Development of New Diffuse Large B Cell Lymphoma Mouse Models
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Handling and Maintenance
2.2. Mouse Strains and Genotyping
2.3. Cell Culture and Maintenance
2.4. Lentiviral Infection with the Luciferase Gene
2.5. DLBCL Cell Tail Vein Injection and In Vivo Bioluminescence Imaging
2.6. Ex Vivo Imaging
2.7. Immunohistochemistry
2.8. Western Blot Analysis
2.9. Cell Proliferation Assay
2.10. qPCR Analysis
2.11. Statistical Analyses
3. Results
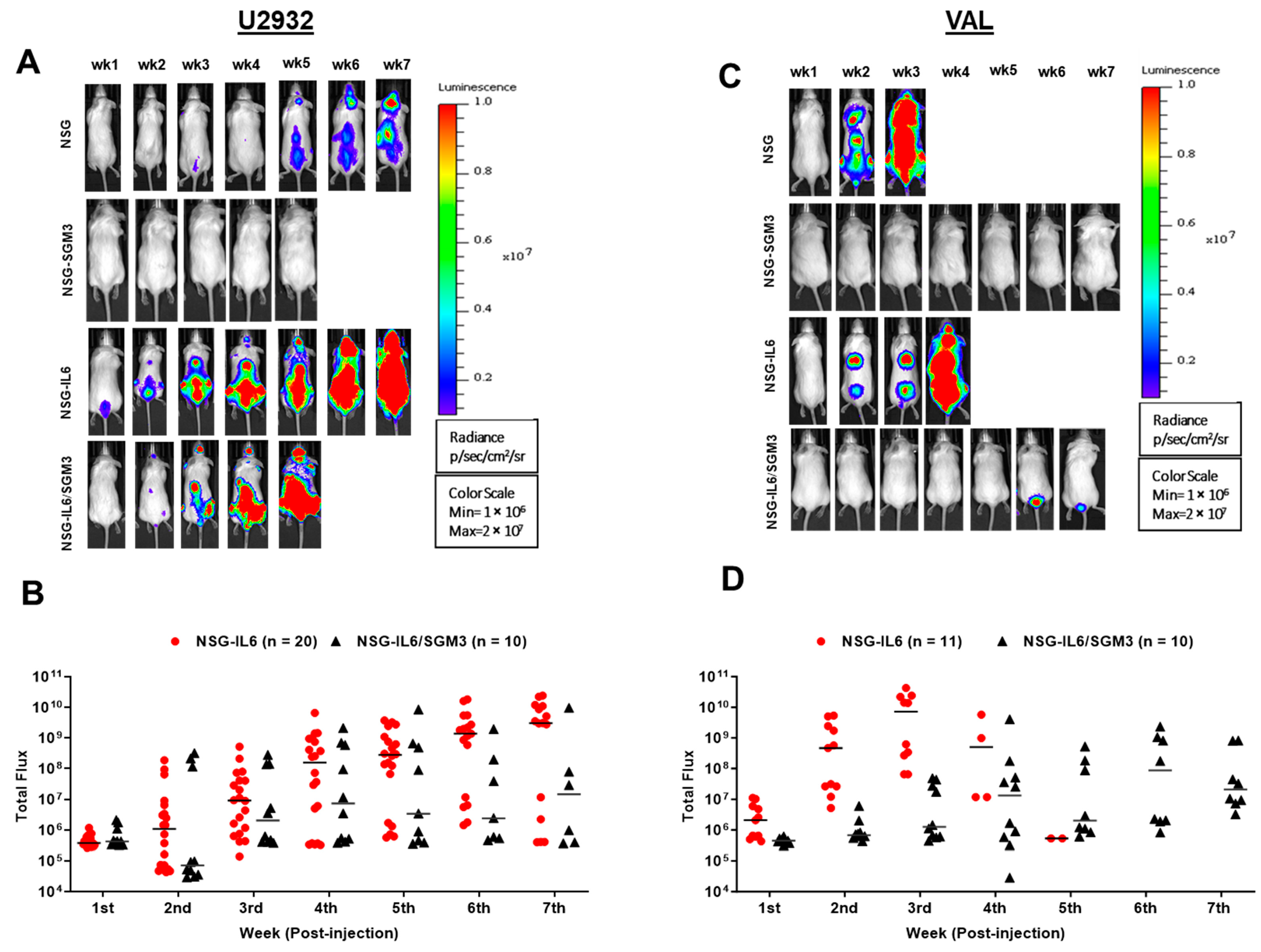
3.1. Luc-Expressing DLBCL Cells Engraft and Expand Rapidly in an Intravenous Xenograft NSG-IL6 Mouse Model
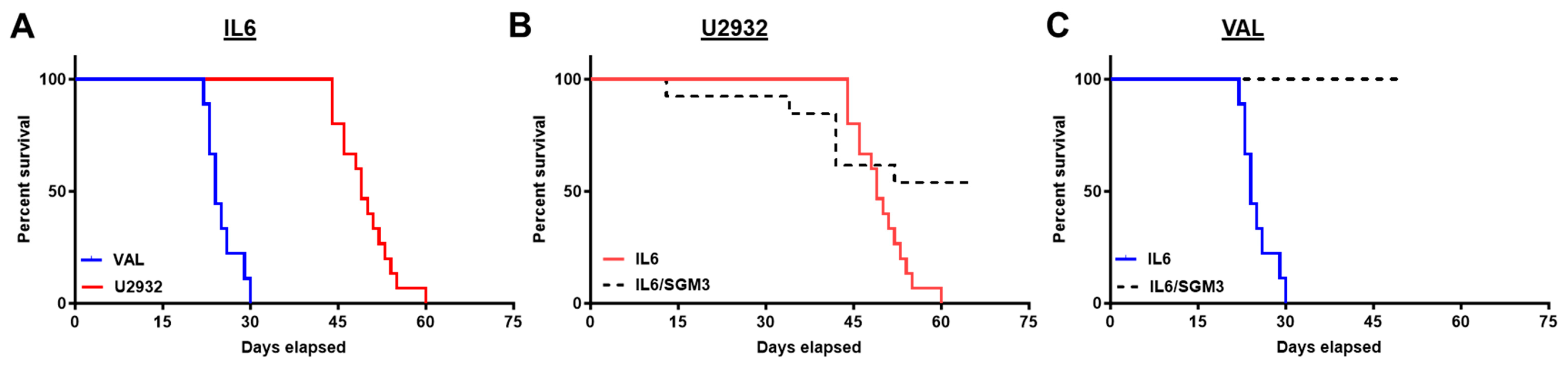
3.2. Recipients of DLBCL Cell Intravenous Xenografts Show Poor Survival Corresponding to Tumor Burden
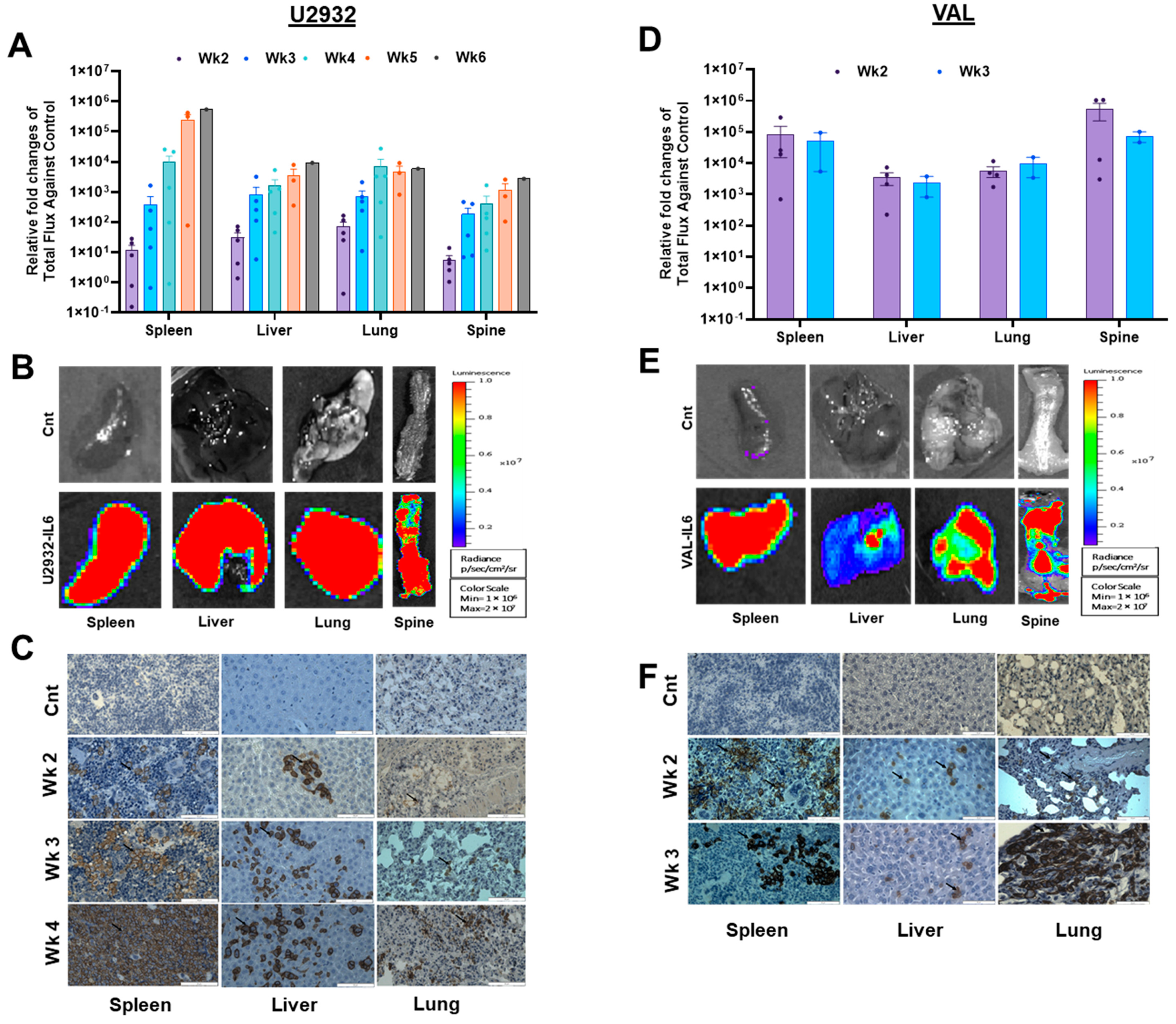
3.3. DLBCL Intravenous Xenografts in NSG-IL6 Infiltrate Various Organs in Disease Progression
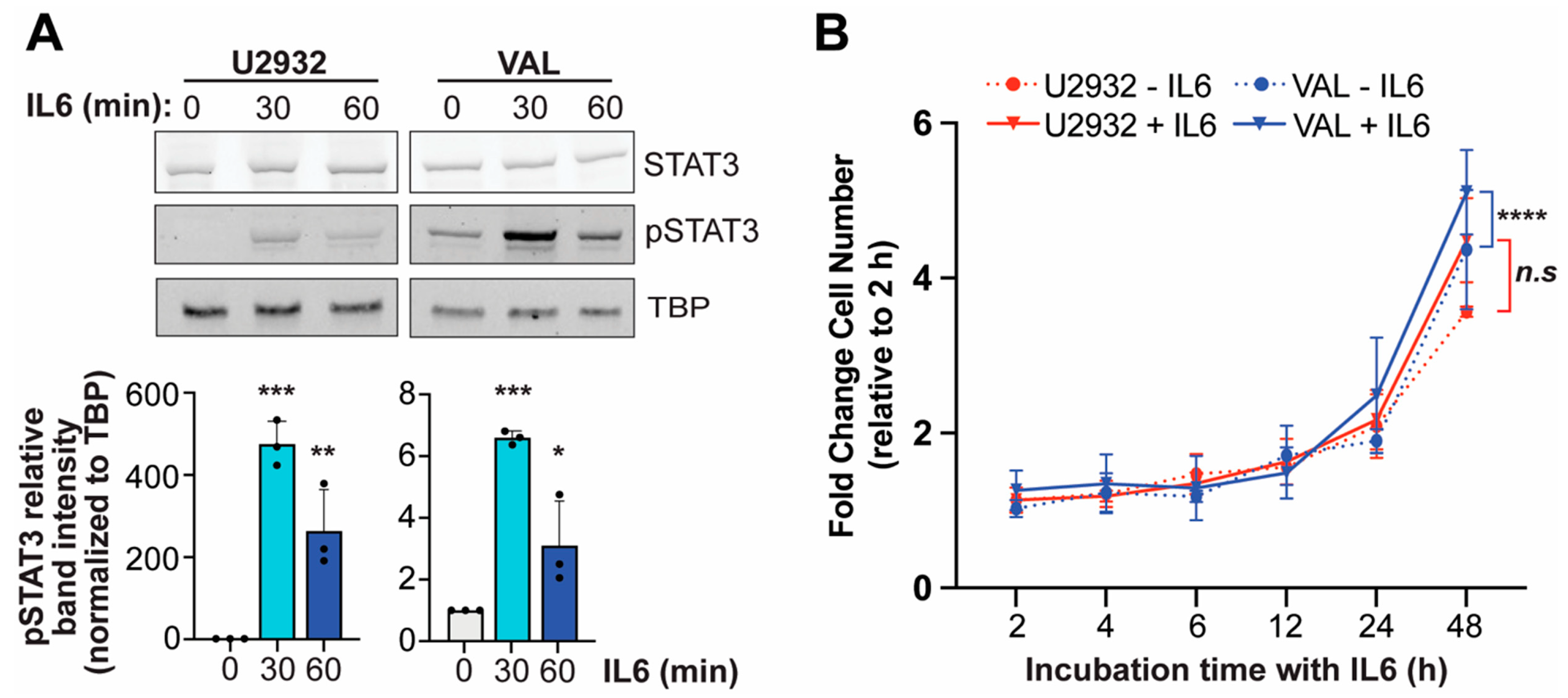
3.4. IL6 Induces Phosphorylation of STAT3 in DLBCL Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sehn, L.H.; Salles, G. Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2021, 384, 842–858. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, G.S.; Czuczman, M.S. ABC, GCB, and Double-Hit Diffuse Large B-Cell Lymphoma: Does Subtype Make a Difference in Therapy Selection? Am. Soc. Clin. Oncol. Educ. Book 2015, 35, e449–e457. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.N. In vivo modeling of diffuse large B cell lymphoma (DLBCL) with the myeloid differentiation primary response gene 88 (MYD88) L265P mutationon. Transl. Cancer Res. 2016, 5 (Suppl. S4), S852–S854. [Google Scholar] [CrossRef]
- Gisselbrecht, C.; Glass, B.; Mounier, N.; Singh Gill, D.; Linch, D.C.; Trneny, M.; Bosly, A.; Ketterer, N.; Shpilberg, O.; Hagberg, H.; et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J. Clin. Oncol. 2010, 28, 4184–4190. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, R.D.; Samant, S.A.; Rosenberg, M.; Silver, L.M.; Cole, M.D. Transgenic N-myc mouse model for indolent B cell lymphoma: Tumor characterization and analysis of genetic alterations in spontaneous and retrovirally accelerated tumors. Oncogene 1998, 17, 2073–2085. [Google Scholar] [CrossRef][Green Version]
- Kovalchuk, A.L.; Qi, C.F.; Torrey, T.A.; Taddesse-Heath, L.; Feigenbaum, L.; Park, S.S.; Gerbitz, A.; Klobeck, G.; Hoertnagel, K.; Polack, A.; et al. Burkitt lymphoma in the mouse. J. Exp. Med. 2000, 192, 1183–1190. [Google Scholar] [CrossRef]
- Field, K.A.; Charoenthongtrakul, S.; Bishop, J.M.; Refaeli, Y. Farnesyl transferase inhibitors induce extended remissions in transgenic mice with mature B cell lymphomas. Mol. Cancer 2008, 7, 39. [Google Scholar] [CrossRef]
- Frank, K.M.; Sharpless, N.E.; Gao, Y.; Sekiguchi, J.M.; Ferguson, D.O.; Zhu, C.; Manis, J.P.; Horner, J.; DePinho, R.A.; Alt, F.W. DNA ligase IV deficiency in mice leads to defective neurogenesis and embryonic lethality via the p53 pathway. Mol. Cell 2000, 5, 993–1002. [Google Scholar] [CrossRef]
- Greenwald, R.J.; Tumang, J.R.; Sinha, A.; Currier, N.; Cardiff, R.D.; Rothstein, T.L.; Faller, D.V.; Denis, G.V. E mu-BRD2 transgenic mice develop B-cell lymphoma and leukemia. Blood 2004, 103, 1475–1484. [Google Scholar] [CrossRef][Green Version]
- Das, R.; Strowig, T.; Verma, R.; Koduru, S.; Hafemann, A.; Hopf, S.; Kocoglu, M.H.; Borsotti, C.; Zhang, L.; Branagan, A.; et al. Microenvironment-dependent growth of preneoplastic and malignant plasma cells in humanized mice. Nat. Med. 2016, 22, 1351–1357. [Google Scholar] [CrossRef]
- Kishimoto, T. IL-6: From laboratory to bedside. Clin. Rev. Allergy Immunol. 2005, 28, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T. Interleukin-6: From basic science to medicine—40 years in immunology. Annu. Rev. Immunol. 2005, 23, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T.; Akira, S.; Taga, T. Interleukin-6 and its receptor: A paradigm for cytokines. Science 1992, 258, 593–597. [Google Scholar] [CrossRef]
- Hashwah, H.; Bertram, K.; Stirm, K.; Stelling, A.; Wu, C.T.; Kasser, S.; Manz, M.G.; Theocharides, A.P.; Tzankov, A.; Muller, A. The IL-6 signaling complex is a critical driver, negative prognostic factor, and therapeutic target in diffuse large B-cell lymphoma. EMBO Mol. Med. 2019, 11, e10576. [Google Scholar] [CrossRef]
- Hammacher, A.; Ward, L.D.; Weinstock, J.; Treutlein, H.; Yasukawa, K.; Simpson, R.J. Structure-function analysis of human IL-6: Identification of two distinct regions that are important for receptor binding. Protein Sci. 1994, 3, 2280–2293. [Google Scholar] [CrossRef]
- Hashwah, H.; Schmid, C.A.; Kasser, S.; Bertram, K.; Stelling, A.; Manz, M.G.; Muller, A. Inactivation of CREBBP expands the germinal center B cell compartment, down-regulates MHCII expression and promotes DLBCL growth. Proc. Natl. Acad. Sci. USA 2017, 114, 9701–9706. [Google Scholar] [CrossRef]
- Rongvaux, A.; Willinger, T.; Martinek, J.; Strowig, T.; Gearty, S.V.; Teichmann, L.L.; Saito, Y.; Marches, F.; Halene, S.; Palucka, A.K.; et al. Development and function of human innate immune cells in a humanized mouse model. Nat. Biotechnol. 2014, 32, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Pasqualucci, L.; Trifonov, V.; Fabbri, G.; Ma, J.; Rossi, D.; Chiarenza, A.; Wells, V.A.; Grunn, A.; Messina, M.; Elliot, O.; et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nat. Genet. 2011, 43, 830–837. [Google Scholar] [CrossRef]
- Amini, R.M.; Berglund, M.; Rosenquist, R.; Von Heideman, A.; Lagercrantz, S.; Thunberg, U.; Bergh, J.; Sundström, C.; Glimelius, B.; Enblad, G. A novel B-cell line (U-2932) established from a patient with diffuse large B-cell lymphoma following Hodgkin lymphoma. Leuk. Lymphoma 2002, 43, 2179–2189. [Google Scholar] [CrossRef]
- Schmitz, R.; Wright, G.W.; Huang, D.W.; Johnson, C.A.; Phelan, J.D.; Wang, J.Q.; Roulland, S.; Kasbekar, M.; Young, R.M.; Shaffer, A.L.; et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2018, 378, 1396–1407. [Google Scholar] [CrossRef]
- Wright, G.W.; Huang, D.W.; Phelan, J.D.; Coulibaly, Z.A.; Roulland, S.; Young, R.M.; Wang, J.Q.; Schmitz, R.; Morin, R.D.; Tang, J.; et al. A Probabilistic Classification Tool for Genetic Subtypes of Diffuse Large B Cell Lymphoma with Therapeutic Implications. Cancer Cell 2020, 37, 551–568.e514. [Google Scholar] [CrossRef]
- Chapuy, B.; Stewart, C.; Dunford, A.J.; Kim, J.; Kamburov, A.; Redd, R.A.; Lawrence, M.S.; Roemer, M.G.M.; Li, A.J.; Ziepert, M.; et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat. Med. 2018, 24, 679–690. [Google Scholar] [CrossRef]
- Kendrick, S.; Muranyi, A.; Gokhale, V.; Hurley, L.H.; Rimsza, L.M. Simultaneous Drug Targeting of the Promoter MYC G-Quadruplex and BCL2 i-Motif in Diffuse Large B-Cell Lymphoma Delays Tumor Growth. J. Med. Chem. 2017, 60, 6587–6597. [Google Scholar] [CrossRef]
- Dyer, M.J.; Lillington, D.M.; Bastard, C.; Tilly, H.; Lens, D.; Heward, J.M.; Stranks, G.; Morilla, R.; Monrad, S.; Guglielmi, P.; et al. Concurrent activation of MYC and BCL2 in B cell non-Hodgkin lymphoma cell lines by translocation of both oncogenes to the same immunoglobulin heavy chain locus. Leukemia 1996, 10, 1198–1208. [Google Scholar] [PubMed]
- Li, W.; Gupta, S.K.; Han, W.; Kundson, R.A.; Nelson, S.; Knutson, D.; Greipp, P.T.; Elsawa, S.F.; Sotomayor, E.M.; Gupta, M. Targeting MYC activity in double-hit lymphoma with MYC and BCL2 and/or BCL6 rearrangements with epigenetic bromodomain inhibitors. J. Hematol. Oncol. 2019, 12, 73. [Google Scholar] [CrossRef]
- Bosch, R.; Moreno, M.J.; Dieguez-Gonzalez, R.; Céspedes, M.V.; Gallardo, A.; Nomdedeu, J.; Pavón, M.A.; Espinosa, I.; Mangues, M.A.; Sierra, J.; et al. Subcutaneous passage increases cell aggressiveness in a xenograft model of diffuse large B cell lymphoma. Clin. Exp. Metastasis 2012, 29, 339–347. [Google Scholar] [CrossRef]
- Wunderlich, M.; Chou, F.S.; Link, K.A.; Mizukawa, B.; Perry, R.L.; Carroll, M.; Mulloy, J.C. AML xenograft efficiency is significantly improved in NOD/SCID-IL2RG mice constitutively expressing human SCF, GM-CSF and IL-3. Leukemia 2010, 24, 1785–1788. [Google Scholar] [CrossRef] [PubMed]
- Billerbeck, E.; Barry, W.T.; Mu, K.; Dorner, M.; Rice, C.M.; Ploss, A. Development of human CD4+FoxP3+ regulatory T cells in human stem cell factor-, granulocyte-macrophage colony-stimulating factor-, and interleukin-3-expressing NOD-SCID IL2Rγ(null) humanized mice. Blood 2011, 117, 3076–3086. [Google Scholar] [CrossRef]
- Janke, L.J.; Imai, D.M.; Tillman, H.; Doty, R.; Hoenerhoff, M.J.; Xu, J.J.; Freeman, Z.T.; Allen, P.; Fowlkes, N.W.; Iacobucci, I.; et al. Development of Mast Cell and Eosinophil Hyperplasia and HLH/MAS-Like Disease in NSG-SGM3 Mice Receiving Human CD34+ Hematopoietic Stem Cells or Patient-Derived Leukemia Xenografts. Vet. Pathol. 2021, 58, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.A.; Slack, G.W.; Savage, K.J.; Connors, J.M.; Ben-Neriah, S.; Rogic, S.; Scott, D.W.; Tan, K.L.; Steidl, C.; Sehn, L.H.; et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J. Clin. Oncol. 2012, 30, 3452–3459. [Google Scholar] [CrossRef]
- Akyurek, N.; Uner, A.; Benekli, M.; Barista, I. Prognostic significance of MYC, BCL2, and BCL6 rearrangements in patients with diffuse large B-cell lymphoma treated with cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab. Cancer 2012, 118, 4173–4183. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehdi, S.H.; Xu, Y.-Z.; Shultz, L.D.; Kim, E.; Lee, Y.G.; Kendrick, S.; Yoon, D. Development of New Diffuse Large B Cell Lymphoma Mouse Models. Cancers 2024, 16, 3006. https://doi.org/10.3390/cancers16173006
Mehdi SH, Xu Y-Z, Shultz LD, Kim E, Lee YG, Kendrick S, Yoon D. Development of New Diffuse Large B Cell Lymphoma Mouse Models. Cancers. 2024; 16(17):3006. https://doi.org/10.3390/cancers16173006
Chicago/Turabian StyleMehdi, Syed Hassan, Ying-Zhi Xu, Leonard D. Shultz, Eunkyung Kim, Yong Gu Lee, Samantha Kendrick, and Donghoon Yoon. 2024. "Development of New Diffuse Large B Cell Lymphoma Mouse Models" Cancers 16, no. 17: 3006. https://doi.org/10.3390/cancers16173006
APA StyleMehdi, S. H., Xu, Y.-Z., Shultz, L. D., Kim, E., Lee, Y. G., Kendrick, S., & Yoon, D. (2024). Development of New Diffuse Large B Cell Lymphoma Mouse Models. Cancers, 16(17), 3006. https://doi.org/10.3390/cancers16173006






