Rationale for the Initiation, Outcomes, and Characteristics of Chemotherapy Following CDK4/6 Inhibitors in Breast Cancer: A Real-World Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Collection
2.3. Study Objectives
2.4. Ethical Considerations
2.5. Statistical Analysis
3. Results
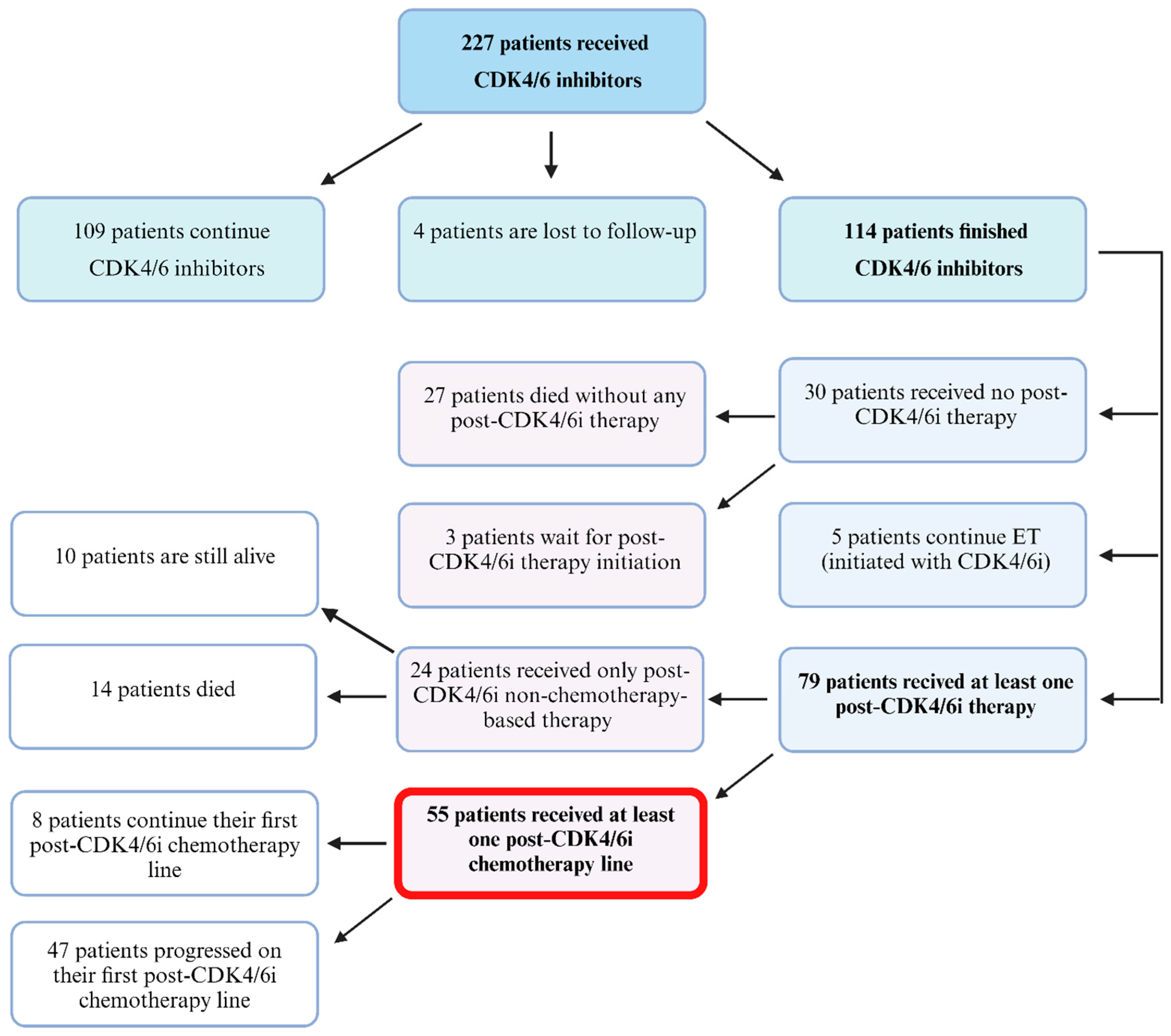
3.1. Patient Selection and Characteristics
3.2. Chemotherapy Regimen Characteristics and Rationale for Initiation
3.3. Factors Influencing Post-CDK4/6i Chemotherapy Initiation
3.4. Chemotherapy Outcomes and Influencing Factors
3.5. Chemotherapy Safety
3.6. Factors Responsible for Post-CDK4/6i Chemotherapy Noninitiation and Group Comparisons
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; André, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef]
- Ghose, A.; Stanway, S.; Sirohi, B.; Mutebi, M.; Adomah, S. Advanced Breast Cancer Care: The Current Situation and Global Disparities. Semin. Oncol. Nurs. 2024, 40, 151551. [Google Scholar] [CrossRef]
- Yersal, O.; Barutca, S. Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J. Clin. Oncol. 2014, 5, 412–424. [Google Scholar] [CrossRef]
- Rakha, E.A.; Tse, G.M.; Quinn, C.M. An update on the pathological classification of breast cancer. Histopathology 2023, 82, 5–16. [Google Scholar] [CrossRef]
- Cicenas, J.; Simkus, J. CDK Inhibitors and FDA: Approved and Orphan. Cancers 2024, 16, 1555. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, F.; Zou, J.; Fang, Y.; Liu, Y.; Li, L.; Hou, J.; Wang, G.; Wang, H.; Lai, X.; et al. Clinical considerations of CDK4/6 inhibitors in HER2 positive breast cancer. Front. Oncol. 2024, 13, 1322078. [Google Scholar] [CrossRef]
- Huang, J.; Zheng, L.; Sun, Z.; Li, J. CDK4/6 inhibitor resistance mechanisms and treatment strategies (Review). Int. J. Mol. Med. 2022, 50, 128. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.L.; Winer, E.P.; et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2018, 29, 1541–1547. [Google Scholar] [CrossRef]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Trédan, O.; Chen, S.-C.; Manso, L.; et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef]
- Rugo, H.S.; Finn, R.S.; Diéras, V.; Ettl, J.; Lipatov, O.; Joy, A.A.; Harbeck, N.; Castrellon, A.; Iyer, S.; Lu, D.R.; et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res. Treat. 2019, 174, 719–729. [Google Scholar] [CrossRef]
- Sledge, G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor-Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy-MONARCH 2: A Randomized Clinical Trial. JAMA Oncol. 2020, 6, 116–124. [Google Scholar] [CrossRef]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.-A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martín, M.; et al. Overall Survival with Ribociclib plus Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2020, 382, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Rugo, H.S.; Im, S.-A.; Slamon, D.J.; Harbeck, N.; Bondarenko, I.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; et al. Overall Survival with Palbociclib and Fulvestrant in Women with HR+/HER2-ABC: Updated Exploratory Analyses of PALOMA-3, a Double-blind, Phase III Randomized Study. Clin. Cancer Res. 2022, 28, 3433–3442. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-S.; Im, S.-A.; Colleoni, M.; Franke, F.; Bardia, A.; Cardoso, F.; Harbeck, N.; Hurvitz, S.; Chow, L.; Sohn, J.; et al. Updated Overall Survival of Ribociclib plus Endocrine Therapy versus Endocrine Therapy Alone in Pre- and Perimenopausal Patients with HR+/HER2− Advanced Breast Cancer in MONALEESA-7: A Phase III Randomized Clinical Trial. Clin. Cancer Res. 2022, 28, 851. [Google Scholar] [CrossRef] [PubMed]
- ESMO Metastatic Breast Cancer Living Guideline|ESMO n.d. Available online: https://www.esmo.org/living-guidelines/esmo-metastatic-breast-cancer-living-guideline/er-positive-her2-negative-breast-cancer (accessed on 4 August 2024).
- Gennari, A.; André, F.; Barrios, C.; Cortés, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer 5 behalf of the ESMO Guidelines Committee. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, K.R.; Schonfeld, R.; Gradishar, W.J.; Lurie, R.H.; Moran, M.S.; Abraham, J.; Agnese, D.; Abramson, V.; Aft, R.; Allison, K.H.; et al. NCCN Guidelines Version 2.2024 Breast Cancer 2024. Available online: www.nccn.org (accessed on 23 April 2024).
- André, F.; Ciruelos, E.M.; Juric, D.; Loibl, S.; Campone, M.; Mayer, I.A.; Rubovszky, G.; Yamashita, T.; Kaufman, B.; Lu, Y.-S.; et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: Final overall survival results from SOLAR-1. Ann. Oncol. 2021, 32, 208–217. [Google Scholar] [CrossRef]
- Rugo, H.S.; Lerebours, F.; Ciruelos, E.; Drullinsky, P.; Borrego, M.R.; Neven, P.; Park, Y.H.; Prat, A.; Bachelot, T.; Juric, D.; et al. Alpelisib (ALP) + fulvestrant (FUL) in patients (pts) with PIK3CA-mutated (mut) hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2–) advanced breast cancer (ABC) previously treated with cyclin-dependent kinase 4/6 inhibitor (CDKi) + aromatase inhibitor (AI): BYLieve study results. J. Clin. Oncol. 2020, 38, 1006. [Google Scholar] [CrossRef]
- Beaver, J.A.; Park, B.H. The BOLERO-2 trial: The addition of everolimus to exemestane in the treatment of postmenopausal hormone receptor-positive advanced breast cancer. Future Oncol. 2012, 8, 651–657. [Google Scholar] [CrossRef]
- Kalinsky, K.; Accordino, M.K.; Chiuzan, C.; Mundi, P.S.; Sakach, E.; Sathe, C.; Ahn, H.; Trivedi, M.S.; Novik, Y.; Tiersten, A.; et al. Randomized Phase II Trial of Endocrine Therapy With or Without Ribociclib After Progression on Cyclin-Dependent Kinase 4/6 Inhibition in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer: MAINTAIN Trial. J. Clin. Oncol. 2023, 41, 4004–4013. [Google Scholar] [CrossRef]
- Mayer, E.L.; Ren, Y.; Wagle, N.; Mahtani, R.; Ma, C.; DeMichele, A.; Cristofanilli, M.; Meisel, J.; Miller, K.D.; Abdou, Y.; et al. PACE: A Randomized Phase II Study of Fulvestrant, Palbociclib, and Avelumab After Progression on Cyclin-Dependent Kinase 4/6 Inhibitor and Aromatase Inhibitor for Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor-Negative Metastatic Breast Cancer. J. Clin. Oncol. 2024, 42, 2050–2060. [Google Scholar] [CrossRef]
- Robson, M.E.; Im, S.-A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Tung, N.; Armstrong, A.; Dymond, M.; et al. OlympiAD extended follow-up for overall survival and safety: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Eur. J. Cancer 2023, 184, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Litton, J.; Hurvitz, S.; Mina, L.; Rugo, H.; Lee, K.-H.; Gonçalves, A.; Diab, S.; Woodward, N.; Goodwin, A.; Yerushalmi, R.; et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: Final overall survival results from the EMBRACA trial. Ann. Oncol. 2020, 31, 1526–1535. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.-B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Obwieszczenia Ministra Zdrowia—Lista Leków Refundowanych—Ministerstwo Zdrowia—Portal Gov.pl n.d. Available online: https://www.gov.pl/web/zdrowie/obwieszczenia-ministra-zdrowia-lista-lekow-refundowanych (accessed on 3 May 2024).
- Nowa Lista Refundacyjna w Onkologii—Kwiecień 2024—Aktualizacja n.d. Available online: https://www.zwrotnikraka.pl/nowa-lista-lekow-refundowanych/ (accessed on 3 May 2024).
- Al Mahmasani, L.; Amhaz, G.; Zeidane, R.A.; Chamseddine, N.; Hatab, T.; Sabbagh, S.; Charafeddine, M.; Assi, H.I. Preferences for the sequencing of first-line systemic treatments in metastatic hormone receptor-positive, HER2-negative breast cancer. Front. Oncol. 2023, 13, 1181375. [Google Scholar] [CrossRef]
- Karacin, C.; Oksuzoglu, B.; Demirci, A.; Keskinkılıç, M.; Baytemür, N.K.; Yılmaz, F.; Selvi, O.; Erdem, D.; Avşar, E.; Paksoy, N.; et al. Efficacy of subsequent treatments in patients with hormone-positive advanced breast cancer who had disease progression under CDK 4/6 inhibitor therapy. BMC Cancer 2023, 23, 136. [Google Scholar] [CrossRef]
- Princic, N.; Aizer, A.; Tang, D.H.; Smith, D.M.; Johnson, W.; Bardia, A. Predictors of systemic therapy sequences following a CDK 4/6 inhibitor-based regimen in post-menopausal women with hormone receptor positive, HEGFR-2 negative metastatic breast cancer. Curr. Med. Res. Opin. 2019, 35, 73–80. [Google Scholar] [CrossRef]
- Püsküllüoğlu, M.; Pieniążek, M.; Rudzińska, A.; Pietruszka, A.; Pacholczak-Madej, R.; Grela-Wojewoda, A.; Ziobro, M. Cisplatin Monotherapy as a Treatment Option for Patients with HER-2 Negative Breast Cancer Experiencing Hepatic Visceral Crisis or Impending Visceral Crisis. Oncol. Ther. 2024, 12, 419–435. [Google Scholar] [CrossRef]
- Martin, J.M.; Handorf, E.A.; Montero, A.J.; Goldstein, L.J. Systemic Therapies Following Progression on First-line CDK4/6-inhibitor Treatment: Analysis of Real-world Data. Oncologist 2022, 27, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Shin, J.; Ahn, J.S.; Park, Y.H.; Im, Y.H. Real World Experience of Second-Line Treatment Strategies after Palbociclib and Letrozole: Overall Survival in Metastatic Hormone Receptor-Positive Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer. Cancers 2023, 15, 3431. [Google Scholar] [CrossRef]
- Rugo, H.S.; Bardia, A.; Marmé, F.; Cortés, J.; Schmid, P.; Loirat, D.; Trédan, O.; Ciruelos, E.; Dalenc, F.; Pardo, P.G.; et al. Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): A randomised, open-label, multicentre, phase 3 trial. Lancet 2023, 402, 1423–1433. [Google Scholar] [CrossRef]
- Xi, J.; Oza, A.; Thomas, S.; Ademuyiwa, F.; Weilbaecher, K.; Suresh, R.; Bose, R.; Cherian, M.; Hernandez-Aya, L.; Frith, A.; et al. Retrospective analysis of treatment patterns and effectiveness of palbociclib and subsequent regimens in metastatic breast cancer. JNCCN J. Natl. Compr. Cancer Netw. 2019, 17, 141–147. [Google Scholar] [CrossRef]
- Masuda, N.; Mukai, H.; Inoue, K.; Rai, Y.; Ohno, S.; Ohtani, S.; Shimizu, C.; Hashigaki, S.; Muramatsu, Y.; Umeyama, Y.; et al. Analysis of subsequent therapy in Japanese patients with hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer who received palbociclib plus endocrine therapy in PALOMA-2 and -3. Breast Cancer 2021, 28, 335–345. [Google Scholar] [CrossRef]
- Alghanmi, H.; Elsamany, S.A.; Elnaghi, K. Outcome of second-line treatment after CDK4/6 inhibitors in hormonal-positive HER-2 negative metastatic breast cancer: Single institution experience. J. Clin. Oncol. 2023, 41, e13053. [Google Scholar] [CrossRef]
- Costa, M.; Valente, A.; Freitas, M.; Almeida, C.; Tavares, N.; Almeida, D.; Caeiro, C.; Augusto, I.; Sousa, I.; Barbosa, M. Advanced breast cancer treatment after CDK4/6– inhibitors—The experience of a Hospital Center. Eur. J. Cancer 2022, 175, S70–S71. [Google Scholar] [CrossRef]
- Giridhar, K.V.; Choong, G.M.; Leon-Ferre, R.A.; O’Sullivan, C.C.; Ruddy, K.J.; Haddad, T.C.; Hobday, T.J.; Peethambaram, P.P.; Moynihan, T.J.; Loprinzi, C.L.; et al. Abstract P6-18-09: Clinical management of metastatic breast cancer (MBC) after CDK 4/6 inhibitors: A retrospective single-institution study. Cancer Res. 2019, 79, P6-18-09. [Google Scholar] [CrossRef]
- Sánchez-Bayona, R.; de Sa, A.L.; Gilarranz, Y.J.; de Torre, A.S.; Alva, M.; Echavarria, I.; Moreno, F.; Tolosa, P.; Lopez, B.H.; de Luna, A.; et al. Everolimus plus endocrine therapy beyond CDK4/6 inhibitors progression for HR+ /HER2− advanced breast cancer: A real-world evidence cohort. Breast Cancer Res. Treat. 2024, 206, 551–559. [Google Scholar] [CrossRef]
- Franzoi, M.A.; Saúde-Conde, R.; Ferreira, S.C.; Eiger, D.; Awada, A.; de Azambuja, E. Clinical outcomes of platinum-based chemotherapy in patients with advanced breast cancer: An 11-year single institutional experience. Breast Off. J. Eur. Soc. Mastol. 2021, 57, 86. [Google Scholar] [CrossRef]
- Yamamura, J.; Kamigaki, S.; Fujita, J.; Osato, H.; Komoike, Y. The Difference in Prognostic Outcomes Between De Novo Stage IV and Recurrent Metastatic Patients with Hormone Receptor-positive, HER2-negative Breast Cancer. In Vivo 2018, 32, 353. [Google Scholar] [CrossRef]
- Brufsky, A.; Liu, X.; Li, B.; McRoy, L.; Chen, C.; Layman, R.M.; Rugo, H.S. Real-world treatment patterns and effectiveness of palbociclib plus an aromatase inhibitor in patients with metastatic breast cancer aged 75 years or older. Front. Oncol. 2023, 13, 1237751. [Google Scholar] [CrossRef]
- Rugo, H.S.; Brufsky, A.; Liu, X.; Li, B.; McRoy, L.; Chen, C.; Layman, R.M.; Cristofanilli, M.; Torres, M.A.; Curigliano, G.; et al. Real-world study of overall survival with palbociclib plus aromatase inhibitor in HR+/HER2− metastatic breast cancer. NPJ Breast Cancer 2022, 8, 114. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Engleton, M.; Davison, M.; Ravaud, P.; Porcher, R.; Boutron, I. Risk of bias in observational studies using routinely collected data of comparative effectiveness research: A meta-research study. BMC Med. 2021, 19, 279. [Google Scholar] [CrossRef]
- Gombos, A.; Goncalves, A.; Curigliano, G.; Bartsch, R.; Kyte, J.; Ignatiadis, M.; Awada, A. How I treat endocrine-dependent metastatic breast cancer. ESMO Open 2023, 8, 100882. [Google Scholar] [CrossRef]
- West, M.T.; Goodyear, S.M.; Hobbs, E.A.; Kaempf, A.; Kartika, T.; Ribkoff, J.; Chun, B.; Mitri, Z.I. Real-World Evaluation of Disease Progression After CDK 4/6 Inhibitor Therapy in Patients With Hormone Receptor-Positive Metastatic Breast Cancer. Oncologist 2023, 28, 682–690. [Google Scholar] [CrossRef]
- Ma, J.; Chan, J.J.; Toh, C.H.; Yap, Y.S. Emerging systemic therapy options beyond CDK4/6 inhibitors for hormone receptor-positive HER2-negative advanced breast cancer. NPJ Breast Cancer 2023, 9, 74. [Google Scholar] [CrossRef]

| Parameter | Total (N = 55) | |
|---|---|---|
| Grade | G1 | 2 (3.64%) |
| G2 | 32 (58.18%) | |
| G3 | 6 (10.91%) | |
| Unknown | 15 (27.27%) | |
| Estrogen Receptor (%) | Mean (SD) | 86.46 (20.37) |
| Range | 20–100 | |
| Progesterone Receptor (%) | Mean (SD) | 62.15 (32.62) |
| Range | 1–100 | |
| HER2 Immunohistochemistry | 0 | 31 (56.36%) |
| 1 | 11 (20.00%) | |
| 2 * | 13 (23.64%) | |
| Ki67 (%) | Mean (SD) | 27.68 (13.12) |
| Range | 10–60 | |
| Stage at diagnosis | IV | 14 (25.45%) |
| I–III | 41 (74.55%) | |
| Visceral metastases | No | 23 (41.82%) |
| Yes | 32 (58.18%) | |
| CNS metastases | No | 53 (96.36%) |
| Yes | 2 (3.64%) | |
| Line of palliative therapy for CDK4/6i | 1st line | 30 (54.55%) |
| 2nd line ** | 25 (45.45%) | |
| Reason for ending CDK4/6i therapy | Toxicity | 2 (3.64%) |
| Disease progression | 47 (85.45%) | |
| Patient’s decision | 1 (1.82%) | |
| Unknown/multiple | 5 (9.09%) | |
| CDK4/6i used | Abemaciclib | 8 (14.55%) |
| Palbociclib | 24 (43.64%) | |
| Ribociclib | 23 (41.82%) | |
| Any chemotherapy in patients history used before CDK4/6i | No | 23 (41.82%) |
| Yes | 32 (58.18%) | |
| Line of palliative treatment chemotherapy was applied for the first time *** | 2nd line | 13 (23.64%) |
| 3rd line | 34 (61.82%) | |
| 4th line | 7 (12.73%) | |
| 5th line | 1 (1.82%) | |
| Line of treatment chemotherapy was applied for the first time following CDK4/6i | 1st line | 38 (69.09%) |
| 2nd line | 15 (27.27%) | |
| 3rd line | 2 (3.64%) | |
| Number of post-CDK4/6i chemotherapy lines received | 1 line | 17 (30.91%) |
| 2 lines | 15 (27.27%) | |
| 3 lines | 3 (5.45%) | |
| At least 1 line **** | 14 (25.45%) | |
| At least 2 lines **** | 5 (9.09%) | |
| At least 3 lines **** | 1 (1.82%) | |
| Parameter | Total (N = 55) | |
|---|---|---|
| Chemotherapy type * | Taxane-based | 17 (30.91%) |
| Anthracycline-based | 16 (29.09%) | |
| Platin-based | 13 (23.64%) | |
| Capecitabine | 9 (16.36%) | |
| Navelbine | 3 (5.45%) | |
| Gemcitabine | 1 (1.82%) | |
| Monotherapy | 26 (47.27%) | |
| Combined therapy | 29 (52.73%) | |
| Patients | Events | Time (from Chemotherapy Start) | |||
|---|---|---|---|---|---|
| 6 Months | 12 Months | 18 Months | Median (Months) | ||
| Median progression-free survival | |||||
| 47 | 44 | 27.90% | 3.32% | 3.32% | 3.02 |
| Median overall survival | |||||
| 36 | 36 | 64.31% | 28.14% | 12.31% | 8.31 |
| Group | Patients | Events | Overall Survival | p | |||
|---|---|---|---|---|---|---|---|
| 6 Months | 12 Months | 18 Months | Median (Months) | ||||
| Chemotherapy | 55 | 36 | 74.96% | 52.64% | 20.01% | 12.39 | p < 0.001 |
| No chemotherapy * | 14 | 14 | 42.86% | 7.14% | >max obs. | 4.24 | |
| Ref. | Year | N | Type of Study | Prior CDK4/6i Treatment | CTH Regimen Characteristics | Outcomes (for CTH Arm) | Comments |
|---|---|---|---|---|---|---|---|
| [34] | 2022 | 1210, of which 249 received CTH | Retrospective cohort study–nationwide database | palbociclib n = 1067 abemaciclib n = 56 ribociclib n = 87 ET agents: anastrozole n = 59 exemestane n = 28 fulvestrant n = 366 letrozole n = 745 tamoxifen n = 12 | The study lacks information on specific CTH regimens. | rwPFS 3.71 mo | Continuation of CDK4/6i was associated with a significantly improved rwPFS compared to CTH. Treatment with fulvestrant monotherapy or everolimus was not observed to have statistically significant benefits in rwPFS compared to CTH. Treatment with everolimus was associated with improved OS compared to CTH, but fulvestrant was not. |
| [31] | 2023 | 609, of which 434 received CTH | Retrospective cohort study | The study lacks information on which CDK4/6i was used. Group A: 1st line CDK4/6i median duration 10 mo Group B: 2nd line CDK4/6i median duration 9 mo Group C: ≥3rd line CDK4/6i median duration 5 mo | 1st line CDK4/6i → CTH n = 126; taxane n = 48 capecitabine n = 37 carboplatin + taxane n = 17 anthracycline + cyclophosphamide n = 12 gemcitabine n = 5 cisplatin + gemcitabine n = 4 taxane + cyclophosphamide n = 3 2nd line CDK4/6i →CTH n = 110; taxane n = 32 capecitabine n = 44 carboplatin + taxane n = 9 anthracycline + cyclophosphamide n = 4 gemcitabine n = 7 cisplatin + gemcitabine n = 10 vinorelbine n = 4 ≥3rd line CDK4/6i → CTH n = 198; taxane n = 43 capecitabine n = 50 carboplatin + taxane n = 23 anthracycline + cyclophosphamide n = 6 gemcitabine n = 24 cisplatin + gemcitabine n = 15 vinorelbine n = 22 eribulin n = 10 ixabepilone n = 3 | mPFS group A: ET 9.5 mo; CTH 5.3 mo group B: ET 6.7 mo; CTH 5.7 mo group C: ET 5.3 mo; CTH 4.0 | Most frequently used CTH were capecitabine and taxanes. No significant difference was found comparing mPFS between ET and CTH groups. CTH was preferred to ET in patients, whose disease progressed shortly after starting CDK4/6i. The shorter median duration of CDK4/6i in patients who received CT compared to ET suggested that this group might have a relatively poor prognosis, which could affect the mPFS in CTH group. |
| [35] | 2023 | 305, of which 80 received CTH | Retrospective cohort study RWD | palbociclib + letrozole | capecitabine n = 47 taxane n = 28 anthracycline n = 3 other CTH n = 2 | mPFS: capecitabine 7.4 mo other CTH (taxane, anthracycline, other CTH) 4.8 mo mOS: capecitabine 42.3 mo other CTH (taxane, anthracycline, other CTH) 23.1 mo | In visceral organ disease progression, capecitabine and cytotoxic CTH had significantly longer mPFS in comparison to fulvestrant. In patients with bone metastasis alone, capecitabine had superior PFS to other second-line regimens. The OS did not differ according to the second-line treatment |
| [36] | 2023 | 543, of which 249 received CTH | Randomized controlled phase 3 trial | CDK4/6i + ET in the CTH arm: 1st line n = 101 2nd line n = 56 3rd line n = 49 ≥4th line n = 62 | CTH arm: eribulin n = 130 vinorelbine n = 63 gemcitabine n = 56 capecitabine n = 22 | mOS 11.2 mo ORR n = 38 Clinical benefit rate n = 60 | All patients received previous CTH in the metastatic setting. SG demonstrated both improved PFS and OS over CTH in the endocrine-resistant, post-CDK4/6i setting. More patients in the SG arm compared to the CTH arm experienced grade 3 adverse events. |
| [32] | 2018 | 525, of which 193 received CTH | Retrospective cohort study | CDK4/6i + ET | capecitabine n = 85 paclitaxel n = 53 gemcitabine n = 24 doxorubicin based n = 15 eribulin n = 12 other n = 4 | No significant endpoints | Patients who transitioned from a CDK4/6i based line to CTH (vs. endocrine, everolimus, or subsequent CDK4/6i) were more likely to have recurrent rapidly progressing disease and were significantly less likely to have the prior CDK4/6i-based line in combination with an AI. |
| [37] | 2019 | 200, of which 70 received CTH | Retrospective cohort study | palbociclib with: letrozole n = 147 fulvestrant n = 50 anastrozole n = 2 tamoxifen n = 1 | capecitabine n = 21 eribulin n = 16 paclitaxel albumin-bound n = 15 other n = 18 | mPFS 4.2 mo mPFS following progression on: 1st line palbociclib not reached, 2nd line palbociclib 4.7 mo; ≥3rd line palbociclib 4.1 mo | The mPFS was similar among patients on capecitabine, eribulin and paclitaxel. The study included 6 patients with HER2-positive tumors. |
| [38] | 2021 | 59, of which 32 received CTH | Retrospective cohort study | PALOMA-2 palbociclib + letrozole PALOMA-3 palbociclib + fulvestrant | PALOMA-2 First line: paclitaxel + bevacizumab n = 2 paclitaxel n = 1 TS n = 1 Second line capecitabine n = 2 capecitabine + fulvestrant n = 1 docetaxel n = 1 eribulin n = 1 PALOMA-3 First line paclitaxel + bevacizumab n = 4 capecitabine n = 1 cyclophosphamide + doxorubicin n = 1 eribulin n = 1 Second line paclitaxel + bevacizumab n = 4 eribulin n = 4 TS-1 n = 3 cyclophosphamide + epirubicin n = 2 capecitabine n = 2 paclitaxel n = 1 | Duration of subsequent therapy: PALOMA 2 First line 6.4 mo Second line 2.4 mo PALOMA-3 First line 3.8 mo Second line 5.8 mo | Compared with patients in PALOMA-2, CTH was used more frequently in PALOMA-3. The efficacy of the implemented CTH which the patients received after palbociclib progression was not assessed. |
| [39] | 2023 | 59 | Retrospective cohort study | palbociclib with AI | The study lacks information on specific CTH regimens. | mPFS 4 mo | CTH only as second line treatment. No information on how many patients received CTH. No significant difference was found in mPFS between CTH and everolimus + ET or ET only. |
| [40] | 2022 | 78, of which 32 received CTH | Retrospective cohort study | 1st line palbociclib (n = 55) with: AI n = 18 fulvestrant n = 18 2nd line palbociclib (n = 23) with: fulvestrant n = 20 | The study lacks information on specific CTH regimens. | Following progression on 1st line palbociclib: mTTF 6 mo CBR 50% following progression on 2nd line palbociclib: mTTF 8 mo CBR 50% | The majority of patients received CTH subsequent to CDK4/6i. |
| [41] | 2019 | 136, of which 14 received CTH | Retrospective cohort study | 1st line palbociclib (n = 81) with: letrozole n = 66 fulvestrant n = 11 2nd line palbociclib (n = 55) with: letrozole n = 16 fulvestrant n = 15 | The study lacks information on specific CTH regimens. | Following progression on 1st line palbociclib: mTTF 4.1 mo following progression on 2nd line palbociclib: mTTF 2.6 mo | No specific comparison between different treatments following CDK4/6i. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Püsküllüoğlu, M.; Ziobro, M.; Lompart, J.; Rudzińska, A.; Zemełka, T.; Jaworska, J.; Ochenduszko, S.; Grela-Wojewoda, A. Rationale for the Initiation, Outcomes, and Characteristics of Chemotherapy Following CDK4/6 Inhibitors in Breast Cancer: A Real-World Cohort Study. Cancers 2024, 16, 2894. https://doi.org/10.3390/cancers16162894
Püsküllüoğlu M, Ziobro M, Lompart J, Rudzińska A, Zemełka T, Jaworska J, Ochenduszko S, Grela-Wojewoda A. Rationale for the Initiation, Outcomes, and Characteristics of Chemotherapy Following CDK4/6 Inhibitors in Breast Cancer: A Real-World Cohort Study. Cancers. 2024; 16(16):2894. https://doi.org/10.3390/cancers16162894
Chicago/Turabian StylePüsküllüoğlu, Miroslawa, Marek Ziobro, Joanna Lompart, Agnieszka Rudzińska, Tomasz Zemełka, Justyna Jaworska, Sebastian Ochenduszko, and Aleksandra Grela-Wojewoda. 2024. "Rationale for the Initiation, Outcomes, and Characteristics of Chemotherapy Following CDK4/6 Inhibitors in Breast Cancer: A Real-World Cohort Study" Cancers 16, no. 16: 2894. https://doi.org/10.3390/cancers16162894
APA StylePüsküllüoğlu, M., Ziobro, M., Lompart, J., Rudzińska, A., Zemełka, T., Jaworska, J., Ochenduszko, S., & Grela-Wojewoda, A. (2024). Rationale for the Initiation, Outcomes, and Characteristics of Chemotherapy Following CDK4/6 Inhibitors in Breast Cancer: A Real-World Cohort Study. Cancers, 16(16), 2894. https://doi.org/10.3390/cancers16162894






