Comparative Analysis of Repeatability in CT Radiomics and Dosiomics Features under Image Perturbation: A Study in Cervical Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Dataset
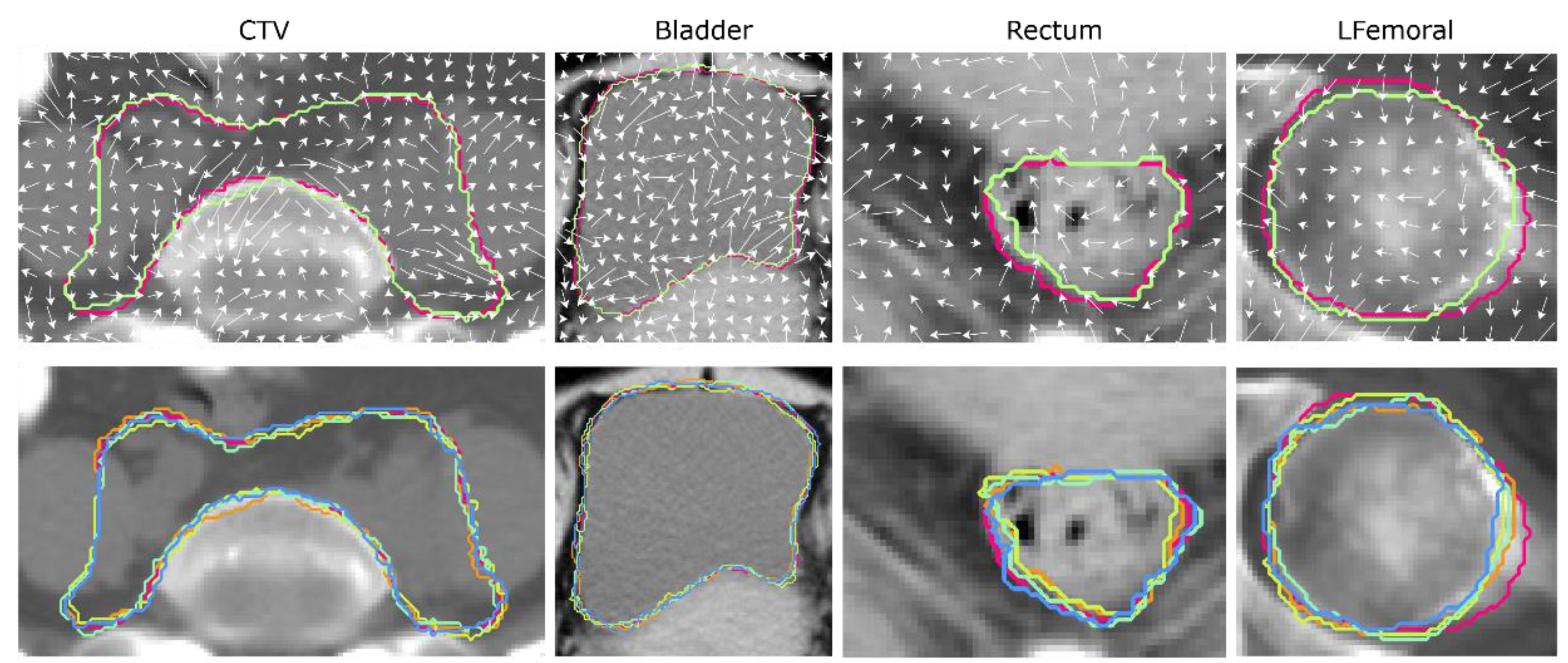
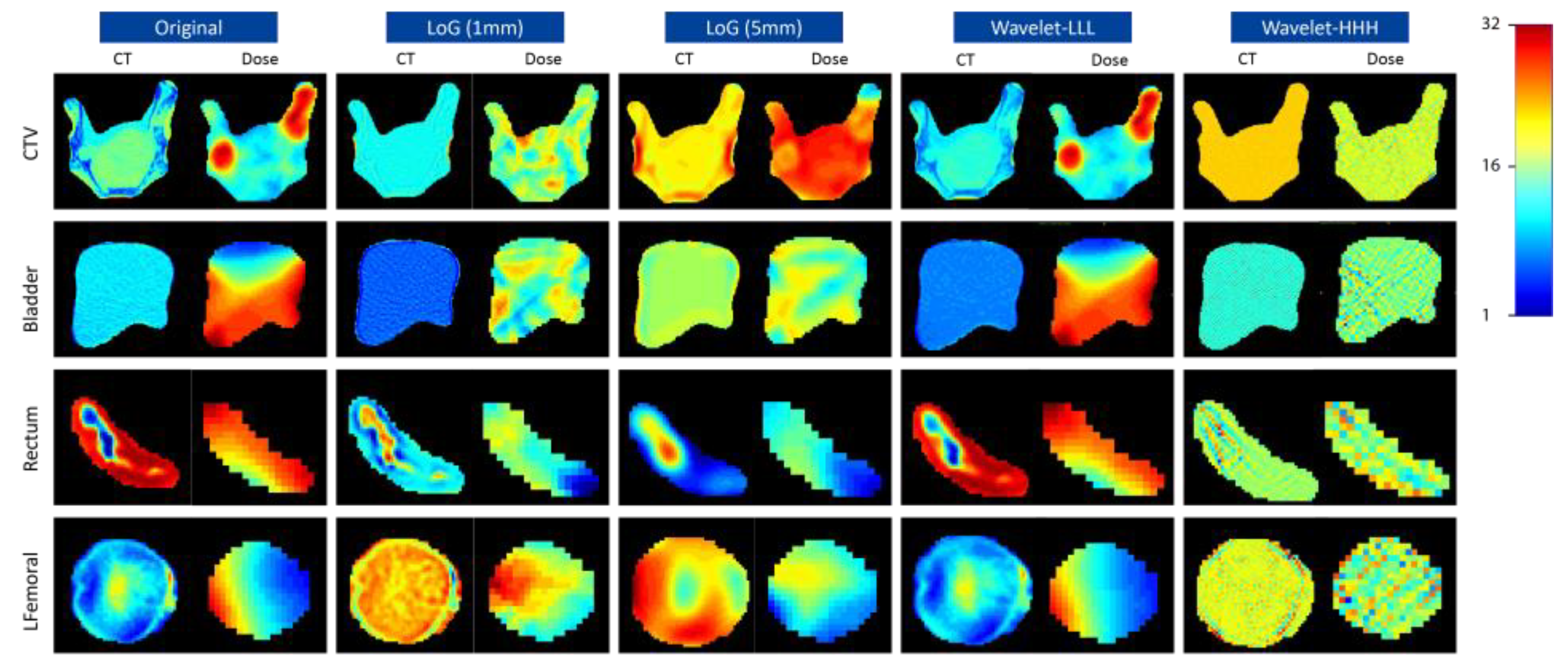
2.2. Image Perturbations
2.3. Radiomics Feature Extraction
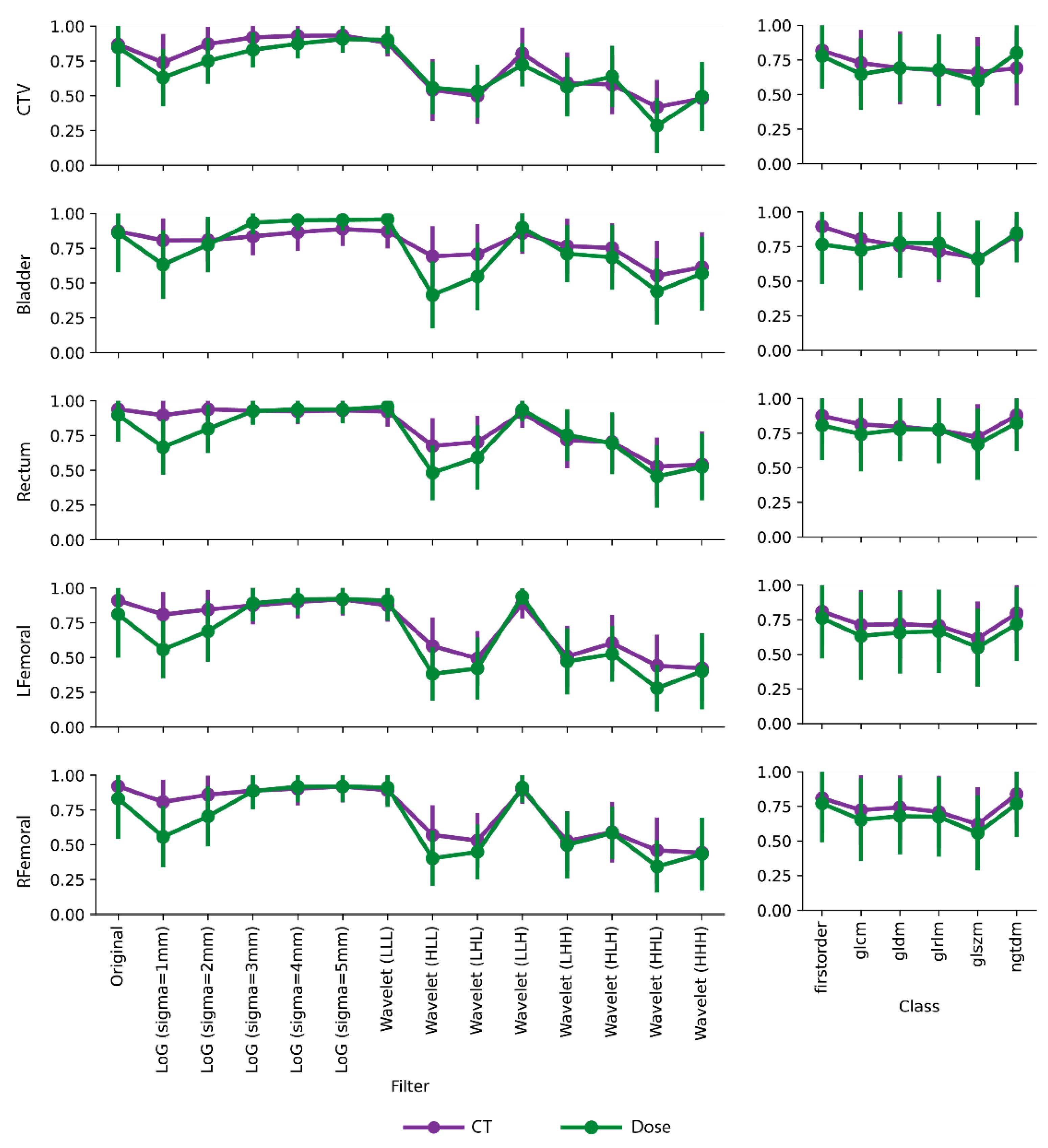
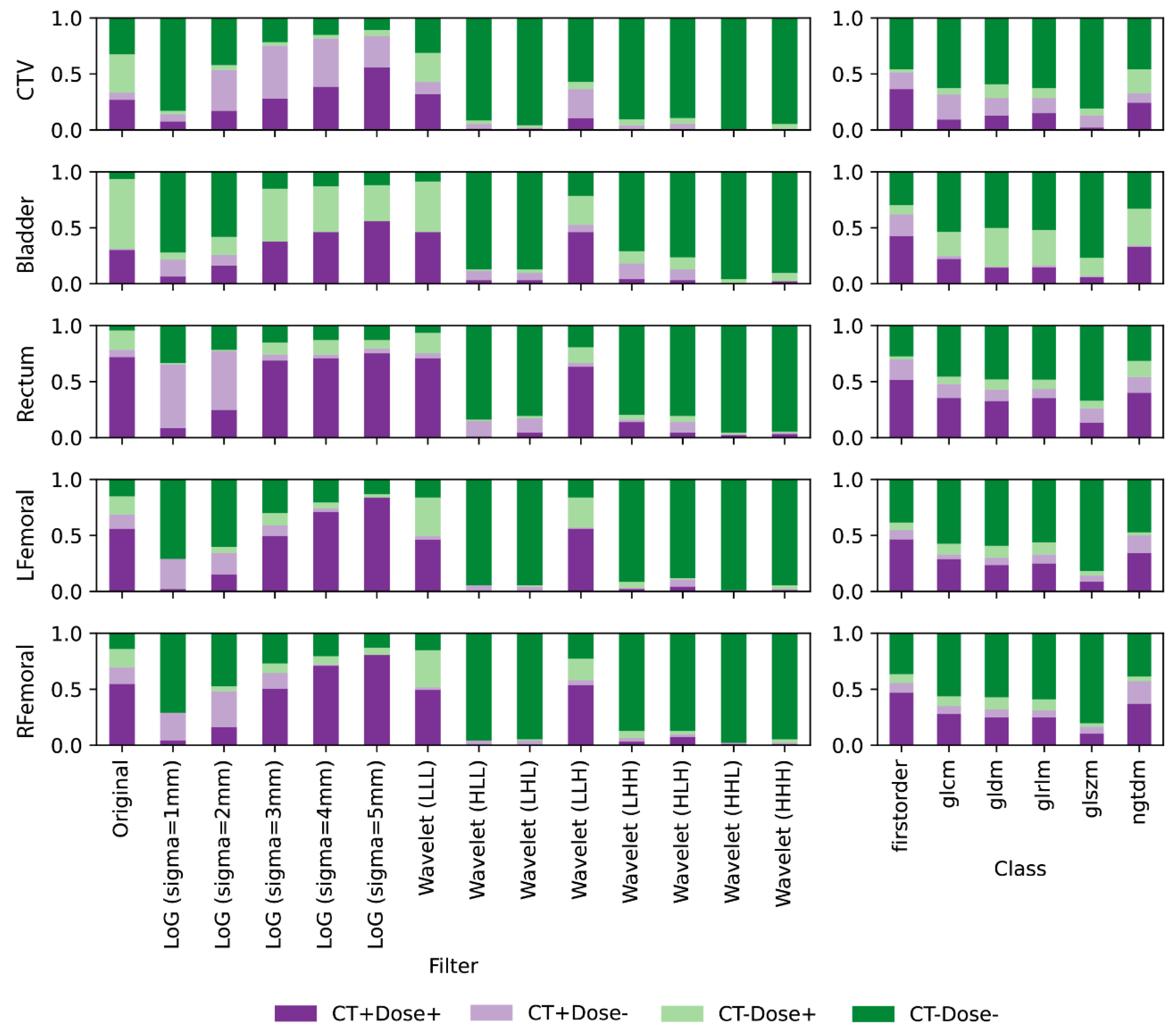
2.4. Feature Repeatability Analysis
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burmeister, C.A.; Khan, S.F.; Schafer, G.; Mbatani, N.; Adams, T.; Moodley, J.; Prince, S. Cervical cancer therapies: Current challenges and future perspectives. Tumour Virus Res. 2022, 13, 200238. [Google Scholar] [CrossRef]
- Scalco, E.; Rizzo, G. Texture analysis of medical images for radiotherapy applications. Br. J. Radiol. 2017, 90, 20160642. [Google Scholar] [CrossRef]
- Meng, J.; Liu, S.L.; Zhu, L.J.; Zhu, L.; Wang, H.H.; Xie, L.; Guan, Y.; He, J.; Yang, X.F.; Zhou, Z.Y. Texture Analysis as Imaging Biomarker for recurrence in advanced cervical cancer treated with CCRT. Sci. Rep. 2018, 8, 11399. [Google Scholar] [CrossRef]
- Pedraza, S.; Seiffert, A.P.; Sarandeses, P.; Muñoz-Lopez, B.; Gómez, E.J.; Sánchez-González, P.; Pérez-Regadera, J.F. The value of metabolic parameters and textural analysis in predicting prognosis in locally advanced cervical cancer treated with chemoradiotherapy. Strahlenther. Onkol. 2022, 198, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Yamada, I.; Oshima, N.; Miyasaka, N.; Wakana, K.; Wakabayashi, A.; Sakamoto, J.; Saida, Y.; Tateishi, U.; Kobayashi, D. Texture Analysis of Apparent Diffusion Coefficient Maps in Cervical Carcinoma: Correlation with Histopathologic Findings and Prognosis. Radiol. Imaging Cancer 2020, 2, e190085. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.P.M.; Granton, P.; Zegers, C.M.L.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef]
- Van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H.J.W.L. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, E104–E107. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Zhang, X.Y.; Cheng, Y.T.; Li, B.; Teng, X.Z.; Zhang, J.; Lam, S.; Zhou, T.; Ma, Z.R.; Sheng, J.B.; et al. Artificial intelligence-driven radiomics study in cancer: The role of feature engineering and modeling. Mil. Med. Res. 2023, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Horvat, N.; Papanikolaou, N.; Koh, D.M. Radiomics Beyond the Hype: A Critical Evaluation Toward Oncologic Clinical Use. Radiol. Artif. Intell. 2024, 6, e230437. [Google Scholar] [CrossRef]
- Jiang, T.; Lau, S.H.; Zhang, J.; Chan, L.C.; Wang, W.; Chan, P.K.; Cai, J.; Wen, C. Radiomics signature of osteoarthritis: Current status and perspective. J. Orthop. Translat. 2024, 45, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Chiappa, V.; Bogani, G.; Interlenghi, M.; Salvatore, C.; Bertolina, F.; Sarpietro, G.; Signorelli, M.; Castiglioni, I.; Raspagliesi, F. The Adoption of Radiomics and machine learning improves the diagnostic processes of women with Ovarian MAsses (the AROMA pilot study). J. Ultrasound 2021, 24, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Chiappa, V.; Interlenghi, M.; Salvatore, C.; Bertolina, F.; Bogani, G.; Ditto, A.; Martinelli, F.; Castiglioni, I.; Raspagliesi, F. Using rADioMIcs and machine learning with ultrasonography for the differential diagnosis of myometRiAL tumors (the ADMIRAL pilot study). Radiomics and differential diagnosis of myometrial tumors. Gynecol. Oncol. 2021, 161, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Chiappa, V.; Bogani, G.; Interlenghi, M.; Vittori Antisari, G.; Salvatore, C.; Zanchi, L.; Ludovisi, M.; Leone Roberti Maggiore, U.; Calareso, G.; Haeusler, E.; et al. Using Radiomics and Machine Learning Applied to MRI to Predict Response to Neoadjuvant Chemotherapy in Locally Advanced Cervical Cancer. Diagnostics 2023, 13, 3139. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Yan, H.; Tian, Y.; Chen, X.Y.; Yan, L.L.; Zhang, T.; Zhou, Z.M.; Wang, L.H.; Dai, J.R. Dosiomics: Extracting 3D Spatial Features From Dose Distribution to Predict Incidence of Radiation Pneumonitis. Front. Oncol. 2019, 9, 269. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Han, P.J.; Hales, R.K.; Voong, K.R.; Noro, K.; Sugiyama, S.; Haller, J.W.; McNutt, T.R.; Lee, J. Multi-view radiomics and dosiomics analysis with machine learning for predicting acute-phase weight loss in lung cancer patients treated with radiotherapy. Phys. Med. Biol. 2020, 65, 195015. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.Q.; Li, Y.B.; Qi, M.K.; Lu, X.Y.; Jia, Q.Y.; Guo, F.T.; Dai, Z.H.; Liu, Y.L.; Chen, C.M.; Zhou, L.H.; et al. Dosiomics improves prediction of locoregional recurrence for intensity modulated radiotherapy treated head and neck cancer cases. Oral Oncol. 2020, 104, 104625. [Google Scholar] [CrossRef] [PubMed]
- Eloyan, A.; Yue, M.S.; Khachatryan, D. Tumor heterogeneity estimation for radiomics in cancer. Stat. Med. 2020, 39, 4704–4723. [Google Scholar] [CrossRef]
- Kang, W.D.; Qiu, X.; Luo, Y.E.; Luo, J.W.; Liu, Y.; Xi, J.Q.; Li, X.; Yang, Z.Q. Application of radiomics-based multiomics combinations in the tumor microenvironment and cancer prognosis. J. Transl. Med. 2023, 21, 598. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, L.E.; Rundo, L.; Gill, A.B.; Hoare, M.; Serrao, E.M.; Sala, E. Robustness of radiomic features in CT images with different slice thickness, comparing liver tumour and muscle. Sci. Rep. 2021, 11, 8262. [Google Scholar] [CrossRef]
- Soleymani, Y.; Jahanshahi, A.R.; Pourfarshid, A.; Khezerloo, D. Reproducibility assessment of radiomics features in various ultrasound scan settings and different scanner vendors. J. Med. Imaging Radiat. 2022, 53, 664–671. [Google Scholar] [CrossRef]
- D’Antonoli, T.A.; Cavallo, A.U.; Vernuccio, F.; Stanzione, A.; Klontzas, M.E.; Cannella, R.; Ugga, L.; Baran, A.; Fanni, S.C.; Petrash, E.; et al. Reproducibility of radiomics quality score: An intra- and inter-rater reliability study. Eur. Radiol. 2024, 34, 2791–2804. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.Z.; Zhang, J.; Zwanenburg, A.; Sun, J.C.; Huang, Y.H.; Lam, S.; Zhang, Y.P.; Li, B.; Zhou, T.; Xiao, H.N.; et al. Building reliable radiomic models using image perturbation. Sci. Rep. 2022, 12, 10035. [Google Scholar] [CrossRef] [PubMed]
- Ubaldi, L.; Saponaro, S.; Giuliano, A.; Talamonti, C.; Retico, A. Deriving quantitative information from multiparametric MRI via Radiomics: Evaluation of the robustness and predictive value of radiomic features in the discrimination of low-grade versus high-grade gliomas with machine learning. Phys. Medica 2023, 107, 102538. [Google Scholar] [CrossRef] [PubMed]
- Jahanshahi, A.; Soleymani, Y.; Ghaziani, M.F.; Khezerloo, D. Radiomics reproducibility challenge in computed tomography imaging as a nuisance to clinical generalization: A mini-review. Egypt. J. Radiol. Nucl. Med. 2023, 54, 83. [Google Scholar] [CrossRef]
- Ibrahim, A.; Primakov, S.; Beuque, M.; Woodruff, H.C.; Halilaj, I.; Wu, G.; Refaee, T.; Granzier, R.; Widaatalla, Y.; Hustinx, R.; et al. Radiomics for precision medicine: Current challenges, future prospects, and the proposal of a new framework. Methods 2021, 188, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lam, S.K.; Teng, X.Z.; Ma, Z.R.; Han, X.Y.; Zhang, Y.P.; Cheung, A.L.Y.; Chau, T.C.; Ng, S.C.Y.; Lee, F.K.H.; et al. Radiomic feature repeatability and its impact on prognostic model generalizability: A multi-institutional study on nasopharyngeal carcinoma patients. Radiother. Oncol. 2023, 183, 109578. [Google Scholar] [CrossRef] [PubMed]
- Small, W.; Bosch, W.R.; Harkenrider, M.M.; Strauss, J.B.; Abu-Rustum, N.; Albuquerque, K.V.; Beriwal, S.; Creutzberg, C.L.; Eifel, P.J.; Erickson, B.A.; et al. NRG Oncology/RTOG Consensus Guidelines for Delineation of Clinical Target Volume for Intensity Modulated Pelvic Radiation Therapy in Postoperative Treatment of Endometrial and Cervical Cancer: An Update. Int. J. Radiat. Oncol. 2021, 109, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Simard, P.Y.; Steinkraus, D.; Platt, J.C. Best practices for convolutional neural networks applied to visual document analysis. In Proceedings of the Seventh International Conference on Document Analysis and Recognition, Edinburgh, UK, 6 August 2003; pp. 958–962. [Google Scholar]
- Whybra, P.; Zwanenburg, A.; Andrearczyk, V.; Schaer, R.; Apte, A.P.; Ayotte, A.; Baheti, B.; Bakas, S.; Bettinelli, A.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Convolutional Filters for Reproducible Radiomics and Enhanced Clinical Insights. Radiology 2024, 310, e231319. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Zhang, J.; Liu, X.; Teng, X.; Huang, Y.-H.; Zhang, X.; Li, J.; Pan, Y.; Sun, J.; Dong, Y.; et al. Comparative Analysis of Repeatability in CT Radiomics and Dosiomics Features under Image Perturbation: A Study in Cervical Cancer Patients. Cancers 2024, 16, 2872. https://doi.org/10.3390/cancers16162872
Ma Z, Zhang J, Liu X, Teng X, Huang Y-H, Zhang X, Li J, Pan Y, Sun J, Dong Y, et al. Comparative Analysis of Repeatability in CT Radiomics and Dosiomics Features under Image Perturbation: A Study in Cervical Cancer Patients. Cancers. 2024; 16(16):2872. https://doi.org/10.3390/cancers16162872
Chicago/Turabian StyleMa, Zongrui, Jiang Zhang, Xi Liu, Xinzhi Teng, Yu-Hua Huang, Xile Zhang, Jun Li, Yuxi Pan, Jiachen Sun, Yanjing Dong, and et al. 2024. "Comparative Analysis of Repeatability in CT Radiomics and Dosiomics Features under Image Perturbation: A Study in Cervical Cancer Patients" Cancers 16, no. 16: 2872. https://doi.org/10.3390/cancers16162872
APA StyleMa, Z., Zhang, J., Liu, X., Teng, X., Huang, Y.-H., Zhang, X., Li, J., Pan, Y., Sun, J., Dong, Y., Li, T., Chan, L. W. C., Chang, A. T. Y., Siu, S. W. K., Cheung, A. L.-Y., Yang, R., & Cai, J. (2024). Comparative Analysis of Repeatability in CT Radiomics and Dosiomics Features under Image Perturbation: A Study in Cervical Cancer Patients. Cancers, 16(16), 2872. https://doi.org/10.3390/cancers16162872





