Lactate Oxidase Disrupts Lactate-Activated RAS and PI3K Oncogenic Signaling
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Measuring Lactate Levels in Breast Epithelial Cell Organoid Culture Medium
2.3. Vector
2.4. RFP-LOX Treatment of Cancer Cell Tumoroids or HMEpiC Organoids
2.5. Migration Assay
2.6. Western Blot
2.7. Protein Immunoprecipitation (IP)
2.8. Real-Time PCR
2.9. SiRNA Knockdown
2.10. Statistics and Analysis
3. Results
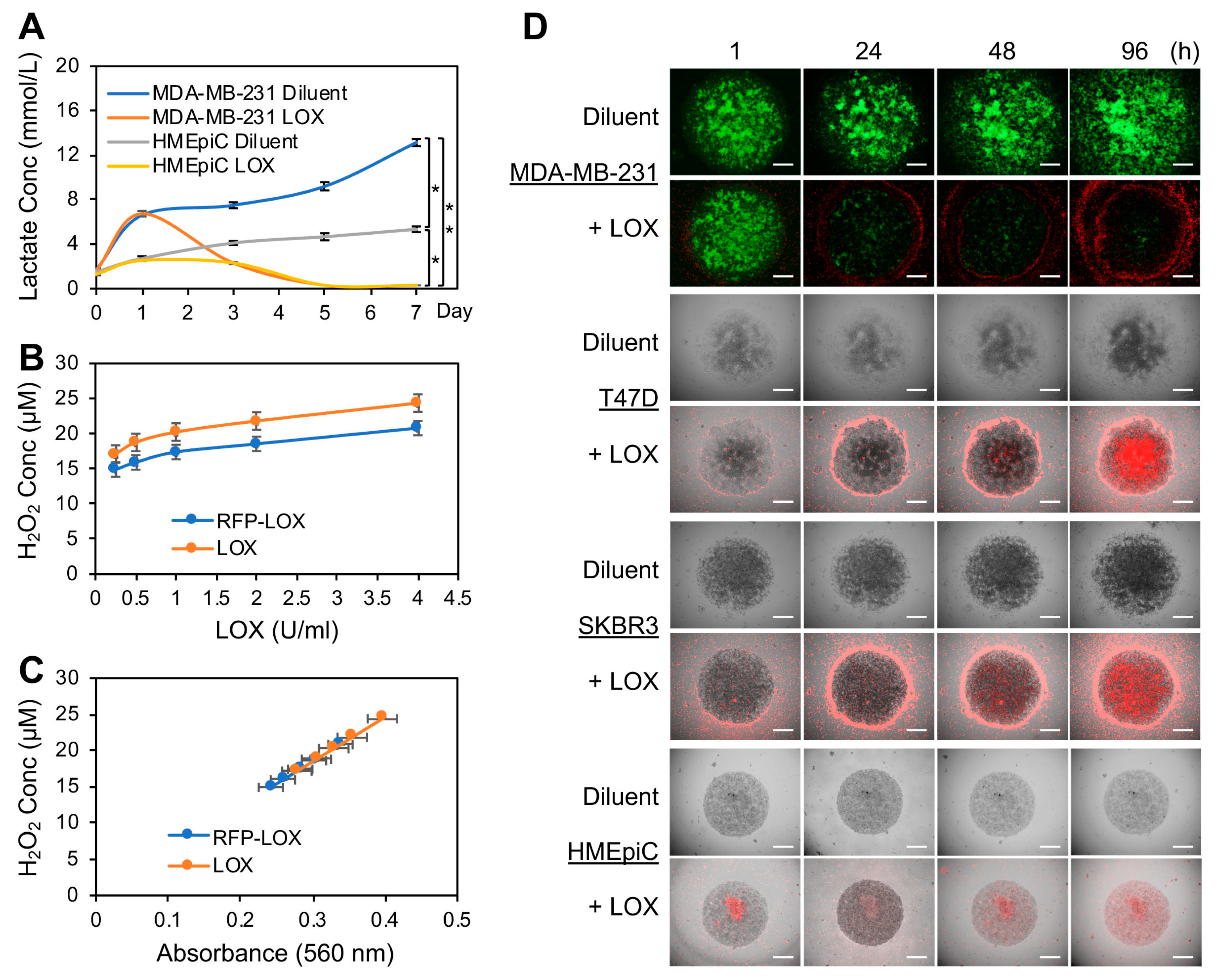
3.1. LOX Depleted the Lactate Produced by BCa Cell Tumoroids Grown in Human Breast ECM 3D Cultures and Inhibited the Tumoroid Growth
3.2. LOX Treatment Impaired Cancer Cell Migration on Native Breast ECM
3.3. Lactate Stimulated HCAR1 Association with the Mediators of the RAS and PI3K Signaling Pathways
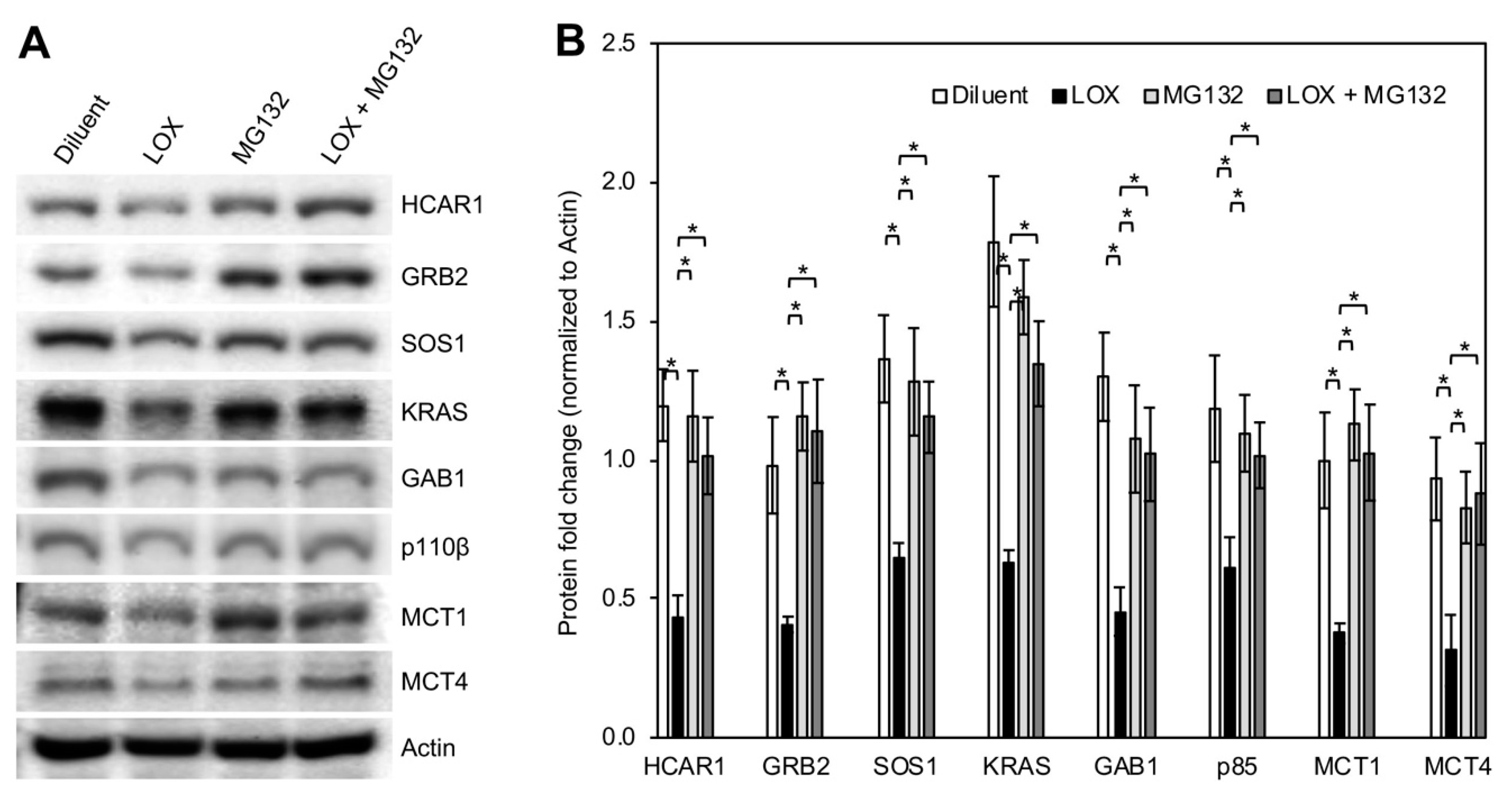
3.4. LOX Treatment Abrogated Lactate-Stimulated HCAR1 and Its Associated Protein Expression as Well as the RAS and the PI3K Signaling
3.5. LOX Treatment Induced Proteasomal Degradation of BCa Cell HCAR1 and Its Associated Proteins as Well as the MCTs
3.6. Downregulation of HCAR1 and Its-Associated Proteins Key for RAS and PI3K Signaling Interrupted Lactate-Stimulated Activation of the Two Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Payen, V.L.; Mina, E.; Van Hee, V.F.; Porporato, P.E.; Sonveaux, P. Monocarboxylate transporters in cancer. Mol. Metab. 2020, 33, 48–66. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.S.; Rogatzki, M.J.; Goodwin, M.L.; Kane, D.A.; Rightmire, Z.; Gladden, L.B. Lactate metabolism: Historical context, prior misinterpretations, and current understanding. Eur. J. Appl. Physiol. 2018, 118, 691–728. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.R.; Ahumada, F.; Garay, F.; Baruzzi, A.M. Amperometric biosensor for direct blood lactate detection. Anal. Chem. 2010, 82, 5568–5572. [Google Scholar] [CrossRef]
- Brizel, D.M.; Schroeder, T.; Scher, R.L.; Walenta, S.; Clough, R.W.; Dewhirst, M.W.; Mueller-Klieser, W. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 349–353. [Google Scholar] [CrossRef]
- Cheung, S.M.; Husain, E.; Masannat, Y.; Miller, I.D.; Wahle, K.; Heys, S.D.; He, J. Lactate concentration in breast cancer using advanced magnetic resonance spectroscopy. Br. J. Cancer 2020, 123, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, K.M.; Scarbrough, P.M.; Ribeiro, A.; Richardson, R.; Yuan, H.; Sonveaux, P.; Landon, C.D.; Chi, J.T.; Pizzo, S.; Schroeder, T.; et al. Catabolism of exogenous lactate reveals it as a legitimate metabolic substrate in breast cancer. PLoS ONE 2013, 8, e75154. [Google Scholar] [CrossRef]
- San-Millan, I.; Brooks, G.A. Reexamining cancer metabolism: Lactate production for carcinogenesis could be the purpose and explanation of the warburg effect. Carcinogenesis 2017, 38, 119–133. [Google Scholar] [CrossRef]
- Brooks, G.A. The science and translation of lactate shuttle theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef]
- Goodwin, M.L.; Gladden, L.B.; Nijsten, M.W.; Jones, K.B. Lactate and cancer: Revisiting the warburg effect in an era of lactate shuttling. Front. Nutr. 2014, 1, 27. [Google Scholar] [CrossRef]
- Caslin, H.L.; Abebayehu, D.; Pinette, J.A.; Ryan, J.J. Lactate is a metabolic mediator that shapes immune cell fate and function. Front. Physiol. 2021, 12, 688485. [Google Scholar] [CrossRef]
- Walenta, S.; Wetterling, M.; Lehrke, M.; Schwickert, G.; Sundfor, K.; Rofstad, E.K.; Mueller-Klieser, W. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 2000, 60, 916–921. [Google Scholar] [PubMed]
- Hayes, C.; Donohoe, C.L.; Davern, M.; Donlon, N.E. The oncogenic and clinical implications of lactate induced immunosuppression in the tumour microenvironment. Cancer Lett. 2021, 500, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Pennington, Z.; Goodwin, M.L.; Westbroek, E.M.; Cottrill, E.; Ahmed, A.K.; Sciubba, D.M. Lactate and cancer: Spinal metastases and potential therapeutic targets (part 2). Ann. Transl. Med. 2019, 7, 221. [Google Scholar] [CrossRef] [PubMed]
- Doherty, J.R.; Cleveland, J.L. Targeting lactate metabolism for cancer therapeutics. J. Clin. Investig. 2013, 123, 3685–3692. [Google Scholar] [CrossRef] [PubMed]
- Pavlides, S.; Whitaker-Menezes, D.; Castello-Cros, R.; Flomenberg, N.; Witkiewicz, A.K.; Frank, P.G.; Casimiro, M.C.; Wang, C.; Fortina, P.; Addya, S.; et al. The reverse warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 2009, 8, 3984–4001. [Google Scholar] [CrossRef] [PubMed]
- Perez-Tomas, R.; Perez-Guillen, I. Lactate in the tumor microenvironment: An essential molecule in cancer progression and treatment. Cancers 2020, 12, 3244. [Google Scholar] [CrossRef]
- Feng, Y.; Xiong, Y.; Qiao, T.; Li, X.; Jia, L.; Han, Y. Lactate dehydrogenase a: A key player in carcinogenesis and potential target in cancer therapy. Cancer Med. 2018, 7, 6124–6136. [Google Scholar] [CrossRef] [PubMed]
- Granchi, C.; Paterni, I.; Rani, R.; Minutolo, F. Small-molecule inhibitors of human ldh5. Future Med. Chem. 2013, 5, 1967–1991. [Google Scholar] [CrossRef] [PubMed]
- Valvona, C.J.; Fillmore, H.L.; Nunn, P.B.; Pilkington, G.J. The regulation and function of lactate dehydrogenase a: Therapeutic potential in brain tumor. Brain Pathol. 2016, 26, 3–17. [Google Scholar] [CrossRef]
- de la Cruz-Lopez, K.G.; Castro-Munoz, L.J.; Reyes-Hernandez, D.O.; Garcia-Carranca, A.; Manzo-Merino, J. Lactate in the regulation of tumor microenvironment and therapeutic approaches. Front. Oncol. 2019, 9, 1143. [Google Scholar] [CrossRef]
- Lee, Y.J.; Shin, K.J.; Park, S.A.; Park, K.S.; Park, S.; Heo, K.; Seo, Y.K.; Noh, D.Y.; Ryu, S.H.; Suh, P.G. G-protein-coupled receptor 81 promotes a malignant phenotype in breast cancer through angiogenic factor secretion. Oncotarget 2016, 7, 70898–70911. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.P.; Bhattacharjee, P.; Ramachandran, S.; Sivaprakasam, S.; Ristic, B.; Sikder, M.O.F.; Ganapathy, V. The lactate receptor gpr81 promotes breast cancer growth via a paracrine mechanism involving antigen-presenting cells in the tumor microenvironment. Oncogene 2020, 39, 3292–3304. [Google Scholar] [CrossRef] [PubMed]
- Roland, C.L.; Arumugam, T.; Deng, D.; Liu, S.H.; Philip, B.; Gomez, S.; Burns, W.R.; Ramachandran, V.; Wang, H.; Cruz-Monserrate, Z.; et al. Cell surface lactate receptor gpr81 is crucial for cancer cell survival. Cancer Res. 2014, 74, 5301–5310. [Google Scholar] [CrossRef] [PubMed]
- de Castro Abrantes, H.; Briquet, M.; Schmuziger, C.; Restivo, L.; Puyal, J.; Rosenberg, N.; Rocher, A.B.; Offermanns, S.; Chatton, J.Y. The lactate receptor hcar1 modulates neuronal network activity through the activation of g(alpha) and g(betagamma) subunits. J. Neurosci. 2019, 39, 4422–4433. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Tunaru, S.; Tang, C.; Muller, M.; Gille, A.; Sassmann, A.; Hanson, J.; Offermanns, S. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through gpr81. Cell Metab. 2010, 11, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Hoque, R.; Farooq, A.; Ghani, A.; Gorelick, F.; Mehal, W.Z. Lactate reduces liver and pancreatic injury in toll-like receptor- and inflammasome-mediated inflammation via gpr81-mediated suppression of innate immunity. Gastroenterology 2014, 146, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Harun-Or-Rashid, M.; Inman, D.M. Reduced ampk activation and increased hcar activation drive anti-inflammatory response and neuroprotection in glaucoma. J. Neuroinflamm. 2018, 15, 313. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K. Biological roles and therapeutic potential of hydroxy-carboxylic acid receptors. Front. Endocrinol. (Lausanne) 2011, 2, 51. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, W.; Wang, M.; Zhang, S.; Li, Y.; Hu, W.; Ren, L.; Luo, S.; Chen, Z. Dual-modal therapeutic role of the lactate oxidase-embedded hierarchical porous zeolitic imidazolate framework as a nanocatalyst for effective tumor suppression. ACS Appl. Mater. Interfaces 2020, 12, 32278–32288. [Google Scholar] [CrossRef]
- Ruud, K.F.; Hiscox, W.C.; Yu, I.; Chen, R.K.; Li, W. Distinct phenotypes of cancer cells on tissue matrix gel. Breast Cancer Res. BCR 2020, 22, 82. [Google Scholar] [CrossRef]
- Leiros, I.; Wang, E.; Rasmussen, T.; Oksanen, E.; Repo, H.; Petersen, S.B.; Heikinheimo, P.; Hough, E. The 2.1 a structure of aerococcus viridans l-lactate oxidase (lox). Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2006, 62, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Maeda-Yorita, K.; Aki, K.; Sagai, H.; Misaki, H.; Massey, V. L-lactate oxidase and l-lactate monooxygenase: Mechanistic variations on a common structural theme. Biochimie 1995, 77, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.X.; Fa, Y.C.; Kempson, I.M.; Tseng, S.J. Repolarization of m2 to m1 macrophages triggered by lactate oxidase released from methylcellulose hydrogel. Bioconjug. Chem. 2019, 30, 2697–2702. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, Y.; Liu, Y.; Zhang, T.; Zhao, Y.; Zang, J.; Yang, Y.; He, R.; Chong, G.; Ruan, S.; et al. Dual closed-loop of catalyzed lactate depletion and immune response to potentiate photothermal immunotherapy. ACS Appl. Mater. Interfaces 2022, 14, 23260–23276. [Google Scholar] [CrossRef] [PubMed]
- Patgiri, A.; Skinner, O.S.; Miyazaki, Y.; Schleifer, G.; Marutani, E.; Shah, H.; Sharma, R.; Goodman, R.P.; To, T.L.; Robert Bao, X.; et al. An engineered enzyme that targets circulating lactate to alleviate intracellular nadh:Nad(+) imbalance. Nat. Biotechnol. 2020, 38, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Yeo, M.; Kang, Y.; Kim, H.J.; Park, S.G.; Jang, E.; Park, S.H.; Kim, E.; Kang, S. Lactate oxidase/catalase-displaying nanoparticles efficiently consume lactate in the tumor microenvironment to effectively suppress tumor growth. J. Nanobiotechnol. 2023, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Shaner, N.C.; Lin, M.Z.; McKeown, M.R.; Steinbach, P.A.; Hazelwood, K.L.; Davidson, M.W.; Tsien, R.Y. Improving the photostability of bright monomeric orange and red fluorescent proteins. Nat. Methods 2008, 5, 545–551. [Google Scholar] [CrossRef]
- Li, W.; Laishram, R.S.; Ji, Z.; Barlow, C.A.; Tian, B.; Anderson, R.A. Star-pap control of bik expression and apoptosis is regulated by nuclear pipkialpha and pkcdelta signaling. Mol. Cell 2012, 45, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Kotoshiba, S.; Berthet, C.; Hilton, M.B.; Kaldis, P. Rb/cdk2/cdk4 triple mutant mice elicit an alternative mechanism for regulation of the g1/s transition. Proc. Natl. Acad. Sci. USA 2009, 106, 486–491. [Google Scholar] [CrossRef]
- Keller, C.R.; Hu, Y.; Ruud, K.F.; VanDeen, A.E.; Martinez, S.R.; Kahn, B.T.; Zhang, Z.; Chen, R.K.; Li, W. Human breast extracellular matrix microstructures and protein hydrogel 3d cultures of mammary epithelial cells. Cancers 2021, 13, 5857. [Google Scholar] [CrossRef]
- Rijal, G.; Li, W. A versatile 3d tissue matrix scaffold system for tumor modeling and drug screening. Sci. Adv. 2017, 3, e1700764. [Google Scholar] [CrossRef]
- Rijal, G.; Wang, J.; Yu, I.; Gang, D.R.; Chen, R.K.; Li, W. Porcine breast extracellular matrix hydrogel for spatial tissue culture. Int. J. Mol. Sci. 2018, 19, 2912. [Google Scholar] [CrossRef] [PubMed]
- Rijal, G.; Li, W. 3d scaffolds in breast cancer research. Biomaterials 2016, 81, 135–156. [Google Scholar] [CrossRef]
- Tang, J.; Meka, A.K.; Theivendran, S.; Wang, Y.; Yang, Y.; Song, H.; Fu, J.; Ban, W.; Gu, Z.; Lei, C.; et al. Openwork@dendritic mesoporous silica nanoparticles for lactate depletion and tumor microenvironment regulation. Angew. Chem. Int. Ed. Engl. 2020, 59, 22054–22062. [Google Scholar] [CrossRef]
- Goetze, K.; Walenta, S.; Ksiazkiewicz, M.; Kunz-Schughart, L.A.; Mueller-Klieser, W. Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int. J. Oncol. 2011, 39, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Downward, J. Targeting ras signalling pathways in cancer therapy. Nat. Rev. Cancer 2003, 3, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Pylayeva-Gupta, Y.; Grabocka, E.; Bar-Sagi, D. Ras oncogenes: Weaving a tumorigenic web. Nat. Rev. Cancer 2011, 11, 761–774. [Google Scholar] [CrossRef]
- Gimple, R.C.; Wang, X. Ras: Striking at the core of the oncogenic circuitry. Front. Oncol. 2019, 9, 965. [Google Scholar] [CrossRef]
- Belov, A.A.; Mohammadi, M. Grb2, a double-edged sword of receptor tyrosine kinase signaling. Sci. Signal. 2012, 5, pe49. [Google Scholar] [CrossRef]
- Lin, C.W.; Nocka, L.M.; Stinger, B.L.; DeGrandchamp, J.B.; Lew, L.J.N.; Alvarez, S.; Phan, H.T.; Kondo, Y.; Kuriyan, J.; Groves, J.T. A two-component protein condensate of the egfr cytoplasmic tail and grb2 regulates ras activation by sos at the membrane. Proc. Natl. Acad. Sci. USA 2022, 119, e2122531119. [Google Scholar] [CrossRef]
- McDonald, C.B.; Seldeen, K.L.; Deegan, B.J.; Bhat, V.; Farooq, A. Assembly of the sos1-grb2-gab1 ternary signaling complex is under allosteric control. Arch. Biochem. Biophys. 2010, 494, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Daub, H.; Wallasch, C.; Lankenau, A.; Herrlich, A.; Ullrich, A. Signal characteristics of g protein-transactivated egf receptor. EMBO J. 1997, 16, 7032–7044. [Google Scholar] [CrossRef] [PubMed]
- Fiaschi, T.; Marini, A.; Giannoni, E.; Taddei, M.L.; Gandellini, P.; De Donatis, A.; Lanciotti, M.; Serni, S.; Cirri, P.; Chiarugi, P. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res. 2012, 72, 5130–5140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Z.; Yu, L.; Peng, W.; Chen, Y.; Zhang, S. Ultrasound-enhanced cascade chemodynamic tumor nanotherapy with lactic acid-enabled hydrogen peroxide self-production. Biomater. Sci. 2023, 11, 1486–1498. [Google Scholar] [CrossRef]
- Morland, C.; Lauritzen, K.H.; Puchades, M.; Holm-Hansen, S.; Andersson, K.; Gjedde, A.; Attramadal, H.; Storm-Mathisen, J.; Bergersen, L.H. The lactate receptor, g-protein-coupled receptor 81/hydroxycarboxylic acid receptor 1: Expression and action in brain. J. Neurosci. Res. 2015, 93, 1045–1055. [Google Scholar] [CrossRef]
- Lockridge, O.; Massey, V.; Sullivan, P.A. Mechanism of action of the flavoenzyme lactate oxidase. J. Biol. Chem. 1972, 247, 8097–8106. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keller, C.R.; Martinez, S.R.; Keltz, A.; Chen, M.; Li, W. Lactate Oxidase Disrupts Lactate-Activated RAS and PI3K Oncogenic Signaling. Cancers 2024, 16, 2817. https://doi.org/10.3390/cancers16162817
Keller CR, Martinez SR, Keltz A, Chen M, Li W. Lactate Oxidase Disrupts Lactate-Activated RAS and PI3K Oncogenic Signaling. Cancers. 2024; 16(16):2817. https://doi.org/10.3390/cancers16162817
Chicago/Turabian StyleKeller, Chandler R., Steve R. Martinez, Alexys Keltz, Michelle Chen, and Weimin Li. 2024. "Lactate Oxidase Disrupts Lactate-Activated RAS and PI3K Oncogenic Signaling" Cancers 16, no. 16: 2817. https://doi.org/10.3390/cancers16162817
APA StyleKeller, C. R., Martinez, S. R., Keltz, A., Chen, M., & Li, W. (2024). Lactate Oxidase Disrupts Lactate-Activated RAS and PI3K Oncogenic Signaling. Cancers, 16(16), 2817. https://doi.org/10.3390/cancers16162817






