Development and Characterization of Syngeneic Orthotopic Transplant Models of Obesity-Responsive Triple-Negative Breast Cancer in C57BL/6J Mice
Abstract
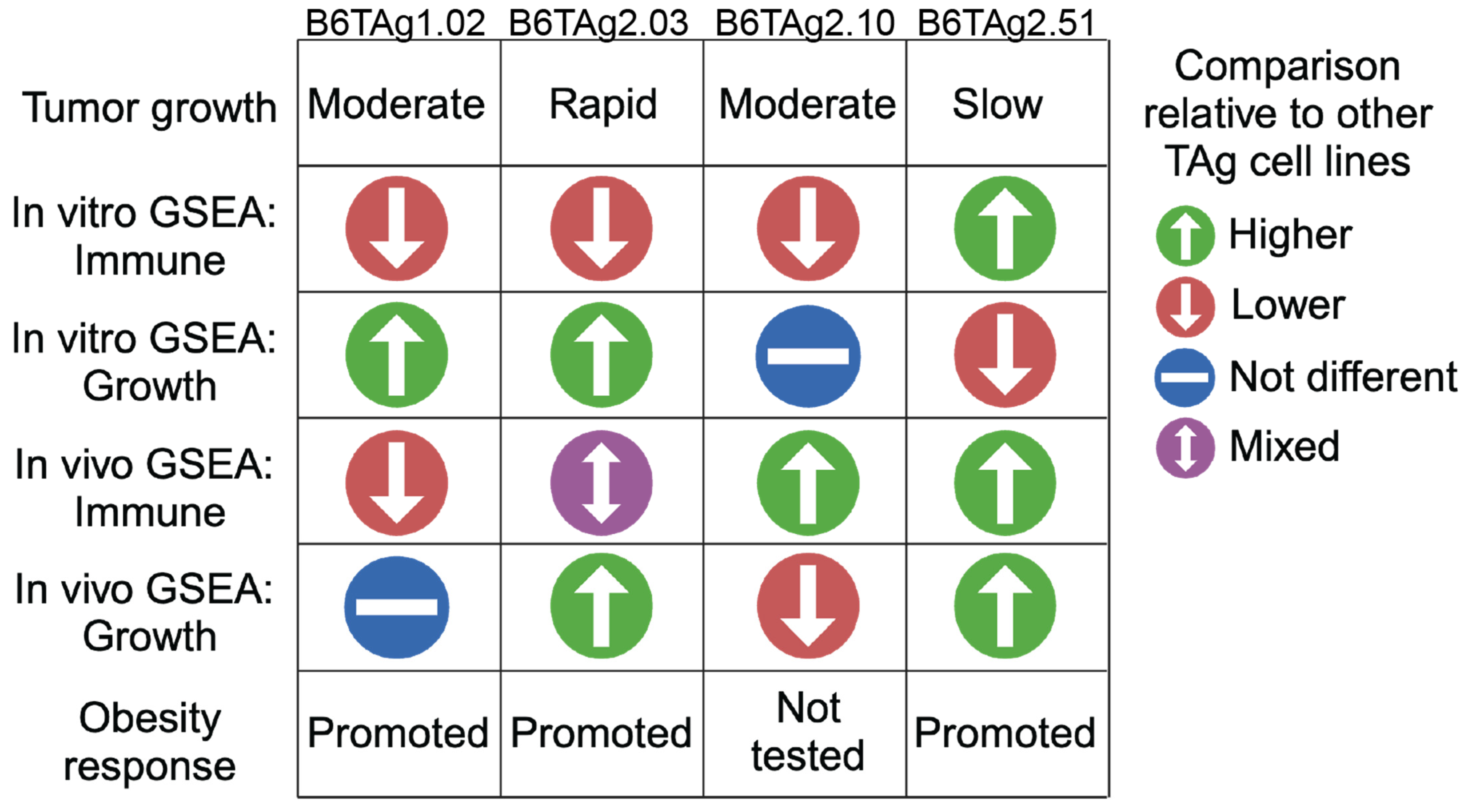
Simple Summary
Abstract
1. Introduction
2. Methods
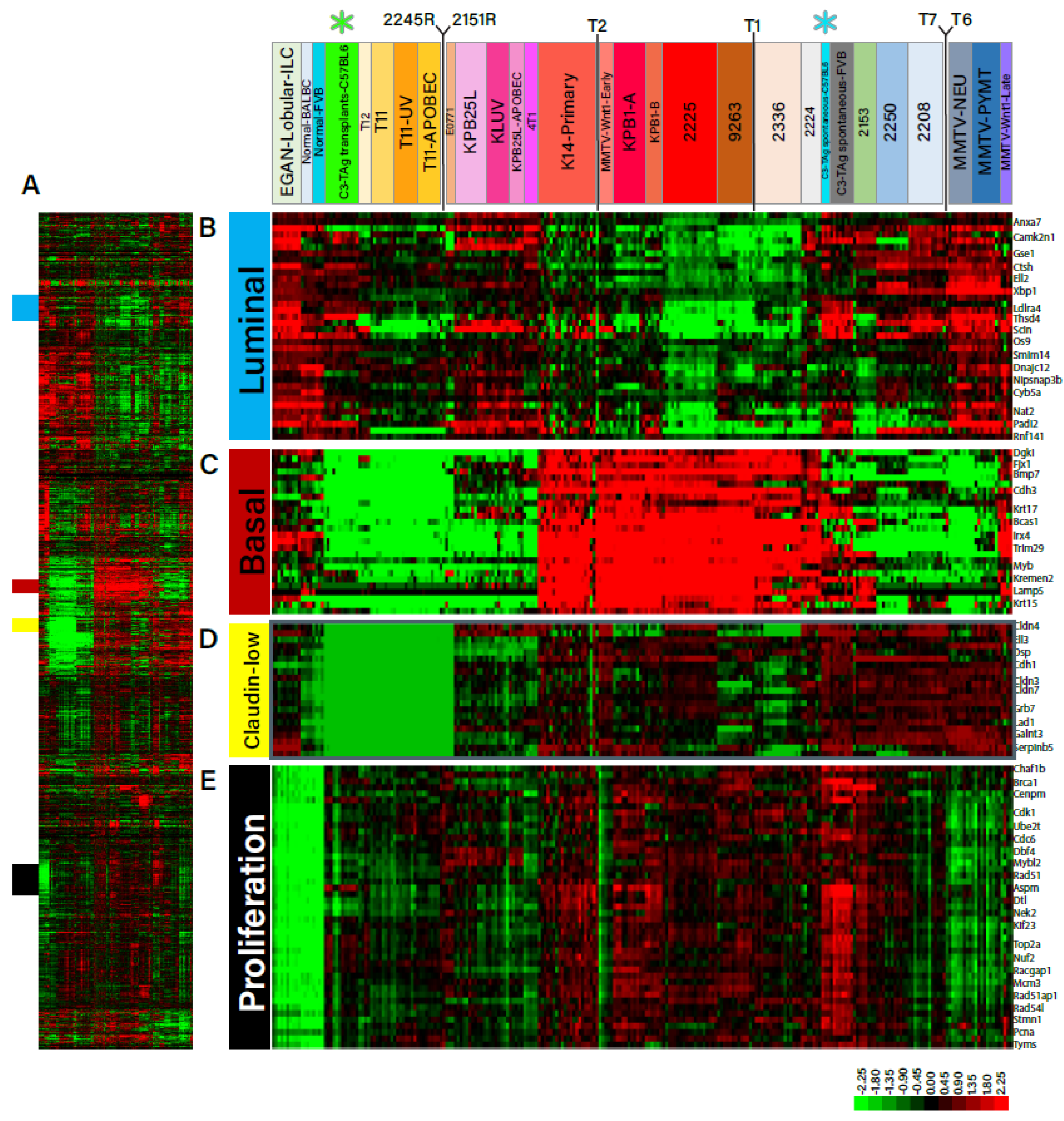
2.1. Generation of B6-C3TAg Mice and Associated Mammary Tumor Cell Lines
2.2. Clonal Cell Line Establishment
2.3. Tumor Cell Transplant Study
2.4. Diet-Induced Obesity Study
2.5. Microarray Transcriptomic Profiling of In Vitro and Transplanted B6TAg Cell Lines
2.6. RNAseq of Spontaneous and Transplanted B6TAg Tumors
2.7. Statistical Analysis
3. Results
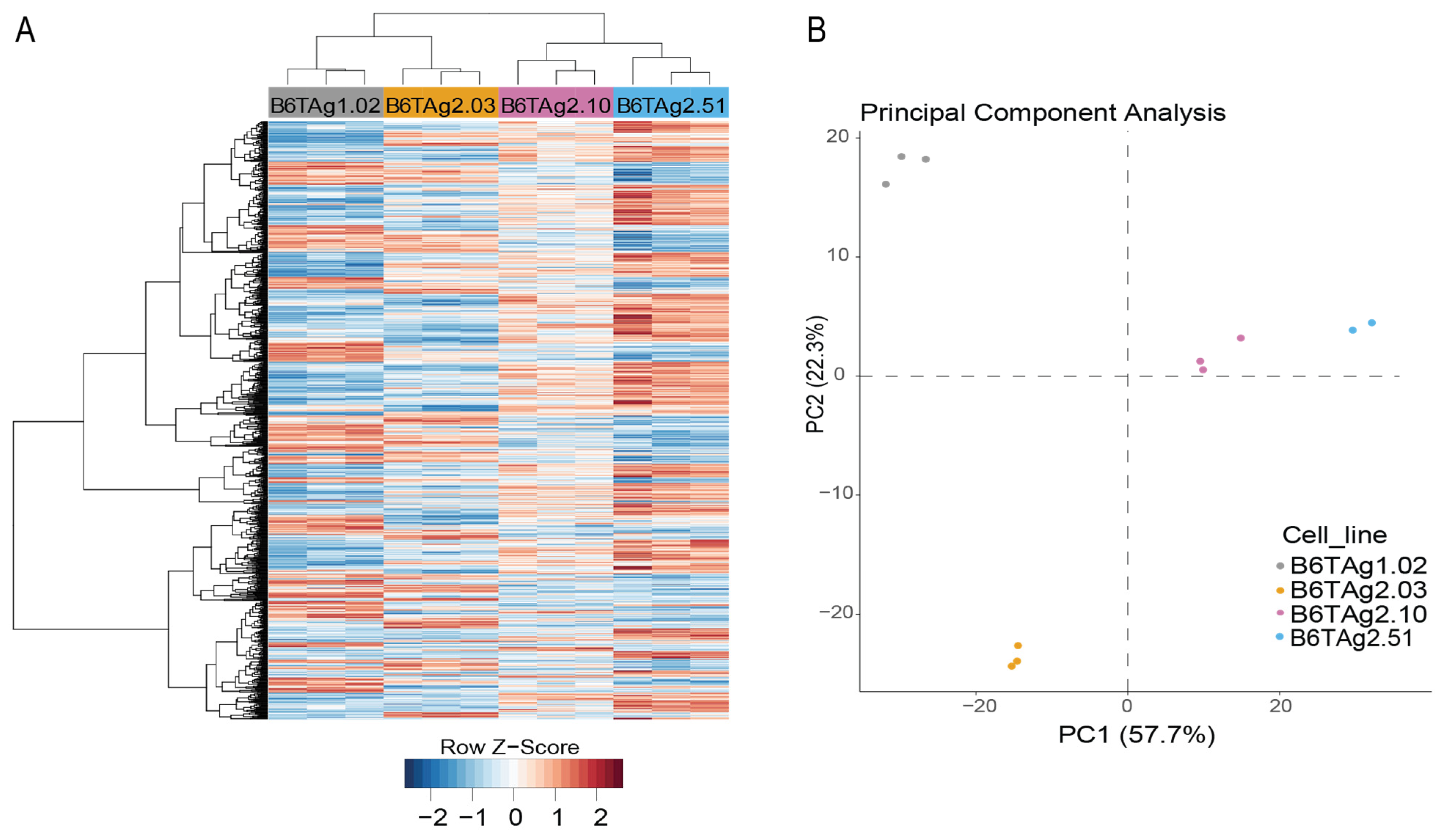
3.1. Generation of B6-C3TAg Mice and Associated TNBC Cell Lines That Harbor Distinct Transcriptomic Profiles
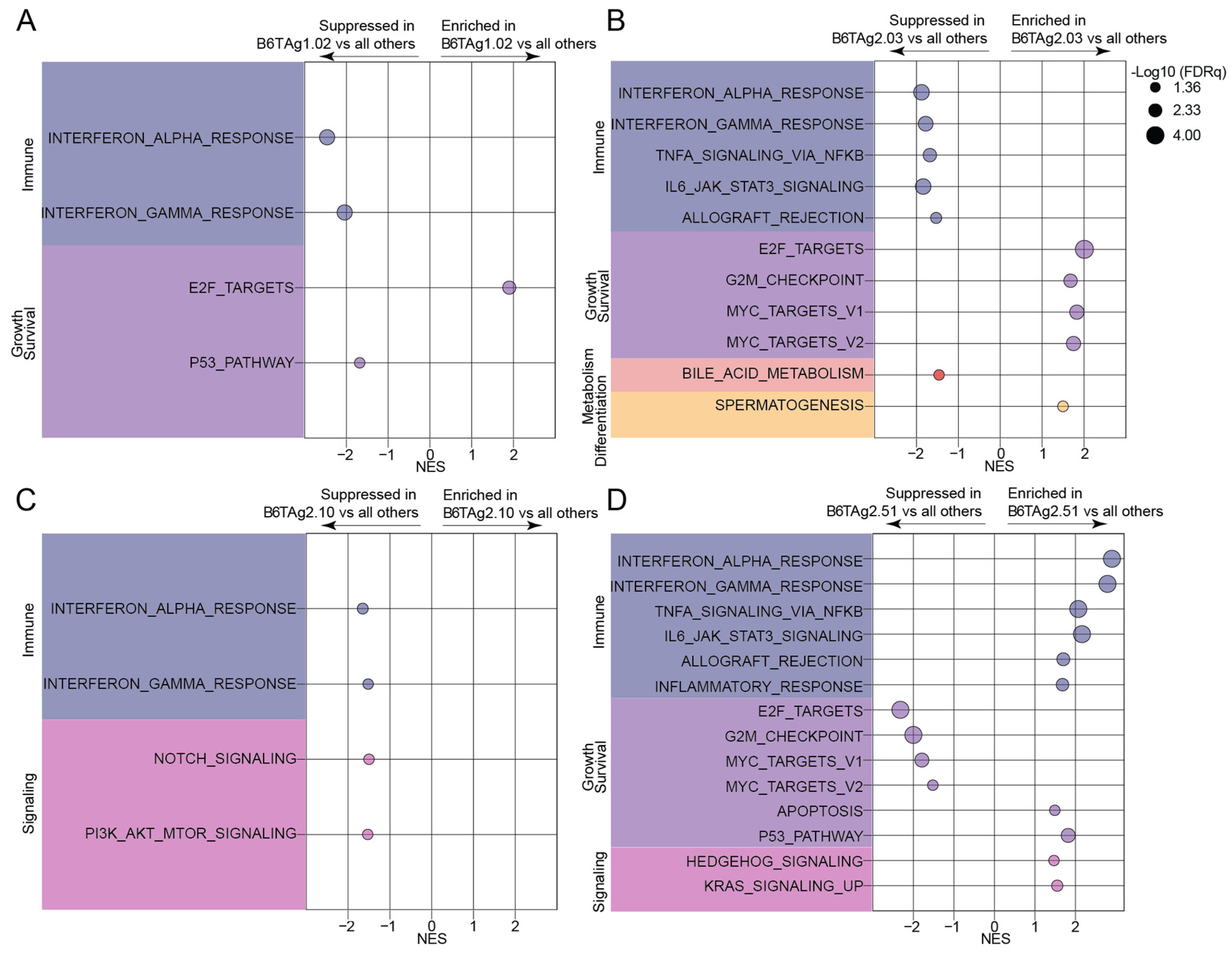
3.2. Immune Response and Growth/Survival-Related Signaling Transcriptomic Signatures Distinguish B6TAg Cells In Vitro
3.3. Orthotopically Transplanted B6TAg Cell Lines Reliably form Tumors in C57BL/6J Mice
3.4. Immune Response, Growth and Survival-Related Signaling, and Steroid Signaling Transcriptomic Signatures Distinguish B6TAg Tumors In Vivo
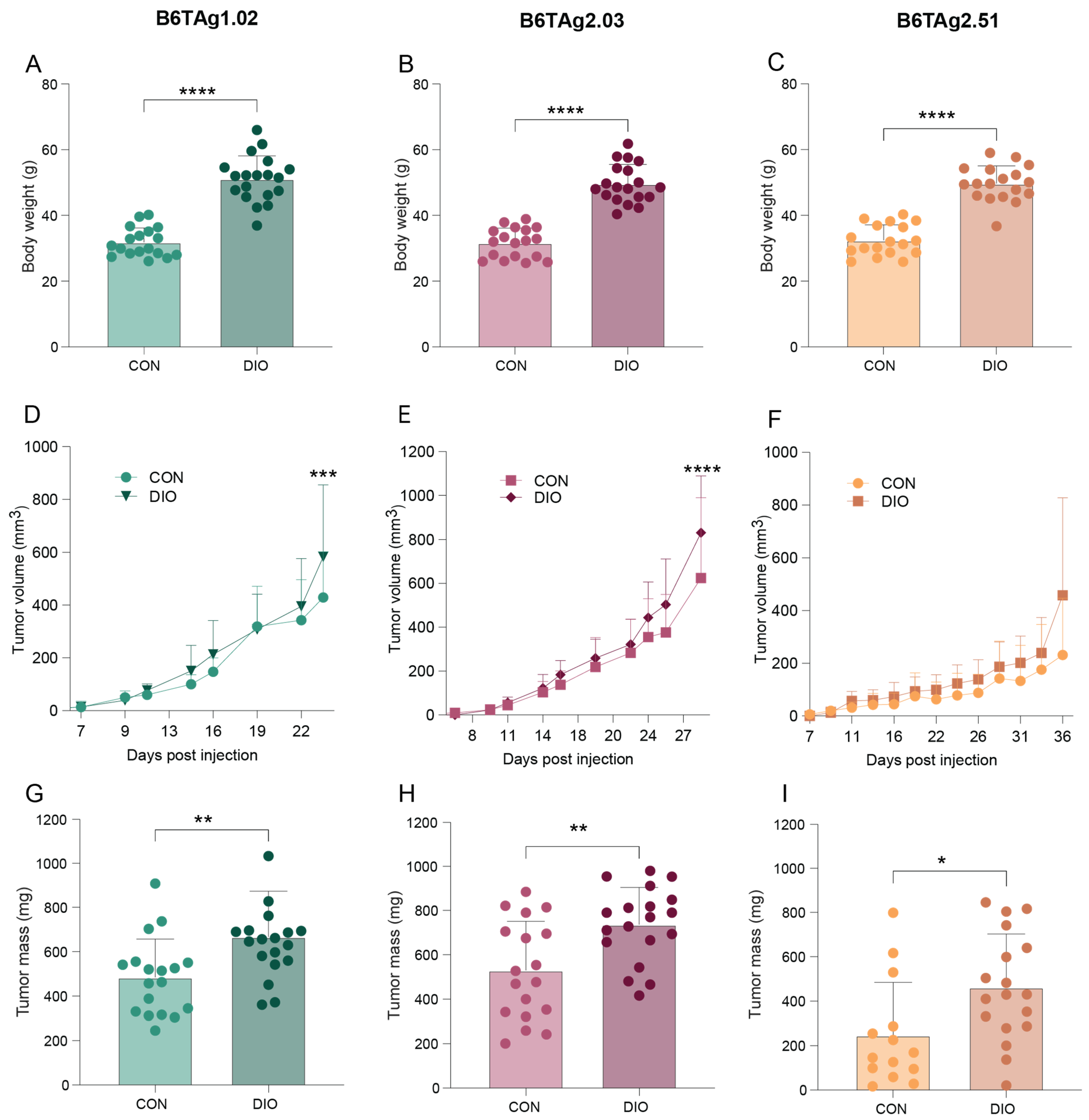
3.5. Orthotopically Transplanted B6TAg Tumor Growth Is Accelerated by High-Fat Diet-Induced Obesity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Orrantia-Borunda, E.; Anchondo-Nunez, P.; Acuna-Aguilar, L.E.; Gomez-Valles, F.O.; Ramirez-Valdespino, C.A. Subtypes of Breast Cancer. In Breast Cancer; Mayrovitz, H.N., Ed.; Exon Publications: Brisbane, Australia, 2022. [Google Scholar]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Abramson, V.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; et al. NCCN Guidelines® Insights: Breast Cancer, Version 4.2023. J. Natl. Compr. Cancer Netw. 2023, 21, 594–608. [Google Scholar] [CrossRef]
- Pfefferle, A.D.; Herschkowitz, J.I.; Usary, J.; Harrell, J.C.; Spike, B.T.; Adams, J.R.; Torres-Arzayus, M.I.; Brown, M.; Egan, S.E.; Wahl, G.M.; et al. Transcriptomic classification of genetically engineered mouse models of breast cancer identifies human subtype counterparts. Genome Biol. 2013, 14, R125. [Google Scholar] [CrossRef]
- Rädler, P.D.; Wehde, B.L.; Triplett, A.A.; Shrestha, H.; Shepherd, J.H.; Pfefferle, A.D.; Rui, H.; Cardiff, R.D.; Perou, C.M.; Wagner, K.-U. Highly metastatic claudin-low mammary cancers can originate from luminal epithelial cells. Nat. Commun. 2021, 12, 3742. [Google Scholar] [CrossRef]
- Berger, E.R.; Iyengar, N.M. Obesity and Energy Balance Considerations in Triple-Negative Breast Cancer. Cancer J. 2021, 27, 17–24. [Google Scholar] [CrossRef]
- Bohm, M.S.; Sipe, L.M.; Pye, M.E.; Davis, M.J.; Pierre, J.F.; Makowski, L. The role of obesity and bariatric surgery-induced weight loss in breast cancer. Cancer Metastasis Rev. 2022, 41, 673–695. [Google Scholar] [CrossRef]
- Glenny, E.M.; Coleman, M.F.; Giles, E.D.; Wellberg, E.A.; Hursting, S.D. Designing Relevant Preclinical Rodent Models for Studying Links Between Nutrition, Obesity, Metabolism, and Cancer. Annu. Rev. Nutr. 2021, 41, 253–282. [Google Scholar] [CrossRef]
- Stribbling, S.M.; Beach, C.; Ryan, A.J. Orthotopic and metastatic tumour models in preclinical cancer research. Pharmacol. Ther. 2024, 257, 108631. [Google Scholar] [CrossRef]
- Green, J.E.; Shibata, M.A.; Yoshidome, K.; Liu, M.L.; Jorcyk, C.; Anver, M.R.; Wigginton, J.; Wiltrout, R.; Shibata, E.; Kaczmarczyk, S.; et al. The C3(1)/SV40 T-antigen transgenic mouse model of mammary cancer: Ductal epithelial cell targeting with multistage progression to carcinoma. Oncogene 2000, 19, 1020–1027. [Google Scholar] [CrossRef]
- Herschkowitz, J.I.; Simin, K.; Weigman, V.J.; Mikaelian, I.; Usary, J.; Hu, Z.; Rasmussen, K.E.; Jones, L.P.; Assefnia, S.; Chandrasekharan, S.; et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007, 8, R76. [Google Scholar] [CrossRef]
- Kim, D.H.; Gutierrez-Aguilar, R.; Kim, H.J.; Woods, S.C.; Seeley, R.J. Increased adipose tissue hypoxia and capacity for angiogenesis and inflammation in young diet-sensitive C57 mice compared with diet-resistant FVB mice. Int. J. Obes. 2013, 37, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Yoshidome, K.; Shibata, M.A.; Maroulakou, I.G.; Liu, M.L.; Jorcyk, C.L.; Gold, L.G.; Welch, V.N.; Green, J.E. Genetic alterations in the development of mammary and prostate cancer in the C3(1)/Tag transgenic mouse model. Int. J. Oncol. 1998, 12, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.C.; Qing, K.; Chen, Y. Diet-induced changes in stearoyl-CoA desaturase 1 expression in obesity-prone and -resistant mice. Obes. Res. 2004, 12, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Devlin, M.J.; Robbins, A.; Cosman, M.N.; Moursi, C.A.; Cloutier, A.M.; Louis, L.; Van Vliet, M.; Conlon, C.; Bouxsein, M.L. Differential effects of high fat diet and diet-induced obesity on skeletal acquisition in female C57BL/6J vs. FVB/NJ Mice. Bone Rep. 2018, 8, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.K.; Hallahan, N.L.; Brown, S.H.; Liu, M.; Mitchell, T.W.; Cooney, G.J.; Turner, N. Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia 2013, 56, 1129–1139. [Google Scholar] [CrossRef]
- Surwit, R.S.; Feinglos, M.N.; Rodin, J.; Sutherland, A.; Petro, A.E.; Opara, E.C.; Kuhn, C.M.; Rebuffe-Scrive, M. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism 1995, 44, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, S.M.; Chiao, L.J.; Nogueira, L.; Usary, J.; Perou, C.M.; Varticovski, L.; Hursting, S.D. Dietary energy balance modulates epithelial-to-mesenchymal transition and tumor progression in murine claudin-low and basal-like mammary tumor models. Cancer Prev. Res. 2012, 5, 930–942. [Google Scholar] [CrossRef] [PubMed]
- O’Flanagan, C.H.; Rossi, E.L.; McDonell, S.B.; Chen, X.; Tsai, Y.H.; Parker, J.S.; Usary, J.; Perou, C.M.; Hursting, S.D. Metabolic reprogramming underlies metastatic potential in an obesity-responsive murine model of metastatic triple negative breast cancer. NPJ Breast Cancer 2017, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Bowers, L.W.; Doerstling, S.S.; Shamsunder, M.G.; Lineberger, C.G.; Rossi, E.L.; Montgomery, S.A.; Coleman, M.F.; Gong, W.; Parker, J.S.; Howell, A.; et al. Reversing the Genomic, Epigenetic, and Triple-Negative Breast Cancer–Enhancing Effects of Obesity. Cancer Prev. Res. 2022, 15, 581–594. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdottir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef]
- Newman, A.M.; Steen, C.B.; Liu, C.L.; Gentles, A.J.; Chaudhuri, A.A.; Scherer, F.; Khodadoust, M.S.; Esfahani, M.S.; Luca, B.A.; Steiner, D.; et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat. Biotechnol. 2019, 37, 773–782. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- de Hoon, M.J.L.; Imoto, S.; Nolan, J.; Miyano, S. Open source clustering software. Bioinformatics 2004, 20, 1453–1454. [Google Scholar] [CrossRef]
- Sena, I.F.G.; Rocha, B.G.S.; Picoli, C.C.; Santos, G.S.P.; Costa, A.C.; Goncalves, B.O.P.; Garcia, A.P.V.; Soltani-Asl, M.; Coimbra-Campos, L.M.C.; Silva, W.N.; et al. C(3)1-TAg in C57BL/6 J background as a model to study mammary tumor development. Histochem. Cell Biol. 2021, 156, 165–182. [Google Scholar] [CrossRef]
- Turbitt, W.J.; Xu, Y.; Sosnoski, D.M.; Collins, S.D.; Meng, H.; Mastro, A.M.; Rogers, C.J. Physical Activity Plus Energy Restriction Prevents 4T1.2 Mammary Tumor Progression, MDSC Accumulation, and an Immunosuppressive Tumor Microenvironment. Cancer Prev. Res. 2019, 12, 493–506. [Google Scholar] [CrossRef]
- Shinde, A.; Wilmanski, T.; Chen, H.; Teegarden, D.; Wendt, M.K. Pyruvate carboxylase supports the pulmonary tropism of metastatic breast cancer. Breast Cancer Res. 2018, 20, 76. [Google Scholar] [CrossRef]
- Kaur, P.; Nagaraja, G.M.; Zheng, H.; Gizachew, D.; Galukande, M.; Krishnan, S.; Asea, A. A mouse model for triple-negative breast cancer tumor-initiating cells (TNBC-TICs) exhibits similar aggressive phenotype to the human disease. BMC Cancer 2012, 12, 120. [Google Scholar] [CrossRef]
- Sugiura, K.; Stock, C.C. Studies in a tumor spectrum. II. The effect of 2,4,6-triethylenimino-s-triazine on the growth of a variety of mouse and rat tumors. Cancer 1952, 5, 979–991. [Google Scholar] [CrossRef]
- Sipe, L.M.; Chaib, M.; Korba, E.B.; Jo, H.; Lovely, M.C.; Counts, B.R.; Tanveer, U.; Holt, J.R.; Clements, J.C.; John, N.A.; et al. Response to immune checkpoint blockade improved in pre-clinical model of breast cancer after bariatric surgery. eLife 2022, 11, 79143. [Google Scholar] [CrossRef]
- Ringel, A.E.; Drijvers, J.M.; Baker, G.J.; Catozzi, A.; García-Cañaveras, J.C.; Gassaway, B.M.; Miller, B.C.; Juneja, V.R.; Nguyen, T.H.; Joshi, S.; et al. Obesity Shapes Metabolism in the Tumor Microenvironment to Suppress Anti-Tumor Immunity. Cell 2020, 183, 1848–1866.e26. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, G.; Liu, J.; Zhang, C.; Liu, Q.; Wang, H.; Chen, T. Comprehensive analysis of the cancer driver genes in breast cancer demonstrates their roles in cancer prognosis and tumor microenvironment. World J. Surg. Oncol. 2021, 19, 273. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carson, M.S.; Rädler, P.D.; Albright, J.; VerHague, M.; Rezeli, E.T.; Roth, D.; French, J.E.; Perou, C.M.; Hursting, S.D.; Coleman, M.F. Development and Characterization of Syngeneic Orthotopic Transplant Models of Obesity-Responsive Triple-Negative Breast Cancer in C57BL/6J Mice. Cancers 2024, 16, 2803. https://doi.org/10.3390/cancers16162803
Carson MS, Rädler PD, Albright J, VerHague M, Rezeli ET, Roth D, French JE, Perou CM, Hursting SD, Coleman MF. Development and Characterization of Syngeneic Orthotopic Transplant Models of Obesity-Responsive Triple-Negative Breast Cancer in C57BL/6J Mice. Cancers. 2024; 16(16):2803. https://doi.org/10.3390/cancers16162803
Chicago/Turabian StyleCarson, Meredith S., Patrick D. Rädler, Jody Albright, Melissa VerHague, Erika T. Rezeli, Daniel Roth, John E. French, Charles M. Perou, Stephen D. Hursting, and Michael F. Coleman. 2024. "Development and Characterization of Syngeneic Orthotopic Transplant Models of Obesity-Responsive Triple-Negative Breast Cancer in C57BL/6J Mice" Cancers 16, no. 16: 2803. https://doi.org/10.3390/cancers16162803
APA StyleCarson, M. S., Rädler, P. D., Albright, J., VerHague, M., Rezeli, E. T., Roth, D., French, J. E., Perou, C. M., Hursting, S. D., & Coleman, M. F. (2024). Development and Characterization of Syngeneic Orthotopic Transplant Models of Obesity-Responsive Triple-Negative Breast Cancer in C57BL/6J Mice. Cancers, 16(16), 2803. https://doi.org/10.3390/cancers16162803






