The Effects of Gynecological Tumor Irradiation on the Immune System
Abstract
Simple Summary
Abstract
1. Introduction
2. Classical Radiobiology: The “5Rs” Model
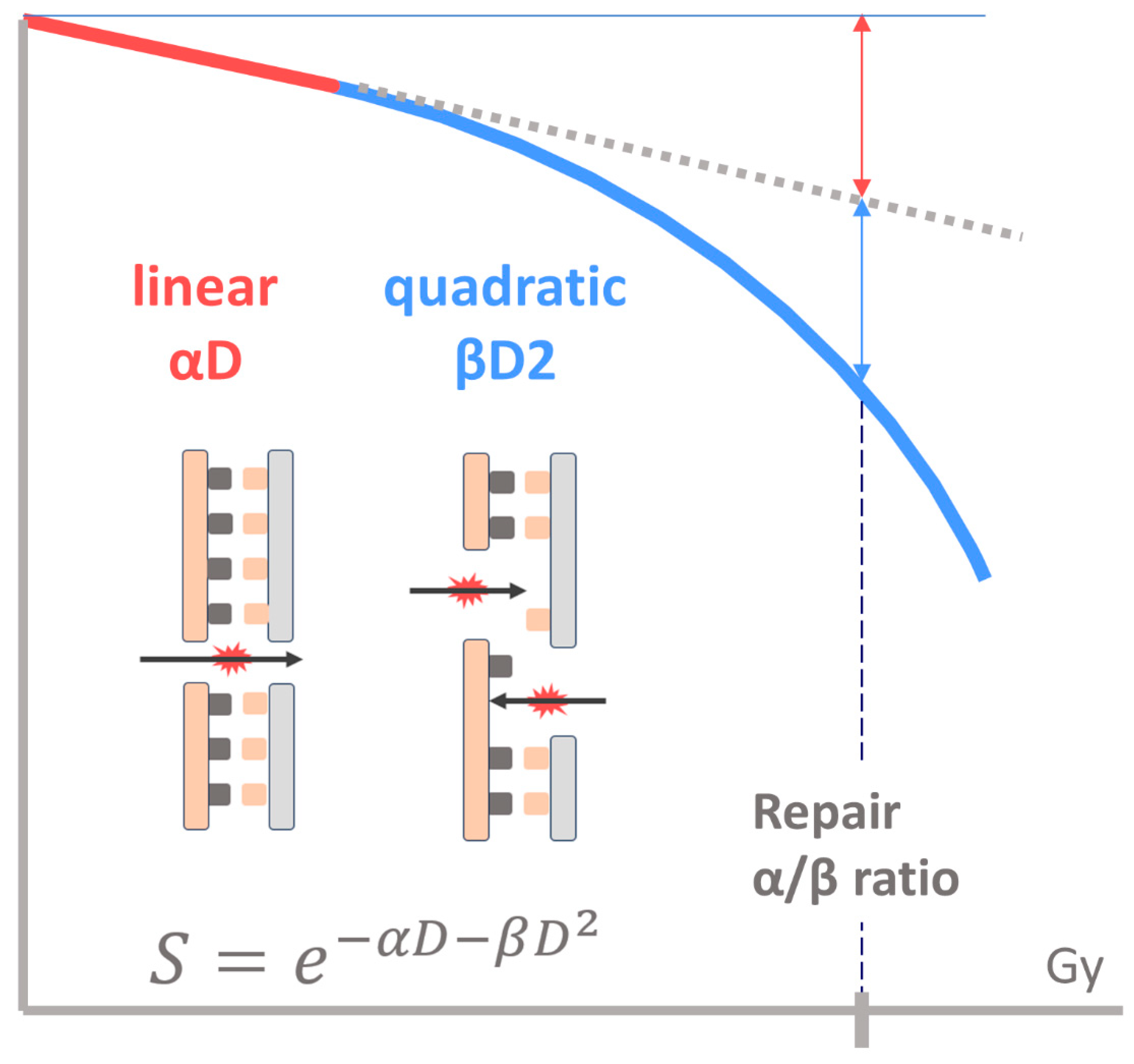
2.1. Linear–Quadratic Model
2.2. Regeneration
2.3. Redistribution
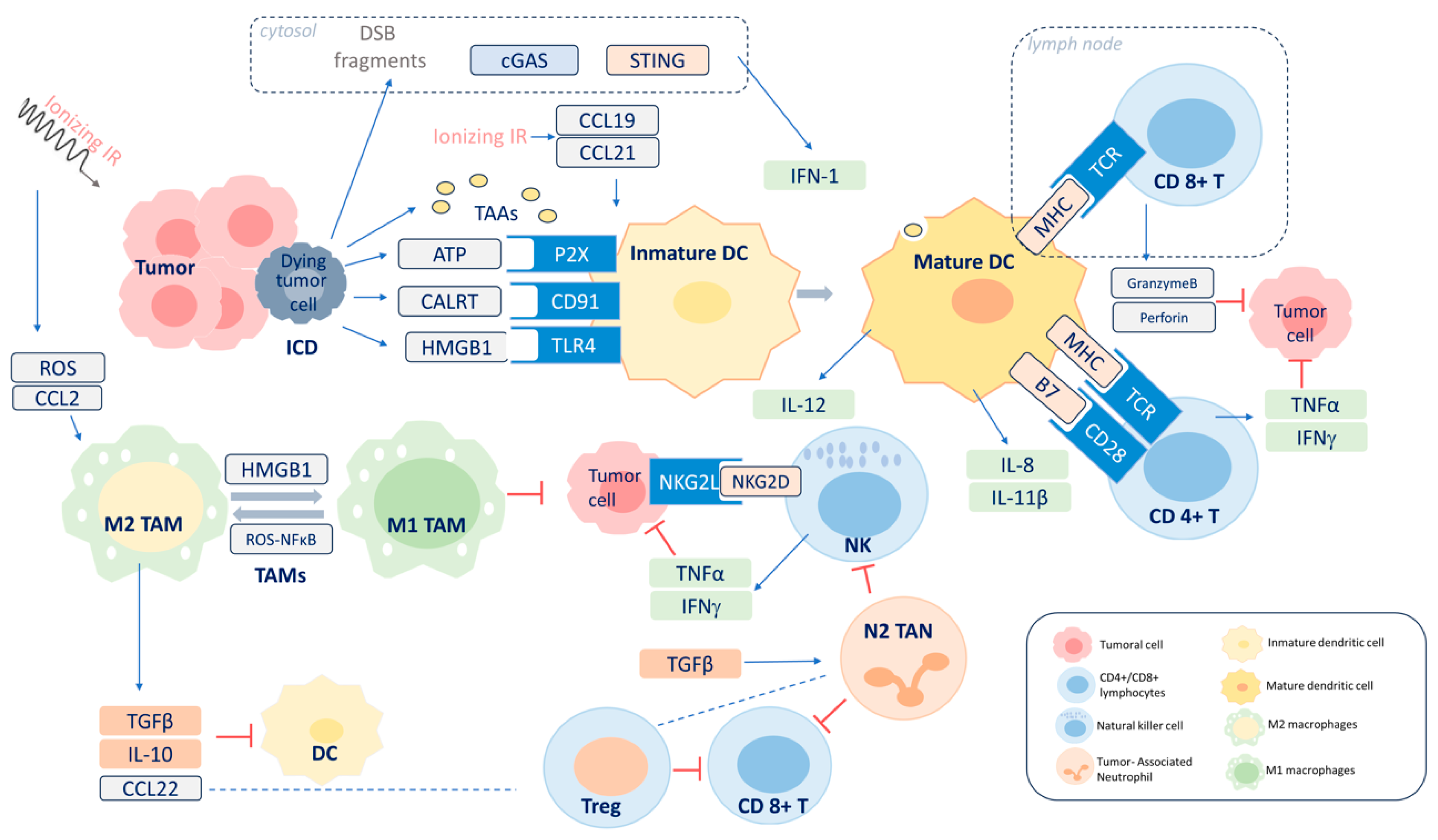
3. Radiation-Induced Immune Response
3.1. Inflammatory Microenvironment
3.2. ICD
3.3. Adaptive Immunity
3.4. AE
3.5. Radiation-Induced Immunosuppression
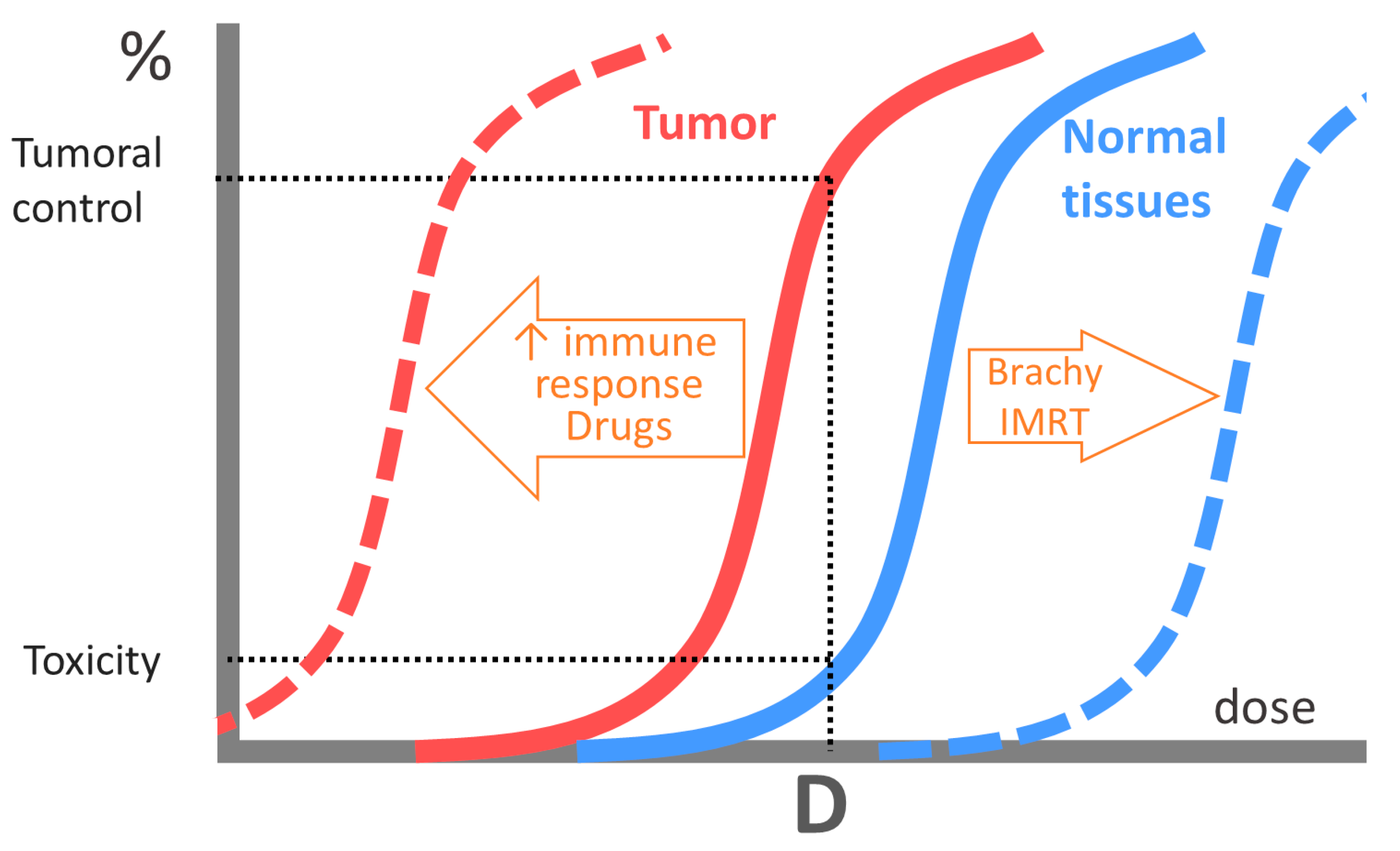
4. Clinical Perspectives
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Raspollini, M.R.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer—Update 2023. Int. J. Gynecol. Cancer 2023, 33, 649–666. [Google Scholar] [CrossRef] [PubMed]
- Cordoba Largo, S.; Rodriguez Rodriguez, I.; Rodriguez Villalba, S.; Najjari Jamal, D.; Anchuelo Latorre, J.; Celada Álvarez, F.; Garcia Cabezas, S.; de la Fuente Alonso, C.; Couselo Paniagua, L.; GINECOR (Spanish Gynaecological Tumors Group of SEOR). Radiation therapy for vulvar cancer: Consensus guidelines of the GINECOR working group of the Spanish Society of Radiation Oncology. Part 1: Clinical recommendations. Clin. Transl. Oncol. 2023, 25, 2153–2168. [Google Scholar] [CrossRef]
- Mahantshetty, U.; Poetter, R.; Beriwal, S.; Grover, S.; Lavanya, G.; Rai, B.; Petric, P.; Tanderup, K.; Carvalho, H.; Hegazy, N.; et al. IBS-GEC ESTRO-ABS recommendations for CT based contouring in image guided adaptive brachytherapy for cervical cancer. Radiother. Oncol. 2021, 160, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef]
- Chambers, L.M.; Jia, X.; Rose, P.G.; AlHilli, M. Impact of treatment modality on overall survival in women with advanced endometrial cancer: A National Cancer Database analysis. Gynecol. Oncol. 2021, 160, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Margioula-Siarkou, C.; Almperis, A.; Gullo, G.; Almperi, E.A.; Margioula-Siarkou, G.; Nixarlidou, E.; Mponiou, K.; Papakotoulas, P.; Sardeli, C.; Guyon, F.; et al. Sentinel Lymph Node Staging in Early-Stage Cervical Cancer: A Comprehensive Review. J. Clin. Med. 2023, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.; The Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Horeweg, N.; Nout, R.A.; Jürgenliemk-Schulz, I.M.; Lutgens, L.C.H.W.; Jobsen, J.J.; Haverkort, M.A.D.; PORTEC Study Group. Molecular Classification Predicts Response to Radiotherapy in the Randomized PORTEC-1 and PORTEC-2 Trials for Early-Stage Endometrioid Endometrial Cancer. J. Clin. Oncol. 2023, 41, 4369–4380. [Google Scholar] [CrossRef]
- Chen, H.; Han, Z.; Luo, Q.; Wang, Y.; Li, Q.; Zhou, L.; Zuo, H. Radiotherapy modulates tumor cell fate decisions: A review. Radiat. Oncol. 2022, 17, 196. [Google Scholar] [CrossRef] [PubMed]
- Steel, G.G.; McMillan, T.J.; Peacock, J.H. The 5Rs of radiobiology. Int. J. Radiat. Biol. 1989, 56, 1045–1048. [Google Scholar] [CrossRef] [PubMed]
- Bernier, J.; Hall, E.J.; Giaccia, A. Radiation oncology: A century of achievements. Nat. Rev. Cancer 2004, 4, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Shibata, A.; Jeggo, P.A. DNA double-strand break repair in a cellular context. Clin. Oncol. (R. Coll. Radiol.) 2014, 26, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Curtin, N.J. DNA repair dysregulation from cancer driver to therapeutic target. Nat. Rev. Cancer. 2012, 12, 801–817. [Google Scholar] [CrossRef] [PubMed]
- Takada, Y.; Someya, M.; Matsumoto, Y.; Satoh, M.; Nakata, K.; Hori, M.; Saito, M.; Hirokawa, N.; Tateoka, K.; Teramoto, M.; et al. Influence of Ku86 and XRCC4 expression in uterine cervical cancer on the response to preoperative radiotherapy. Med. Mol. Morphol. 2016, 49, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Someya, M.; Tsuchiya, T.; Fukushima, Y.; Hasegawa, T.; Hori, M.; Kitagawa, M.; Gocho, T.; Mafune, S.; Ikeuchi, Y.; Hirohashi, Y.; et al. Prediction of treatment response from the microenvironment of tumor immunity in cervical cancer patients treated with chemoradiotherapy. Med. Mol. Morphol. 2021, 54, 245–252. [Google Scholar] [CrossRef]
- Saygili, U.; Gorkay, I.B.; Koyuncuoglu, M.; Gol, M.; Uslu, T.; Erten, O. The relationship between expression of Ku70 and survival in irradiated patients with endometrial carcinoma. Gynecol. Oncol. 2004, 95, 518–522. [Google Scholar] [CrossRef]
- Fowler, J.F. The linear-quadratic formula and progress in fractionated radiotherapy. Br. J. Radiol. 1989, 62, 679–694. [Google Scholar] [CrossRef]
- Jones, L.; Hoban, P.; Metcalfe, P. The use of the linear quadratic model in radiotherapy: A review. Australas. Phys. Eng. Sci. Med. 2001, 24, 132–146. [Google Scholar] [CrossRef]
- Swamidas, J.; Mahantshetty, U. Prescribing, Recording, and Reporting Brachytherapy for Cancer of the Cervix. J. ICRU 2013, 13, 1–10. [Google Scholar] [CrossRef]
- Sood, B.; Garg, M.; Avadhani, J.; Gorla, G.; Malhotra, H.; Guha, C.; Deore, S.; Vikram, B. Predictive value of linear-quadratic model in the treatment of cervical cancer using high-dose-rate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Mazeron, R.; Castelnau-Marchand, P.; Dumas, I.; del Campo, E.R.; Kom, L.K.; Martinetti, F.; Farha, G.; Tailleur, A.; Morice, P.; Chargari, C.; et al. Impact of treatment time and dose escalation on local control in locally advanced cervical cancer treated by chemoradiation and image-guided pulsed-dose rate adaptive brachytherapy. Radiother. Oncol. 2015, 114, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Tanderup, K.; Fokdal, L.U.; Sturdza, A.; Haie-Meder, C.; Mazeron, R.; van Limbergen, E.; Jürgenliemk-Schulz, I.; Petric, P.; Hoskin, P.; Dörr, W.; et al. Effect of tumor dose, volume and overall treatment time on local control after radiochemotherapy including MRI guided brachytherapy of locally advanced cervical cancer. Radiother. Oncol. 2016, 120, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.R.; Marcus, R.B., Jr.; Sombeck, M.D.; Mendenhall, W.M.; Morgan, L.S.; Freeman, D.E.; Million, R.R. Radiotherapy alone for carcinoma of the vagina: The importance of overall treatment time. Int. J. Radiat. Oncol. Biol. Phys. 1994, 29, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Chow, B.; Warkentin, B.; Menon, G. Radiobiological dose calculation parameters for cervix cancer brachytherapy: A systematic review. Brachytherapy 2019, 18, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Mayr, N.A.; Gao, M.; Lo, S.S.; Wang, J.Z.; Jia, G.; Yuh, W.T. Onset time of tumor repopulation for cervical cancer: First evidence from clinical data. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Dale, R.G. The application of the linear-quadratic dose-effect equation to fractionated and protracted radiotherapy. Br. J. Radiol. 1985, 58, 515–528. [Google Scholar] [CrossRef]
- Fowler, J.F. 21 years of biologically effective dose. Br. J. Radiol. 2010, 83, 554–568. [Google Scholar] [CrossRef]
- Kastan, M.B.; Bartek, J. Cell-cycle checkpoints and cancer. Nature 2004, 432, 316–323. [Google Scholar] [CrossRef]
- Harms, W.; Weber, K.J.; Ehemann, V.; Zuna, I.; Debus, J.; Peschke, P. Differential effects of CLDR and PDR brachytherapy on cell cycle progression in a syngeneic rat prostate tumour model. Int. J. Radiat. Biol. 2006, 82, 191–196. [Google Scholar] [CrossRef]
- Sinclair, W.K.; Morton, R.A. X-Ray and ultraviolet sensitivity of synchronized Chinese hamster cells at various stages of the cell cycle. Biophys. J. 1965, 5, 1–25. [Google Scholar] [CrossRef]
- Benlloch, R.; Castejón, R.; Rosado, S.; Coronado, M.J.; Sánchez, P.; Romero, J. In vitro radiosensitization by eribulin in human cancer cell lines. Rep. Pract. Oncol. Radiother. 2022, 27, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, J.P.; Meyer, J.J.; Marks, L.B. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin. Radiat. Oncol. 2008, 18, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.J. The linear-quadratic model is an appropriate methodology for determining isoeffective doses at large doses per fraction. Semin. Radiat. Oncol. 2008, 18, 234–239. [Google Scholar] [CrossRef]
- Barker, H.E.; Paget, J.T.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer. 2015, 15, 409–425. [Google Scholar] [CrossRef]
- Boustani, J.; Grapin, M.; Laurent, P.A.; Apetoh, L.; Mirjolet, C. The 6th R of Radiobiology: Reactivation of Anti-Tumor Immune Response. Cancers 2019, 11, 860. [Google Scholar] [CrossRef] [PubMed]
- Ladbury, C.; Germino, E.; Novak, J.; Liu, J.; Horne, Z.; Dyer, B.; Glaser, S. Combination radiation and immunotherapy in gynecologic malignancies—A comprehensive review. Transl. Cancer Res. 2021, 10, 2609–2619. [Google Scholar] [CrossRef]
- Taghizadeh-Hesary, F. “Reinforcement” by Tumor Microenvironment: The Seventh “R” of Radiobiology. Int. J. Radiat. Oncol. Biol. Phys. 2024, 119, 727–733. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Chen, D.; Yu, J. Radiotherapy combined with immunotherapy: The dawn of cancer treatment. Signal Transduct. Target. Ther. 2022, 7, 258. [Google Scholar] [CrossRef]
- Hallahan, D.E.; Spriggs, D.R.; Beckett, M.A.; Kufe, D.W.; Weichselbaum, R.R. Increased tumor necrosis factor alpha mRNA after cellular exposure to ionizing radiation. Proc. Natl. Acad. Sci. USA 1989, 86, 10104–10107. [Google Scholar] [CrossRef] [PubMed]
- Schaue, D.; McBride, W.H. Links between innate immunity and normal tissue radiobiology. Radiat. Res. 2010, 173, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Ho, A.S.; Chang, C.C.; Sie, Z.L.; Peng, C.L.; Chang, J.; Cheng, C.C. Radiotherapy enhances CXCR3highCD8+ T cell activation through inducing IFNγ-mediated CXCL10 and ICAM-1 expression in lung cancer cells. Cancer Immunol. Immunother. 2023, 72, 1865–1880. [Google Scholar] [CrossRef] [PubMed]
- Lugade, A.A.; Sorensen, E.W.; Gerber, S.A.; Moran, J.P.; Frelinger, J.G.; Lord, E.M. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J. Immunol. 2008, 180, 3132–3139. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Barber, G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 2008, 455, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Ma, Z.; Barber, G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 2009, 461, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liang, H.; Xu, M.; Yang, X.; Burnette, B.; Arina, A.; Li, X.D.; Mauceri, H.; Beckett, M.; Darga, T.; et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity 2014, 41, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Ferguson, T.; Zitvogel, L.; Kroemer, G. Immunogenic and tolerogenic cell death. Nat. Rev. Immunol. 2009, 9, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buque, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017, 17, 97–111. [Google Scholar] [CrossRef]
- Golden, E.B.; Frances, D.; Pellicciotta, I.; Demaria, S.; Helen Barcellos-Hoff, M.; Formenti, S.C. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology 2014, 3, e28518. [Google Scholar] [CrossRef]
- Gameiro, S.R.; Jammeh, M.L.; Wattenberg, M.M.; Tsang, K.Y.; Ferrone, S.; Hodge, J.W. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget 2014, 5, 403–416. [Google Scholar] [CrossRef]
- Wang, L.; He, L.; Bao, G.; He, X.; Fan, S.; Wang, H. Ionizing Radiation Induces HMGB1 Cytoplasmic Translocation and Extracellular Release. Guo Ji Fang She Yi Xue He Yi Xue Za Zhi 2016, 40, 91–99. [Google Scholar] [PubMed]
- Kawai, T.; Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.K.; Sköld, A.E.; Mohanram, V.; Persson, C.; Johansson, U.; Spetz, A.L. Activated apoptotic cells induce dendritic cell maturation via engagement of Toll-like receptor 4 (TLR4), dendritic cell-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin (DC-SIGN), and β2 integrins. J. Biol. Chem. 2012, 287, 13731–13742. [Google Scholar] [CrossRef]
- Kulzer, L.; Rubner, Y.; Deloch, L.; Allgäuer, A.; Frey, B.; Fietkau, R.; Dörrie, J.; Schaft, N.; Gaipl, U.S. Norm- and hypo-fractionated radiotherapy is capable of activating human dendritic cells. J. Immunotoxicol. 2014, 11, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Son, Y.O.; Park, S.W.; Bae, J.H.; Chung, J.S.; Kim, H.H.; Chung, B.S.; Kim, S.H.; Kang, C.D. Increase of NKG2D ligands and sensitivity to NK cell-mediated cytotoxicity of tumor cells by heat shock and ionizing radiation. Exp. Mol. Med. 2006, 38, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Linares, I.; Berenguer Frances, M.Á.; Cañas, R.; Najjari, D.; Gutiérrez, C.; Marín, S.; Comas, S.; Guedea, F.; Pujol, M. Brachytherapy for targeting the immune system in cervical cancer patients. BMC Immunol. 2023, 24, 23. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, O.S.; Zheng, Y.; Nakamura, K.; Attridge, K.; Manzotti, C.; Schmidt, E.M.; Baker, J.; Jeffery, L.E.; Kaur, S.; Briggs, Z.; et al. Trans-endocytosis of CD80 and CD86: A molecular basis for the cell-extrinsic function of CTLA-4. Science 2011, 332, 600–603. [Google Scholar] [CrossRef]
- Borst, J.; Ahrends, T.; Bąbała, N.; Melief, C.J.M.; Kastenmüller, W. CD4+ T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2018, 18, 635–647. [Google Scholar] [CrossRef]
- Wan, S.; Pestka, S.; Jubin, R.G.; Lyu, Y.L.; Tsai, Y.C.; Liu, L.F. Chemotherapeutics and radiation stimulate MHC class I expression through elevated interferon-beta signaling in breast cancer cells. PLoS ONE 2012, 7, e32542. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, S.; Wang, B.; Kawashima, N.; Braunstein, S.; Badura, M.; Cameron, T.O.; Babb, J.S.; Schneider, R.J.; Formenti, S.C.; Dustin, M.L.; et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J. Immunol. 2008, 181, 3099–3107. [Google Scholar] [CrossRef]
- Piersma, S.J.; Jordanova, E.S.; van Poelgeest, M.I.; Kwappenberg, K.M.; van der Hulst, J.M.; Drijfhout, J.W.; Melief, C.J.; Kenter, G.G.; Fleuren, G.J.; Offringa, R.; et al. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007, 67, 354–361. [Google Scholar] [CrossRef] [PubMed]
- de Vos van Steenwijk, P.J.; Ramwadhdoebe, T.H.; Goedemans, R.; Doorduijn, E.M.; van Ham, J.J.; Gorter, A.; van Hall, T.; Kuijjer, M.L.; van Poelgeest, M.I.; van der Burg, S.H.; et al. Tumor-infiltrating CD14-positive myeloid cells and CD8-positive T-cells prolong survival in patients with cervical carcinoma. Int. J. Cancer. 2013, 133, 2884–2894. [Google Scholar] [CrossRef]
- Jordanova, E.S.; Gorter, A.; Ayachi, O.; Prins, F.; Durrant, L.G.; Kenter, G.G.; van der Burg, S.H.; Fleuren, G.J. Human leukocyte antigen class I, MHC class I chain-related molecule A, and CD8+/regulatory T-cell ratio: Which variable determines survival of cervical cancer patients? Clin. Cancer Res. 2008, 14, 2028–2035. [Google Scholar] [CrossRef]
- Demaria, S.; Ng, B.; Devitt, M.L.; Babb, J.S.; Kawashima, N.; Liebes, L.; Formenti, S.C. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 2004, 8, 62–70. [Google Scholar] [CrossRef]
- Abuodeh, Y.; Venkat, P.; Kim, S. Systematic review of case reports on the abscopal effect. Curr. Probl. Cancer. 2016, 40, 25–37. [Google Scholar] [CrossRef]
- Takaya, M.; Niibe, Y.; Tsunoda, S.; Jobo, T.; Imai, M.; Kotani, S.; Unno, N.; Hayakawa, K. Abscopal effect of radiation on toruliform para-aortic lymph node metastases of advanced uterine cervical carcinoma—A case report. Anticancer. Res. 2007, 27, 499–503. [Google Scholar] [PubMed]
- Ando, K.; Fujita, H.; Hosoi, A.; Ma, L.; Wakatsuki, M.; Seino, K.I.; Kakimi, K.; Imai, T.; Shimokawa, T.; Nakano, T. Intravenous dendritic cell administration enhances suppression of lung metastasis induced by carbon-ion irradiation. J. Radiat. Res. 2017, 58, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Demaria, S.; Kawashima, N.; Yang, A.M.; Devitt, M.L.; Babb, J.S.; Allison, J.P.; Formenti, S.C. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 2005, 11, 728–734. [Google Scholar] [CrossRef]
- Zhu, L.; Hu, S.; Chen, Q.; Zhang, H.; Fu, J.; Zhou, Y.; Bai, Y.; Pan, Y.; Shao, C. Macrophage contributes to radiation-induced anti-tumor abscopal effect on transplanted breast cancer by HMGB1/TNF-α signaling factors. Int. J. Biol. Sci. 2021, 17, 926–941. [Google Scholar] [CrossRef] [PubMed]
- Vanpouille-Box, C.; Diamond, J.M.; Pilones, K.A.; Zavadil, J.; Babb, J.S.; Formenti, S.C.; Barcellos-Hoff, M.H.; Demaria, S. TGFβ Is a Master Regulator of Radiation Therapy-Induced Antitumor Immunity. Cancer Res. 2015, 75, 2232–2242. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, T.; Wang, Y.; Lu, D.; Du, J.; Feng, X.; Zhou, H.; Liu, N.; Zhu, H.; Qin, S.; et al. ICAM-1 orchestrates the abscopal effect of tumor radiotherapy. Proc. Natl. Acad. Sci. USA 2021, 118, e2010333118. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wu, M.O.; De la Maza, L.; Yun, Z.; Yu, J.; Zhao, Y.; Cho, J.; de Perrot, M. Targeting the inhibitory receptor CTLA-4 on T cells increased abscopal effects in murine mesothelioma model. Oncotarget 2015, 6, 12468–12480. [Google Scholar] [CrossRef]
- Formenti, S.C.; Rudqvist, N.P.; Golden, E.; Cooper, B.; Wennerberg, E.; Lhuillier, C.; Vanpouille-Box, C.; Friedman, K.; Ferrari de Andrade, L.; Wucherpfennig, K.W.; et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat. Med. 2018, 24, 1845–1851. [Google Scholar] [CrossRef]
- Ji, D.; Song, C.; Li, Y.; Xia, J.; Wu, Y.; Jia, J.; Cui, X.; Yu, S.; Gu, J. Combination of radiotherapy and suppression of Tregs enhances abscopal antitumor effect and inhibits metastasis in rectal cancer. J. Immunother. Cancer. 2020, 8, e000826. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Gao, Y.; Li, J.; Gao, F.; Zhang, J.; Li, L.; Feng, X.; Zuo, D.; Jin, X.; Chen, W.; et al. Killing two birds with one stone: Abscopal effect mechanism and its application prospect in radiotherapy. Crit. Rev. Oncol. Hematol. 2024, 196, 104325. [Google Scholar] [CrossRef] [PubMed]
- Kachikwu, E.L.; Iwamoto, K.S.; Liao, Y.P.; DeMarco, J.J.; Agazaryan, N.; Economou, J.S.; McBride, W.H.; Schaue, D. Radiation enhances regulatory T cell representation. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1128–1135. [Google Scholar] [CrossRef]
- Li, C.; Jiang, P.; Wei, S.; Xu, X.; Wang, J. Regulatory T cells in tumor microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Mol. Cancer. 2020, 19, 116. [Google Scholar] [CrossRef]
- Burnette, B.; Weichselbaum, R.R. Radiation as an immune modulator. Semin. Radiat. Oncol. 2013, 23, 273–280. [Google Scholar] [CrossRef]
- Heylmann, D.; Rödel, F.; Kindler, T.; Kaina, B. Radiation sensitivity of human and murine peripheral blood lymphocytes, stem and progenitor cells. Biochim. Biophys. Acta 2014, 1846, 121–129. [Google Scholar] [CrossRef]
- Qinfeng, S.; Depu, W.; Xiaofeng, Y.; Shah, W.; Hongwei, C.; Yili, W. In situ observation of the effects of local irradiation on cytotoxic and regulatory T lymphocytes in cervical cancer tissue. Radiat. Res. 2013, 179, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Marciscano, A.E.; Ghasemzadeh, A.; Nirschl, T.R.; Theodros, D.; Kochel, C.M.; Francica, B.J.; Muroyama, Y.; Anders, R.A.; Sharabi, A.B.; Velarde, E.; et al. Elective Nodal Irradiation Attenuates the Combinatorial Efficacy of Stereotactic Radiation Therapy and Immunotherapy. Clin. Cancer Res. 2018, 24, 5058–5071. [Google Scholar] [CrossRef]
- Yovino, S.; Kleinberg, L.; Grossman, S.A.; Narayanan, M.; Ford, E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: Modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Investig. 2013, 31, 140–144. [Google Scholar] [CrossRef]
- Liu, C.; Lu, J.; Tian, H.; Du, W.; Zhao, L.; Feng, J.; Yuan, D.; Li, Z. Increased expression of PD-L1 by the human papillomavirus 16 E7 oncoprotein inhibits anticancer immunity. Mol. Med. Rep. 2017, 15, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Iijima, M.; Okonogi, N.; Nakajima, N.I.; Morokoshi, Y.; Kanda, H.; Yamada, T.; Kobayashi, Y.; Banno, K.; Wakatsuki, M.; Yamada, S.; et al. Significance of PD-L1 expression in carbon-ion radiotherapy for uterine cervical adeno/adenosquamous carcinoma. J. Gynecol. Oncol. 2020, 31, e19. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, T.; Someya, M.; Takada, Y.; Hasegawa, T.; Kitagawa, M.; Fukushima, Y.; Gocho, T.; Hori, M.; Nakata, K.; Hirohashi, Y.; et al. Association between radiotherapy-induced alteration of programmed death ligand 1 and survival in patients with uterine cervical cancer undergoing preoperative radiotherapy. Strahlenther. Onkol. 2020, 196, 725–735. [Google Scholar] [CrossRef]
- Miyata, Y.; Ogo, E.; Abe, T.; Hirata, H.; Tsuda, N.; Ushijima, K.; Kawahara, A.; Akiba, J.; Obara, H.; Kakuma, T. Dynamics in the expression of programmed death ligand 1 and cluster of differentiation 163 in the tumor microenvironment of uterine cervical cancer: A single-center retrospective study. Radiat. Oncol. 2023, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Reddy, O.L.; Shintaku, P.I.; Moatamed, N.A. Programmed death-ligand 1 (PD-L1) is expressed in a significant number of the uterine cervical carcinomas. Diagn. Pathol. 2017, 12, 45. [Google Scholar] [CrossRef]
- Monk, B.J.; Colombo, N.; Tewari, K.S.; Dubot, C.; Caceres, M.V.; Hasegawa, K.; Shapira-Frommer, R.; Salman, P.; Yañez, E.; Gümüş, M.; et al. First-Line Pembrolizumab + Chemotherapy Versus Placebo + Chemotherapy for Persistent, Recurrent, or Metastatic Cervical Cancer: Final Overall Survival Results of KEYNOTE-826. J. Clin. Oncol. 2023, 36, 5505–5511. [Google Scholar] [CrossRef]
- Dewan, M.Z.; Galloway, A.E.; Kawashima, N.; Dewyngaert, J.K.; Babb, J.S.; Formenti, S.C.; Demaria, S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 2009, 15, 5379–5388. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.D.; Drake, C.G.; Scher, H.I.; Fizazi, K.; Bossi, A.; van den Eertwegh, A.J.; Krainer, M.; Houede, N.; Santos, R.; Mahammedi, H.; et al. CA184-043 Investigators. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014, 15, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Mayadev, J.S.; Enserro, D.; Lin, Y.G.; Da Silva, D.M.; Lankes, H.A.; Aghajanian, C.; Ghamande, S.; Moore, K.N.; Kennedy, V.A.; Fracasso, P.M.; et al. Sequential Ipilimumab After Chemoradiotherapy in Curative-Intent Treatment of Patients with Node-Positive Cervical Cancer. JAMA Oncol. 2020, 6, 92–99. [Google Scholar] [CrossRef]
- Da Silva, D.M.; Enserro, D.M.; Mayadev, J.S.; Skeate, J.G.; Matsuo, K.; Pham, H.Q.; Lankes, H.A.; Moxley, K.M.; Ghamande, S.A.; Lin, Y.G.; et al. Immune Activation in Patients with Locally Advanced Cervical Cancer Treated with Ipilimumab Following Definitive Chemoradiation (GOG-9929). Clin. Cancer Res. 2020, 26, 5621–5630. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; See, A.P.; Phallen, J.; Jackson, C.M.; Belcaid, Z.; Ruzevick, J.; Durham, N.; Meyer, C.; Harris, T.J.; Albesiano, E.; et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Dovedi, S.J.; Illidge, T.M. The antitumor immune response generated by fractionated radiation therapy may be limited by tumor cell adaptive resistance and can be circumvented by PD-L1 blockade. Oncoimmunology 2015, 4, e1016709. [Google Scholar] [CrossRef]
- Lorusso, D.; Xiang, Y.; Hasegawa, K.; Scambia, G.; Leiva, M.; Ramos-Elias, P.; Acevedo, A.; Sukhin, V.; Cloven, N.; Pereira de Santana Gomes, A.J.; et al. Pembrolizumab or placebo with chemoradiotherapy followed by pembrolizumab or placebo for newly diagnosed, high-risk, locally advanced cervical cancer (ENGOT-cx11/GOG-3047/KEYNOTE-A18): A randomised, double-blind, phase 3 clinical trial. Lancet 2024, 403, 1341–1350. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero Fernandez, J.; Cordoba Largo, S.; Benlloch Rodriguez, R.; Gil Haro, B. The Effects of Gynecological Tumor Irradiation on the Immune System. Cancers 2024, 16, 2804. https://doi.org/10.3390/cancers16162804
Romero Fernandez J, Cordoba Largo S, Benlloch Rodriguez R, Gil Haro B. The Effects of Gynecological Tumor Irradiation on the Immune System. Cancers. 2024; 16(16):2804. https://doi.org/10.3390/cancers16162804
Chicago/Turabian StyleRomero Fernandez, Jesus, Sofia Cordoba Largo, Raquel Benlloch Rodriguez, and Beatriz Gil Haro. 2024. "The Effects of Gynecological Tumor Irradiation on the Immune System" Cancers 16, no. 16: 2804. https://doi.org/10.3390/cancers16162804
APA StyleRomero Fernandez, J., Cordoba Largo, S., Benlloch Rodriguez, R., & Gil Haro, B. (2024). The Effects of Gynecological Tumor Irradiation on the Immune System. Cancers, 16(16), 2804. https://doi.org/10.3390/cancers16162804






