Epidemiology, Diagnostics, and Therapy of Oral Cancer—Update Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Overview of Oral Cancer
Oral Potentially Malignant Disorders (OPMD)
3. Classification
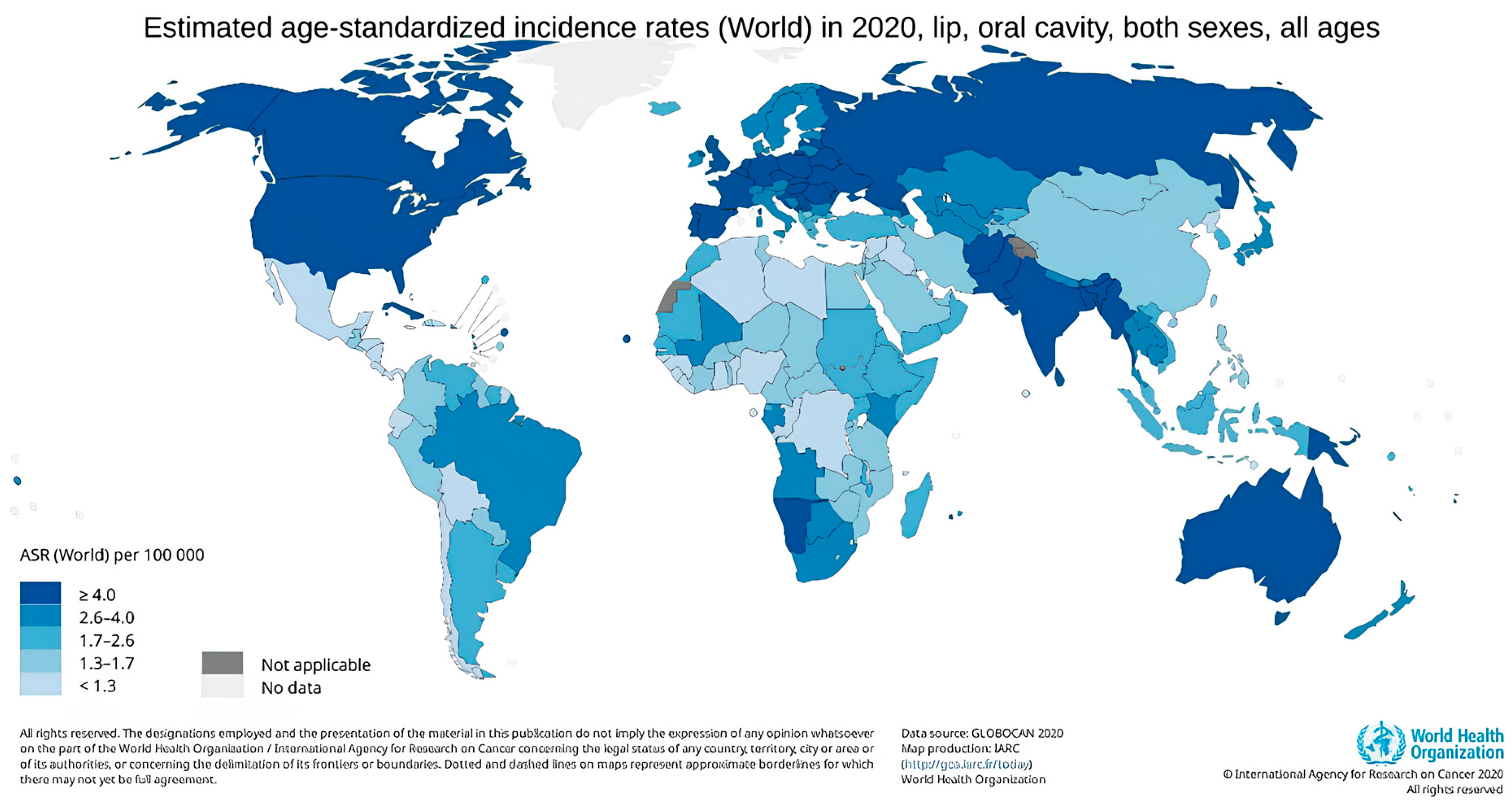
4. Epidemiology
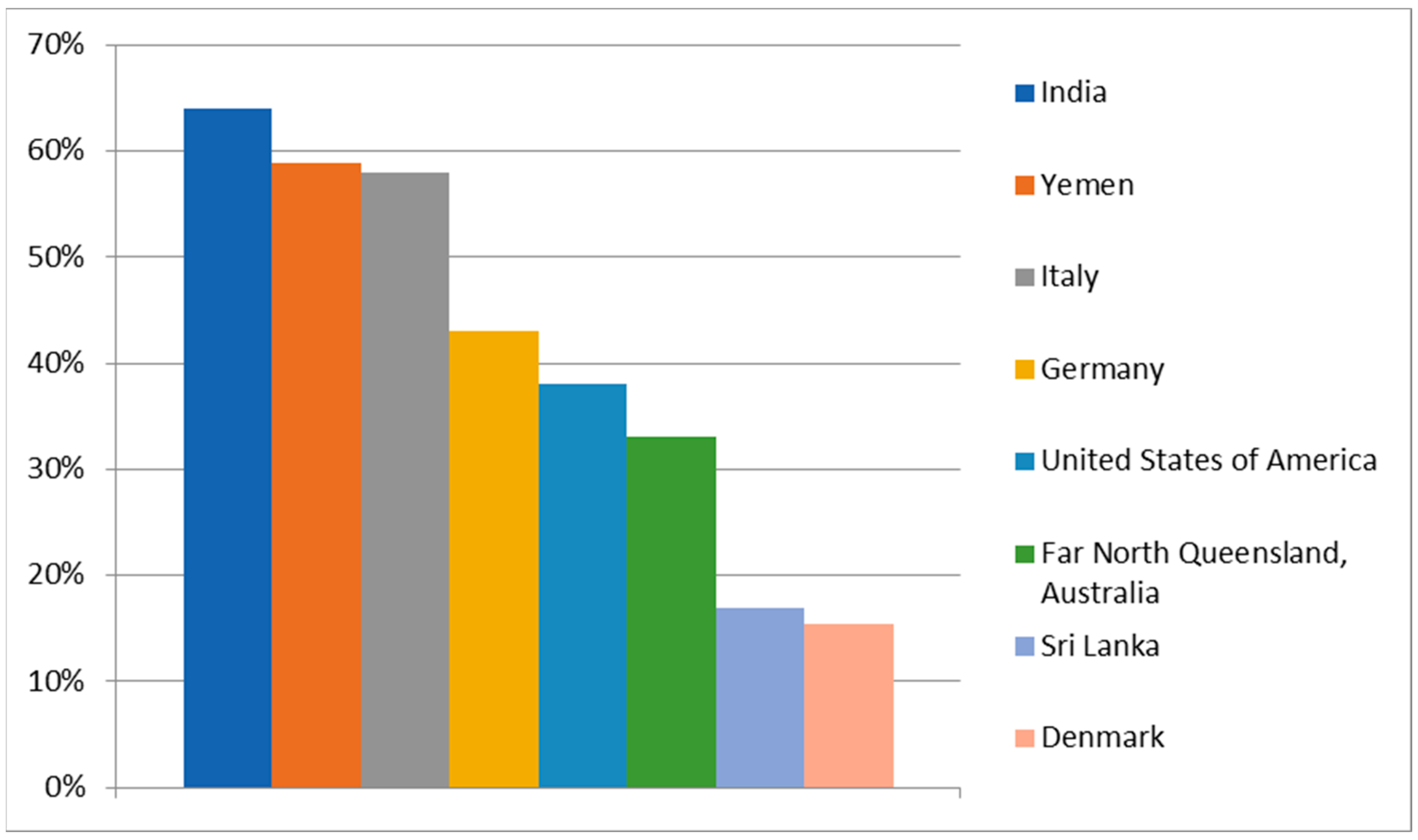
5. Risk Factors
5.1. Alcohol
5.2. Smoking
5.3. HPV
5.4. Diet
5.5. Oral Hygiene
6. Diagnostics
6.1. Vital Tissue Staining
6.2. Optical Imaging
6.3. Oral Cytology
6.4. Salivary Biomarkers
6.5. Artificial Intelligence
6.6. Colposcopy
6.7. Spectroscopy
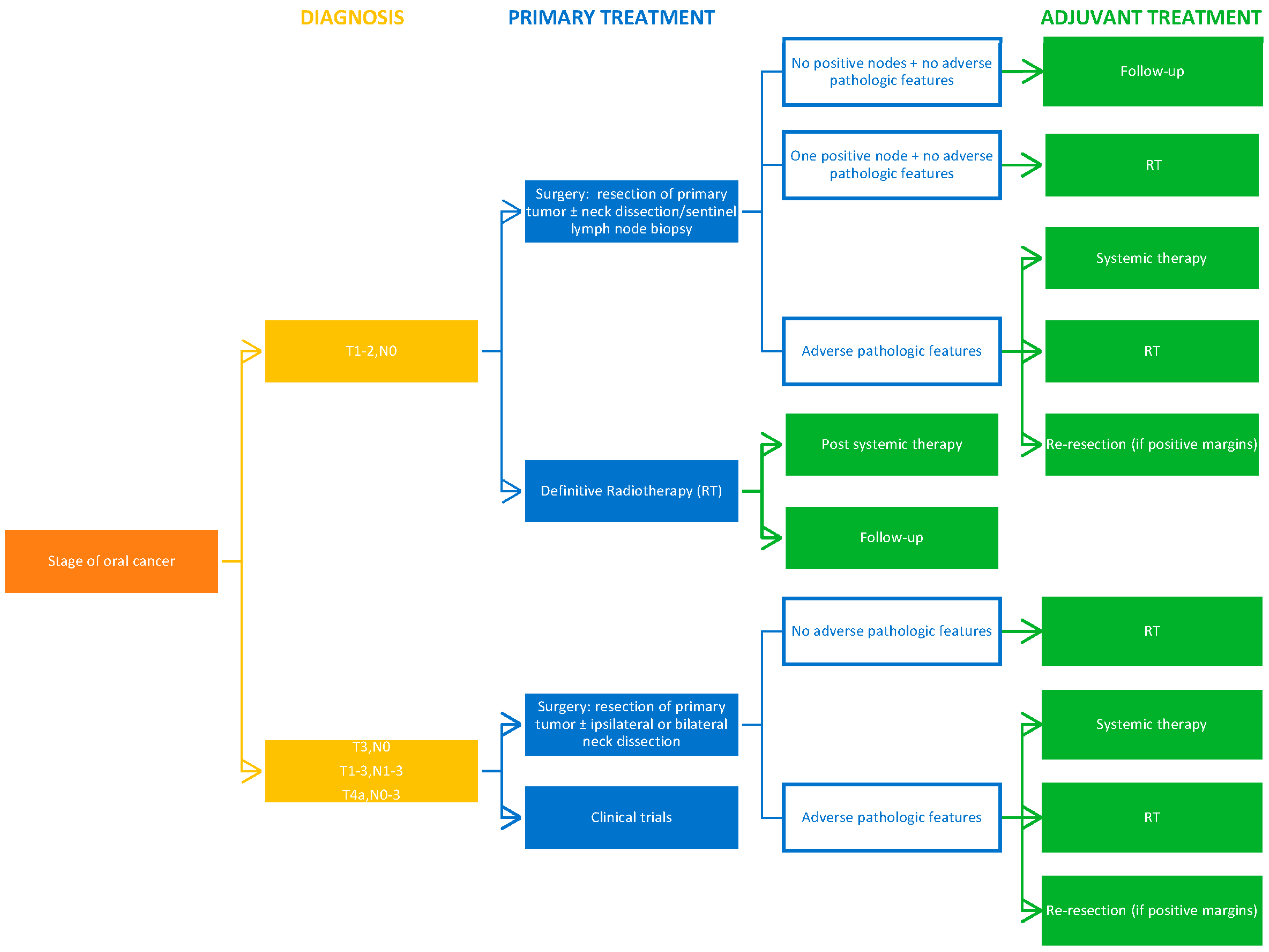
7. Treatment
7.1. Surgery
7.2. Radiotherapy
7.3. Chemotherapy
7.4. Immunotherapy
7.4.1. Immunotherapy Strategies under Clinical Trials
7.4.2. Immunotherapy and Chemotherapy Combination
7.5. Novel Treatment
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Speight, P.M.; Epstein, J.; Kujan, O.; Lingen, M.W.; Nagao, T.; Ranganathan, K.; Vargas, P. Screening for Oral Cancer—A Perspective from the Global Oral Cancer Forum. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 123, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Campos, N.G.; Tsu, V.; Jeronimo, J.; Regan, C.; Resch, S.; Clark, A.; Sy, S.; Kim, J.J. Health Impact of Delayed Implementation of Cervical Cancer Screening Programs in India: A Modeling Analysis. Int. J. Cancer 2019, 144, 687–696. [Google Scholar] [CrossRef]
- Srivastava, A.N.; Misra, J.S.; Srivastava, S.; Das, B.C.; Gupta, S. Cervical Cancer Screening in Rural India: Status & Current Concepts. Indian J. Med. Res. 2018, 148, 687–696. [Google Scholar]
- Bashar, M.D.A.; Aggarwal, A.K.; Valecha, D. A Successful Model of Cancer Screening in Low-Resource Settings: Findings of an Integrated Cancer Screening Camp from a Rural Setting of North India. Oncol. J. India 2020, 4, 83–86. [Google Scholar] [CrossRef]
- Mesher, D.; Panwar, K.; Thomas, S.L.; Edmundson, C.; Choi, Y.H.; Beddows, S.; Soldan, K. The Impact of the National HPV Vaccination Program in England Using the Bivalent HPV Vaccine: Surveillance of Type-Specific HPV in Young Females, 2010–2016. J. Infect. Dis. 2018, 218, 911–921. [Google Scholar] [CrossRef]
- Ribeiro, M.F.A.; Oliveira, M.C.M.; Leite, A.C.; Bruzinga, F.F.B.; Mendes, P.A.; Grossmann, S.d.M.C.; de Araújo, V.E.; Souto, G.R. Assessment of Screening Programs as a Strategy for Early Detection of Oral Cancer: A Systematic Review. Oral Oncol. 2022, 130, 105936. [Google Scholar] [CrossRef]
- Zhou, X.-H.; Huang, Y.; Yuan, C.; Zheng, S.-G.; Zhang, J.-G.; Lv, X.-M.; Zhang, J. A Survey of the Awareness and Knowledge of Oral Cancer among Residents in Beijing. BMC Oral Health 2022, 22, 367. [Google Scholar] [CrossRef]
- Kawecki, M.M.; Nedeva, I.R.; Iloya, J.; Macfarlane, T. V Mouth Cancer Awareness in General Population: Results from Grampian Region of Scotland, United Kingdom. J. Oral Maxillofac. Res. 2019, 10, e3. [Google Scholar] [CrossRef]
- Cancer Research UK. Head and Neck Cancers Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/head-and-neck-cancers (accessed on 15 December 2023).
- Gupta, B.; Bray, F.; Kumar, N.; Johnson, N.W. Associations between Oral Hygiene Habits, Diet, Tobacco and Alcohol and Risk of Oral Cancer: A Case–Control Study from India. Cancer Epidemiol. 2017, 51, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Oral Health Foundation. The State of Mouth Cancer UK Report 2022. Available online: https://www.dentalhealth.org/thestateofmouthcancer (accessed on 15 December 2023).
- Tan, Y.; Wang, Z.; Xu, M.; Li, B.; Huang, Z.; Qin, S.; Nice, E.C.; Tang, J.; Huang, C. Oral Squamous Cell Carcinomas: State of the Field and Emerging Directions. Int. J. Oral Sci. 2023, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- Nandini, D.; Rao, R.; Hosmani, J.; Khan, S.; Patil, S. Novel Therapies in the Management of Oral Cancer: An Update. Dis. Mon. 2020, 66, 101036. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Yang, J.-Y.; Lee, D.-M.; Lee, J.-Y.; Hwang, D.-S.; Ryu, M.-H.; Kim, U.-K. Retrospective Analysis on Prognosis of Oral Cancer Patients According to Surgical Approaches for Effective Cancer Ablation: Swing Approach versus Visor Approach. Maxillofac. Plast. Reconstr. Surg. 2024, 46, 15. [Google Scholar] [CrossRef]
- Eskander, A.; Dziegielewski, P.T.; Patel, M.R.; Jethwa, A.R.; Pai, P.S.; Silver, N.L.; Sajisevi, M.; Sanabria, A.; Doweck, I.; Khariwala, S.S. Oral Cavity Cancer Surgical and Nodal Management: A Review from the American Head and Neck Society. JAMA Otolaryngol. Head Neck Surg. 2024, 150, 172–178. [Google Scholar] [CrossRef]
- Jitender, S.; Sarika, G.; Varada, H.R.; Omprakash, Y.; Mohsin, K. Screening for Oral Cancer. J. Exp. Ther. Oncol. 2016, 11, 303–307. [Google Scholar]
- Muller, S.; Tilakaratne, W.M. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Tumours of the Oral Cavity and Mobile Tongue. Head Neck Pathol. 2022, 16, 54–62. [Google Scholar] [CrossRef]
- El-Naggar, A.K.; Chan, J.K.C.; Rubin Grandis, J.; Slootweg, P.J. WHO Classification of Head and Neck Tumours; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral Potentially Malignant Disorders: A Consensus Report from an International Seminar on Nomenclature and Classification, Convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef]
- de Azevedo, A.B.; Dos Santos, T.C.R.B.; Lopes, M.A.; Pires, F.R. Oral Leukoplakia, Leukoerythroplakia, Erythroplakia and Actinic Cheilitis: Analysis of 953 Patients Focusing on Oral Epithelial Dysplasia. J. Oral Pathol. Med. 2021, 50, 829–840. [Google Scholar] [CrossRef]
- Van der Waal, I. Oral Leukoplakia, the Ongoing Discussion on Definition and Terminology. Med. Oral Patol. Oral Cir. Bucal. 2015, 20, e685. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Johnson, N.W.; Van der Waal, I. Nomenclature and Classification of Potentially Malignant Disorders of the Oral Mucosa. J. Oral Pathol. Med. 2007, 36, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Saldivia-Siracusa, C.; González-Arriagada, W.A. Difficulties in the Prognostic Study of Oral Leukoplakia: Standardisation Proposal of Follow-up Parameters. Front. Oral Health 2021, 2, 614045. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.; Barbieri, C.; Warnakulasuriya, S.; Martins, M.; Salazar, F.; Pacheco, J.-J.; Vescovi, P.; Meleti, M. Type of Surgical Treatment and Recurrence of Oral Leukoplakia: A Retrospective Clinical Study. Med. Oral Patol. Oral Cir. Bucal. 2017, 22, e520. [Google Scholar] [CrossRef] [PubMed]
- Maymone, M.B.C.; Greer, R.O.; Kesecker, J.; Sahitya, P.C.; Burdine, L.K.; Cheng, A.-D.; Maymone, A.C.; Vashi, N.A. Premalignant and Malignant Oral Mucosal Lesions: Clinical and Pathological Findings. J. Am. Acad. Dermatol. 2019, 81, 59–71. [Google Scholar] [CrossRef]
- Tovaru, S.; Costache, M.; Perlea, P.; Caramida, M.; Totan, C.; Warnakulasuriya, S.; Parlatescu, I. Oral Leukoplakia: A Clinicopathological Study and Malignant Transformation. Oral Dis. 2023, 29, 1454–1463. [Google Scholar] [CrossRef]
- Favia, G.; Capodiferro, S.; Limongelli, L.; Tempesta, A.; Maiorano, E. Malignant Transformation of Oral Proliferative Verrucous Leukoplakia: A Series of 48 Patients with Suggestions for Management. Int. J. Oral Maxillofac. Surg. 2021, 50, 14–20. [Google Scholar] [CrossRef]
- Bagan, J.; Murillo-Cortes, J.; Poveda-Roda, R.; Leopoldo-Rodado, M.; Bagan, L. Second Primary Tumors in Proliferative Verrucous Leukoplakia: A Series of 33 Cases. Clin. Oral Investig. 2020, 24, 1963–1969. [Google Scholar] [CrossRef]
- Shanahan, D.; Cowie, R.; Rogers, H.; Staines, K. Oral Hairy Leukoplakia in Healthy Immunocompetent Patients: A Small Case Series. Oral Maxillofac. Surg. 2018, 22, 335–339. [Google Scholar] [CrossRef]
- Wils, L.J.; Poell, J.B.; Evren, I.; Koopman, M.S.; Brouns, E.R.E.A.; de Visscher, J.G.A.M.; Brakenhoff, R.H.; Bloemena, E. Incorporation of Differentiated Dysplasia Improves Prediction of Oral Leukoplakia at Increased Risk of Malignant Progression. Mod. Pathol. 2020, 33, 1033–1040. [Google Scholar] [CrossRef]
- Pires, F.R.; Barreto, M.E.Z.; Nunes, J.G.R.; Carneiro, N.S.; de Azevedo, A.B.; dos Santos, T.C.R.B. Oral Potentially Malignant Disorders: Clinical-Pathological Study of 684 Cases Diagnosed in a Brazilian Population. Med. Oral Patol. Oral Cir. Bucal. 2020, 25, e84. [Google Scholar]
- Patait, M.; Nikate, U.; Saraf, K.; Singh, P.; Jadhav, V. Oral Erythroplakia–A Case Report. Int. J. Appl. Dental Sci. 2016, 2, 79–82. [Google Scholar]
- Hallikeri, K.; Naikmasur, V.; Guttal, K.; Shodan, M.; Chennappa, N.K. Prevalence of Oral Mucosal Lesions among Smokeless Tobacco Usage: A Cross-Sectional Study. Indian J. Cancer 2018, 55, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.H.; Iyer, N.G.; Tan, M.; Edgren, G. Changing Epidemiology of Oral Squamous Cell Carcinoma of the Tongue: A Global Study. Head Neck 2017, 39, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Dhanuthai, K.; Rojanawatsirivej, S.; Thosaporn, W.; Kintarak, S.; Subarnbhesaj, A.; Darling, M.; Kryshtalskyj, E.; Chiang, C.P.; Shin, H.I.; Choi, S.Y.; et al. Oral Cancer: A Multicenter Study. Med. Oral Patol. Oral Cir. Bucal. 2018, 23, e23–e29. [Google Scholar] [CrossRef]
- Wong, T.S.C.; Wiesenfeld, D. Oral Cancer. Aust. Dent. J. 2018, 63, S91–S99. [Google Scholar] [CrossRef]
- Farhood, Z.; Simpson, M.; Ward, G.M.; Walker, R.J.; Osazuwa-Peters, N. Does Anatomic Subsite Influence Oral Cavity Cancer Mortality? A SEER Database Analysis. Laryngoscope 2019, 129, 1400–1406. [Google Scholar] [CrossRef]
- Huang, J.; Chan, S.C.; Ko, S.; Lok, V.; Zhang, L.; Lin, X.; Lucero-Prisno, D.E.; Xu, W.; Zheng, Z.J.; Elcarte, E.; et al. Disease Burden, Risk Factors, and Trends of Lip, Oral Cavity, Pharyngeal Cancers: A Global Analysis. Cancer Med. 2023, 12, 18153–18164. [Google Scholar] [CrossRef]
- Mehanna, H.; Beech, T.; Nicholson, T.; El-Hariry, I.; McConkey, C.; Paleri, V.; Roberts, S. Prevalence of Human Papillomavirus in Oropharyngeal and Nonoropharyngeal Head and Neck Cancer--Systematic Review and Meta-Analysis of Trends by Time and Region. Head Neck 2013, 35, 747–755. [Google Scholar] [CrossRef]
- Bosetti, C.; Carioli, G.; Santucci, C.; Bertuccio, P.; Gallus, S.; Garavello, W.; Negri, E.; La Vecchia, C. Global Trends in Oral and Pharyngeal Cancer Incidence and Mortality. Int. J. Cancer 2020, 147, 1040–1049. [Google Scholar] [CrossRef]
- Homann, N.; Jousimies-Somer, H.; Jokelainen, K.; Heine, R.; Salaspuro, M. High Acetaldehyde Levels in Saliva after Ethanol Consumption: Methodological Aspects and Pathogenetic Implications. Carcinogenesis 1997, 18, 1739–1743. [Google Scholar] [CrossRef]
- Seitz, H.K.; Stickel, F. Acetaldehyde as an Underestimated Risk Factor for Cancer Development: Role of Genetics in Ethanol Metabolism. Genes Nutr. 2010, 5, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, M.T.; Salaspuro, M. Local Acetaldehyde-An Essential Role in Alcohol-Related Upper Gastrointestinal Tract Carcinogenesis. Cancers 2018, 10, 11. [Google Scholar] [CrossRef]
- Elango, J.K.; Sundaram, K.R.; Gangadharan, P.; Subhas, P.; Peter, S.; Pulayath, C.; Kuriakose, M.A. Factors Affecting Oral Cancer Awareness in a High-Risk Population in India. Asian Pac. J. Cancer Prev. 2009, 10, 627–630. [Google Scholar] [PubMed]
- Al-Maweri, S.A.; Addas, A.; Tarakji, B.; Abbas, A.; Al-Shamiri, H.M.; Alaizari, N.A.; Shugaa-Addin, B. Public Awareness and Knowledge of Oral Cancer in Yemen. Asian Pac. J. Cancer Prev. 2015, 15, 10861–10865. [Google Scholar] [CrossRef]
- Nocini, R.; Capocasale, G.; Marchioni, D.; Zotti, F. A Snapshot of Knowledge about Oral Cancer in Italy: A 505 Person Survey. Int. J. Environ. Res. Public Health 2020, 17, 4889. [Google Scholar] [CrossRef] [PubMed]
- Hertrampf, K.; Wenz, H.J.; Koller, M.; Wiltfang, J. Public Awareness about Prevention and Early Detection of Oral Cancer: A Population-Based Study in Northern Germany. J. Cranio-Maxillofac. Surg. 2012, 40, e82–e86. [Google Scholar] [CrossRef]
- Wiseman, K.P.; Klein, W.M.P. Evaluating Correlates of Awareness of the Association between Drinking Too Much Alcohol and Cancer Risk in the United States. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 1195–1201. [Google Scholar] [CrossRef]
- Formosa, J.; Jenner, R.; Nguyen-Thi, M.D.; Stephens, C.; Wilson, C.; Ariyawardana, A. Awareness and Knowledge of Oral Cancer and Potentially Malignant Oral Disorders among Dental Patients in Far North Queensland, Australia. Asian Pac. J. Cancer Prev. 2015, 16, 4429–4434. [Google Scholar] [CrossRef]
- Thomsen, K.L.; Christensen, A.S.P.; Meyer, M.K.H. Awareness of Alcohol as a Risk Factor for Cancer: A Population-Based Cross-Sectional Study among 3000 Danish Men and Women. Prev. Med. Rep. 2020, 19, 101156. [Google Scholar] [CrossRef]
- Ariyawardana, A.; Vithanaarachchi, N. Awareness of Oral Cancer and Precancer among Patients Attending a Hospital in Sri Lanka. Asian Pac. J. Cancer Prev. 2005, 6, 58–61. [Google Scholar]
- Zhang, Y.; He, J.; He, B.; Huang, R.; Li, M. Effect of Tobacco on Periodontal Disease and Oral Cancer. Tob. Induc. Dis. 2019, 17, 40. [Google Scholar] [CrossRef]
- WHO Global Report on Trends in Prevalence of Tobacco Use 2000–2025, Third Edition. Available online: https://www.who.int/publications/i/item/who-global-report-on-trends-in-prevalence-of-tobacco-use-2000-2025-third-edition (accessed on 13 December 2023).
- Hecht, S.S. Tobacco Carcinogens, Their Biomarkers and Tobacco-Induced Cancer. Nat. Rev. Cancer 2003, 3, 733–744. [Google Scholar] [CrossRef] [PubMed]
- WHO. List of Classifications—IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Available online: https://monographs.iarc.who.int/list-of-classifications/ (accessed on 13 December 2023).
- Cole, A.G.; Aleyan, S.; Battista, K.; Leatherdale, S.T. Trends in Youth E-Cigarette and Cigarette Use between 2013 and 2019: Insights from Repeat Cross-Sectional Data from the COMPASS Study. Can. J. Public Health 2021, 112, 60–69. [Google Scholar] [CrossRef] [PubMed]
- McNeill, A.; Brose, L.; Calder, R.; Robson, D.; Bauld, L.; Dockrell, M. E-Cigarette Regulation in the United States and the United Kingdom: Two Countries Divided by a Common Language. Am. J. Public Health 2019, 109, E26–E27. [Google Scholar] [CrossRef]
- Javed, F.; Abduljabbar, T.; Vohra, F.; Malmstrom, H.; Rahman, I.; Romanos, G.E. Comparison of Periodontal Parameters and Self-Perceived Oral Symptoms Among Cigarette Smokers, Individuals Vaping Electronic Cigarettes, and Never-Smokers. J. Periodontol. 2017, 88, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Goniewicz, M.L.; Knysak, J.; Gawron, M.; Kosmider, L.; Sobczak, A.; Kurek, J.; Prokopowicz, A.; Jablonska-Czapla, M.; Rosik-Dulewska, C.; Havel, C.; et al. Levels of Selected Carcinogens and Toxicants in Vapour from Electronic Cigarettes. Tob. Control 2014, 23, 133–139. [Google Scholar] [CrossRef]
- Williams, M.; Villarreal, A.; Bozhilov, K.; Lin, S.; Talbot, P. Metal and Silicate Particles Including Nanoparticles Are Present in Electronic Cigarette Cartomizer Fluid and Aerosol. PLoS ONE 2013, 8, e57987. [Google Scholar] [CrossRef]
- Tzortzi, A.; Kapetanstrataki, M.; Evangelopoulou, V.; Beghrakis, P. A Systematic Literature Review of E-Cigarette-Related Illness and Injury: Not Just for the Respirologist. Int. J. Environ. Res. Public Health 2020, 17, 2248. [Google Scholar] [CrossRef]
- Welz, C.; Canis, M.; Schwenk-Zieger, S.; Becker, S.; Stucke, V.; Ihler, F.; Baumeister, P. Cytotoxic and Genotoxic Effects of Electronic Cigarette Liquids on Human Mucosal Tissue Cultures of the Oropharynx. J. Environ. Pathol. Toxicol. Oncol. 2016, 35, 343–354. [Google Scholar] [CrossRef]
- WHO. Human Papillomavirus and Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/human-papilloma-virus-and-cancer (accessed on 13 December 2023).
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global Burden of Cancer Attributable to Infections in 2018: A Worldwide Incidence Analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef]
- Ndiaye, C.; Mena, M.; Alemany, L.; Arbyn, M.; Castellsagué, X.; Laporte, L.; Bosch, F.X.; de Sanjosé, S.; Trottier, H. HPV DNA, E6/E7 MRNA, and P16INK4a Detection in Head and Neck Cancers: A Systematic Review and Meta-Analysis. Lancet Oncol. 2014, 15, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide Burden of Cancer Attributable to HPV by Site, Country and HPV Type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Rautava, J.; Willberg, J.; Louvanto, K.; Wideman, L.; Syrjänen, K.; Grénman, S.; Syrjänen, S. Prevalence, Genotype Distribution and Persistence of Human Papillomavirus in Oral Mucosa of Women: A Six-Year Follow-up Study. PLoS ONE 2012, 7, e42171. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beachler, D.C.; Sugar, E.A.; Margolick, J.B.; Weber, K.M.; Strickler, H.D.; Wiley, D.J.; Cranston, R.D.; Burk, R.D.; Minkoff, H.; Reddy, S.; et al. Risk Factors for Acquisition and Clearance of Oral Human Papillomavirus Infection Among HIV-Infected and HIV-Uninfected Adults. Am. J. Epidemiol. 2015, 181, 40–53. [Google Scholar] [CrossRef]
- Marur, S.; D’Souza, G.; Westra, W.H.; Forastiere, A.A. HPV-Associated Head and Neck Cancer: A Virus-Related Cancer Epidemic. Lancet Oncol. 2010, 11, 781–789. [Google Scholar] [CrossRef]
- Kaur, G.; Yap, T.; Ramani, R.; McCullough, M.; Singh, A. Assessing Bias in the Causal Role of HPV in Oral Cancer: A Systematic Review and Meta-Analysis. Oral Dis. 2024. [Google Scholar] [CrossRef]
- Hua, R.; Liang, G.; Yang, F. Meta-Analysis of the Association between Dietary Inflammatory Index (DII) and Upper Aerodigestive Tract Cancer Risk. Medicine 2020, 99, E19879. [Google Scholar] [CrossRef]
- Rodríguez-Molinero, J.; Migueláñez-Medrán, B.D.C.; Puente-Gutiérrez, C.; Delgado-Somolinos, E.; Carreras-Presas, C.M.; Fernández-Farhall, J.; López-Sánchez, A.F. Association between Oral Cancer and Diet: An Update. Nutrients 2021, 13, 1299. [Google Scholar] [CrossRef]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef]
- Chang, C.-C.; Lee, W.-T.; Lee, Y.-C.; Huang, C.-C.; Ou, C.-Y.; Lin, Y.-H.; Huang, J.-S.; Wong, T.-Y.; Chen, K.-C.; Hsiao, J.-R.; et al. Investigating the Association between Diet and Risk of Head and Neck Cancer in Taiwan. Oncotarget 2017, 8, 98865–98875. [Google Scholar] [CrossRef]
- Esquivel-Chirino, C.; Bolaños-Carrillo, M.A.; Carmona-Ruiz, D.; Lopéz-Macay, A.; Hernández-Sánchez, F.; Montés-Sánchez, D.; Escuadra-Landeros, M.; Gaitán-Cepeda, L.A.; Maldonado-Frías, S.; Yáñez-Ocampo, B.R.; et al. The Protective Role of Cranberries and Blueberries in Oral Cancer. Plants 2023, 12, 2330. [Google Scholar] [CrossRef] [PubMed]
- McClain, K.M.; Bradshaw, P.T.; Khankari, N.K.; Gammon, M.D.; Olshan, A.F. Fish/Shellfish Intake and the Risk of Head and Neck Cancer. Eur. J. Cancer Prev. 2019, 28, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Ansary, J.; Forbes-Hernández, T.Y.; Gil, E.; Cianciosi, D.; Zhang, J.; Elexpuru-Zabaleta, M.; Simal-Gandara, J.; Giampieri, F.; Battino, M. Potential Health Benefit of Garlic Based on Human Intervention Studies: A Brief Overview. Antioxidants 2020, 9, 619. [Google Scholar] [CrossRef]
- Mukherjee, D.; Krishnan, A. Therapeutic Potential of Curcumin and Its Nanoformulations for Treating Oral Cancer. World J. Methodol. 2023, 13, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Gaonkar, P.P.; Patankar, S.R.; Tripathi, N.; Sridharan, G. Oral Bacterial Flora and Oral Cancer: The Possible Link? J. Oral Maxillofac. Pathol. 2018, 22, 234. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Y.; Zhang, L. Role of the Microbiome in Oral Cancer Occurrence, Progression and Therapy. Microb. Pathog. 2022, 169, 105638. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, J.; Zhou, B. Toothbrushing Frequency and Gastric and Upper Aerodigestive Tract Cancer Risk: A Meta-Analysis. Eur. J. Clin. Investig. 2021, 51, e13478. [Google Scholar] [CrossRef]
- Su, Y.F.; Chen, Y.J.; Tsai, F.T.; Li, W.C.; Hsu, M.L.; Wang, D.H.; Yang, C.C. Current Insights into Oral Cancer Diagnostics. Diagnostics 2021, 11, 1287. [Google Scholar] [CrossRef]
- Irani, S. New Insights into Oral Cancer-Risk Factors and Prevention: A Review of Literature. Int. J. Prev. Med. 2020, 11, 182–190. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Y.; Huang, M.; Huang, Z.; Wang, Q.; Qing, L.; Li, L.; Xu, S.; Jia, B. Current Status, Opportunities, and Challenges of Exosomes in Oral Cancer Diagnosis and Treatment. Int. J. Nanomed. 2022, 17, 2679–2705. [Google Scholar] [CrossRef]
- Abdul, N.S. Role of Advanced Diagnostic Aids in the Detection of Potentially Malignant Disorders and Oral Cancer at an Early Stage. Cureus 2023, 15, e34113. [Google Scholar] [CrossRef] [PubMed]
- Abati, S.; Bramati, C.; Bondi, S.; Lissoni, A.; Trimarchi, M. Oral Cancer and Precancer: A Narrative Review on the Relevance of Early Diagnosis. Int. J. Environ. Res. Public Health 2020, 17, 9160. [Google Scholar] [CrossRef] [PubMed]
- Muthu, K.; Vaishnavi, V.; Sivadas, G. Warning Signs and Symptoms of Oral Cancer and Its Differential Diagnosis. J. Young Pharm. 2018, 10, 138. [Google Scholar] [CrossRef]
- Ramesh, R.; Sadasivan, A. Oral Squamous Cell Carcinoma Masquerading as Gingival Overgrowth. Eur. J. Dent. 2017, 11, 390–394. [Google Scholar] [CrossRef]
- George, A.K. Abnormal tooth mobility due to carcinoma of the gingiva and underlying alveolar bone-a case report. Indian Dent. Assoc. Thiruvalla 2019, 1000, 7–9. [Google Scholar]
- Bora, P.; Singh, S.; Sharma, K.; Singh, N.; Singh, P. Advanced diagnostic aids in oral pathology. Int. J. Adv. Res. 2022, 10, 511–517. [Google Scholar] [CrossRef]
- Parakh, M.K.; Ulaganambi, S.; Ashifa, N.; Premkumar, R.; Jain, A.L. Oral Potentially Malignant Disorders: Clinical Diagnosis and Current Screening Aids: A Narrative Review. Eur. J. Cancer Prev. 2020, 29, 65–72. [Google Scholar] [CrossRef]
- Fedele, S. Diagnostic Aids in the Screening of Oral Cancer. Head Neck Oncol. 2009, 1, 5. [Google Scholar] [CrossRef]
- McCullough, M.J.; Prasad, G.; Farah, C.S. Oral Mucosal Malignancy and Potentially Malignant Lesions: An Update on the Epidemiology, Risk Factors, Diagnosis and Management. Aust. Dent. J. 2010, 55 (Suppl. S1), 61–65. [Google Scholar] [CrossRef]
- Nikolov, N.; Karaslavova, E.; Yaneva, B. Effectiveness of Velscope and Vizilite plus Systems in Diagnostics of Oral Lesions. Acta Medica Bulg. 2021, 48, 1. [Google Scholar] [CrossRef]
- Jaitley, S.; Agarwal, P.; Upadhyay, R. Role of Oral Exfoliative Cytology in Predicting Premalignant Potential of Oral Submucous Fibrosis: A Short Study. J. Cancer Res. Ther. 2015, 11, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Umapathy, V.R.; Natarajan, P.M.; Swamikannu, B. Comprehensive Review on Development of Early Diagnostics on Oral Cancer with a Special Focus on Biomarkers. Appl. Sci. 2022, 12, 4926. [Google Scholar] [CrossRef]
- Mehrotra, R.; Mishra, S.; Singh, M.; Singh, M. The Efficacy of Oral Brush Biopsy with Computer-Assisted Analysis in Identifying Precancerous and Cancerous Lesions. Head Neck Oncol. 2011, 3, 39. [Google Scholar] [CrossRef] [PubMed]
- Ademola Adeola, H.; Holmes, H.; Oluwaseyi Temilola, D. Diagnostic Potential of Salivary Exosomes in Oral Cancer. In Oral Cancer—Current Concepts and Future Perspectives; IntechOpen: London, UK, 2022. [Google Scholar]
- Kaur, J.; Jacobs, R.; Huang, Y.; Salvo, N.; Politis, C. Salivary Biomarkers for Oral Cancer and Pre-Cancer Screening: A Review. Clin. Oral Investig. 2018, 22, 633–640. [Google Scholar] [CrossRef]
- Goldoni, R.; Scolaro, A.; Boccalari, E.; Dolci, C.; Scarano, A.; Inchingolo, F.; Ravazzani, P.; Muti, P.; Tartaglia, G. Malignancies and Biosensors: A Focus on Oral Cancer Detection through Salivary Biomarkers. Biosensors 2021, 11, 396. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Rees, T.; Wright, J. A Review of Research on Salivary Biomarkers for Oral Cancer Detection. Clin. Transl. Med. 2014, 3, e3. [Google Scholar] [CrossRef]
- García-Pola, M.; Pons-Fuster, E.; Suárez-Fernández, C.; Seoane-Romero, J.; Romero-Méndez, A.; López-Jornet, P. Role of Artificial Intelligence in the Early Diagnosis of Oral Cancer. A Scoping Review. Cancers 2021, 13, 4600. [Google Scholar] [CrossRef]
- Khanagar, S.B.; Naik, S.; Al Kheraif, A.A.; Vishwanathaiah, S.; Maganur, P.C.; Alhazmi, Y.; Mushtaq, S.; Sarode, S.C.; Sarode, G.S.; Zanza, A.; et al. Application and Performance of Artificial Intelligence Technology in Oral Cancer Diagnosis and Prediction of Prognosis: A Systematic Review. Diagnostics 2021, 11, 1004. [Google Scholar] [CrossRef]
- Hegde, S.; Ajila, V.; Zhu, W.; Zeng, C. Artificial Intelligence in Early Diagnosis and Prevention of Oral Cancer. Asia Pac. J. Oncol. Nurs. 2022, 9, 100133. [Google Scholar] [CrossRef]
- Bisht, S.R.; Mishra, P.; Yadav, D.; Rawal, R.; Mercado-Shekhar, K.P. Current and Emerging Techniques for Oral Cancer Screening and Diagnosis: A Review. Prog. Biomed. Eng. 2021, 3, 199–209. [Google Scholar] [CrossRef]
- Alhazmi, A.; Alhazmi, Y.; Makrami, A.; Masmali, A.; Salawi, N.; Masmali, K.; Patil, S. Application of Artificial Intelligence and Machine Learning for Prediction of Oral Cancer Risk. J. Oral Pathol. Med. 2021, 50, 444–450. [Google Scholar] [CrossRef]
- Mahmood, H.; Shaban, M.; Rajpoot, N.; Khurram, S.A. Artificial Intelligence-Based Methods in Head and Neck Cancer Diagnosis: An Overview. Br. J. Cancer 2021, 124, 1934–1940. [Google Scholar] [CrossRef] [PubMed]
- Khanagar, S.B.; Alkadi, L.; Alghilan, M.A.; Kalagi, S.; Awawdeh, M.; Bijai, L.K.; Vishwanathaiah, S.; Aldhebaib, A.; Singh, O.G. Application and Performance of Artificial Intelligence (AI) in Oral Cancer Diagnosis and Prediction Using Histopathological Images: A Systematic Review. Biomedicines 2023, 11, 1612. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Abbasi, M.S.; Zuberi, F.; Qamar, W.; Halim, M.S.B.; Maqsood, A.; Alam, M.K. Artificial Intelligence Techniques: Analysis, Application, and Outcome in Dentistry—A Systematic Review. Biomed. Res. Int. 2021, 2021, 9751564. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A. Colposcopy—A Novel Diagnostic Technique for Oral Mucosal Lesions. J. Clin. Diagn. Res. 2014, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Ujwala, N.; Singh, N.A.; Milind, N.; Prafulla, P.; Vidhya, P.; Bhushan, B.; Qahar, Q.A.; Abhijeet, W. Colposcopy in Pre-Malignant Lesions and Oral Squamous Cell Carcinoma: Linking Threads of Clinical, Histopathological and Colposcopic Inferences. J. Cancer Res. Ther. 2016, 12, 295–301. [Google Scholar] [CrossRef]
- Costa, S.; Panta, P. Colposcopy: A Direct Oral Microscopy for Oral Cancer and Precancer. In Oral Cancer Detection: Novel Strategies and Clinical Impact; Springer International Publishing: Berlin/Heildelberg, Germany, 2019; pp. 205–216. ISBN 9783319612553. [Google Scholar]
- Drogoszewska, B.; Chomik, P.; Michcik, A.; Polcyn, A. A Standard Picture of Healthy Oral Mucosae by Direct Oral Microscopy. Postep. Dermatol. Alergol. 2013, 30, 159–164. [Google Scholar] [CrossRef]
- Mahmoud, S.A.M.; Latif, M.K.A.; Dahmoush, H.M.; Hussein, E.A. Diagnostic Accuracy of Colposcopic Examination in Patients with Oral Dysplastic Lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 692–699. [Google Scholar] [CrossRef]
- Jeng, M.J.; Sharma, M.; Sharma, L.; Chao, T.Y.; Huang, S.F.; Chang, L.B.; Wu, S.L.; Chow, L. Raman Spectroscopy Analysis for Optical Diagnosis of Oral Cancer Detection. J. Clin. Med. 2019, 8, 1313. [Google Scholar] [CrossRef]
- Maryam, S.; Nogueira, M.S.; Gautam, R.; Krishnamoorthy, S.; Venkata Sekar, S.K.; Kho, K.W.; Lu, H.; Ni Riordain, R.; Feeley, L.; Sheahan, P.; et al. Label-Free Optical Spectroscopy for Early Detection of Oral Cancer. Diagnostics 2022, 12, 2896. [Google Scholar] [CrossRef]
- Kawecki, A. Nowotwory nabłonkowe narządów głowy i szyi—Co nowego w ostatnich lat-ach? Oncol. Clin. Pract. 2013, 9, 18. (In Polish) [Google Scholar]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Anzai, Y.; Brizel, D.M.; Bruce, J.Y.; Busse, P.M.; Caudell, J.J.; Cmelak, A.J. Head and Neck Cancers, Version 4.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2024, 18, 873–898. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.V.; Sharan, R.; Manikantan, K.; Clark, G.M.; Chatterjee, S.; Mallick, I.; Roy, P.; Arun, P. Redefining Adequate Margins in Oral Squamous Cell Carcinoma: Outcomes from Close and Positive Margins. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Aaboubout, Y.; Hove, I.T.; Smits, R. Specimen-driven Intraoperative Assessment of Resection Margins Should Be Standard of Care for Oral Cancer Patients. Oral Dis. 2021, 27, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Helliwell, T.; Woolgar, J.A. Standards and Minimum Datasets for Reporting Common Cancers. In Minimum Dataset for Head and Neck Histopathology Reports; The Royal College of Pathologists: London, UK, 1998. [Google Scholar]
- Singh, A.; Qayyumi, B.; Chaturvedi, P. An Update on Surgical Margins in the Head Neck Squamous Cell Carcinoma: Assessment, Clinical Outcome, and Future Directions. Curr. Oncol. Rep. 2020, 22, 82. [Google Scholar] [CrossRef]
- Zanoni, D.K.; Migliacci, J.C.; Xu, B.; Katabi, N.; Montero, P.H.; Ganly, I.; Shah, J.P.; Wong, R.J.; Ghossein, R.A.; Patel, S.G. A proposal to redefine close surgical margins in squamous cell carcinoma of the oral tongue. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 555. [Google Scholar] [CrossRef]
- de Koning, S.G.B.; Schaeffers, A.; Schats, W.; van den Brekel, M.W.M.; Ruers, T.J.M.; Karakullukcu, M.B. Assessment of the Deep Resection Margin during Oral Cancer Surgery: A Systematic Review. Eur. J. Surg. Oncol. 2021, 47, 2220–2232. [Google Scholar] [CrossRef]
- Smits, R.W.H.; van Lanschot, C.G.F.; Aaboubout, Y.; de Ridder, M.; Hegt, V.N.; Barroso, E.M.; Meeuwis, C.A.; Sewnaik, A.; Hardillo, J.A.; Monserez, D.; et al. Intraoperative Assessment of the Resection Specimen Facilitates Achievement of Adequate Margins in Oral Carcinoma. Front. Oncol. 2020, 10, 614593. [Google Scholar] [CrossRef]
- Mair, M.; Nair, D.; Nair, S.; Dutta, S.; Garg, A.; Malik, A.; Mishra, A.; Shetty KS, R.; Chaturvedi, P. Intraoperative Gross Examination vs. Frozen Section for Achievement of Adequate Margin in Oral Cancer Surgery. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 123, 544–549. [Google Scholar] [CrossRef]
- Buchakjian, M.R.; Ginader, T.; Tasche, K.K.; Pagedar, N.A.; Smith, B.J.; Sperry, S.M. Independent Predictors of Prognosis Based on Oral Cavity Squamous Cell Carcinoma Surgical Margins. Otolaryngol. Head Neck Surg. 2018, 159, 675–682. [Google Scholar] [CrossRef]
- Abbas, S.A.; Ikram, M.; Tariq, M.U.; Raheem, A.; Saeed, J. Accuracy of frozen sections in oral cancer resections, an experience of a tertiary care hospital. PubMed 2017, 67, 806–809. [Google Scholar]
- Bulbul, M.G.; Tarabichi, O.; Sethi, R.K.; Parikh, A.S.; Varvares, M.A. Does Clearance of Positive Margins Improve Local Control in Oral Cavity Cancer? A Meta-Analysis. Otolaryngol. Head Neck Surg. 2019, 161, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Voskuil, F.; de Jongh, S.; Hooghiemstra, W.T.R.; Linssen, M.D.; Steinkamp, P.J.; de Visscher, S.A.H.J.; Schepman, K.P.; Elias, S.G.; Meersma, G.J.; Jonker, P.K.C.; et al. Fluorescence-Guided Imaging for Resection Margin Evaluation in Head and Neck Cancer Patients Using Cetuximab-800CW: A Quantitative Dose-Escalation Study. Theranostics 2020, 10, 3994–4005. [Google Scholar] [CrossRef] [PubMed]
- de Wit, J.; van Schaik, J.; Voskuil, F. Comparison of Narrow Band and Fluorescence Molecular Imaging to Improve Intraoperative Tumour Margin Assessment in Oral Cancer Surgery. Oral Oncol. 2022, 134, 106099. [Google Scholar] [CrossRef]
- Tirelli, G.; Gatto, A.; Bonini, P.; Tofanelli, M. Prognostic Indicators of Improved Survival and Quality of Life in Surgically Treated Oral Cancer. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 31–40. [Google Scholar] [CrossRef]
- Quinlan-Davidson, S.; Mohamed, A. Outcomes of Oral Cavity Cancer Patients Treated with Surgery Followed by Postoperative Intensity Modulated Radiation Therapy. Oral Oncol. 2017, 72, 90–97. [Google Scholar] [CrossRef]
- Huang, S.; Hahn, E.; Chiosea, S.; Xu, Z.; Li, J. The Role of Adjuvant (Chemo-) Radiotherapy in Oral Cancers in the Contemporary Era. Oral Oncol. 2020, 102, 104563. [Google Scholar] [CrossRef]
- Hosni, A.; Chiu, K.; Huang, S.; Xu, W.; Huang, J. Non-Operative Management for Oral Cavity Carcinoma: Definitive Radiation Therapy as a Potential Alternative Treatment Approach. Radiother. Oncol. 2021, 154, 70–75. [Google Scholar] [CrossRef]
- Goetz, C.; Raschka, J.; Wolff, K.-D.; Kolk, A.; Bissinger, O. Hospital Based Quality of Life in Oral Cancer Surgery. Cancers 2020, 12, 2152. [Google Scholar] [CrossRef]
- Wang, C.-P.; Liao, L.-J.; Chiang, C.-J.; Hsu, W.-L.; Kang, C.-J.; Wang, C.-C.; Chen, P.-R.; Chen, T.-C.; Huang, W.-W.; Chien, C.-Y. Patients with Oral Cancer Do Not Undergo Surgery as Primary Treatment: A Population-Based Study in Taiwan. J. Formos. Med. Assoc. 2020, 119, 392–398. [Google Scholar] [CrossRef]
- Ellis, M.A.; Graboyes, E.M.; Wahlquist, A.E.; Neskey, D.M.; Kaczmar, J.M.; Schopper, H.K.; Sharma, A.K.; Morgan, P.F.; Nguyen, S.A.; Day, T.A. Primary Surgery vs. Radiotherapy for Early Stage Oral Cavity Cancer. Otolaryngol. Head Neck Surg. 2018, 158, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Alterio, D.; Marvaso, G.; Ferrari, A.; Volpe, S.; Orecchia, R. Modern Radiotherapy for Head and Neck Cancer. Semin. Oncol. 2019, 43, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Lapeyre, M.; Racadot, S.; Renard, S.; Biau, J. Radiotherapy for Oral Cavity Cancers. Cancer Radiother. 2022, 26, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Lane, C.; Myers, C.; Jiang, D.; Cooke, A.; Kerr, P. Selected Functional Outcomes in Advanced Oral Cancer: Comparison of Surgery Alone versus Surgery with Postoperative Radiotherapy. Head Neck 2021, 44, 710–721. [Google Scholar] [CrossRef]
- Baudelet, M.; Van Den Steen, L.; Tomassen, P.; Bonte, K.; Deron, P.; Huvenne, W.; Rottey, S.; De Neve, W.; Sundahl, N.; Van Nuffelen, G.; et al. Very Late Xerostomia, Dysphagia, and Neck Fibrosis after Head and Neck Radiotherapy. Head Neck 2019, 41, 3594–3603. [Google Scholar] [CrossRef]
- Noronha, V.; Joshi, A.; Patil, V.; Agarwal, J. Once-a-Week versus Once-Every-3-Weeks Cisplatin Chemoradiation for Locally Advanced Head and Neck Cancer: A Phase III Randomized Noninferiority Trial. J. Clin. Oncol. 2018, 36, 1064–1072. [Google Scholar] [CrossRef]
- Atashi, F.; Vahed, N.; Emamverdizadeh, P.; Fattahi, S.; Paya, L. Drug Resistance against 5-Fluorouracil and Cisplatin in the Treatment of Head and Neck Squamous Cell Carcinoma: A Systematic Review. J. Dent. Res. Dent. Clin. Dent. Prospect. 2021, 15, 219. [Google Scholar] [CrossRef]
- Zhang, Z.; Qiu, N.; Yin, J.; Zhang, J.; Liu, H.; Guo, W.; Liu, M.; Liu, T.; Chen, D.; Luo, K. SRGN Crosstalks with YAP to Maintain Chemoresistance and Stemness in Breast Cancer Cells by Modulating HDAC2 Expression. Theranostics 2020, 10, 4290. [Google Scholar] [CrossRef]
- Minami, K.; Ueda, N.; Ishimoto, K.; Tsujiuchi, T. Lysophosphatidic Acid Receptor-2 (LPA 2)-Mediated Signaling Enhances Chemoresistance in Melanoma Cells Treated with Anticancer Drugs. Mol. Cell. Biochem. 2020, 469, 89–95. [Google Scholar] [CrossRef]
- Arai, K.; Eguchi, T.; Rahman, M.M.; Sakamoto, R.; Masuda, N.; Nakatsura, T.; Calderwood, S.K.; Kozaki, K.; Itoh, M. A novel high-throughput 3D screening system for EMT inhibitors: A pilot screening discovered the EMT inhibitory activity of CDK2 inhibitor SU9516. PLoS ONE 2016, 11, e0162394. [Google Scholar] [CrossRef]
- Hartner, L. Chemotherapy for oral cancer. Dent. Clin. N. Am. 2018, 62, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Spiotto, M.T.; Jefferson, G.; Wenig, B.; Markiewicz, M.; Weichselbaum, R.R.; Koshy, M. Differences in survival with surgery and postoperative radiotherapy compared with definitive chemoradiotherapy for oral cavity cancer. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 691. [Google Scholar] [CrossRef] [PubMed]
- Tangthongkum, M.; Kirtsreesakul, V.; Supanimitjaroenporn, P.; Leelasawatsuk, P. Treatment Outcome of Advance Staged Oral Cavity Cancer: Concurrent Chemoradiotherapy Compared with Primary Surgery. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 2567–2572. [Google Scholar] [CrossRef]
- Foster, C.C.; Melotek, J.M.; Brisson, R.J.; Seiwert, T.Y.; Cohen, E.E.W.; Stenson, K.M.; Blair, E.A.; Portugal, L.; Gooi, Z.; Agrawal, N.; et al. Definitive Chemoradiation for Locally-Advanced Oral Cavity Cancer: A 20-Year Experience. Oral Oncol. 2018, 80, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Owosho, A.A.; Tsai, C.J.; Lee, R.S.; Freymiller, H.; Kadempour, A.; Varthis, S.; Sax, A.Z.; Rosen, E.B.; Yom, S.K.; Randazzo, J. The Prevalence and Risk Factors Associated with Osteoradionecrosis of the Jaw in Oral and Oropharyngeal Cancer Patients Treated with Intensity-Modulated Radiation Therapy (IMRT): The Memorial Sloan Kettering Cancer Center Experience. Oral Oncol. 2017, 64, 44–51. [Google Scholar] [CrossRef]
- Dzioba, A.; Aalto, D.; Papadopoulos-Nydam, G.; Seikaly, H.; Rieger, J.; Wolfaardt, J.; Osswald, M.; Harris, J.R.; O’Connell, D.A.; Lazarus, C. Functional and Quality of Life Outcomes after Partial Glossectomy: A Multi-Institutional Longitudinal Study of the Head and Neck Research Network. J. Otolaryngol. Head Neck Surg. 2017, 46, 56. [Google Scholar] [CrossRef]
- Rudresha, A.H.; Chaudhuri, T.; Lakshmaiah, K.C.; Babu, K.G.; Dasappa, L.; Jacob, L.A.; Babu, M.C.S.; Lokesh, K.N.; Rajeev, L.K. Induction Chemotherapy in Technically Unresectable Locally Advanced T4a Oral Cavity Squamous Cell Cancers: Experience from a Regional Cancer Center of South India. Indian J. Med. Paediatr. Oncol. 2017, 38, 490–494. [Google Scholar] [CrossRef]
- Abdelmeguid, A.S.; Silver, N.L.; Boonsripitayanon, M.; Glisson, B.S.; Ferrarotto, R.; Gunn, G.B.; Phan, J.; Gillenwater, A.M.; Hanna, E.Y. Role of Induction Chemotherapy for Oral Cavity Squamous Cell Carcinoma. Cancer 2021, 127, 3107–3112. [Google Scholar] [CrossRef]
- Rajendra, A.; Noronha, V.; Joshi, A.; Patil, V.M.; Menon, N.; Prabhash, K. Palliative Chemotherapy in Head and Neck Cancer: Balancing between Beneficial and Adverse Effects. Expert Rev. Anticancer Ther. 2020, 20, 17–29. [Google Scholar] [CrossRef]
- Kandipalli, S. Impact of Metronomic Chemotherapy on Quality of Life in Recurrent, Residual and Metastatic Head & Neck Cancers. J. Med. Sci. Clin. Res. 2018, 6, 64–72. [Google Scholar] [CrossRef]
- Parikh, P.M.; Hingmire, S.S.; Deshmukh, C.D. Selected Current Data on Metronomic Therapy (and Its Promise) from India. S. Asian J. Cancer 2016, 5, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.; Noronha, V.; Dhumal, S.B.; Joshi, A.; Menon, N.; Bhattacharjee, A.; Kulkarni, S.; Ankathi, S.K.; Mahajan, A.; Sable, N. Low-Cost Oral Metronomic Chemotherapy versus Intravenous Cisplatin in Patients with Recurrent, Metastatic, Inoperable Head and Neck Carcinoma: An Open-Label, Parallel-Group, Non-Inferiority, Randomised, Phase 3 Trial. Lancet Glob. Health 2020, 8, e1213–e1222. [Google Scholar] [CrossRef]
- Hsieh, M.Y.; Chen, G.; Chang, D.C.; Chien, S.Y.; Chen, M.K. The Impact of Metronomic Adjuvant Chemotherapy in Patients with Advanced Oral Cancer. Ann. Surg. Oncol. 2018, 25, 2091–2097. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Huang, J.; Qiao, B.; Lam, A. Immune Checkpoint Pathways in Immunotherapy for Head and Neck Squamous Cell Carcinoma. Int. J. Oral Sci. 2020, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.P.; Bhaskaran, M.K.; George, A.L.; Thirutheri, A.; Somasundaran, M.; Pavithran, A. Immunotherapy in oral cancer. J. Pharm. Bioallied Sci. 2019, 11, 107. [Google Scholar] [CrossRef]
- Ferris, R.L.; Licitra, L.; Fayette, J.; Even, C.; Blumenschein, G.; Harrington, K.J.; Guigay, J.; Vokes, E.E.; Saba, N.F.; Haddad, R.; et al. Nivolumab in Patients with Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck: Efficacy and Safety in CheckMate 141 by Prior Cetuximab Use. Clin. Cancer Res. 2019, 25, 5221–5230. [Google Scholar] [CrossRef] [PubMed]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; De Castro, G.; Psyrri, A.; Rotllan, N.B.; Neupane, P.C.; Bratland, A.; et al. KEYNOTE-048: Phase III Study of First-Line Pembrolizumab (P) for Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma (R/M HNSCC). Ann. Oncol. 2018, 29, viii729. [Google Scholar] [CrossRef]
- Couey, M.A.; Leidner, R.S.; Young, S.W.; Bell, R.B. Immunotherapy in Oral Cancer: A Fourth Dimension of Cancer Treatment. In Improving Outcomes in Oral Cancer: A Clinical and Translational Update; Springer: Berlin/Heidelberg, Germany, 2019; pp. 129–154. [Google Scholar] [CrossRef]
- Licitra, L.F.; Haddad, R.I.; Even, C.; Tahara, M.; Dvorkin, M.; Ciuleanu, T.-E.; Clement, P.M.; Mesia, R.; Kutukova, S.I.; Zholudeva, L.; et al. EAGLE: A Phase 3, Randomized, Open-Label Study of Durvalumab (D) with or without Tremelimumab (T) in Patients (Pts) with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma (R/M HNSCC). J. Clin. Oncol. 2019, 37, 6012. [Google Scholar] [CrossRef]
- Liu, M.; Wang, X.; Wang, L.; Ma, X.; Gong, Z.; Zhang, S.; Li, Y. Targeting the IDO1 Pathway in Cancer: From Bench to Bedside. J. Hematol. Oncol. 2018, 11, 100. [Google Scholar] [CrossRef]
- Tamimi, A.; Tamimi, A.; Sorkheh, F.; Asl, S.M.; Ghafari, A.; Karimi, A.G.; Erabi, G.; Pourmontaseri, H.; Deravi, N. Monoclonal Antibodies for the Treatment of Squamous Cell Carcinoma: A Literature Review. Cancer Rep. 2023, 6, e1802. [Google Scholar] [CrossRef]
- Miyamoto, S.; Hirohashi, Y.; Morita, R.; Miyazaki, A.; Ogi, K.; Kanaseki, T.; Kentaro, I.; Shirakawa, J.; Tsukahara, T.; Murai, A.; et al. Exploring Olfactory Receptor Family 7 Subfamily C Member 1 as a Novel Oral Cancer Stem Cell Target for Immunotherapy. Cancer Sci. 2023, 114, 3496–3508. [Google Scholar] [CrossRef] [PubMed]
- Ribbat-Idel, J.; Perner, S.; Kuppler, P.; Klapper, L.; Krupar, R.; Watermann, C.; Paulsen, F.-O.; Offermann, A.; Bruchhage, K.-L.; Wollenberg, B. Immunologic “Cold” Squamous Cell Carcinomas of the Head and Neck Are Associated with an Unfavorable Prognosis. Front. Med. 2021, 8, 622330. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Geng, H.; Liu, Y.; Liu, L.; Chen, Y.; Wu, F.; Liu, Z.; Ling, S.; Wang, Y.; Zhou, L. Hot and Cold Tumors: Immunological Features and the Therapeutic Strategies. MedComm 2023, 4, e343. [Google Scholar] [CrossRef] [PubMed]
- Fuereder, T.; Minichsdorfer, C.; Mittlboeck, M.; Wagner, C. Pembrolizumab plus Docetaxel for the Treatment of Recurrent/Metastatic Head and Neck Cancer: A Prospective Phase I/II Study. Oral Oncol. 2022, 124, 105634. [Google Scholar] [CrossRef]
- Harrington, K.; Cohen, E.; Siu, L.; Rischin, D.; Licitra, L. P-94 Pembrolizumab plus Lenvatinib vs. Chemotherapy and Lenvatinib Monotherapy for Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma That Progressed. Oral Oncol. 2021, 118, 10–11. [Google Scholar] [CrossRef]
- Nör, J.E.; Gutkind, J.S. Head and Neck Cancer in the New Era of Precision Medicine. J. Dent. Res. 2018, 97, 601–602. [Google Scholar] [CrossRef]
- Wang, X.; Li, N.; Meng, J.; Wen, N. The Use of Topical ALA-Photodynamic Therapy Combined with Induction Chemotherapy for Locally Advanced Oral Squamous Cell Carcinoma. Am. J. Otolaryngol. 2021, 42, 103112. [Google Scholar] [CrossRef]
- Nauta, J.M.; van Leengoed, H.L.L.M.; Star, W.M.; Roodenburg, J.L.N.; Witjes, M.J.H.; Vermey, A. Photodynamic Therapy of Oral Cancer A Review of Basic Mechanisms and Clinical Applications. Eur. J. Oral Sci. 1996, 104, 69–81. [Google Scholar] [CrossRef]
- Lambert, A.; Nees, L.; Nuyts, S.; Clement, P.; Meulemans, J.; Delaere, P.; Vander Poorten, V. Photodynamic Therapy as an Alternative Therapeutic Tool in Functionally Inoperable Oral and Oropharyngeal Carcinoma: A Single Tertiary Center Retrospective Cohort Analysis. Front. Oncol. 2021, 11, 626394. [Google Scholar] [CrossRef]
- Santos, L.L.; Oliveira, J.; Monteiro, E.; Santos, J.; Sarmento, C. Treatment of Head and Neck Cancer with Photodynamic Therapy with Redaporfin: A Clinical Case Report. Case Rep. Oncol. 2018, 11, 769–776. [Google Scholar] [CrossRef]
- van Doeveren, T.E.M.; Karakullukçu, M.B.; van Veen, R.L.P.; Lopez-Yurda, M.; Schreuder, W.H.; Tan, I.B. Adjuvant Photodynamic Therapy in Head and Neck Cancer after Tumor-positive Resection Margins. Laryngoscope 2018, 128, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Ohba, S.; Egashira, K.; Asahina, I. The Effect of Photodynamic Therapy with Talaporfin Sodium, a Second-Generation Photosensitizer, on Oral Squamous Cell Carcinoma: A Series of Eight Cases. Photodiagnosis Photodyn. Ther. 2018, 21, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Burcher, J.T.; DeLiberto, L.K.; Allen, A.M.; Kilpatrick, K.L.; Bishayee, A. Bioactive phytocompounds for oral cancer prevention and treatment: A comprehensive and critical evaluation. Med. Res. Rev. 2023, 43, 2025–2085. [Google Scholar] [CrossRef]
- Beutler, J.A. Natural Products as a Foundation for Drug Discovery. Curr. Protoc. Pharmacol. 2019, 86, e67. [Google Scholar] [CrossRef] [PubMed]
- Fuad, A.S.M.; Amran, N.A.; Nasruddin, N.S.; Burhanudin, N.A.; Dashper, S.; Arzmi, M.H. The Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics in Oral Cancer Management. Probiotics Antimicrob. Proteins 2022, 15, 1298–1311. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh, Z.; Pouralibaba, F.; Khosroushahi, A.Y. The Prophylactic Effect of Acetobacter Syzygii Probiotic Species against Squamous Cell Carcinoma. J. Dent. Res. Dent. Clin. Dent. Prospects 2017, 11, 208. [Google Scholar] [PubMed]
- Asoudeh-Fard, A.; Barzegari, A.; Dehnad, A.; Bastani, S.; Golchin, A.; Omidi, Y. Lactobacillus Plantarum Induces Apoptosis in Oral Cancer KB Cells through Upregulation of PTEN and Downregulation of MAPK Signalling Pathways. Bioimpacts 2017, 7, 193. [Google Scholar] [CrossRef]
- Miyaguchi, J.; Shiga, K.; Ogawa, K.; Suzuki, F.; Katagiri, K.; Saito, D.; Ikeda, A.; Horii, A.; Watanabe, M.; Igimi, S. Treatment with Lactobacillus Retards the Tumor Growth of Head and Neck Squamous Cell Carcinoma Cells Inoculated in Mice. Tohoku J. Exp. Med. 2018, 245, 269–275. [Google Scholar] [CrossRef]
- Arbab Soleimani, N.; Khosravi, A. Investigating the Effects of Lactobacillus Plantarum Strain ATCC 8014 on Gene Expression of NF-ĸB, TLR-4, and BCL-2 in Oral Rat Cancer Induced by 4-Nitroquioline 1-Oxide. Jorjani Biomed. J. 2022, 10, 12–20. [Google Scholar]
- Kaur, K.; Topchyan, P.; Kozlowska, A.K.; Ohanian, N.; Chiang, J.; Maung, P.O.; Park, S.-H.; Ko, M.-W.; Fang, C.; Nishimura, I. Super-Charged NK Cells Inhibit Growth and Progression of Stem-like/Poorly Differentiated Oral Tumors in Vivo in Humanized BLT Mice; Effect on Tumor Differentiation and Response to Chemotherapeutic Drugs. Oncoimmunology 2018, 7, e1426518. [Google Scholar] [CrossRef]
- Capote-Moreno, A.; Ramos, E.; Egea, J.; López-Muñoz, F.; Gil-Martín, E.; Romero, A. Potential of Melatonin as Adjuvant Therapy of Oral Cancer in the Era of Epigenomics. Cancers 2019, 11, 1712. [Google Scholar] [CrossRef] [PubMed]
- D’souza, S.; Addepalli, V. Preventive Measures in Oral Cancer: An Overview. Biomed. Pharmacother. 2018, 107, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Dholariya, S.; Singh, R.; Patel, K. Melatonin: Emerging Player in the Management of Oral Cancer. Crit. Rev. Oncog. 2023, 28, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-K.; Ryu, M.-H.; Hwang, D.-S.; Kim, G.-C.; Jang, M.-A.; Kim, U.-K. Effects of Melatonin Receptor Expression on Prognosis and Survival in Oral Squamous Cell Carcinoma Patients. Int. J. Oral Maxillofac. Surg. 2022, 51, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, M.; Clarke, D.; Johnson, N.; McMillan, N. Can Gene Editing and Silencing Technologies Play a Role in the Treatment of Head and Neck Cancer? Oral Oncol. 2017, 68, 9–19. [Google Scholar] [CrossRef]
- Usman, S.; Jamal, A.; Teh, M.-T.; Waseem, A. Major Molecular Signaling Pathways in Oral Cancer Associated with Therapeutic Resistance. Front. Oral Health 2021, 1, 603160. [Google Scholar] [CrossRef]
- Alexandra, T.; Marina, I.M.; Daniela, M.; Ioana, S.I.; Maria, B.; Radu, R.; Maria, T.A.; Tudor, S.; Maria, G. Autophagy—A Hidden but Important Actor on Oral Cancer Scene. Int. J. Mol. Sci. 2020, 21, 9325. [Google Scholar] [CrossRef]
- Marquard, F.E.; Jücker, M. PI3K/AKT/MTOR Signaling as a Molecular Target in Head and Neck Cancer. Biochem. Pharmacol. 2020, 172, 113729. [Google Scholar] [CrossRef]
- Harsha, C.; Banik, K.; Ang, H.L.; Girisa, S.; Vikkurthi, R.; Parama, D.; Rana, V.; Shabnam, B.; Khatoon, E.; Kumar, A.P. Targeting AKT/MTOR in Oral Cancer: Mechanisms and Advances in Clinical Trials. Int. J. Mol. Sci. 2020, 21, 3285. [Google Scholar] [CrossRef]
- Patel, J.; Nguyen, S.A.; Ogretmen, B.; Gutkind, J.S.; Nathan, C.; Day, T. MTOR Inhibitor Use in Head and Neck Squamous Cell Carcinoma: A Meta-analysis on Survival, Tumor Response, and Toxicity. Laryngoscope Investig. Otolaryngol. 2020, 5, 243–255. [Google Scholar] [CrossRef]
- Jung, K.; Kang, H.; Mehra, R. Targeting Phosphoinositide 3-Kinase (PI3K) in Head and Neck Squamous Cell Carcinoma (HNSCC). Cancers Head Neck 2018, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Bendell, J.C.; Varghese, A.M.; Hyman, D.M.; Bauer, T.M.; Pant, S.; Callies, S.; Lin, J.; Martinez, R.; Wickremsinhe, E.; Fink, A. A First-in-Human Phase 1 Study of LY3023414, an Oral PI3K/MTOR Dual Inhibitor, in Patients with Advanced Cancer. Clin. Cancer Res. 2018, 24, 3253–3262. [Google Scholar] [CrossRef] [PubMed]
- Janku, F. Phosphoinositide 3-Kinase (PI3K) Pathway Inhibitors in Solid Tumors: From Laboratory to Patients. Cancer Treat. Rev. 2017, 59, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.; Ganapathy, D.M.; Ameya, K.P.; Sekar, D.; Kaliaperumal, K. Expression Analysis of Nuclear Factor Kappa B (NF-ΚB) in Oral Squamous Cell Carcinoma. Oral Oncol. Rep. 2024, 10, 100481. [Google Scholar] [CrossRef]
- Das, R.; Mehta, D.K.; Dhanawat, M. Medicinal Plants in Cancer Treatment: Contribution of Nuclear Factor-Kappa B (NF-KB) Inhibitors. Mini Rev. Med. Chem. 2022, 22, 1938–1962. [Google Scholar] [CrossRef]
- Reyes, M.; Peña-Oyarzun, D.; Maturana, A.; Torres, V.A. Nuclear Localization of β-Catenin and Expression of Target Genes Are Associated with Increased Wnt Secretion in Oral Dysplasia. Oral Oncol. 2019, 94, 58–67. [Google Scholar] [CrossRef]
- Bai, Y.; Sha, J.; Kanno, T. The Role of Carcinogenesis-Related Biomarkers in the Wnt Pathway and Their Effects on Epithelial–Mesenchymal Transition (EMT) in Oral Squamous Cell Carcinoma. Cancers 2020, 12, 555. [Google Scholar] [CrossRef]
- Reyes, M.; Flores, T.; Betancur, D.; Peña-Oyarzún, D.; Torres, V.A. Wnt/β-Catenin Signaling in Oral Carcinogenesis. Int. J. Mol. Sci. 2020, 21, 4682. [Google Scholar] [CrossRef]
- Cierpikowski, P.; Lis-Nawara, A.; Bar, J. Sonic Hedgehog Is a Novel Prognostic Biomarker in Patients with Oral Squamous Cell Carcinoma. Neoplasma 2021, 68, 867–874. [Google Scholar] [CrossRef]
- Zhao, X.-W.; Zhou, J.-P.; Bi, Y.-L.; Wang, J.-Y.; Yu, R.; Deng, C.; Wang, W.-K.; Li, X.-Z.; Huang, R.; Zhang, J. The Role of MAPK Signaling Pathway in Formation of EMT in Oral Squamous Carcinoma Cells Induced by TNF-α. Mol. Biol. Rep. 2019, 46, 3149–3156. [Google Scholar] [CrossRef]
- Nigam, K.; Srivastav, R.K. Notch Signaling in Oral Pre-Cancer and Oral Cancer. Med. Oncol. 2021, 38, 139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Bao, C.; Zhang, X.; Lin, X.; Pan, D.; Chen, Y. Knockdown of LncRNA LEF1-AS1 Inhibited the Progression of Oral Squamous Cell Carcinoma (OSCC) via Hippo Signaling Pathway. Cancer Biol. Ther. 2019, 20, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Coletta, R.D.; Yeudall, W.A.; Salo, T. Grand Challenges in Oral Cancers. Front. Oral Health 2020, 1, 3. [Google Scholar] [CrossRef] [PubMed]

| Localization | Histogenesis | Type |
|---|---|---|
| Oral cavity and mobile tongue tumors | Non-neoplastic lesions |
|
| Epithelial tumors |
| |
| Tumors of uncertain histogenesis |
| |
| Oropharyngeal tumors | Benign lesions |
|
| Epithelial tumors |
|
| Bacteria | Role in Oral Cancer |
|---|---|
| Fusobacterium nucleatum |
|
| Neisseria |
|
| Porphyromonas gingivalis |
|
| Prevotella intermedia |
|
| Pseudomonas aeruginosa |
|
| Treponema denticola |
|
| Lactobacillus |
|
| Clinical Symptoms That May Indicate Oral Cancer |
|---|
| Diagnostic Method | Pros | Cons |
|---|---|---|
| Vital tissue staining |
|
|
| Optical imaging |
|
|
| Oral cytology |
|
|
| Salivary biomarkers |
|
|
| Artificial intelligence |
|
|
| Colposcopy |
|
|
| Spectroscopy |
|
|
| Type of Immunotherapy | Description |
|---|---|
| Immune checkpoint inhibitors | Blockage of PD-1, PD-L1, CTLA-4, IDO1, TIM-3, LAG-3, TIGIT, VISTA, and their combinations [164,169,170]. |
| Targeted monoclocal antibodies | Monoclonal antibodies targeting the receptors responsible for the pathogenesis and progression of oral cancer, e.g., EGFR, HER2, HER3, IGFR, VEGF, and CD44 [171]. |
| Gene therapy | OR7C1 gene expressed in oral cancer cells located within the tumor can be a stem cell target [172]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kijowska, J.; Grzegorczyk, J.; Gliwa, K.; Jędras, A.; Sitarz, M. Epidemiology, Diagnostics, and Therapy of Oral Cancer—Update Review. Cancers 2024, 16, 3156. https://doi.org/10.3390/cancers16183156
Kijowska J, Grzegorczyk J, Gliwa K, Jędras A, Sitarz M. Epidemiology, Diagnostics, and Therapy of Oral Cancer—Update Review. Cancers. 2024; 16(18):3156. https://doi.org/10.3390/cancers16183156
Chicago/Turabian StyleKijowska, Julia, Julia Grzegorczyk, Katarzyna Gliwa, Aleksandra Jędras, and Monika Sitarz. 2024. "Epidemiology, Diagnostics, and Therapy of Oral Cancer—Update Review" Cancers 16, no. 18: 3156. https://doi.org/10.3390/cancers16183156
APA StyleKijowska, J., Grzegorczyk, J., Gliwa, K., Jędras, A., & Sitarz, M. (2024). Epidemiology, Diagnostics, and Therapy of Oral Cancer—Update Review. Cancers, 16(18), 3156. https://doi.org/10.3390/cancers16183156






