by the Host Immune System and Supports the Bacterial Infection of Gingival Tissue. Int. J. Mol. Sci. 2020, 21, 1984. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Wang, H.; Retuerto, M.; Zhang, H.; Burkey, B.; Ghannoum, M.A.; Eng, C. Bacteriome and Mycobiome Associations in Oral Tongue Cancer. Oncotarget 2017, 8, 97273–97289. [Google Scholar] [CrossRef]
- Di Cosola, M.; Cazzolla, A.P.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Santacroce, L. Candida Albicans and Oral Carcinogenesis. A Brief Review. J. Fungi 2021, 7, 476. [Google Scholar] [CrossRef]
- Ayuningtyas, N.F.; Mahdani, F.Y.; Pasaribu, T.A.S.; Chalim, M.; Ayna, V.K.P.; Santosh, A.B.R.; Santacroce, L.; Surboyo, M.D.C. Role of Candida Albicans in Oral Carcinogenesis. Pathophysiology 2022, 29, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Talapko, J.; Juzbašić, M.; Matijević, T.; Pustijanac, E.; Bekić, S.; Kotris, I.; Škrlec, I. Candida Albicans-The Virulence Factors and Clinical Manifestations of Infection. J. Fungi 2021, 7, 79. [Google Scholar] [CrossRef]
- Ilkhanizadeh-Qomi, M.; Nejatbakhsh, S.; Jahanshiri, Z.; Razzaghi-Abyaneh, M. Aspartyl Proteinase and Phospholipase Activities of Candida Albicans Isolated From Oropharyngeal Candidiasis in Head and Neck Cancer Patients. Jundishapur. J. Microbiol. 2020, 13, e105200. [Google Scholar] [CrossRef]
- Mogavero, S.; Höfs, S.; Lauer, A.N.; Müller, R.; Brunke, S.; Allert, S.; Gerwien, F.; Groth, S.; Dolk, E.; Wilson, D.; et al. Candidalysin Is the Hemolytic Factor of Candida Albicans. Toxins 2022, 14, 874. [Google Scholar] [CrossRef]
- Richardson, J.P.; Brown, R.; Kichik, N.; Lee, S.; Priest, E.; Mogavero, S.; Maufrais, C.; Wickramasinghe, D.N.; Tsavou, A.; Kotowicz, N.K.; et al. Candidalysins Are a New Family of Cytolytic Fungal Peptide Toxins. MBio 2022, 13, e0351021. [Google Scholar] [CrossRef]
- Nikou, S.A.; Zhou, C.; Griffiths, J.S.; Kotowicz, N.K.; Coleman, B.M.; Green, M.J.; Moyes, D.L.; Gaffen, S.L.; Naglik, J.R.; Parker, P.J. The Candida Albicans Toxin Candidalysin Mediates Distinct Epithelial Inflammatory Responses through P38 and EGFR-ERK Pathways. Sci. Signal. 2022, 15, eabj6915. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and Tumor Progression: Signaling Pathways and Targeted Intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Engku Nasrullah Satiman, E.A.F.; Ahmad, H.; Ramzi, A.B.; Abdul Wahab, R.; Kaderi, M.A.; Wan Harun, W.H.A.; Dashper, S.; McCullough, M.; Arzmi, M.H. The Role of Candida Albicans Candidalysin ECE1 Gene in Oral Carcinogenesis. J. Oral Pathol. Med. 2020, 49, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Vadovics, M.; Ho, J.; Igaz, N.; Alföldi, R.; Rakk, D.; Veres, E.; Szücs, B.; Horváth, M.; Tóth, R.; Szücs, A.; et al. Candida Albicans Enhances the Progression of Oral Squamous Cell Carcinoma In Vitro and In Vivo. MBio 2021, 13, e0314421. [Google Scholar] [CrossRef]
- Khanam, A.; Hithamani, G.; Naveen, J.; Pradeep, S.R.; Barman, S.; Srinivasan, K. Management of Invasive Infections in Diabetes Mellitus: A Comprehensive Review. Biologics 2023, 3, 40–71. [Google Scholar] [CrossRef]
- Thompson, A.; Orr, S.J. Emerging IL-12 Family Cytokines in the Fight against Fungal Infections. Cytokine 2018, 111, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, K.; Ahangari, H.; Chapeland-Leclerc, F.; Ruprich-Robert, G.; Tarhriz, V.; Dilmaghani, A. Role of Fungal Infections in Carcinogenesis and Cancer Development: A Literature Review. Adv. Pharm. Bull. 2022, 12, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, P.P.; Mallya, S.; Dongari-Bagtzoglou, A. A Novel Immunocompetent Murine Model for Candida Albicans-Promoted Oral Epithelial Dysplasia. Med. Mycol. 2009, 47, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Fadhil Ali Malaa, S.; Abd Ali Abd Aun Jwad, B.; Khalis Al-Masoudi, H. Assessment of Entamoeba Gingivalis and Trichomonas Tenax in Patients with Chronic Diseases and Its Correlation with Some Risk Factors. Arch. Razi Inst. 2022, 77, 77–82. [Google Scholar] [CrossRef]
- Sun, J.; Tang, Q.; Yu, S.; Xie, M.; Xie, Y.; Chen, G.; Chen, L. Role of the oral microbiota in cancer evolution and progression. Cancer Med. 2020, 9, 6306–6321. [Google Scholar] [CrossRef]
| Gram + Cocci | Gram + Bacilli | Gram − Cocci | Gram − Bacilli |
|---|---|---|---|
| Abiotrophia Peptostreptococcus Streptococcus | Actinomyces | Moraxella | Campylobacter |
| Bifidobacterium | Neisseria | Capnocytophaga | |
| Corynebacterium | Veillonella | Desulfobacter | |
| Stomatococcus | Eubacterium | Desulfovibrio | |
| Lactobacillus | Eikenella | ||
| Propionibacterium | Fusobacterium | ||
| Pseudoramibacter | Hemophilus | ||
| Rothia | Leptotrichia | ||
| Prevotella | |||
| Selemonas | |||
| Simonsiella | |||
| Treponema | |||
| Wolinella |
| No. | Mechanisms by Which Biofilm Promotes the Formation of Cancer | Ref. |
|---|---|---|
| 1. | Biofilm can directly affect the immune response of the host, thus creating a favorable environment for the development of cancer. | [66] |
| 2. | It can trigger chronic inflammation that leads to DNA damage and thus promotes the growth of cancer cells. | [82] |
| 3. | Bacteria inside the biofilm can secrete toxins that have carcinogenic effects. | [83] |
| 4. | Bacteria found in the tumor microenvironment (tumor microbiome) influence cancer progression. | [84] |
| 5. | Bacteria in the biofilm can significantly change the metabolism of the host. | [70] |
| Bacteria | Oncogenic Effects | Ref. |
|---|---|---|
| F. nucleatum P. gingivalis | Inducing infected cells to produce inflammatory cytokines or growth factors Induction of epithelial-to-mesenchymal transition Increase in proliferation Establishment of a tumor-promoting immune environment induction of chemoresistance, Induction of epithelial-to-mesenchymal transition Release of mutagenic substances Upregulation of cell survival factors Stimulation of cell invasion Suppression of antitumor immune response Initiation of tumor angiogenesis Enhancement of cellular stemness | [99,126] |
| Parasite | Representation of Parasites in the Oral Cavity |
|---|---|
| Entamoeba gingivalis | Highly prevalent in people with diabetes |
| Trichomonas tenax | Low in thyroid disorders Highly prevalent in people with hypertension |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talapko, J.; Erić, S.; Meštrović, T.; Stipetić, M.M.; Juzbašić, M.; Katalinić, D.; Bekić, S.; Muršić, D.; Flam, J.; Belić, D.; et al. The Impact of Oral Microbiome Dysbiosis on the Aetiology, Pathogenesis, and Development of Oral Cancer. Cancers 2024, 16, 2997. https://doi.org/10.3390/cancers16172997
Talapko J, Erić S, Meštrović T, Stipetić MM, Juzbašić M, Katalinić D, Bekić S, Muršić D, Flam J, Belić D, et al. The Impact of Oral Microbiome Dysbiosis on the Aetiology, Pathogenesis, and Development of Oral Cancer. Cancers. 2024; 16(17):2997. https://doi.org/10.3390/cancers16172997
Chicago/Turabian StyleTalapko, Jasminka, Suzana Erić, Tomislav Meštrović, Marinka Mravak Stipetić, Martina Juzbašić, Darko Katalinić, Sanja Bekić, Dora Muršić, Josipa Flam, Dino Belić, and et al. 2024. "The Impact of Oral Microbiome Dysbiosis on the Aetiology, Pathogenesis, and Development of Oral Cancer" Cancers 16, no. 17: 2997. https://doi.org/10.3390/cancers16172997
APA StyleTalapko, J., Erić, S., Meštrović, T., Stipetić, M. M., Juzbašić, M., Katalinić, D., Bekić, S., Muršić, D., Flam, J., Belić, D., Lešić, D., Fureš, R., Markanović, M., & Škrlec, I. (2024). The Impact of Oral Microbiome Dysbiosis on the Aetiology, Pathogenesis, and Development of Oral Cancer. Cancers, 16(17), 2997. https://doi.org/10.3390/cancers16172997