Radioimmunotheragnosis in Cancer Research
Abstract
Simple Summary
Abstract
1. Introduction
2. Antibodies in ImmunoPET and RIT
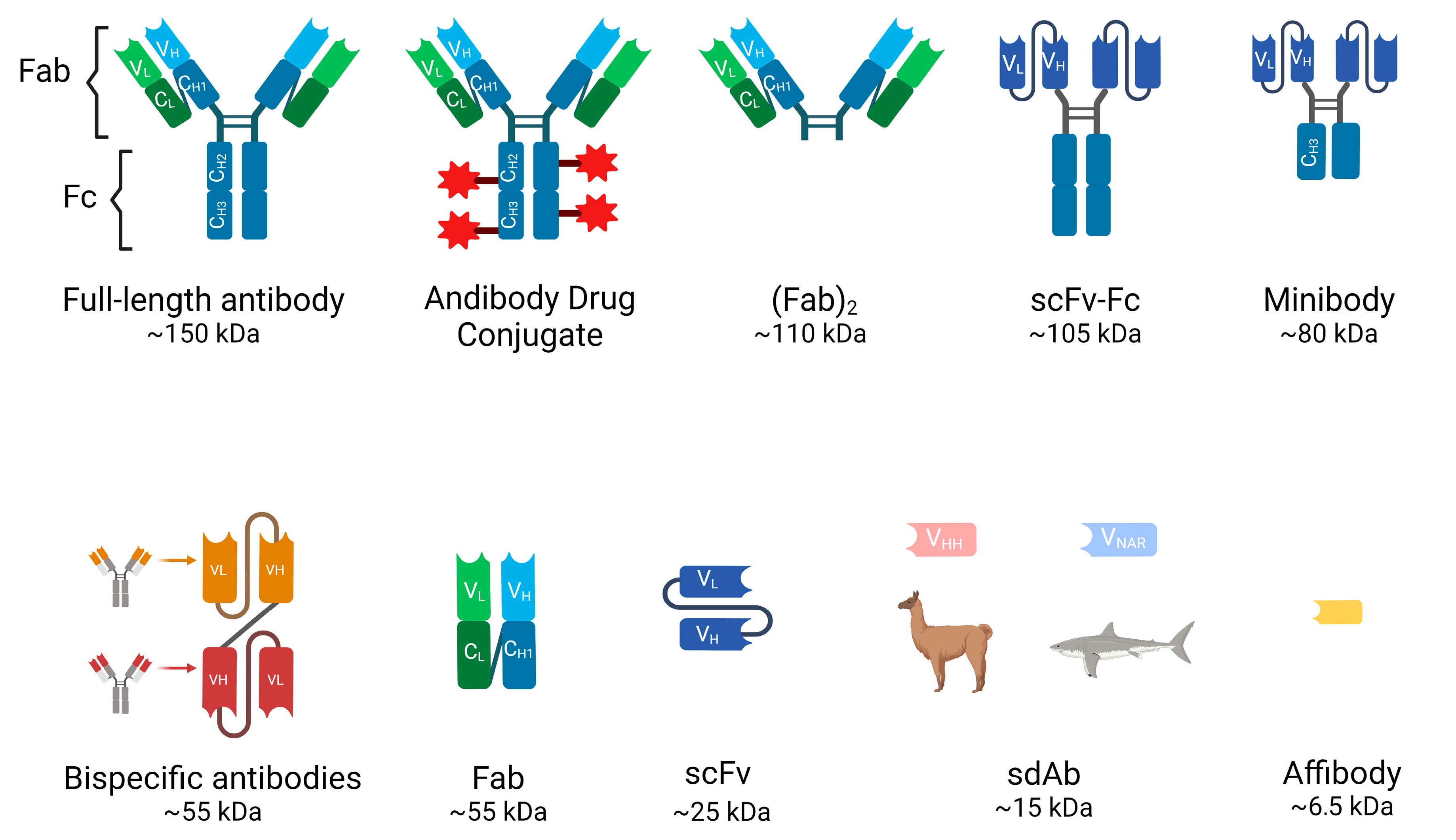
3. Full-Length Antibodies (Abs)
4. Antibody Fragments
5. Isotopes
6. Commonly Used Radioactive Isotopes in ImmunoPET
- 89Zr (Zirconium-89)
- 64Cu (Copper-64)
- 68Ga (Gallium-68)
- 124I (Iodine-124)
- 86Y (Yttrium-86)
- 18F (Fluorine-18)
7. Commonly Used Radioactive Isotopes in Radioimmunotherapy
- 131I (Iodine-131)
- 90Y (Yttrium-90)
- 177Lu (Lutetium-177)
- 188Re (Rhenium-188)
- 211At (Astatine-211)
- 225Ac (Actinium-225)
- 213Bi (Bismuth 213)
8. Factors Influencing the Choice of Isotope
9. Pretargeted ImmunoPET and Radioimmunotherapy (PRIT): General Strategy
10. Radioimmunotheragnosis Applications in Cancer
11. Hematological Malignancies
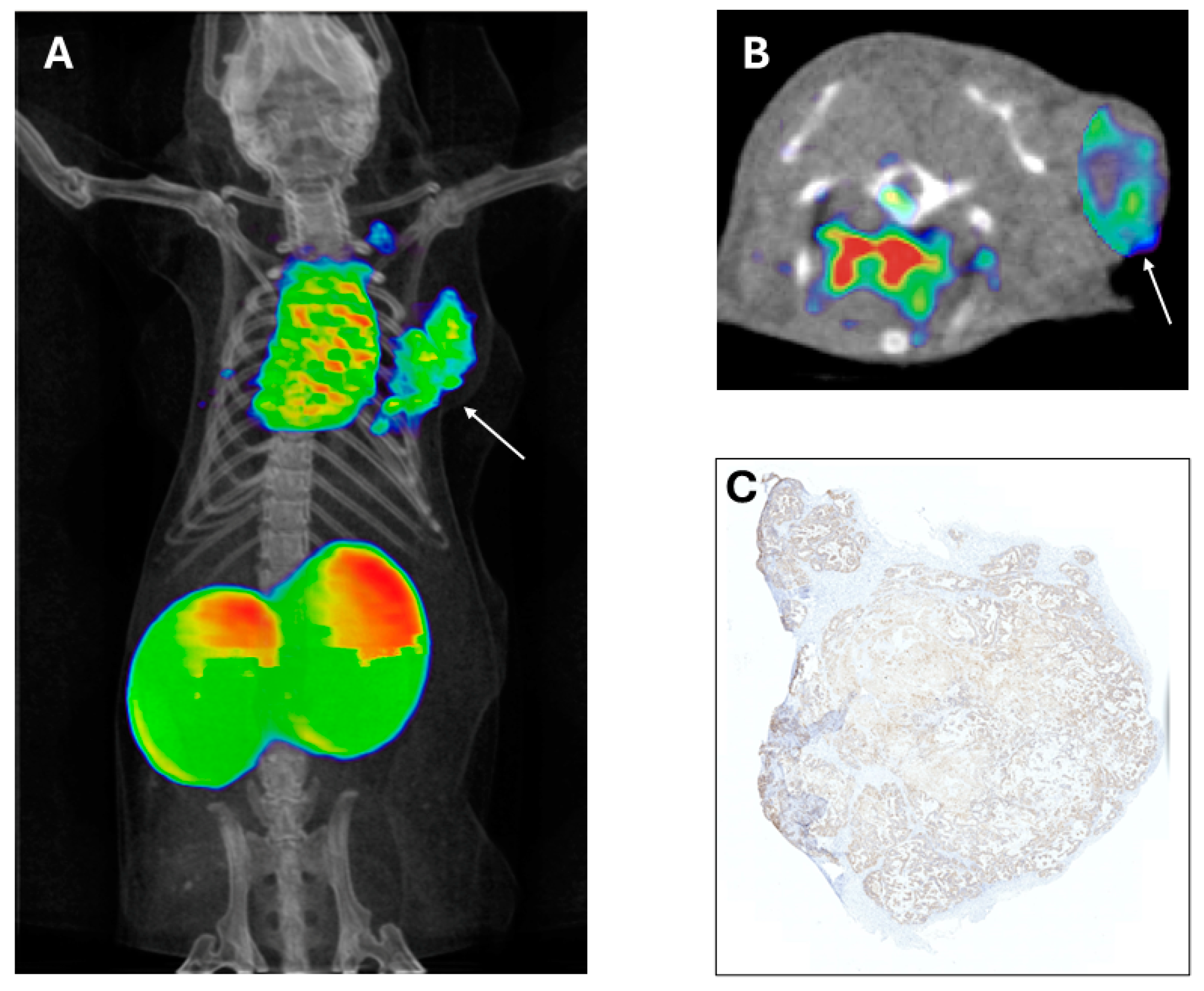
12. Breast Cancer
13. Prostate Cancer
14. Hepatocellular Carcinoma
15. Colorectal Cancer
16. Lung Cancer
17. Others
18. Dosimetry
19. Future
20. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wei, W.; Rosenkrans, Z.T.; Liu, J.; Huang, G.; Luo, Q.Y.; Cai, W. ImmunoPET: Concept, Design, and Applications. Chem. Rev. 2020, 120, 3787. [Google Scholar]
- Rondon, A.; Rouanet, J.; Degoul, F. Radioimmunotherapy in Oncology: Overview of the Last Decade Clinical Trials. Cancers 2021, 13, 5570. [Google Scholar] [CrossRef]
- Bodei, L.; Herrmann, K.; Schöder, H.; Scott, A.M.; Lewis, J.S. Radiotheranostics in oncology: Current challenges and emerging opportunities. Nat. Rev. Clin. Oncol. 2022, 19, 534–550. [Google Scholar] [CrossRef] [PubMed]
- Mulero, F. Editorial: ImmunoPET imaging in disease diagnosis and therapy assessment. Front. Med. 2023, 10, 1231525. [Google Scholar] [CrossRef]
- Wei, W.; Jiang, D.; Lee, H.J.; Li, M.; Kutyreff, C.J.; Engle, J.W.; Liu, J.; Cai, W. Development and Characterization of CD54-Targeted ImmunoPET Imaging in Solid Tumors. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2765. [Google Scholar]
- Carter, L.M.; Poty, S.; Sharma, S.K.; Lewis, J.S. Preclinical optimization of antibody-based radiopharmaceuticals for cancer imaging and radionuclide therapy-Model, vector, and radionuclide selection. J. Label. Comp. Radiopharm. 2018, 61, 611–635. [Google Scholar] [CrossRef]
- Turner, J.H. An introduction to the clinical practice of theranostics in oncology. Br. J. Radiol. 2018, 91, 20180440. [Google Scholar] [CrossRef]
- Rahmim, A.; Zaidi, H. PET versus SPECT: Strengths, limitations and challenges. Nucl. Med. Commun. 2008, 29, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Mulero, F. ImmunoPET in oncology. Rev. Española Med. Nucl. Imagen Mol. (Engl. Ed.) 2022, 41, 332–339. [Google Scholar] [CrossRef]
- Dewulf, J.; Adhikari, K.; Vangestel, C.; Wyngaert, T.V.D.; Elvas, F. Development of Antibody Immuno-PET/SPECT Radiopharmaceuticals for Imaging of Oncological Disorders-An Update. Cancers 2020, 12, 1868. [Google Scholar] [CrossRef]
- Harsini, S.; Alavi, A.; Rezaei, N. Introduction on Nuclear Medicine and Immunology; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–13. [Google Scholar] [CrossRef]
- Petronis, J.D.; Regan, F.; Lin, K. Indium-111 capromab pendetide (ProstaScint) imaging to detect recurrent and metastatic prostate cancer. Clin. Nucl. Med. 1998, 23, 672–677. [Google Scholar]
- Deb, N.; Goris, M.; Trisler, K.; Fowler, S.; Saal, J.; Ning, S.; Becker, M.; Marquez, C.; Knox, S. Treatment of hormone-refractory prostate cancer with 90Y-CYT-356 monoclonal antibody. Clin. Cancer Res. 1996, 2, 1289–1297. [Google Scholar] [PubMed]
- Hu, M.; Chen, P.; Wang, J.; Scollard, D.A.; Vallis, K.A.; Reilly, R.M. 123I-labeled HIV-1 tat peptide radioimmunoconjugates are imported into the nucleus of human breast cancer cells and functionally interact in vitro and in vivo with the cyclin-dependent kinase inhibitor, p21(WAF-1/Cip-1). Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, B.; Kersemans, V.; McLarty, K.; Tran, L.; Vallis, K.A.; Reilly, R.M. In vivo monitoring of intranuclear p27(kip1) protein expression in breast cancer cells during trastuzumab (Herceptin) therapy. Nucl. Med. Biol. 2009, 36, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.C.; Torres, J.B.; Goldin, R.; Mosley, M.; Dias, G.M.; Bravo, L.C.; Kersemans, V.; Allen, P.D.; Mukherjee, S.; Smart, S.; et al. Early Detection in a Mouse Model of Pancreatic Cancer by Imaging DNA Damage Response Signaling. J. Nucl. Med. 2020, 61, 1006–1013. [Google Scholar] [CrossRef]
- Woof, J.M.; Burton, D.R. Human antibody-Fc receptor interactions illuminated by crystal structures. Nat. Rev. Immunol. 2004, 4, 89–99. [Google Scholar] [CrossRef]
- Manafi-Farid, R.; Ataeinia, B.; Ranjbar, S.; Jamshidi Araghi, Z.; Moradi, M.M.; Pirich, C.; Beheshti, M. ImmunoPET: Antibody-Based PET Imaging in Solid Tumors. Front. Med. 2022, 9, 916693. [Google Scholar] [CrossRef]
- Olafsen, T.; Wu, A. Antibody Vectors for Imaging. Semin. Nucl. Med. 2010, 40, 167–181. [Google Scholar] [CrossRef]
- Ahamadi-Fesharaki, R.; Fateh, A.; Vaziri, F.; Solgi, G.; Siadat, S.D.; Mahboudi, F.; Rahimi-Jamnani, F. Single-Chain Variable Fragment-Based Bispecific Antibodies: Hitting Two Targets with One Sophisticated Arrow. Mol. Ther. Oncolytics 2019, 14, 38–56. [Google Scholar] [CrossRef]
- Huehls, A.M.; Coupet, T.A.; Sentman, C.L. Bispecific T-cell engagers for cancer immunotherapy. Immunol. Cell Biol. 2015, 93, 290–296. [Google Scholar] [CrossRef]
- Bargou, R.; Leo, E.; Zugmaier, G.; Klinger, M.; Goebeler, M.; Knop, S.; Noppeney, R.; Viardot, A.; Hess, G.; Schuler, M.; et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 2008, 321, 974–977. [Google Scholar] [CrossRef]
- Salvador, J.P.; Vilaplana, L.; Marco, M.P. Nanobody: Outstanding features for diagnostic and therapeutic applications. Anal. Bioanal. Chem. 2019, 411, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Pang, Y.; Li, L.; Pang, Y.; Zhang, J.; Wang, X.; Raes, G. Applications of nanobodies in brain diseases. Front. Immunol. 2022, 13, 978513. [Google Scholar] [CrossRef]
- Alamoudi, A.O. Radiomics, aptamers and nanobodies: New insights in cancer diagnostics and imaging. Hum. Antibodies 2021, 29, 1–15. [Google Scholar] [CrossRef]
- Mulero, F.; Oteo, M.; Garaulet, G.; Magro, N.; Rebollo, L.; Medrano, G.; Santiveri, C.; Romero, E.; Sellek, R.E.; Margolles, Y.; et al. Development of anti-membrane type 1-matrix metalloproteinase nanobodies as immunoPET probes for triple negative breast cancer imaging. Front. Med. 2022, 9, 1058455. [Google Scholar] [CrossRef]
- Wei, W.; Younis, M.H.; Lan, X.; Liu, J.; Cai, W. Single-Domain Antibody Theranostics on the Horizon. J. Nucl. Med. 2022, 63, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Arbabi-Ghahroudi, M. Camelid Single-Domain Antibodies: Promises and Challenges as Lifesaving Treatments. Int. J. Mol. Sci. 2022, 23, 5009. [Google Scholar] [CrossRef]
- Sulea, T. Humanization of Camelid Single-Domain Antibodies. Methods Mol. Biol. 2022, 2446, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, M.; Skerra, A. Engineered protein scaffolds as next-generation antibody therapeutics. Curr. Opin. Chem. Biol. 2009, 13, 245–255. [Google Scholar] [CrossRef]
- Altai, M.; Wållberg, H.; Orlova, A.; Rosestedt, M.; Hosseinimehr, S.J.; Tolmachev, V.; Ståhl, S. Order of amino acids in C-terminal cysteine-containing peptide-based chelators influences cellular processing and biodistribution of 99mTc-labeled recombinant Affibody molecules. Amino Acids 2012, 42, 1975–1985. [Google Scholar] [CrossRef]
- Orlova, A.; Jonsson, A.; Rosik, D.; Lundqvist, H.; Lindborg, M.; Abrahmsen, L.; Ekblad, C.; Frejd, F.Y.; Tolmachev, V. Site-Specific Radiometal Labeling and Improved Biodistribution Using ABY-027, A Novel HER2-Targeting Affibody Molecule–Albumin-Binding Domain Fusion Protein. J. Nucl. Med. 2013, 54, 961–968. [Google Scholar] [CrossRef]
- Sörensen, J.; Sandberg, D.; Sandström, M.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Åström, G.; Lubberink, M.; Garske-Román, U.; Carlsson, J.; et al. First-in-human molecular imaging of HER2 expression in breast cancer metastases using the 111In-ABY-025 affibody molecule. J. Nucl. Med. 2014, 55, 730–735. [Google Scholar] [CrossRef]
- Baum, R.P.; Prasad, V.; Muller, D.; Schuchardt, C.; Orlova, A.; Wennborg, A.; Tolmachev, V.; Feldwisch, J. Molecular imaging of HER2-expressing malignant tumors in breast cancer patients using synthetic 111In- or 68Ga-labeled affibody molecules. J. Nucl. Med. 2010, 51, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Masters, J.C.; Nickens, D.J.; Xuan, D.; Shazer, R.L.; Amantea, M. Clinical toxicity of antibody drug conjugates: A meta-analysis of payloads. Investig. New Drugs 2018, 36, 121–135. [Google Scholar] [CrossRef]
- Carmon, K.S.; Azhdarinia, A. Application of Immuno-PET in Antibody–Drug Conjugate Development. Mol. Imaging 2018, 17, 1536012118801223. [Google Scholar] [CrossRef]
- Cahuzac, H.; Devel, L. Analytical Methods for the Detection and Quantification of ADCs in Biological Matrices. Pharmaceuticals 2020, 13, 462. [Google Scholar] [CrossRef] [PubMed]
- Ulaner, G.A.; Hyman, D.M.; Lyashchenko, S.K.; Lewis, J.S.; Carrasquillo, J.A. 89Zr-Trastuzumab PET/CT for Detection of Human Epidermal Growth Factor Receptor 2-Positive Metastases in Patients with Human Epidermal Growth Factor Receptor 2-Negative Primary Breast Cancer. Clin. Nucl. Med. 2017, 42, 912–917. [Google Scholar] [CrossRef]
- Bensch, F.; Brouwers, A.H.; Lub-de Hooge, M.N.; de Jong, J.R.; van der Vegt, B.; Sleijfer, S.; de Vries, E.G.E.; Schröder, C.P. (89)Zr-trastuzumab PET supports clinical decision making in breast cancer patients, when HER2 status cannot be determined by standard work up. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2300–2306. [Google Scholar] [CrossRef]
- van Asselt, S.J.; Oosting, S.F.; Brouwers, A.H.; Bongaerts, A.H.; de Jong, J.R.; Lub-de Hooge, M.N.; Oude Munnink, T.H.; Fiebrich, H.B.; Sluiter, W.J.; Links, T.P.; et al. Everolimus Reduces (89)Zr-Bevacizumab Tumor Uptake in Patients with Neuroendocrine Tumors. J. Nucl. Med. 2014, 55, 1087–1092. [Google Scholar] [CrossRef]
- Niemeijer, A.N.; Leung, D.; Huisman, M.C.; Bahce, I.; Hoekstra, O.S.; van Dongen, G.; Boellaard, R.; Du, S.; Hayes, W.; Smith, R.; et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat. Commun. 2018, 9, 4664. [Google Scholar] [CrossRef]
- Malih, S.; Lin, W.; Tang, Z.; DeLuca, M.C.; Engle, J.W.; Alirezapour, B.; Cai, W.; Rasaee, M.J. Noninvasive PET imaging of tumor PD-L1 expression with (64)Cu-labeled Durvalumab. Am. J. Nucl. Med. Mol. Imaging 2024, 14, 31–40. [Google Scholar] [CrossRef]
- Larson, S.M.; Carrasquillo, J.A.; Cheung, N.-K.V.; Press, O.W. Radioimmunotherapy of human tumours. Nat. Rev. Cancer 2015, 15, 347–360. [Google Scholar]
- Xavier, C.; Vaneycken, I.; D’huyvetter, M.; Heemskerk, J.; Keyaerts, M.; Vincke, C.; Devoogdt, N.; Muyldermans, S.; Lahoutte, T.; Caveliers, V. Synthesis, Preclinical Validation, Dosimetry, and Toxicity of 68Ga-NOTA-Anti-HER2 Nanobodies for IPET Imaging of HER2 Receptor Expression in Cancer. J. Nucl. Med. 2013, 54, 776. [Google Scholar]
- de Lucas, A.G.; Schuhmacher, A.J.; Oteo, M.; Romero, E.; Camara, J.A.; de Martino, A.; Arroyo, A.G.; Morcillo, M.A.; Squatrito, M.; Martinez-Torrecuadrada, J.L.; et al. Targeting MT1-MMP as an ImmunoPET-Based Strategy for Imaging Gliomas. PLoS ONE 2016, 11, e0158634. [Google Scholar] [CrossRef]
- Carrasquillo, J.A.; Pandit-Taskar, N.; O’Donoghue, J.A.; Humm, J.L.; Zanzonico, P.; Smith-Jones, P.M.; Divgi, C.R.; Pryma, D.A.; Ruan, S.; Kemeny, N.E.; et al. (124)I-huA33 antibody PET of colorectal cancer. J. Nucl. Med. 2011, 52, 1173–1180. [Google Scholar] [CrossRef]
- Samnick, S.; Al-Momani, E.; Schmid, J.S.; Mottok, A.; Buck, A.K.; Lapa, C. Initial Clinical Investigation of [18F]Tetrafluoroborate PET/CT in Comparison to [124I]Iodine PET/CT for Imaging Thyroid Cancer. Clin. Nucl. Med. 2018, 43, 162–167. [Google Scholar] [CrossRef]
- Cheal, S.M.; Xu, H.; Guo, H.-f.; Patel, M.; Punzalan, B.; Fung, E.K.; Lee, S.-g.; Bell, M.; Singh, M.; Jungbluth, A.A. Theranostic pretargeted radioimmunotherapy of internalizing solid tumor antigens in human tumor xenografts in mice: Curative treatment of HER2-positive breast carcinoma. Theranostics 2018, 8, 5106. [Google Scholar] [PubMed]
- Nayak, T.K.; Garmestani, K.; Baidoo, K.E.; Milenic, D.E.; Brechbiel, M.W. PET imaging of tumor angiogenesis in mice with VEGF-A-targeted (86)Y-CHX-A″-DTPA-bevacizumab. Int. J. Cancer 2011, 128, 920–926. [Google Scholar] [CrossRef]
- Wong, K.J.; Baidoo, K.E.; Nayak, T.K.; Garmestani, K.; Brechbiel, M.W.; Milenic, D.E. In Vitro and In Vivo Pre-Clinical Analysis of a F(ab’)(2) Fragment of Panitumumab for Molecular Imaging and Therapy of HER1 Positive Cancers. EJNMMI Res. 2011, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- McBride, W.J.; Sharkey, R.M.; Karacay, H.; D’Souza, C.A.; Rossi, E.A.; Laverman, P.; Chang, C.H.; Boerman, O.C.; Goldenberg, D.M. A novel method of 18F radiolabeling for PET. J. Nucl. Med. 2009, 50, 991–998. [Google Scholar] [CrossRef]
- Cleeren, F.; Lecina, J.; Ahamed, M.; Raes, G.; Devoogdt, N.; Caveliers, V.; McQuade, P.; Rubins, D.J.; Li, W.; Verbruggen, A.; et al. Al(18)F-Labeling Of Heat-Sensitive Biomolecules for Positron Emission Tomography Imaging. Theranostics 2017, 7, 2924–2939. [Google Scholar] [CrossRef]
- Grillo-López, A.J. Zevalin: The first radioimmunotherapy approved for the treatment of lymphoma. Expert. Rev. Anticancer Ther. 2002, 2, 485–493. [Google Scholar] [CrossRef]
- Friedberg, J.W.; Fisher, R.I. Iodine-131 tositumomab (Bexxar): Radioimmunoconjugate therapy for indolent and transformed B-cell non-Hodgkin’s lymphoma. Expert. Rev. Anticancer Ther. 2004, 4, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Rieger, K.; De Filippi, R.; Lindén, O.; Viardot, A.; Hess, G.; Lerch, K.; Neumeister, P.; Stroux, A.; Peuker, C.A.; Pezzutto, A.; et al. 90-yttrium-ibritumomab tiuxetan as first-line treatment for follicular lymphoma: Updated efficacy and safety results at an extended median follow-up of 9.6 years. Ann. Hematol. 2022, 101, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Jia, A.Y.; Kashani, R.; Zaorsky, N.G.; Spratt, D.E.; Kiess, A.P.; Michalski, J.M.; Zoberi, J.E.; Kim, H.; Baumann, B.C. Lutetium-177 DOTATATE: A Practical Review. Pr. Radiat. Oncol. 2022, 12, 305–311. [Google Scholar] [CrossRef]
- D’Huyvetter, M.; Vincke, C.; Xavier, C.; Aerts, A.; Impens, N.; Baatout, S.; De Raeve, H.; Muyldermans, S.; Caveliers, V.; Devoogdt, N. Targeted radionuclide therapy with A 177Lu-labeled anti-HER2 nanobody. Theranostics 2014, 4, 708. [Google Scholar]
- Tagawa, S.T.; Milowsky, M.I.; Morris, M.; Vallabhajosula, S.; Christos, P.; Akhtar, N.H.; Osborne, J.; Goldsmith, S.J.; Larson, S.; Taskar, N.P. Phase II study of lutetium-177–labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2013, 19, 5182–5191. [Google Scholar]
- Liu, C.-M.; Chang, C.-H.; Chang, Y.-J.; Hsu, C.-W.; Chen, L.-C.; Chen, H.-L.; Ho, C.-L.; Yu, C.-Y.; Chang, T.-J.; Chiang, T.-C.; et al. Preliminary evaluation of acute toxicity of (188) Re-BMEDA-liposome in rats. J. Appl. Toxicol. 2010, 30, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, J.; Gui, J.; Liu, C.; Wang, Y.; Zhang, G.; Kuai, D.; Wu, Y.; Liu, Z.; Zuo, C.; et al. Efficacy and safety of (188)Re-HEDP in lung cancer patients with bone metastases: A randomized, multicenter, multiple-dose phase IIa study. Int. J. Clin. Oncol. 2021, 26, 1212–1220. [Google Scholar] [CrossRef]
- Albertsson, P.; Bäck, T.; Bergmark, K.; Hallqvist, A.; Johansson, M.; Aneheim, E.; Lindegren, S.; Timperanza, C.; Smerud, K.; Palm, S. Astatine-211 based radionuclide therapy: Current clinical trial landscape. Front. Med. 2022, 9, 1076210. [Google Scholar] [CrossRef]
- Bidkar, A.P.; Zerefa, L.; Yadav, S.; VanBrocklin, H.F.; Flavell, R.R. Actinium-225 targeted alpha particle therapy for prostate cancer. Theranostics 2024, 14, 2969–2992. [Google Scholar] [CrossRef]
- Jurcic, J.G. Clinical Studies with Bismuth-213 and Actinium-225 for Hematologic Malignancies. Curr. Radiopharm. 2018, 11, 192–199. [Google Scholar] [CrossRef]
- de Kruijff, R.M.; Wolterbeek, H.T.; Denkova, A.G. A Critical Review of Alpha Radionuclide Therapy-How to Deal with Recoiling Daughters? Pharmaceuticals 2015, 8, 321–336. [Google Scholar] [CrossRef]
- Hertz, S.; Roberts, A.; Evans, R.D. Radioactive Iodine as an Indicator in the Study of Thyroid Physiology. Proc. Soc. Exp. Biol. Med. 1938, 38, 510–513. [Google Scholar] [CrossRef]
- Witzig, T.E.; Gordon, L.I.; Cabanillas, F.; Czuczman, M.S.; Emmanouilides, C.; Joyce, R.; Pohlman, B.L.; Bartlett, N.L.; Wiseman, G.A.; Padre, N. Randomized controlled trial of yttrium-90–labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J. Clin. Oncol. 2002, 20, 2453–2463. [Google Scholar]
- Leahy, M.F.; Seymour, J.F.; Hicks, R.J.; Turner, J.H. Multicenter phase II clinical study of iodine-131-rituximab radioimmunotherapy in relapsed or refractory indolent non-Hodgkin’s lymphoma. J. Clin Oncol. 2006, 24, 4418–4425. [Google Scholar] [CrossRef]
- Krasniqi, A.; D’Huyvetter, M.; Xavier, C.; Van der Jeught, K.; Muyldermans, S.; Van Der Heyden, J.; Lahoutte, T.; Tavernier, J.; Devoogdt, N. Theranostic Radiolabeled Anti-CD20 SdAb for Targeted Radionuclide Therapy of Non-Hodgkin Lymphoma. Mol. Cancer Ther. 2017, 16, 2828–2939. [Google Scholar]
- Gomes Marin, J.F.; Nunes, R.F.; Coutinho, A.M.; Zaniboni, E.C.; Costa, L.B.; Barbosa, F.G.; Queiroz, M.A.; Cerri, G.G.; Buchpiguel, C.A. Theranostics in Nuclear Medicine: Emerging and Re-emerging Integrated Imaging and Therapies in the Era of Precision Oncology. Radiographics 2020, 40, 1715–1740. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; Orgun, N.N.; Jones, J.C.; Hylarides, M.D.; Pagel, J.M.; Hamlin, D.K.; Wilbur, D.; Lin, Y.; Fisher, D.R.; Kenoyer, A.L. A preclinical model of CD38-pretargeted radioimmunotherapy for plasma cell malignancies. Cancer Res. 2014, 74, 1179–1189. [Google Scholar] [PubMed]
- O’Steen, S.; Comstock, M.L.; Orozco, J.J.; Hamlin, D.K.; Wilbur, D.S.; Jones, J.C.; Kenoyer, A.; Nartea, M.E.; Lin, Y.; Miller, B.W. The α-emitter astatine-211 targeted to CD38 can eradicate multiple myeloma in a disseminated disease model. Blood J. Am. Soc. Hematol. 2019, 134, 1247–1256. [Google Scholar]
- Green, D.J.; O’Steen, S.; Lin, Y.; Comstock, M.L.; Kenoyer, A.L.; Hamlin, D.K.; Wilbur, D.S.; Fisher, D.R.; Nartea, M.; Hylarides, M.D. CD38-bispecific antibody pretargeted radioimmunotherapy for multiple myeloma and other B-cell malignancies. Blood J. Am. Soc. Hematol. 2018, 131, 611–620. [Google Scholar]
- bastienJamet, B.; Bailly, C.; Carlier, T.; Touzeau, C.; Nanni, C.; Zamagni, E.; Barré, L.; Michaud, A.-V.; Chérel, M.; Moreau, P. Interest of pet imaging in multiple myeloma. Front. Med. 2019, 6, 69. [Google Scholar]
- Perik, P.J.; Lub-De Hooge, M.N.; Gietema, J.A.; van der Graaf, W.T.; de Korte, M.A.; Jonkman, S.; Kosterink, J.G.; van Veldhuisen, D.J.; Sleijfer, D.T.; Jager, P.L.; et al. Indium-111-labeled trastuzumab scintigraphy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J. Clin Oncol. 2006, 24, 2276–2282. [Google Scholar] [CrossRef] [PubMed]
- D’Huyvetter, M.; De Vos, J.; Xavier, C.; Pruszynski, M.; Sterckx, Y.G.; Massa, S.; Raes, G.; Caveliers, V.; Zalutsky, M.R.; Lahoutte, T. 131I-labeled anti-HER2 camelid sdAb as a theranostic tool in cancer treatment. Clin. Cancer Res. 2017, 23, 6616–6628. [Google Scholar]
- Magro, N.; Oteo, M.; Romero, E.; Ibáñez-Moragues, M.; Lujan, V.M.; Martínez, L.; Vela, O.; López-Melero, M.E.; Arroyo, A.G.; Garaulet, G.; et al. Target engagement of an anti-MT1-MMP antibody for triple-negative breast cancer PET imaging and beta therapy. Nucl. Med. Biol. 2024, 136, 108930. [Google Scholar] [CrossRef]
- Pretze, M.; Reffert, L.; Diehl, S.; Schönberg, S.O.; Wängler, C.; Hohenberger, P.; Wängler, B. GMP-compliant production of [(68)Ga]Ga-NeoB for positron emission tomography imaging of patients with gastrointestinal stromal tumor. EJNMMI Radiopharm. Chem. 2021, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Montemagno, C.; Raes, F.; Ahmadi, M.; Bacot, S.; Debiossat, M.; Leenhardt, J.; Boutonnat, J.; Orlandi, F.; Barbato, D.; Tedesco, M.; et al. In Vivo Biodistribution and Efficacy Evaluation of NeoB, a Radiotracer Targeted to GRPR, in Mice Bearing Gastrointestinal Stromal Tumor. Cancers 2021, 13, 1051. [Google Scholar] [CrossRef] [PubMed]
- Gruber, L.; Jiménez-Franco, L.D.; Decristoforo, C.; Uprimny, C.; Glatting, G.; Hohenberger, P.; Schoenberg, S.O.; Reindl, W.; Orlandi, F.; Mariani, M.; et al. MITIGATE-NeoBOMB1, a Phase I/IIa Study to Evaluate Safety, Pharmacokinetics, and Preliminary Imaging of (68)Ga-NeoBOMB1, a Gastrin-Releasing Peptide Receptor Antagonist, in GIST Patients. J. Nucl. Med. 2020, 61, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Dalm, S.U.; Bakker, I.L.; de Blois, E.; Doeswijk, G.N.; Konijnenberg, M.W.; Orlandi, F.; Barbato, D.; Tedesco, M.; Maina, T.; Nock, B.A.; et al. 68Ga/177Lu-NeoBOMB1, a Novel Radiolabeled GRPR Antagonist for Theranostic Use in Oncology. J. Nucl. Med. 2017, 58, 293–299. [Google Scholar] [CrossRef]
- Bander, N.H. Technology insight: Monoclonal antibody imaging of prostate cancer. Nat. Clin. Pract. Urol. 2006, 3, 216–225. [Google Scholar]
- Liu, H.; Moy, P.; Kim, S.; Xia, Y.; Rajasekaran, A.; Navarro, V.; Knudsen, B.; Bander, N.H. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res. 1997, 57, 3629–3634. [Google Scholar]
- Holland, J.P.; Divilov, V.; Bander, N.H.; Smith-Jones, P.M.; Larson, S.M.; Lewis, J.S. 89Zr-DFO-J591 for ImmunoPET of Prostate-Specific Membrane Antigen Expression In Vivo. J. Nucl. Med. 2010, 51, 1293. [Google Scholar]
- Fung, E.K.; Cheal, S.M.; Fareedy, S.B.; Punzalan, B.; Beylergil, V.; Amir, J.; Chalasani, S.; Weber, W.A.; Spratt, D.E.; Veach, D.R.; et al. Targeting of radiolabeled J591 antibody to PSMA-expressing tumors: Optimization of imaging and therapy based on non-linear compartmental modeling. EJNMMI Res. 2016, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Lütje, S.; Gerrits, D.; Molkenboer-Kuenen, J.D.; Herrmann, K.; Fracasso, G.; Colombatti, M.; Boerman, O.C.; Heskamp, S. Characterization of Site-Specifically Conjugated Monomethyl Auristatin E–And Duocarmycin-Based Anti-PSMA Antibody–Drug Conjugates for Treatment of PSMA-Expressing Tumors. J. Nucl. Med. 2018, 59, 494–501. [Google Scholar]
- Kratochwil, C.; Schmidt, K.; Afshar-Oromieh, A.; Bruchertseifer, F.; Rathke, H.; Morgenstern, A.; Haberkorn, U.; Giesel, F.L. Targeted alpha therapy of mCRPC: Dosimetry estimate of (213)Bismuth-PSMA-617. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 31–37. [Google Scholar] [CrossRef] [PubMed]
- González, G.P.; García, I.G.; González, J.G.; Sánchez, L.P.; Mirabal, M.V.; Marín, C.C.; Ruiz, F.L.G.; Iglesias, E.G.; de Queralta, R.L.; Toirac, R.R. Phase I clinical trial of the 131I-Labeled anticarcinoembryonic antigen CIGB-M3 multivalent antibody fragment. Cancer Biother. Radiopharm. 2011, 26, 353–363. [Google Scholar]
- Wu, L.; Yang, Y.-F.; Ge, N.-J.; Shen, S.-Q.; Liang, J.; Wang, Y.; Zhou, W.-P.; Shen, F.; Wu, M.-C. Hepatic artery injection of 131 I-labelled metuximab combined with chemoembolization for intermediate hepatocellular carcinoma: A prospective nonrandomized study. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1306–1315. [Google Scholar]
- Meyer, T.; Gaya, A.; Dancey, G.; Stratford, M.; Othman, S.; Sharma, S.; Wellsted, D.; Taylor, J.; Stirling, J.; Poupard, L.; et al. A Phase I Trial of Radioimmunotherapy with I-131-A5B7 Anti-CEA Antibody in Combination with Combretastatin-A4-Phosphate in Advanced Gastrointestinal Carcinomas. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 4484–4492. [Google Scholar] [CrossRef]
- Rossi, E.A.; Goldenberg, D.M.; Cardillo, T.M.; McBride, W.J.; Sharkey, R.M.; Chang, C.-H. Stably tethered multifunctional structures of defined composition made by the dock and lock method for use in cancer targeting. Proc. Natl. Acad. Sci. USA 2006, 103, 6841–6846. [Google Scholar]
- Schoffelen, R.; Boerman, O.C.; Goldenberg, D.M.; Sharkey, R.M.; van Herpen, C.M.; Franssen, G.M.; McBride, W.J.; Chang, C.-H.; Rossi, E.A.; van der Graaf, W.T. Development of an imaging-guided CEA-pretargeted radionuclide treatment of advanced colorectal cancer: First clinical results. Br. J. Cancer 2013, 109, 934–942. [Google Scholar]
- Lawhn-Heath, C.; Hope, T.A.; Martinez, J.; Fung, E.K.; Shin, J.; Seo, Y.; Flavell, R.R. Dosimetry in radionuclide therapy: The clinical role of measuring radiation dose. Lancet Oncol. 2022, 23, e75–e87. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.N.; Visser, O.J.; Vosjan, M.J.; van Lingen, A.; Hoekstra, O.S.; Zijlstra, J.M.; Huijgens, P.C.; van Dongen, G.A.; Lubberink, M. Biodistribution, radiation dosimetry and scouting of 90 Y-ibritumomab tiuxetan therapy in patients with relapsed B-cell non-Hodgkin’s lymphoma using 89 Zr-ibritumomab tiuxetan and PET. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 512–520. [Google Scholar]
- Eberlein, U.; Cremonesi, M.; Lassmann, M. Individualized Dosimetry for Theranostics: Necessary, Nice to Have, or Counterproductive? J. Nucl. Med. 2017, 58, 97s–103s. [Google Scholar] [CrossRef]
- Zaidi, H.; Alavi, A.; El Naqa, I. Novel quantitative PET techniques for clinical decision support in oncology. Semin. Nucl. Med. 2018, 48, 548–564. [Google Scholar]
- Dimitrakopoulou-Strauss, A.; Pan, L.; Sachpekidis, C. Long axial field of view (LAFOV) PET-CT: Implementation in static and dynamic oncological studies. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 3354–3362. [Google Scholar] [CrossRef]
- Nelson, A.L.; Reichert, J.M. Development trends for therapeutic antibody fragments. Nat. Biotechnol. 2009, 27, 331–337. [Google Scholar]
- Massa, S.; Xavier, C.; Muyldermans, S.; Devoogdt, N. Emerging site-specific bioconjugation strategies for radioimmunotracer development. Expert. Opin. Drug Deliv. 2016, 13, 1149–1163. [Google Scholar]
- Herrero Álvarez, N.; Bauer, D.; Hernández-Gil, J.; Lewis, J.S. Recent advances in radiometals for combined imaging and therapy in cancer. ChemMedChem 2021, 16, 2909–2941. [Google Scholar]
- Lapi, S.E.; Scott, P.J.H.; Scott, A.M.; Windhorst, A.D.; Zeglis, B.M.; Abdel-Wahab, M.; Baum, R.P.; Buatti, J.M.; Giammarile, F.; Kiess, A.P.; et al. Recent advances and impending challenges for the radiopharmaceutical sciences in oncology. Lancet Oncol. 2024, 25, e236–e249. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.M.; Wolchok, J.D.; Old, L.J. Antibody therapy of cancer. Nat. Rev. Cancer 2012, 12, 278–287. [Google Scholar] [PubMed]
- Valkenburg, K.C.; De Groot, A.E.; Pienta, K.J. Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 2018, 15, 366–381. [Google Scholar] [PubMed]

| Common Use | Radioactive Isotopes | Half-Life | Emission | |
|---|---|---|---|---|
| ImmunoPET | 89Zr | Zirconium-89 | 3.3 days | β+ |
| 64Cu | Copper-64 | 12.7 h | β | |
| 68Ga | Gallium-68 | 68 min | β+ | |
| 124I | Iodine-124 | 4.2 days | β+ | |
| 86Y | Yttrium-86 | 14.7 h | β+ | |
| 18F | Fluorine-18 | 110 min | β+ | |
| Radioimmunotherapy | 131I | Iodine-131 | 8 days | β and ɣ |
| 90Y | Yttrium-90 | 2.7 days | β | |
| 177Lu | Lutetium-177 | 6.7 days | β and ɣ | |
| 188Re | Rhenium-188 | 16.9 h | β and ɣ | |
| 211At | Astatine-211 | 7.2 h | α | |
| 225Ac | Actinium-225 | 10 days | α | |
| 213Bi | Bismuth-213 | 45 min | α | |
| Target Antigen | Tracer Name | Patient Population | Trial Phase | |
|---|---|---|---|---|
| Carbonic anhydrase IX (CAIX) | 177Lu-girentuximab mAb (Girentuximab) | Clear Cell Renal Cell Carcinoma | phase II | |
| 131I-cG250 mAb | Kidney cancer | phase II | ||
| Carcinoembryonic antigen (CEA) | 131I-CIGB-M3 scFv | CEA+ colorectal cancer | phase I | |
| 131I-A5B7 mAb | Gastrointestinal carcinoma | phase I | ||
| 131I-labetuzumab mAb | Chronic myelogenous leukemia | phase II | ||
| Clusters of differentiation (CD) | CD20 | 90Y-Rituximab mAb | B-cell Non-Hodgkin lymphoma | phase I |
| 90Y-ibritumomab tiuxetan mAb | CD20+ Non-Hodgkin lymphoma and B-cell lymphoma | phase II/phase III | ||
| CD22 | 227Th-labeled CD22-targeting antibody mAb | CD22+ Non-Hodgkin lymphoma | phase I | |
| 111In/90Y-epratuzumab mAb | Non-Hodgkin lymphoma | phase II | ||
| CD33 | 225Ac-lintuzumab mAb (Lintuzumab) | Acute myeloid leukemia | phase I | |
| CD37 | 177Lu-lilotomab satetraxetan mAb (Lilotomab) | Non-Hodgkin lymphoma | phase II | |
| CD44 | 186Re-bivatuzumab mAb | Head and neck squamous cell carcinoma | phase I | |
| CD45 | 131I-BC8 mAb (Apamistamab) | Acute myeloid leukemia | phase I | |
| Epidermal growth factor receptor (EGFR) family | HER2 | 212Pb-trastuzumab mAb | Peritoneal carcinomatosis | phase I |
| 89Zr/177Lu-trastuzumab mAb | Breast cancer | phase I | ||
| Fibroblast activation protein (FAP) | 131I-sibrotuzumab mAb | 131I-sibrotuzumab mAb | phase I | |
| 131I-mAbF19 mAb | 131I-mAbF19 mAb | phase I | ||
| Prostate-specific membrane antigen (PSMA) | 111In/177Lu-J591 mAb (Rosopatamab) | 111In/177Lu-J591 mAb | phase II | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garaulet, G.; Báez, B.B.; Medrano, G.; Rivas-Sánchez, M.; Sánchez-Alonso, D.; Martinez-Torrecuadrada, J.L.; Mulero, F. Radioimmunotheragnosis in Cancer Research. Cancers 2024, 16, 2896. https://doi.org/10.3390/cancers16162896
Garaulet G, Báez BB, Medrano G, Rivas-Sánchez M, Sánchez-Alonso D, Martinez-Torrecuadrada JL, Mulero F. Radioimmunotheragnosis in Cancer Research. Cancers. 2024; 16(16):2896. https://doi.org/10.3390/cancers16162896
Chicago/Turabian StyleGaraulet, Guillermo, Bárbara Beatriz Báez, Guillermo Medrano, María Rivas-Sánchez, David Sánchez-Alonso, Jorge L. Martinez-Torrecuadrada, and Francisca Mulero. 2024. "Radioimmunotheragnosis in Cancer Research" Cancers 16, no. 16: 2896. https://doi.org/10.3390/cancers16162896
APA StyleGaraulet, G., Báez, B. B., Medrano, G., Rivas-Sánchez, M., Sánchez-Alonso, D., Martinez-Torrecuadrada, J. L., & Mulero, F. (2024). Radioimmunotheragnosis in Cancer Research. Cancers, 16(16), 2896. https://doi.org/10.3390/cancers16162896







