Detection of Hepatocellular Carcinoma in an Orthotopic Patient-Derived Xenograft with an Epithelial Cell Adhesion Molecule-Specific Peptide
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Chemicals
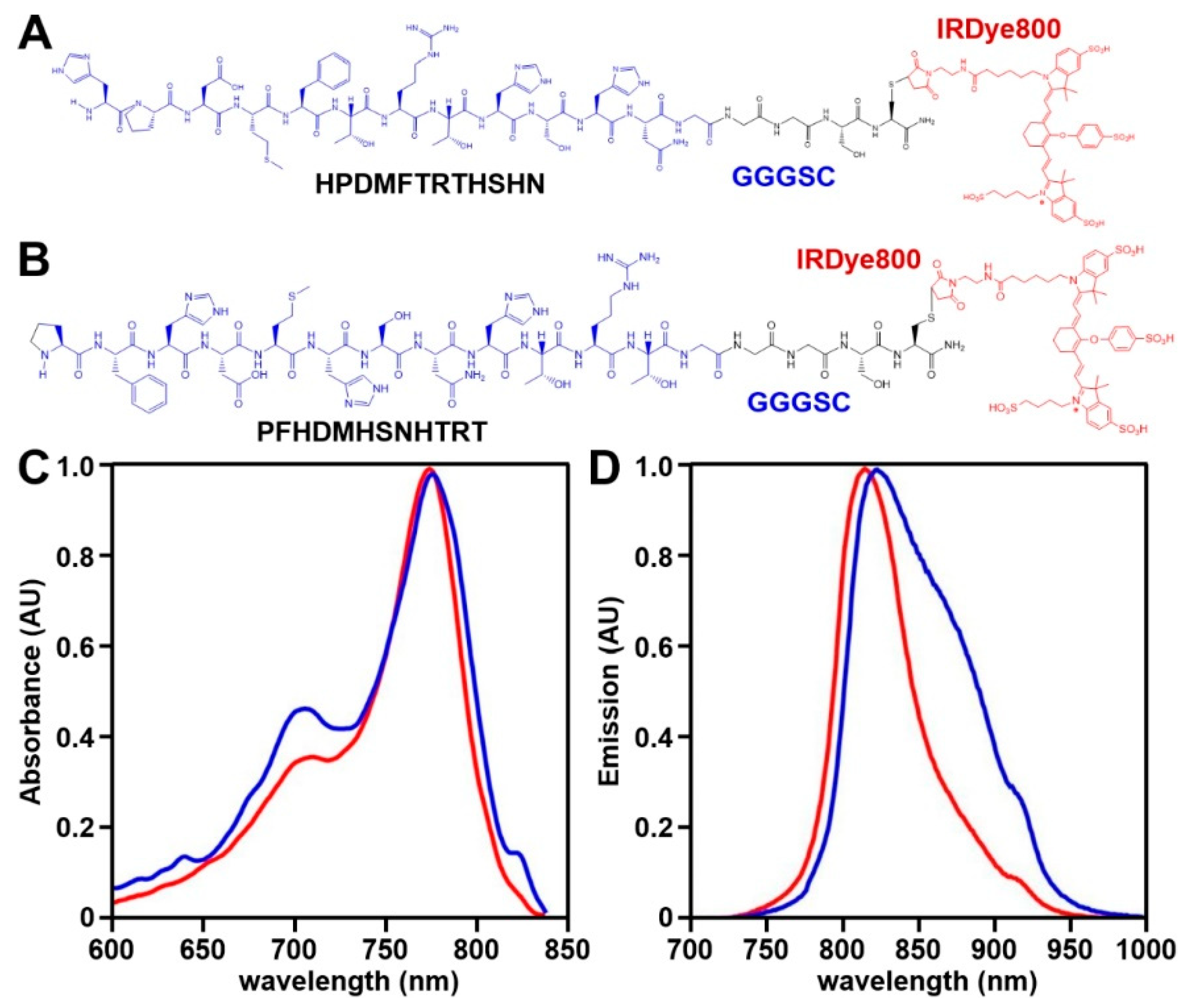
2.2. NIR Peptide Specific for EpCAM
2.2.1. Peptide Selection
2.2.2. Spectral Measurements
2.3. Validation of Specific Peptide Binding and Peptide Characterization
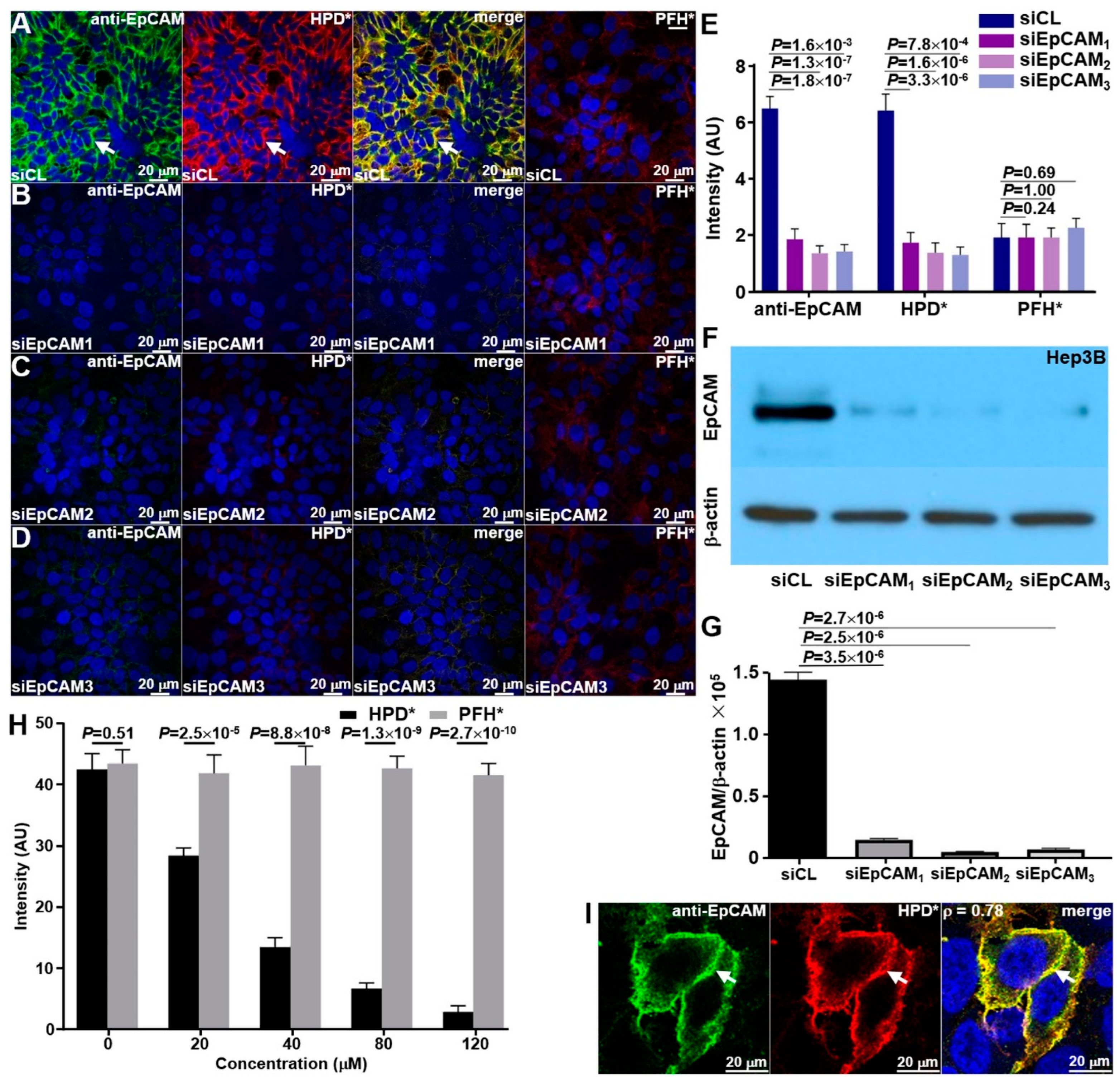
2.3.1. siRNA Knockdown
2.3.2. Binding Competition
2.3.3. Binding Affinity and Binding Onset
2.3.4. Serum Stability
2.4. Orthotopic HCC Patient-Derived Xenograft (PDX) Model
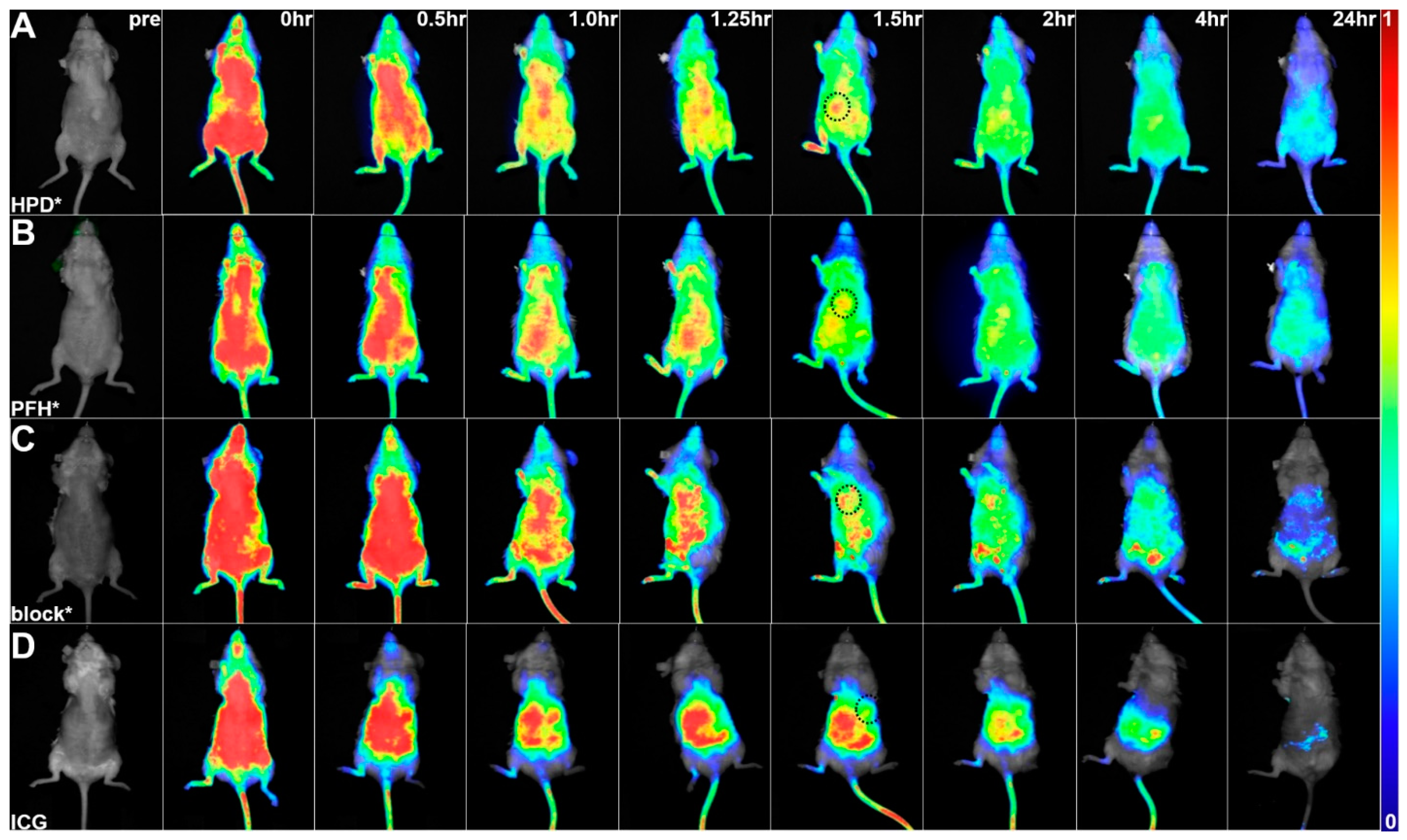
2.5. In Vivo Whole-Body Imaging
2.6. Laparoscopic Imaging
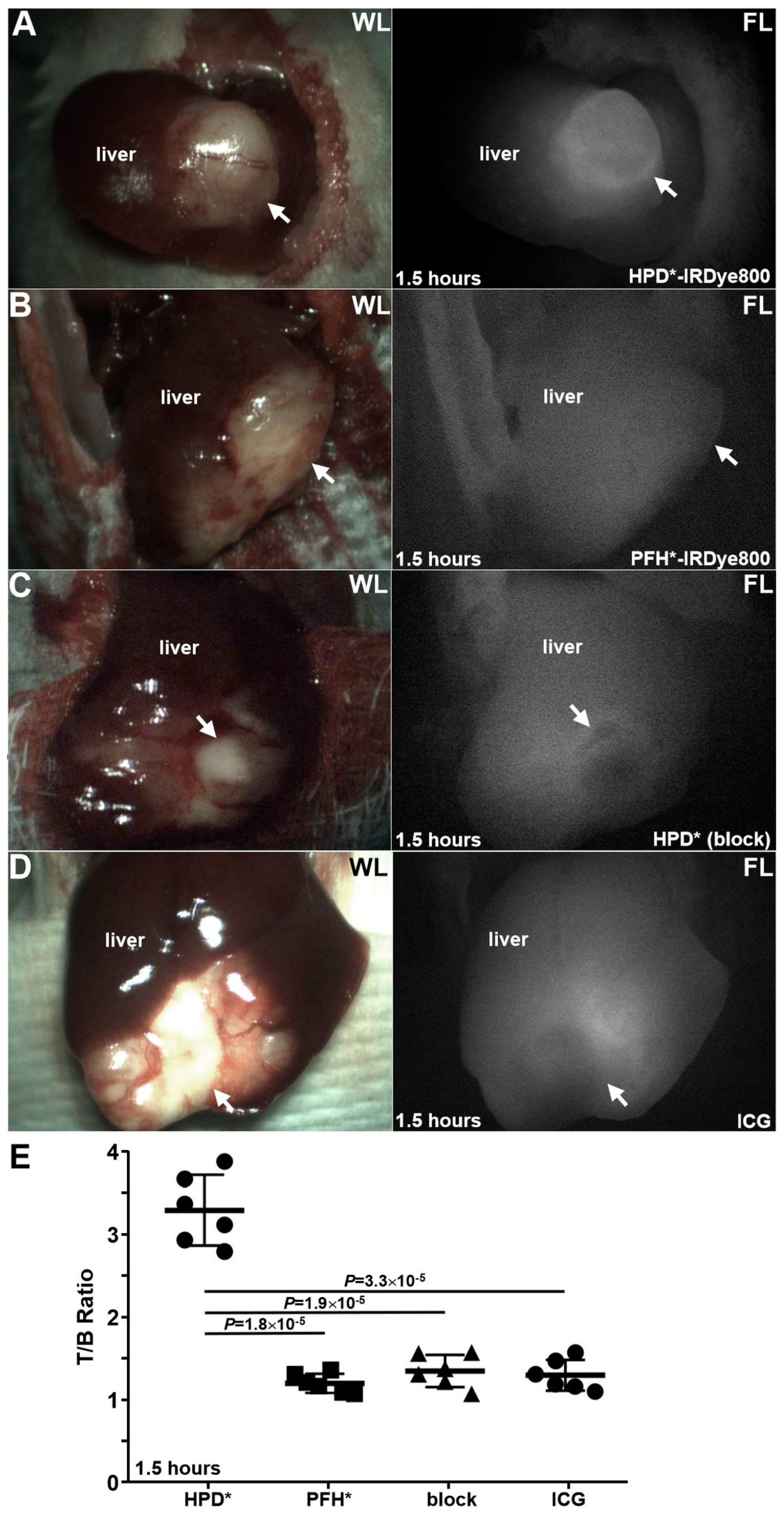
2.7. Animal Necropsy
2.8. Ex Vivo Peptide Validation with Human Liver Specimens
3. Results
3.1. NIR Peptide Specific for EpCAM
3.2. Specific Peptide Binding
3.2.1. Knockdown of siRNA
3.2.2. Binding Competition
3.2.3. Binding Co-Localization
3.3. Peptide Characterization
3.3.1. Binding Affinity and Onset
3.3.2. Serum Stability
3.4. In Vivo Whole-Body Imaging
3.5. Laparoscopy
3.5.1. Detection of Primary Tumor
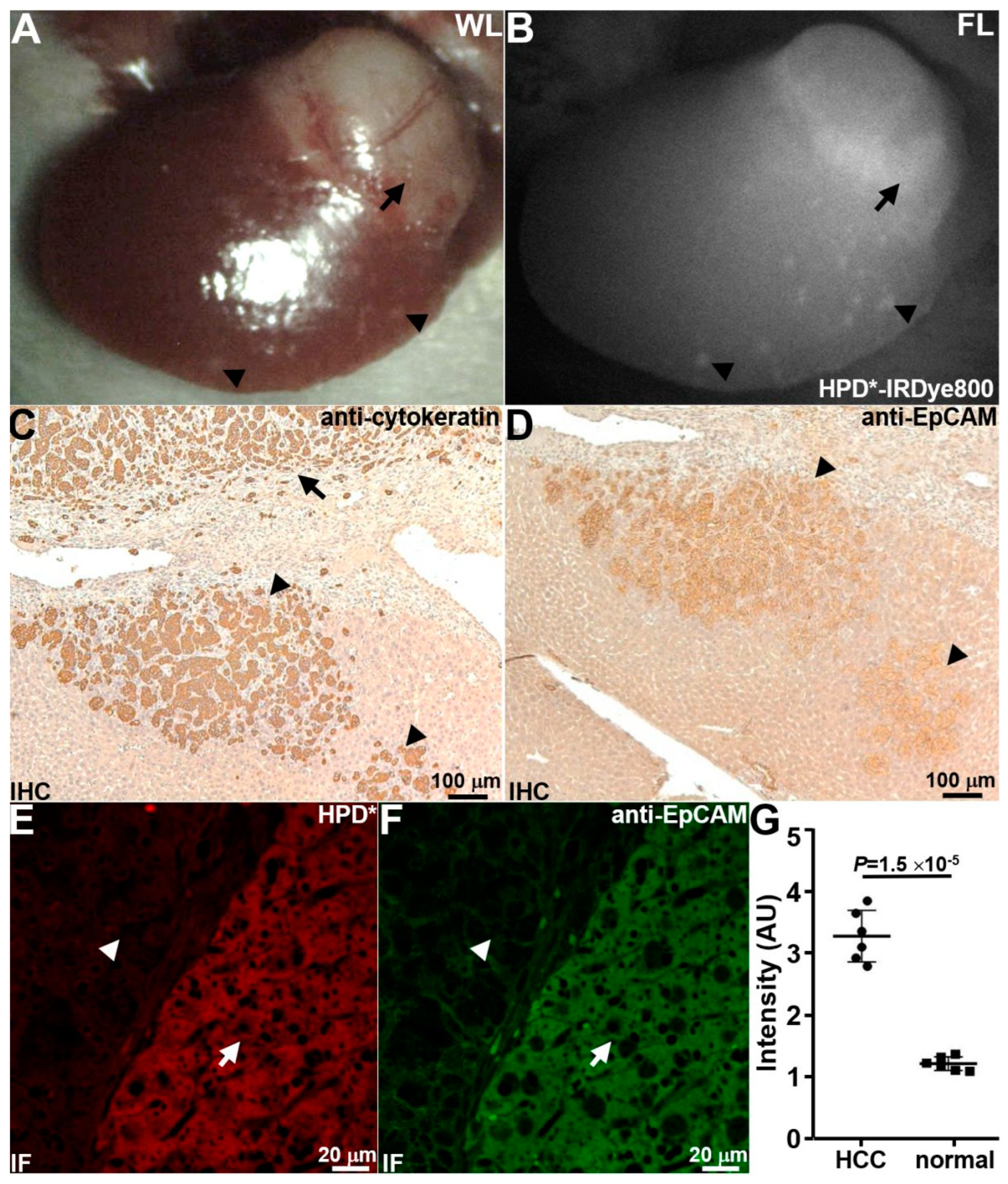
3.5.2. Detection of Local Micrometastases
3.5.3. Detection of Distant Micrometastases
3.6. Peptide Biodistribution
3.7. Animal Necropsy
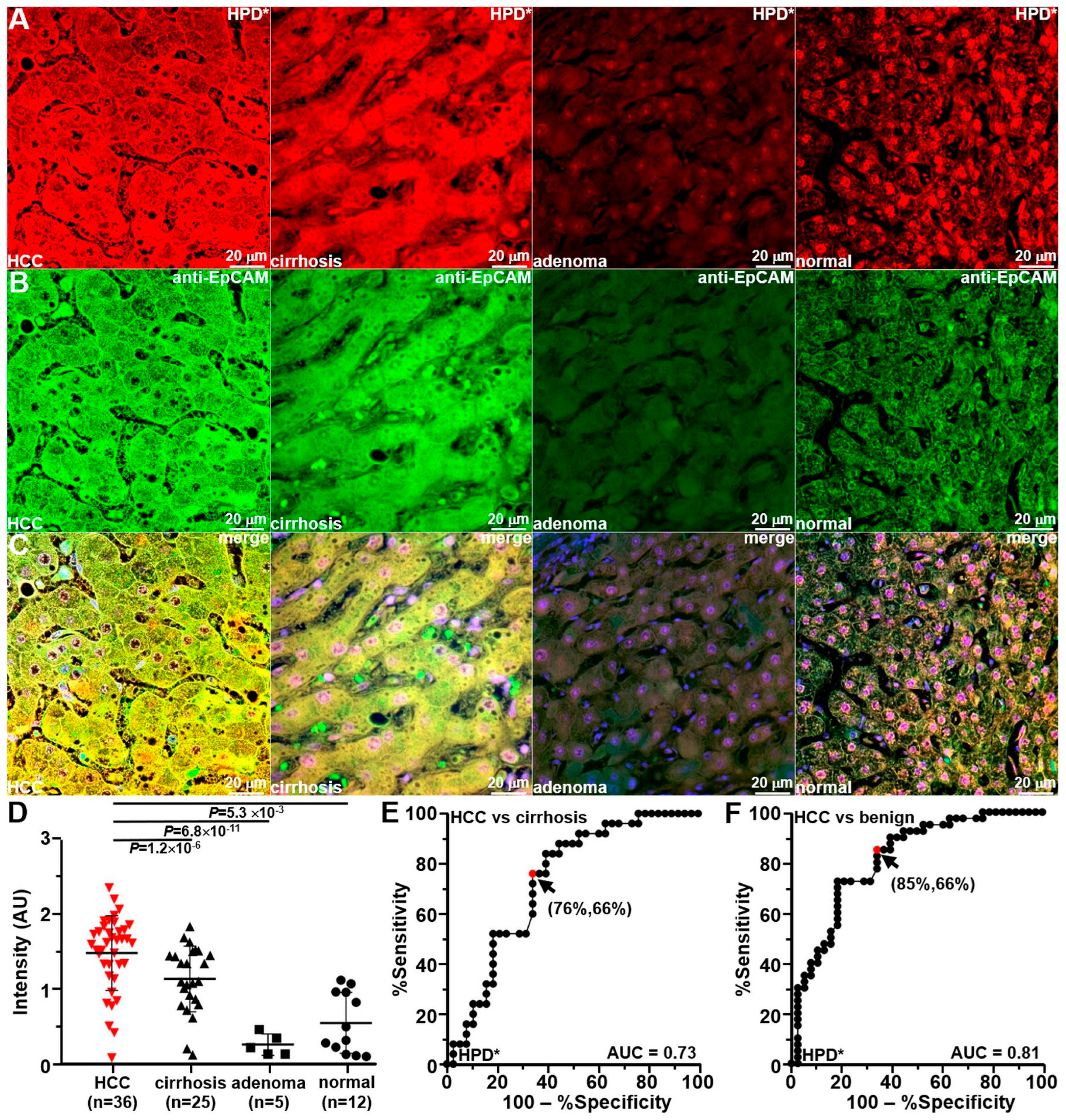
3.8. Ex Vivo Peptide Validation with Human Liver Specimens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ozakyol, A. Global Epidemiology of Hepatocellular Carcinoma (HCC Epidemiology). J. Gastrointest. Cancer 2017, 48, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, C.B.; Lim, C.S.; Sirlin, C.B.; McGrath, T.A.; Salameh, J.P.; Bashir, M.R.; Tang, A.; Singal, A.G.; Costa, A.F.; Fowler, K.; et al. Accuracy of the Liver Imaging Reporting and Data System in Computed Tomography and Magnetic Resonance Image Analysis of Hepatocellular Carcinoma or Overall Malignancy—A Systematic Review. Gastroenterology 2019, 156, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed]
- Pinto Marques, H.; Gomes da Silva, S.; De Martin, E.; Agopian, V.G.; Martins, P.N. Emerging biomarkers in HCC patients: Current status. Int. J. Surg. 2020, 82, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Takai, A.; Eso, Y.; Kinoshita, K.; Manabe, T.; Seno, H.; Chiba, T.; Marusawa, H. Proliferating EpCAM-Positive Ductal Cells in the Inflamed Liver Give Rise to Hepatocellular Carcinoma. Cancer Res. 2017, 77, 6131–6143. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Ji, J.; Budhu, A.; Forgues, M.; Yang, W.; Wang, H.Y.; Jia, H.; Ye, Q.; Qin, L.X.; Wauthier, E.; et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology 2009, 136, 1012–1024. [Google Scholar] [CrossRef]
- Murakata, A.; Tanaka, S.; Mogushi, K.; Yasen, M.; Noguchi, N.; Irie, T.; Kudo, A.; Nakamura, N.; Tanaka, H.; Arii, S. Gene expression signature of the gross morphology in hepatocellular carcinoma. Ann. Surg. 2011, 253, 94–100. [Google Scholar] [CrossRef]
- Zhou, L.; Zhu, Y. The EpCAM overexpression is associated with clinicopathological significance and prognosis in hepatocellular carcinoma patients: A systematic review and meta-analysis. Int. J. Surg. 2018, 56, 274–280. [Google Scholar] [CrossRef]
- Vasanthakumar, S.; Sasikala, P.; Padma, M.; Balachandar, V.; Venkatesh, B.; Ganesan, S. EpCAM as a novel therapeutic target for hepatocellular carcinoma. J. Oncol. Sci. 2017, 3, 71–76. [Google Scholar]
- Su, R.; Nan, H.; Guo, H.; Ruan, Z.; Jiang, L.; Song, Y.; Nan, K. Associations of components of PTEN/AKT/mTOR pathway with cancer stem cell markers and prognostic value of these biomarkers in hepatocellular carcinoma. Hepatol. Res. 2016, 46, 1380–1391. [Google Scholar] [CrossRef]
- Xu, M.; Qian, G.; Xie, F.; Shi, C.; Yan, L.; Yu, L.; Zheng, T.; Wei, L.; Yang, J. Expression of epithelial cell adhesion molecule associated with elevated ductular reactions in hepatocellar carcinoma. Clin. Res. Hepatol. Gastroenterol. 2014, 38, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Ding, T.; Guo, Z.W.; Yu, X.J.; Hu, Y.Z.; Zheng, L.; Xu, J. Expression pattern of tumour-associated antigens in hepatocellular carcinoma: Association with immune infiltration and disease progression. Br. J. Cancer 2013, 109, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.W.; Tong, J.H.; Chan, S.L.; Lai, P.B.; To, K.F. Expression of stemness markers (CD133 and EpCAM) in prognostication of hepatocellular carcinoma. Histopathology 2014, 64, 935–950. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.M.; Huang, T.; Yang, S.L.; Zheng, X.M.; Chen, G.G.; Zhang, T. Peritumoral EpCAM Is an Independent Prognostic Marker after Curative Resection of HBV-Related Hepatocellular Carcinoma. Dis. Markers 2017, 1, 8495326. [Google Scholar]
- Noh, C.K.; Wang, H.J.; Kim, C.M.; Kim, J.; Yoon, S.Y.; Lee, G.H.; Cho, H.J.; Yang, M.J.; Kim, S.S.; Hwang, J.C.; et al. EpCAM as a Predictive Marker of Tumor Recurrence and Survival in Patients Who Underwent Surgical Resection for Hepatocellular Carcinoma. Anticancer Res. 2018, 38, 4101–4109. [Google Scholar] [CrossRef]
- Bae, J.S.; Noh, S.J.; Jang, K.Y.; Park, H.S.; Chung, M.J.; Park, C.K.; Moon, W.S. Expression and role of epithelial cell adhesion molecule in dysplastic nodule and hepatocellular carcinoma. Int. J. Oncol. 2012, 41, 2150–2158. [Google Scholar] [CrossRef]
- Lima, L.D.P.; Machado, C.J.; Rodrigues, J.B.S.R.; Vasconcellos, L.S.; Junior, E.P.; Vidigal, P.V.T.; Resende, V. Immunohistochemical Coexpression of Epithelial Cell Adhesion Molecule and Alpha-Fetoprotein in Hepatocellular Carcinoma. Can. J. Gastroenterol. Hepatol. 2018, 2018, 5970852. [Google Scholar] [CrossRef]
- Baeuerle, P.A.; Gires, O. EpCAM (CD326) finding its role in cancer. Br. J. Cancer 2007, 96, 417–423. [Google Scholar] [CrossRef]
- Yamashita, T.; Budhu, A.; Forgues, M.; Wang, X.W. Activation of hepatic stem cell marker EpCAM by Wnt-beta-catenin signaling in hepatocellular carcinoma. Cancer Res. 2007, 67, 10831–10839. [Google Scholar] [CrossRef] [PubMed]
- Maetzel, D.; Denzel, S.; Mack, B.; Canis, M.; Went, P.; Benk, M.; Kieu, C.; Papior, P.; Baeuerle, P.A.; Munz, M.; et al. Nuclear signalling by tumour-associated antigen EpCAM. Nat. Cell. Biol. 2009, 11, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.C.; Yang, J.Y.; Yan, L.N. Relevant markers of cancer stem cells indicate a poor prognosis in hepatocellular carcinoma patients: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2013, 25, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, T.; Cacciaguerra, A.B.; Abe, Y.; Dalla Bona, E.; Nicolini, D.; Mocchegiani, F.; Kabeshima, Y.; Vivarelli, M.; Wakabayashi, G.; Kitagawa, Y. Indocyanine green fluorescence navigation in liver surgery: A systematic review on dose and timing of administration. Ann. Surg. 2022, 275, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Cui, Y.; Anderson, C.F.; Zhang, C.; Li, Y.; Wang, R.; Cui, H. Peptide-based nanoprobes for molecular imaging and disease diagnostics. Chem. Soc. Rev. 2018, 47, 3490–3529. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hu, Z. Targeting Peptide-Based Probes for Molecular Imaging and Diagnosis. Adv. Mater. 2019, 31, e1804827. [Google Scholar] [CrossRef] [PubMed]
- de Serres, M.; Ellis, B.; Dillberger, J.E.; Rudolph, S.K.; Hutchins, J.T.; Boytos, C.M.; Weigl, D.L.; DePrince, R.B. Immunogenicity of thrombopoietin mimetic peptide GW395058 in BALB/c mice and New Zealand white rabbits: Evaluation of the potential for thrombopoietin neutralizing antibody production in man. Stem Cells 1999, 17, 203–209. [Google Scholar] [CrossRef]
- Tangri, S.; Mothé, B.R.; Eisenbraun, J.; Sidney, J.; Southwood, S.; Briggs, K.; Zinckgraf, J.; Bilsel, P.; Newman, M.; Chesnut, R.; et al. Rationally engineered therapeutic proteins with reduced immunogenicity. J. Immunol. 2005, 174, 3187–3196. [Google Scholar] [CrossRef]
- Li, M.; Anastassiades, C.P.; Joshi, B.; Komarck, C.M.; Piraka, C.; Elmunzer, B.J.; Turgeon, D.K.; Johnson, T.D.; Appelman, H.; Beer, D.G.; et al. Affinity peptide for targeted detection of dysplasia in Barrett’s esophagus. Gastroenterology 2010, 139, 1472–1480. [Google Scholar] [CrossRef]
- Macindoe, G.; Mavridis, L.; Venkatraman, V.; Devignes, M.D.; Ritchie, D.W. HexServer: An FFT-based protein docking server powered by graphics processors. Nucleic Acids Res. 2010, 38 (Suppl. S2), W445–W449. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Chen, J.; Roudier, M.M.; Vessella, R.L.; Lantry, L.E.; Nunn, A.D. In vitro binding evaluation of 177Lu-AMBA, a novel 177Lu-labeled GRP-R agonist for systemic radiotherapy in human tissues. Clin. Exp. Metastasis 2009, 26, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, A.M.; Harmon, C.; Whelan, S.; O’Brien, E.C.; O’Reilly, V.P.; Crotty, P.; Kelly, P.; Ryan, M.; Hickey, F.B.; O’Farrelly, C.; et al. Myeloid Engraftment in Humanized Mice: Impact of Granulocyte-Colony Stimulating Factor Treatment and Transgenic Mouse Strain. Stem Cells Dev. 2016, 25, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Shultz, L.D.; Lyons, B.L.; Burzenski, L.M.; Gott, B.; Chen, X.; Chaleff, S.; Kotb, M.; Gillies, S.D.; King, M.; Mangada, J.; et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 2005, 174, 6477–6489. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Meng, X.; Chang, T.S.; Feng, S.; Lee, M.; Jaiswal, S.; Choi, E.K.; Tran, L.; Jiang, H.; Wang, T.D. Multi-modal imaging for uptake of peptide ligand specific for CD44 by hepatocellular carcinoma. Photoacoustics 2022, 26, 100355. [Google Scholar] [CrossRef] [PubMed]
- van Driel, P.B.; Boonstra, M.C.; Prevoo, H.A.; van de Giessen, M.; Snoeks, T.J.; Tummers, Q.R.; Keereweer, S.; Cordfunke, R.A.; Fish, A.; van Eendenburg, J.D.; et al. EpCAM as multi-tumour target for near-infrared fluorescence guided surgery. BMC Cancer 2016, 16, 884. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Tang, Y.; Huang, W.; Zhao, Y.; Gao, X.; Gu, Y. Multi-modal imaging probe for EpCAM overexpressed in breast cancer. Talanta 2022, 250, 123715. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Kang, X.; He, L.; Zhou, J.; Zhou, J.; Sturm, M.B.; Beer, D.G.; Kuick, R.; Nancarrow, D.J.; Appelman, H.D.; et al. Identification of Tumor Specific Peptide as EpCAM Ligand and Its Potential Diagnostic and Therapeutic Clinical Application. Mol. Pharm. 2019, 16, 2199–2213. [Google Scholar] [CrossRef]
- Yim, S.Y.; Lee, J.S. Genomic Perspective on Mouse Liver Cancer Models. Cancers 2019, 11, 1648. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Tian, D.A.; Li, P.Y.; He, X.X. Mouse models of liver cancer: Progress and recommendations. Oncotarget 2015, 6, 23306–23322. [Google Scholar] [CrossRef]
- Jones, A.D.; Wilton, J.C. Can intra-operative fluorescence play a significant role in hepatobiliary surgery? Eur. J. Surg. Oncol. 2017, 43, 1622–1627. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. J. Control. Release 2012, 64, 138–144. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Chi, C.; Li, D.; Zhang, J.; Niu, G.; Lv, F.; Wang, J.; Che, W.; Zhang, L.; Ji, N.; et al. Resection and survival data from a clinical trial of glioblastoma multiforme-specific IRDye800-BBN fluorescence-guided surgery. Bioeng. Trans. Med. 2021, 6, e10182. [Google Scholar] [CrossRef]
- Lai, X.; Tang, J.; ElSayed, M.E. Recent advances in proteolytic stability for peptide, protein, and antibody drug discovery. Expert Opin. Drug Discov. 2021, 16, 1467–1482. [Google Scholar] [CrossRef] [PubMed]
- Sheard, D.E.; Li, W.; O’Brien-Simpson, N.M.; Separovic, F.; Wade, J.D. Peptide Multimerization as Leads for Therapeutic Development. Biologics 2022, 2, 15–44. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Feng, S.; Chang, T.-S.; Zhang, R.; Jaiswal, S.; Choi, E.-Y.K.; Duan, Y.; Jiang, H.; Wang, T.D. Detection of Hepatocellular Carcinoma in an Orthotopic Patient-Derived Xenograft with an Epithelial Cell Adhesion Molecule-Specific Peptide. Cancers 2024, 16, 2818. https://doi.org/10.3390/cancers16162818
Wu X, Feng S, Chang T-S, Zhang R, Jaiswal S, Choi E-YK, Duan Y, Jiang H, Wang TD. Detection of Hepatocellular Carcinoma in an Orthotopic Patient-Derived Xenograft with an Epithelial Cell Adhesion Molecule-Specific Peptide. Cancers. 2024; 16(16):2818. https://doi.org/10.3390/cancers16162818
Chicago/Turabian StyleWu, Xiaoli, Shuo Feng, Tse-Shao Chang, Ruoliu Zhang, Sangeeta Jaiswal, Eun-Young K. Choi, Yuting Duan, Hui Jiang, and Thomas D. Wang. 2024. "Detection of Hepatocellular Carcinoma in an Orthotopic Patient-Derived Xenograft with an Epithelial Cell Adhesion Molecule-Specific Peptide" Cancers 16, no. 16: 2818. https://doi.org/10.3390/cancers16162818
APA StyleWu, X., Feng, S., Chang, T.-S., Zhang, R., Jaiswal, S., Choi, E.-Y. K., Duan, Y., Jiang, H., & Wang, T. D. (2024). Detection of Hepatocellular Carcinoma in an Orthotopic Patient-Derived Xenograft with an Epithelial Cell Adhesion Molecule-Specific Peptide. Cancers, 16(16), 2818. https://doi.org/10.3390/cancers16162818






