Phase I Clinical Evaluation of Designed Ankyrin Repeat Protein [99mTc]Tc(CO)3-(HE)3-Ec1 for Visualization of EpCAM-Expressing Lung Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Radiopharmaceutical
2.3. Evaluation of Safety and Tolerability
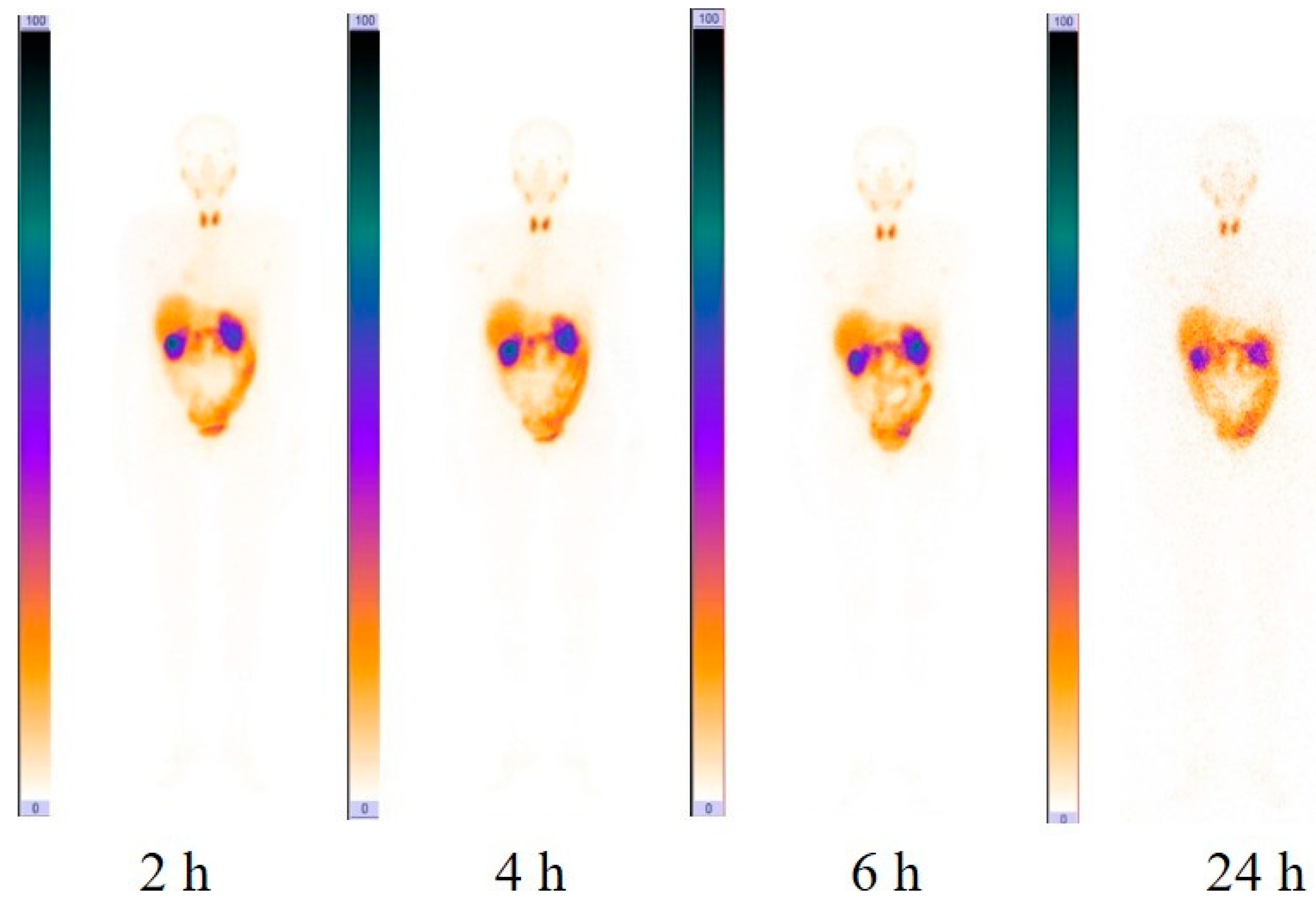
2.4. Imaging Protocol
2.5. Evaluation of Distribution and Dosimetry
2.6. Immunohistochemical Detection of EpCAM Expression
2.7. Statistics
3. Results
3.1. Safety and Tolerability
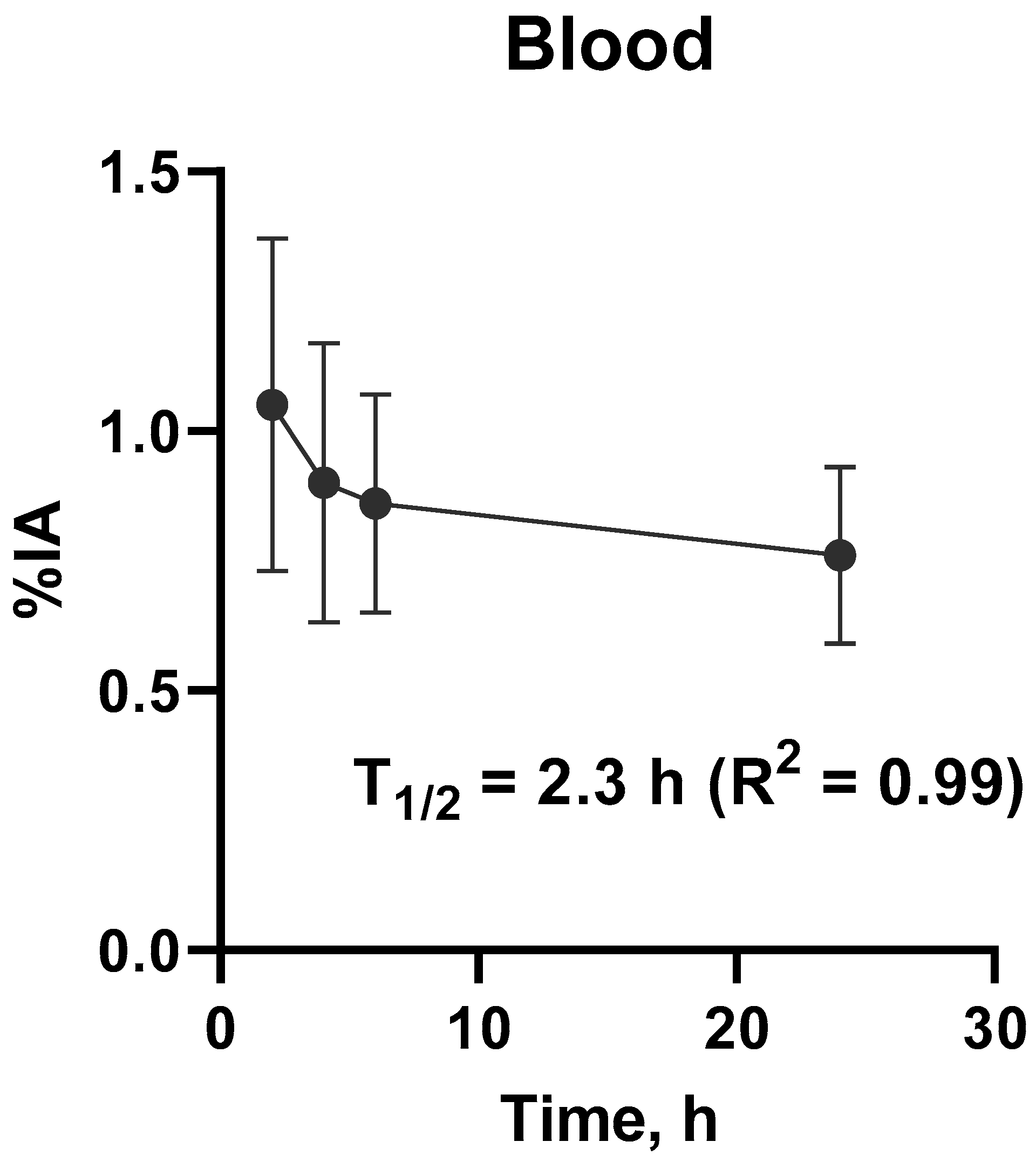
3.2. Evaluation of Distribution and Dosimetry
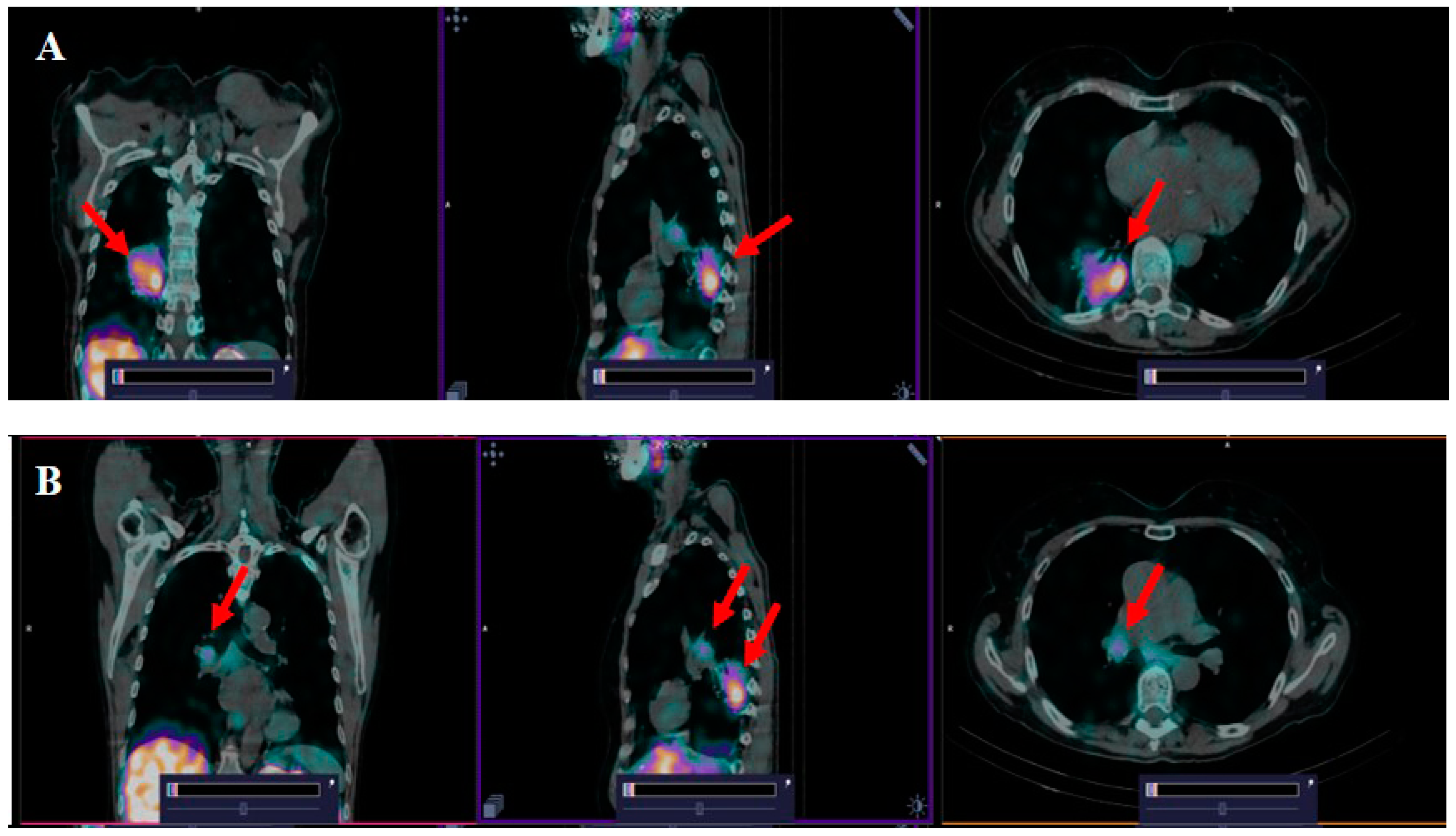
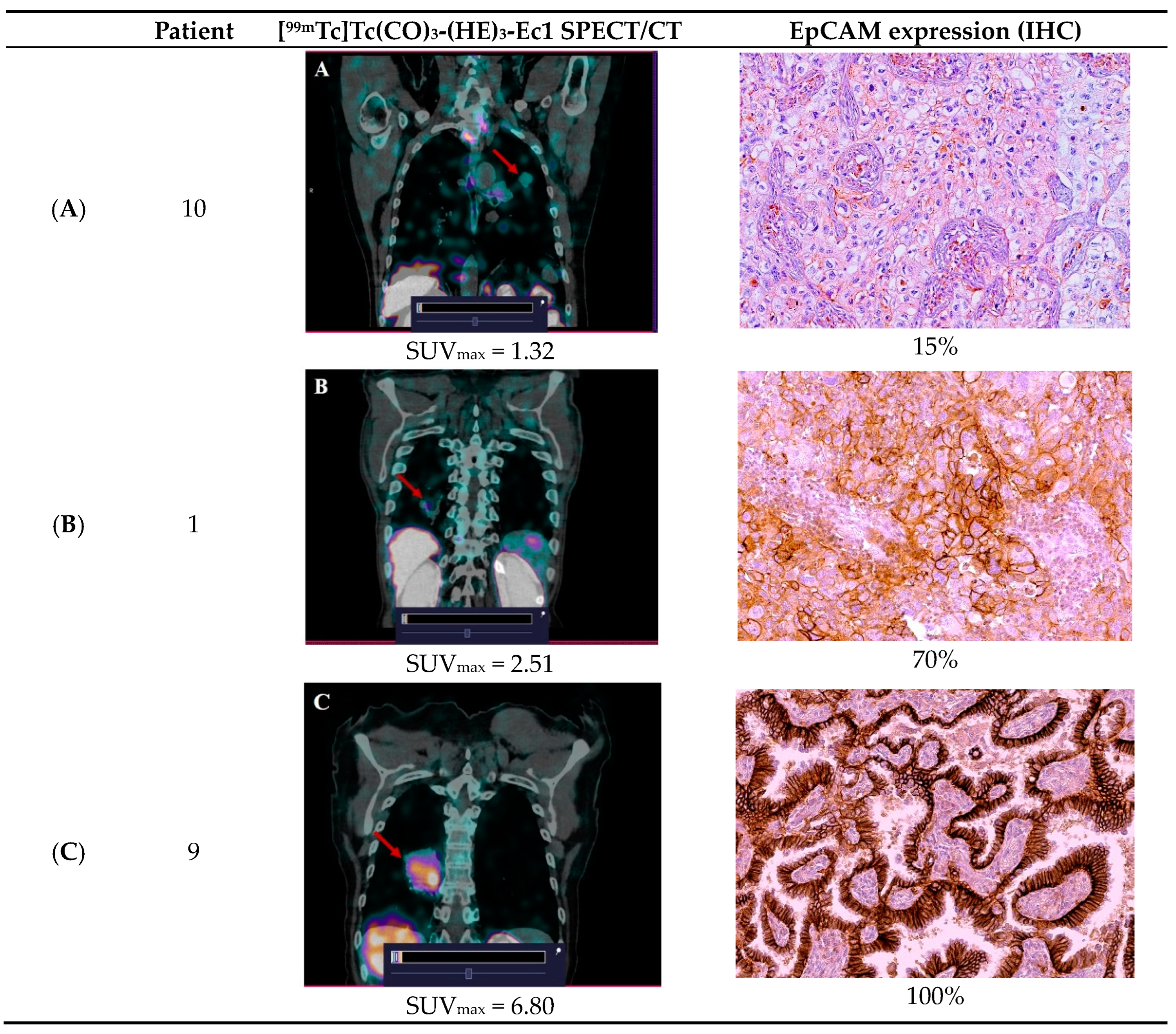
3.3. [99mTc]Tc(CO)3-(HE)3-Ec1 SPECT/CT Imaging of Primary Lung Tumors and Lymph Node Lesions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, G.S.; Baldwin, D.R. Recent advances in the management of lung cancer. Clin. Med. 2018, 18 (Suppl. S2), s41–s46. [Google Scholar] [CrossRef]
- Bade, B.C.; Dela Cruz, C.S. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef]
- Alduais, Y.; Zhang, H.; Fan, F.; Chen, J.; Chen, B. Non-small cell lung cancer (NSCLC): A review of risk factors, diagnosis, and treatment. Medicine 2023, 102, e32899. [Google Scholar] [CrossRef]
- Pignon, J.P.; Tribodet, H.; Scagliotti, G.V.; Douillard, J.Y.; Shepherd, F.A.; Stephens, R.J.; Dunant, A.; Torri, V.; Rosell, R.; Seymour, L.; et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 3552–3559. [Google Scholar] [CrossRef]
- Koprowski, H.; Steplewski, Z.; Mitchell, K.; Herlyn, M.; Herlyn, D.; Fuhrer, P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somat. Cell Genet. 1979, 5, 957–971. [Google Scholar] [CrossRef]
- Litvinov, S.V.; Balzar, M.; Winter, M.J.; Bakker, H.A.; Briaire-de Bruijn, I.H.; Prins, F.; Fleuren, G.J.; Warnaar, S.O. Epithelial cell adhesion molecule (Ep-CAM) modulates cell-cell interactions mediated by classic cadherins. J. Cell Biol. 1997, 139, 1337–1348. [Google Scholar] [CrossRef]
- Eyvazi, S.; Farajnia, S.; Dastmalchi, S.; Kanipour, F.; Zarredar, H.; Bandehpour, M. Antibody Based EpCAM Targeted Therapy of Cancer, Review and Update. Curr. Cancer Drug Targets 2018, 18, 857–868. [Google Scholar] [CrossRef]
- Gires, O.; Pan, M.; Schinke, H.; Canis, M.; Baeuerle, P.A. Expression and function of epithelial cell adhesion molecule EpCAM: Where are we after 40 years? Cancer Metastasis Rev. 2020, 39, 969–987. [Google Scholar] [CrossRef]
- Knödler, M.; Körfer, J.; Kunzmann, V.; Trojan, J.; Daum, S.; Schenk, M.; Kullmann, F.; Schroll, S.; Behringer, D.; Stahl, M.; et al. Randomised phase II trial to investigate catumaxomab (anti-EpCAM × anti-CD3) for treatment of peritoneal carcinomatosis in patients with gastric cancer. Br. J. Cancer 2018, 119, 296–302. [Google Scholar] [CrossRef]
- Ruf, P.; Kluge, M.; Jäger, M.; Burges, A.; Volovat, C.; Heiss, M.M.; Hess, J.; Wimberger, P.; Brandt, B.; Lindhofer, H. Pharmacokinetics, immunogenicity and bioactivity of the therapeutic antibody catumaxomab intraperitoneally administered to cancer patients. Br. J. Clin. Pharmacol. 2010, 69, 617–625. [Google Scholar] [CrossRef]
- Menz, A.; Lony, N.; Lennartz, M.; Dwertmann Rico, S.; Schlichter, R.; Kind, S.; Reiswich, V.; Viehweger, F.; Dum, D.; Luebke, A.M.; et al. Epithelial Cell Adhesion Molecule (EpCAM) Expression in Human Tumors: A Comparison with Pan-Cytokeratin and TROP2 in 14,832 Tumors. Diagnostics 2024, 14, 1044. [Google Scholar] [CrossRef]
- Tolmachev, V.; Bodenko, V.; Orlova, A.; Schulga, A.; Deyev, S.M.; Vorobyeva, A. Visualization of epithelial cell adhesion molecule-expressing renal cell carcinoma xenografts using designed ankyrin repeat protein Ec1 labelled with 99mTc and 125I. Oncol. Lett. 2023, 25, 12. [Google Scholar] [CrossRef]
- Stefan, N.; Martin-Killias, P.; Wyss-Stoeckle, S.; Honegger, A.; Zangemeister-Wittke, U.; Plückthun, A. DARPins recognizing the tumor-associated antigen EpCAM selected by phage and ribosome display and engineered for multivalency. J. Mol. Biol. 2011, 413, 826–843. [Google Scholar] [CrossRef]
- Tolmachev, V.M.; Chernov, V.I.; Deyev, S.M. Targeted nuclear medicine. Seek and destroy. Russ. Chem. Rev. 2022, 91, RCR5034. [Google Scholar] [CrossRef]
- Plückthun, A. Designed ankyrin repeat proteins (DARPins): Binding proteins for research, diagnostics, and therapy. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 489–511. [Google Scholar] [CrossRef]
- Vorobyeva, A.; Bezverkhniaia, E.; Konovalova, E.; Schulga, A.; Garousi, J.; Vorontsova, O.; Abouzayed, A.; Orlova, A.; Deyev, S.; Tolmachev, V. Radionuclide Molecular Imaging of EpCAM Expression in Triple-Negative Breast Cancer Using the Scaffold Protein DARPin Ec1. Molecules 2020, 25, 4719. [Google Scholar] [CrossRef]
- Deyev, S.M.; Vorobyeva, A.; Schulga, A.; Abouzayed, A.; Günther, T.; Garousi, J.; Konovalova, E.; Ding, H.; Gräslund, T.; Orlova, A.; et al. Effect of a radiolabel biochemical nature on tumor-targeting properties of EpCAM-binding engineered scaffold protein DARPin Ec1. Int. J. Biol. Macromol. 2020, 145, 216–225. [Google Scholar] [CrossRef]
- Chernov, V.; Dudnikova, E.; Zelchan, R.; Medvedeva, A.; Rybina, A.; Bragina, O.; Goldberg, V.; Muravleva, A.; Sörensen, J.; Tolmachev, V. Phase I Clinical Trial Using [99mTc]Tc-1-thio-D-glucose for Diagnosis of Lymphoma Patients. Pharmaceutics 2022, 14, 1274. [Google Scholar] [CrossRef]
- Li, G.; Suzuki, H.; Asano, T.; Tanaka, T.; Suzuki, H.; Kaneko, M.K.; Kato, Y. Development of a Novel Anti-EpCAM Monoclonal Antibody for Various Applications. Antibodies 2022, 11, 41. [Google Scholar] [CrossRef]
- Li, G.; Suzuki, H.; Ohishi, T.; Asano, T.; Tanaka, T.; Yanaka, M.; Nakamura, T.; Yoshikawa, T.; Kawada, M.; Kaneko, M.K.; et al. Antitumor activities of a defucosylated anti-EpCAM monoclonal antibody in colorectal carcinoma xenograft models. Int. J. Mol. Med. 2023, 51, 18. [Google Scholar] [CrossRef]
- Satofuka, H.; Wang, Y.; Yamazaki, K.; Hamamichi, S.; Fukuhara, T.; Rafique, A.; Osako, N.; Kanazawa, I.; Endo, T.; Miyake, N.; et al. Characterization of human anti-EpCAM antibodies for developing an antibody-drug conjugate. Sci. Rep. 2023, 13, 4225. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Karschnia, P.; Cadilha, B.L.; Dede, S.; Lorenz, M.; Seewaldt, N.; Nikolaishvili, E.; Müller, K.; Blobner, J.; Teske, N.; et al. In vivo dynamics and anti-tumor effects of EpCAM-directed CAR T-cells against brain metastases from lung cancer. Oncoimmunology 2023, 12, 2163781. [Google Scholar] [CrossRef] [PubMed]
- Bragina, O.; Chernov, V.; Larkina, M.; Rybina, A.; Zelchan, R.; Garbukov, E.; Oroujeni, M.; Loftenius, A.; Orlova, A.; Sörensen, J.; et al. Phase I clinical evaluation of 99mTc-labeled Affibody molecule for imaging HER2 expression in breast cancer. Theranostics 2023, 13, 4858–4871. [Google Scholar] [CrossRef] [PubMed]
- Tolmachev, V.; Orlova, A.; Andersson, K. Methods for radiolabelling of monoclonal antibodies. Methods Mol. Biol. 2014, 1060, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.F.; Li, J.; Yu, L.C.; Wu, Y.; Tang, X.P.; Hu, Y.M.; Chen, Y.C. Prognostic value of EpCAM/MUC1 mRNA-positive cells in non-small cell lung cancer patients. Tumour Biol. 2014, 35, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Breitz, H.B.; Sullivan, K.; Nelp, W.B. Imaging lung cancer with radiolabeled antibodies. Semin. Nucl. Med. 1993, 23, 127–132. [Google Scholar] [CrossRef]
- Kalofonos, H.P.; Sivolapenko, G.B.; Courtenay-Luck, N.S.; Snook, D.E.; Hooker, G.R.; Winter, R.; McKenzie, C.G.; Taylor-Papadimitriou, J.J.; Lavender, P.J.; Epenetos, A.A. Antibody guided targeting of non-small cell lung cancer using 111In-labeled HMFG1 F(ab’)2 fragments. Cancer Res. 1988, 48, 1977–1984. [Google Scholar] [PubMed]
- Kosterink, J.G.; de Jonge, M.W.; Smit, E.F.; Piers, D.A.; Kengen, R.A.; Postmus, P.E.; Shochat, D.; Groen, H.J.; The, H.T.; de Leij, L. Pharmacokinetics and scintigraphy of indium-111-DTPA-MOC-31 in small-cell lung carcinoma. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1995, 36, 2356–2362. [Google Scholar]
- Friedman, S.; Sullivan, K.; Salk, D.; Nelp, W.B.; Griep, R.J.; Johnson, D.H.; Blend, M.J.; Aye, R.; Suppers, V.; Abrams, P.G. Staging non-small cell carcinoma of the lung using technetium-99m-labeled monoclonal antibodies. Hematol./Oncol. Clin. N. Am. 1990, 4, 1069–1078. [Google Scholar]
- Vansant, J.P.; Johnson, D.H.; O’Donnell, D.M.; Stewart, J.R.; Sonin, A.H.; McCook, B.M.; Powers, T.A.; Salk, D.J.; Frist, W.H.; Sandler, M.P. Staging lung carcinoma with a Tc-99m labeled monoclonal antibody. Clin. Nucl. Med. 1992, 17, 431–438. [Google Scholar] [CrossRef]
| Patient | Age (y) | Sex | Clinical Stage | Diagnosis (Histopathology) |
|---|---|---|---|---|
| 1 | 71 | Male | T3N0M0 | PLC |
| 2 | 58 | Male | T3N0M0 | IA |
| 3 | 46 | Male | T3N1M0 | MDSCC |
| 4 | 45 | Male | T3N1M0 | LDSCC |
| 5 | 71 | Male | T2N0M0 | LDSCC |
| 6 | 36 | Male | T2N0M0 | LDSCC |
| 7 | 42 | Male | T2N0M0 | MDSCC |
| 8 | 72 | Male | T3N1M0 | MDSCC |
| 9 | 68 | Female | T2N1M0 | NMA |
| 10 | 46 | Male | T2N0M0 | IA |
| 11 | 47 | Female | T1N2M0 | MDSC |
| 12 | 45 | Female | T2N1M0 | NMA |
| Time | Kidney | Liver | Lung |
|---|---|---|---|
| 2 h | 32.24 ± 9.63 | 10.38 ± 1.80 | 3.05 ± 0.86 |
| 4 h | 30.68 ± 9.30 | 10.27 ± 1.83 | 2.82 ± 0.78 |
| 6 h | 29.55 ± 8.83 | 10.02 ± 1.65 | 2.67 ± 0.78 |
| 24 h | 22.1 ± 5.58 | 9.12 ± 1.55 | 2.51 ± 0.80 |
| Site | Absorbed Dose |
|---|---|
| Adrenals | 0.017 ± 0.004 |
| Brain | 0.001 ± 0.000 |
| Breasts | 0.002 ± 0.000 |
| Gallbladder Wall | 0.010 ± 0.002 |
| LLI Wall | 0.006 ± 0.002 |
| Small Intestine | 0.007 ± 0.002 |
| Stomach Wall | 0.005 ± 0.001 |
| ULI Wall | 0.006 ± 0.002 |
| Heart Wall | 0.005 ± 0.001 |
| Kidneys | 0.113 ± 0.044 |
| Liver | 0.013 ± 0.002 |
| Lungs | 0.005 ± 0.001 |
| Muscle | 0.003 ± 0.001 |
| Ovaries | 0.004 ± 0.001 |
| Pancreas | 0.010 ± 0.002 |
| Red Marrow | 0.005 ± 0.004 |
| Osteogenic Cells | 0.007 ± 0.001 |
| Skin | 0.003 ± 0.003 |
| Spleen | 0.008 ± 0.004 |
| Testes | 0.006 ± 0.003 |
| Thymus | 0.004 ± 0.001 |
| Thyroid | 0.056 ± 0.012 |
| Urinary Bladder Wall | 0.009 ± 0.008 |
| Prostate | 0.005 ± 0.002 |
| Total body | 0.004 ± 0.001 |
| Effective Dose Equivalent (mSv/MBq) | 0.015 ± 0.004 |
| Effective Dose (mSv/MBq) | 0.011 ± 0.003 |
| Patients | SUVmax Tumor | SUVmax Lymph Node | SUVTumor/Background 2 h | SUVTumor/Background 4 h | SUVTumor/Background 6 h |
|---|---|---|---|---|---|
| 1. | 2.51 | - | 7.17 | 6.4 | 7 |
| 2. | 2.8 | - | 23.3 | 13.2 | 13.4 |
| 3. | 2.29 | 0.76 | 10.9 | 10.3 | 14.4 |
| 4. | 1.42 | 0.79 | 8.35 | 8.3 | 6.5 |
| 5. | 1.1 | - | 6.88 | 7.2 | 10.4 |
| 6. | 3.23 | - | 7.17 | 5 | 12.3 |
| 7. | 2.83 | - | 14.9 | 15.4 | 16.5 |
| 8. | 1.16 | 0.94 | 9.7 | 10.5 | 9.4 |
| 9. | 6.8 | 2.47 | 27.2 | 22 | 26.3 |
| 10. | 1.32 | - | 12 | 7.6 | 2.3 |
| 11. | 2.73 | 1.75 | 15.2 | 5.7 | 6.4 |
| 12. | 2.66 | 1.82 | 20.5 | 13.3 | 13.2 |
| Mean ± SD | 2.57 ± 1.52 | 1.2 ± 0.5 | 13.6 ± 6.81 | 10.4 ± 4.91 | 11.5 ± 6.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelchan, R.; Chernov, V.; Medvedeva, A.; Rybina, A.; Bragina, O.; Mishina, E.; Larkina, M.; Varvashenya, R.; Fominykh, A.; Schulga, A.; et al. Phase I Clinical Evaluation of Designed Ankyrin Repeat Protein [99mTc]Tc(CO)3-(HE)3-Ec1 for Visualization of EpCAM-Expressing Lung Cancer. Cancers 2024, 16, 2815. https://doi.org/10.3390/cancers16162815
Zelchan R, Chernov V, Medvedeva A, Rybina A, Bragina O, Mishina E, Larkina M, Varvashenya R, Fominykh A, Schulga A, et al. Phase I Clinical Evaluation of Designed Ankyrin Repeat Protein [99mTc]Tc(CO)3-(HE)3-Ec1 for Visualization of EpCAM-Expressing Lung Cancer. Cancers. 2024; 16(16):2815. https://doi.org/10.3390/cancers16162815
Chicago/Turabian StyleZelchan, Roman, Vladimir Chernov, Anna Medvedeva, Anastasia Rybina, Olga Bragina, Elizaveta Mishina, Mariia Larkina, Ruslan Varvashenya, Anastasia Fominykh, Alexey Schulga, and et al. 2024. "Phase I Clinical Evaluation of Designed Ankyrin Repeat Protein [99mTc]Tc(CO)3-(HE)3-Ec1 for Visualization of EpCAM-Expressing Lung Cancer" Cancers 16, no. 16: 2815. https://doi.org/10.3390/cancers16162815
APA StyleZelchan, R., Chernov, V., Medvedeva, A., Rybina, A., Bragina, O., Mishina, E., Larkina, M., Varvashenya, R., Fominykh, A., Schulga, A., Konovalova, E., Vorobyeva, A., Orlova, A., Tashireva, L., Deyev, S. M., & Tolmachev, V. (2024). Phase I Clinical Evaluation of Designed Ankyrin Repeat Protein [99mTc]Tc(CO)3-(HE)3-Ec1 for Visualization of EpCAM-Expressing Lung Cancer. Cancers, 16(16), 2815. https://doi.org/10.3390/cancers16162815








