The Anticancer Activity of Monosaccharides: Perspectives and Outlooks
Abstract
Simple Summary
Abstract
1. Introduction
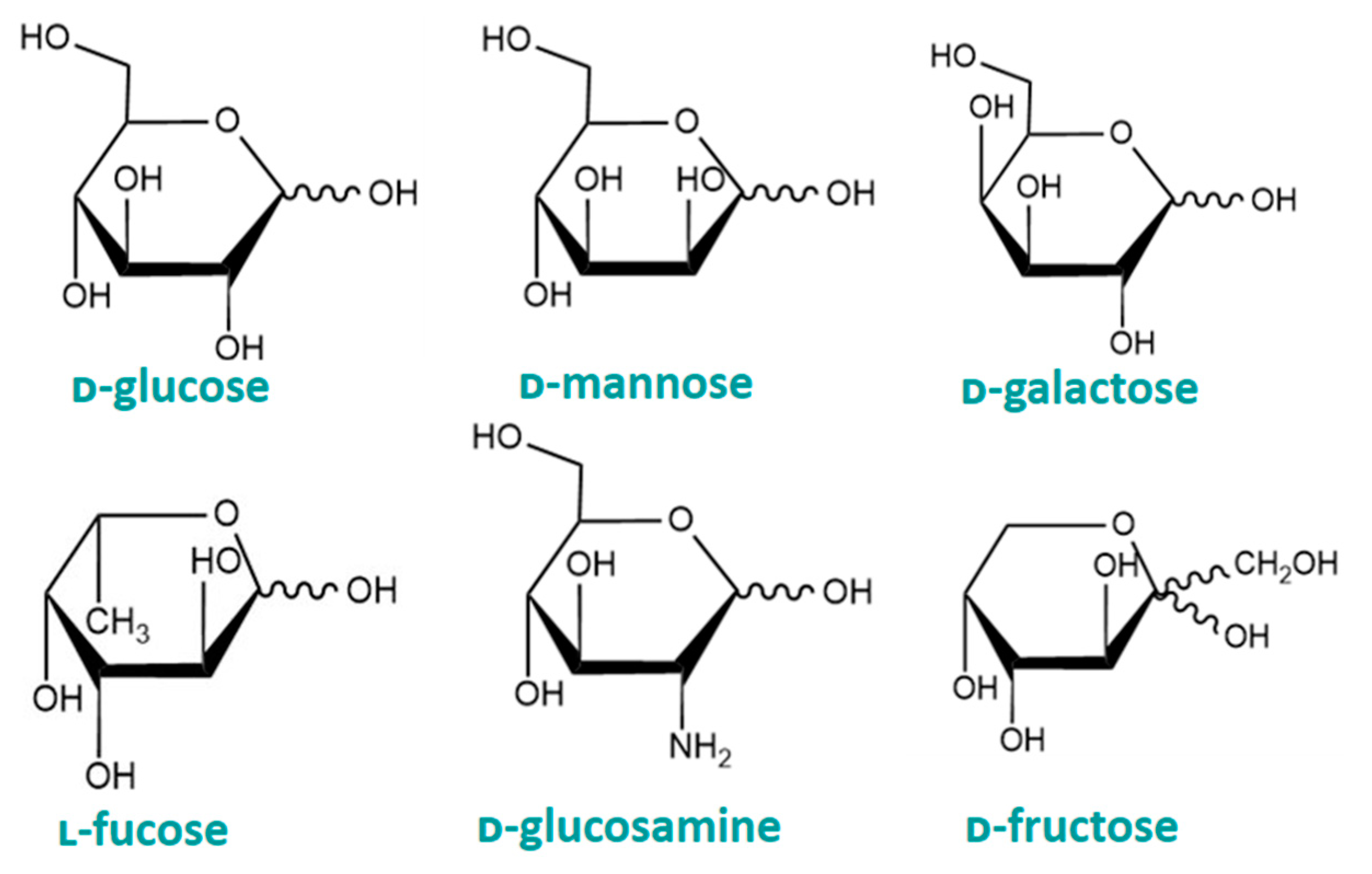
2. Common Monosaccharides
2.1. ᴅ-Mannose
2.2. ᴅ-Glucosamine
2.3. ᴅ-Galactose
2.4. ᴅ-Fructose
2.5. ʟ-Fucose
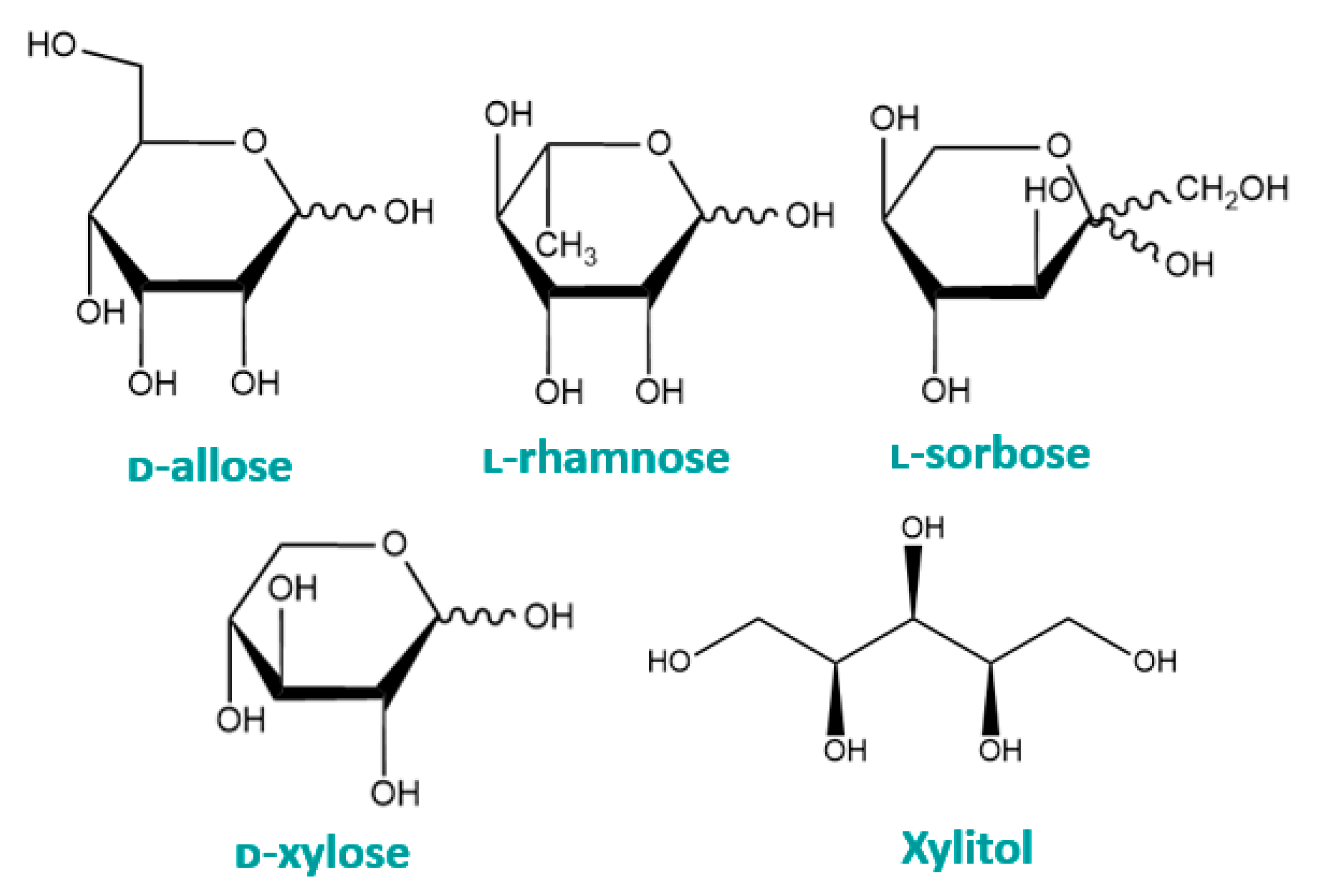
3. Rare Monosaccharides
3.1. Xylitol
3.2. ᴅ-Allose
3.3. ʟ-Sorbose
3.4. ʟ-Rhamnose
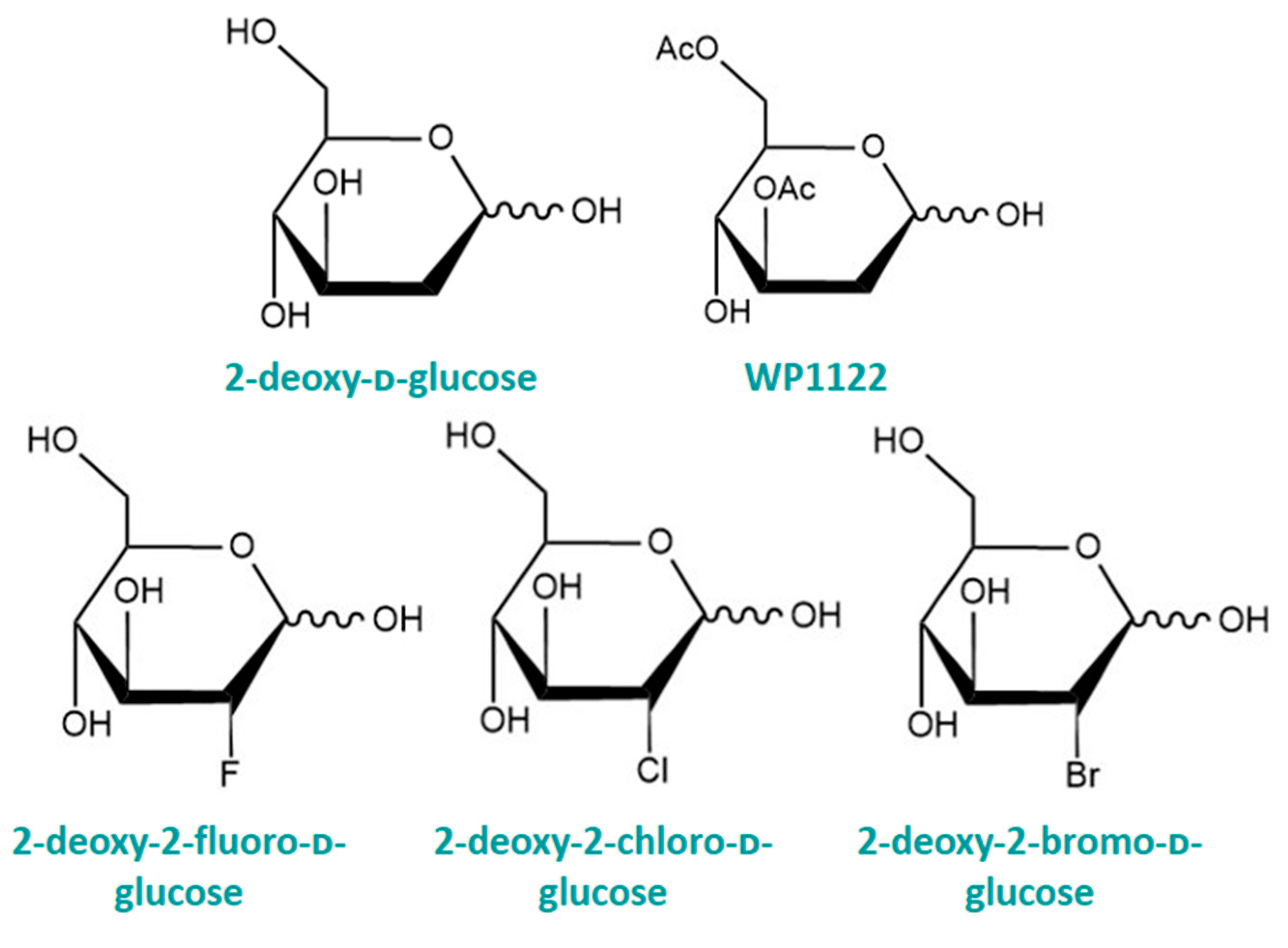
4. Structurally Modified Monosaccharides
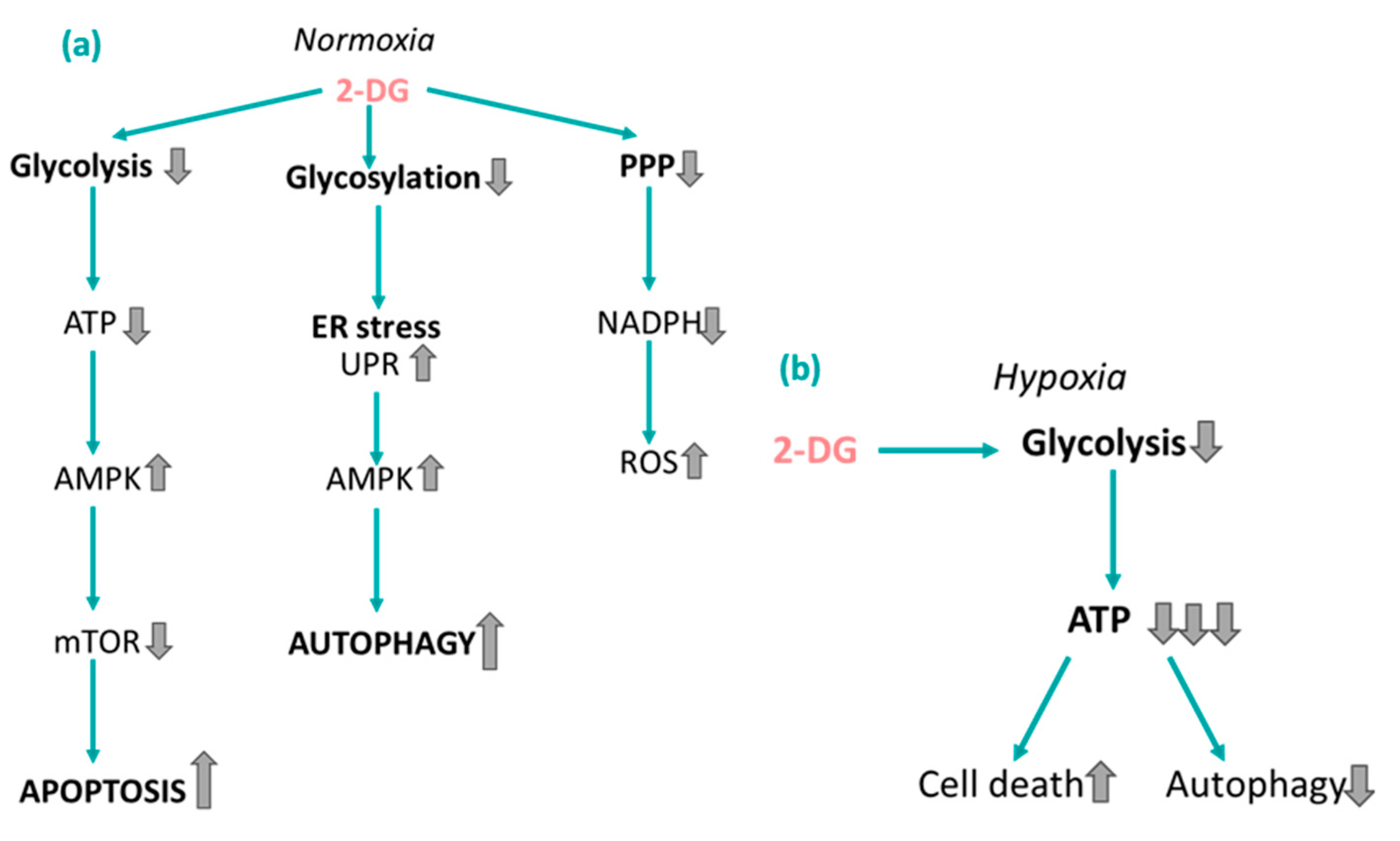
4.1. 2-Deoxy-ᴅ-glucose
4.2. Halogenated 2-DG
4.3. WP1122—O-Acetylated 2-DG
5. Monosaccharides as Potentiators of Chemotherapy
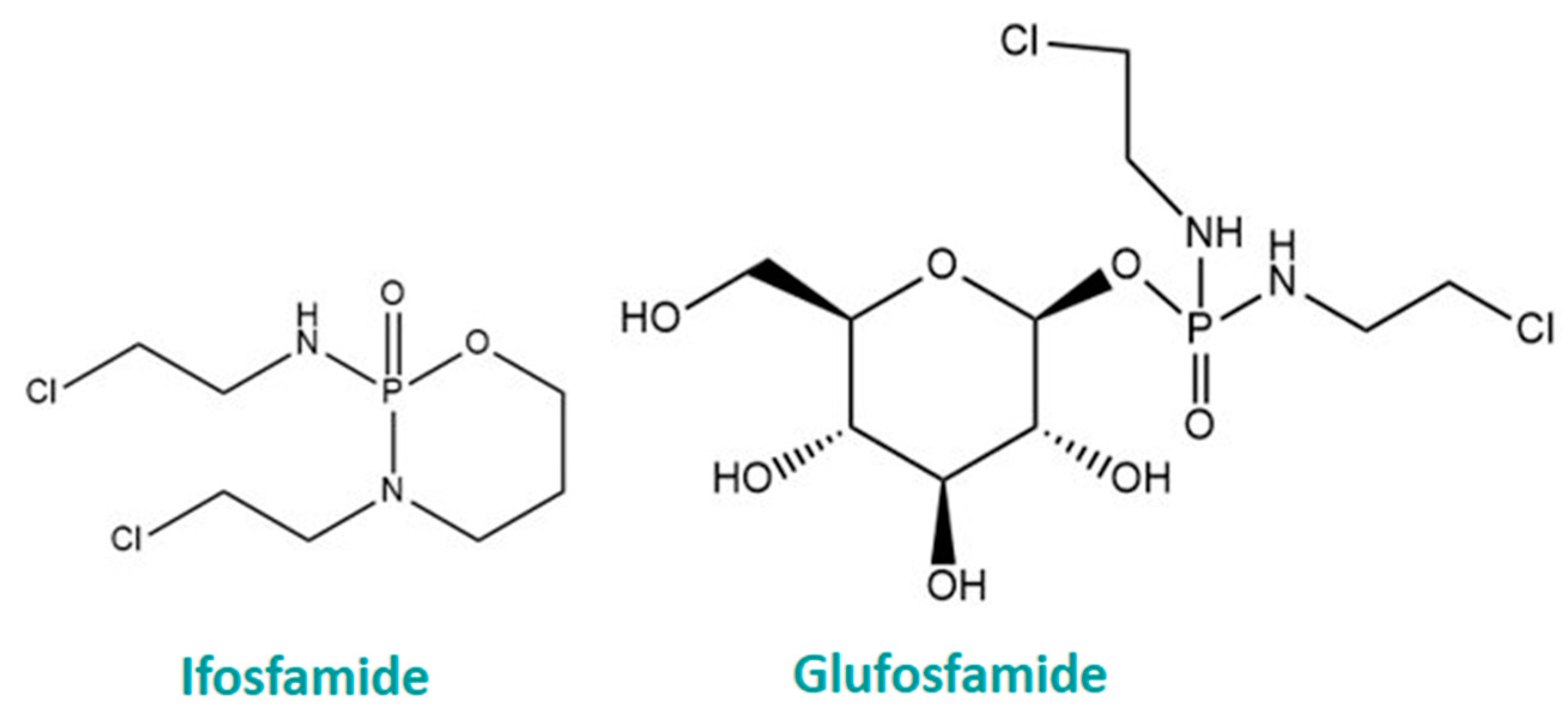
6. Glycoconjugation
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Abbreviations | Definition |
| AKT1 | AKT Serine/Threonine Kinase 1 |
| AMPK | AMP-activated protein kinase |
| AR | Aldose reductase |
| ASGP | Asialoglycoprotein |
| ATP | Adenosine triphosphate |
| BBB | Blood–brain barrier |
| BAX | Bcl-2-associated X protein |
| BCl-2 | B-cell leukaemia/lymphoma 2 protein |
| CDK4 | Cyclin-dependent kinase 4 |
| CDK6 | Cyclin-dependent kinase 6 |
| CRC | Colorectal cancer |
| ER | Endoplasmic reticulum |
| FK | Fructokinase |
| FOXO | Forkhead box transcription factors |
| F6P | Fructose-6-phosphate |
| FUTs | Fucosyltransferases |
| Gal-dox | Galactose-conjugated doxorubicin |
| Gal-Pt | Galactose-conjugated oxaliplatin |
| GBM | Glioblastoma |
| GlcN | Glucosamine |
| Gluc-PAX | Glucose-conjugated paclitaxel |
| GLUT | Glucose transporter |
| HCFS | High-fructose corn syrup |
| HK | Hexokinase |
| IARC | International Agency for Research on Cancer |
| KHK | Ketohexokinase |
| LDH | Lactate dehydrogenase |
| LLOs | Lipid linked oligosaccharides |
| M6P | Mannose-6-phosphate |
| MTD | Maximum tolerated dose |
| mTOR | Mammalian target of rapamycin |
| NADH | Nicotinamide adenine dinucleotide phosphate hydrogen |
| NAD+ | Nicotinamide adenine dinucleotide phosphate |
| Nrf2 | Erythroid 2-related factor 2 |
| OXPHOS | Oxidative phosphorylation |
| PET | Positron emission tomography |
| PFK | Phosphofructokinase |
| PGI | Phosphogluco isomerase |
| PMI | Phosphomannose isomerase |
| PSAT1 | Phosphoserine aminotransferase |
| Rha-lip | Rhamnose-functionalised liposomes |
| ROS | Reactive oxygen species |
| SLC2A5 | Solute carrier family 2 member 5 |
| STAT | Signal transducer and activator of transcription |
| S1P | Sorbose-1-phosphate |
| TUDCA | Tauroursodeoxycholic acid |
| TXNIP | Thioredoxin-interacting protein |
| UPR | Unfolded protein response |
| WP1122 | 3,6-di-O-acetyl-2-deoxy-ᴅ-glucose |
| 2-BDG | 2-bromo-2-deoxy-ᴅ-glucose |
| 2-CDG | 2-chloro-2-deoxy-ᴅ-glucose |
| 2-DG | 2-deoxy-ᴅ-glucose |
| 2DG-6P | 2-deoxy-ᴅ-glucose-6-phosphate |
| 2-FDG/2-18FDG | 2-deoxy-2(18)fluoro-ᴅ-glucose |
| 2-FDG-PAX | 2-FDG-conjugated paclitaxel |
| 2-XDG | Halogenated 2-deoxy-ᴅ-glucose |
| Cell Line | Tissue or Cell Type |
| A549 | Human lung carcinoma |
| Caco-2 | Human colon carcinoma |
| Ca9-22 | Human gingival squamous carcinoma |
| Caki-1 | Human kidney carcinoma |
| CAL-27 | Human squamous carcinoma |
| C6 | Murine glioma |
| DU145 | Human prostate carcinoma |
| HCT-15 | Human colon carcinoma |
| HCT-116 | Human colon carcinoma |
| HcoEpic | Human colonic epithelial cells |
| HeLa | Human cervical carcinoma |
| HepG2 | Human liver carcinoma |
| HL-60 | Human promyelocytic leukaemia |
| HSC-3 | Human tongue squamous carcinoma |
| HT-29 | Human colon carcinoma |
| Huh-7 | Human liver carcinoma |
| HUVEC | Human umbilical vein endothelium |
| H460 | Human lung carcinoma |
| KP-4 | Human pancreatic carcinoma |
| K562 | Human myelogenous leukaemia |
| L1210 | Human lymphocytic leukaemia |
| MCF-7 | Human breast carcinoma |
| MCF-7/DOX | Human breast carcinoma (doxorubicin-resistant) |
| MDA-MB-468 | Human breast carcinoma |
| MDA-MB-231 | Human breast carcinoma |
| MeWo | Human melanoma |
| MOLT-4F | Human lymphoblastic leukaemia |
| NCI-H23 | Human lung carcinoma |
| N2a | Murine neuroblastoma |
| PANC-1 | Human pancreatic epithelioid carcinoma |
| PC-3 | Human adenocarcinoma |
| RKO | Human colon carcinoma |
| Saos-2 | Human osteosarcoma |
| Sarcoma 37 | Murine soft tissue sarcoma |
| SH-SY5Y | Human neuroblastoma |
| SK-MEL-2 | Human melanoma |
| SK-OV-3 | Human ovarian carcinoma |
| U2OS | Human osteosarcoma |
| U-87MG | Human glioblastoma |
| U-937 | Human lymphoma |
| Walker 256 | Murine breast carcinoma |
| MIA-PaCa-2 | Human pancreatic carcinoma |
| 4T1 | Murine breast/mammary gland |
| 786-O | Human kidney carcinoma |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Hanahan, D.; Robert, A. Weinberg, Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Zheng, H.C. The molecular mechanisms of chemoresistance in cancers. Oncotarget 2017, 8, 59950–59964. [Google Scholar] [CrossRef]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Ortega, A.D.; Sánchez-Aragó, M.; Giner-Sánchez, D.; Sánchez-Cenizo, L.; Willers, I.; Cuezva, J.M. Glucose avidity of carcinomas. Cancer Lett. 2009, 276, 125–135. [Google Scholar] [CrossRef]
- Cunha, A.; Rocha, A.C.; Barbosa, F.; Baião, A.; Silva, P.; Sarmento, B.; Queirós, O. Glycolytic Inhibitors Potentiated the Activity of Paclitaxel and Their Nanoencapsulation Increased Their Delivery in a Lung Cancer Model. Pharmaceutics 2022, 14, 2021. [Google Scholar] [CrossRef]
- Nowak, N.; Kulma, A.; Gutowicz, J. Up-regulation of Key Glycolysis Proteins in Cancer Development. Open Life Sci. 2018, 13, 569–581. [Google Scholar] [CrossRef]
- Warburg, O.; Wind, F.; Negelein, E. The Metabolism of Tumors in the Body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef]
- Pajak, B.; Siwiak, E.; Sołtyka, M.; Priebe, A.; Zieliński, R.; Fokt, I.; Ziemniak, M.; Jaśkiewicz, A.; Borowski, R.; Domoradzki, T.; et al. 2-Deoxy-d-Glucose and Its Analogs: From Diagnostic to Therapeutic Agents. Int. J. Mol. Sci. 2019, 21, 234. [Google Scholar] [CrossRef]
- Hossain, F.; Andreana, P.R. Developments in Carbohydrate-Based Cancer Therapeutics. Pharmaceuticals 2019, 12, 84. [Google Scholar] [CrossRef]
- Zhao, Y.; Dunmall, L.S.C.; Cheng, Z.; Wang, Y.; Si, L. Natural products targeting glycolysis in cancer. Front. Pharmacol. 2022, 13, 1036502. [Google Scholar] [CrossRef]
- Barchi, J.J., Jr. Emerging roles of carbohydrates and glycomimetics in anticancer drug design. Curr. Pharm. Des. 2000, 6, 485–501. [Google Scholar] [CrossRef]
- Zhao, M.; Wei, F.; Sun, G.; Wen, Y.; Xiang, J.; Su, F.; Zhan, L.; Nian, Q.; Chen, Y.; Zeng, J. Natural compounds targeting glycolysis as promising therapeutics for gastric cancer: A review. Front. Pharmacol. 2022, 13, 1004383. [Google Scholar] [CrossRef]
- Stylianopoulos, C.L. Carbohydrates: Chemistry and Classification. In Encyclopedia of Human Nutrition, 2nd ed.; Elsevier: San Diego, CA, USA, 2005; pp. 303–309. [Google Scholar]
- Herman, R.H. Mannose metabolism. I. Am. J. Clin. Nutr. 1971, 24, 488–498. [Google Scholar] [CrossRef]
- Alton, G.; Hasilik, M.; Niehues, R.; Panneerselvam, K.; Etchison, J.R.; Fana, F.; Freeze, H.H. Direct utilization of mannose for mammalian glycoprotein biosynthesis. Glycobiology 1998, 8, 285–295. [Google Scholar] [CrossRef]
- Bunn, H.F.; Higgins, P.J. Reaction of Monosaccharides with Proteins: Possible Evolutionary Significance. Science 1981, 213, 222–224. [Google Scholar] [CrossRef]
- Mueckler, M.; Thorens, B. The SLC2 (GLUT) family of membrane transporters. Mol. Aspects Med. 2013, 34, 121–138. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, H.; Gui, Y.; Zhan, Q.; Li, S.; Qiao, W.; Tong, A. Mannose Treatment: A Promising Novel Strategy to Suppress Inflammation. Front. Immunol. 2021, 12, 756920. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, S.; He, B. Mannose shows antitumour properties against lung cancer via inhibiting proliferation, promoting cisplatin-mediated apoptosis and reducing metastasis. Mol. Med. Rep. 2020, 22, 2957–2965. [Google Scholar] [CrossRef]
- Gonzalez, P.S.; O’prey, J.; Cardaci, S.; Barthet, V.J.A.; Sakamaki, J.-I.; Beaumatin, F.; Roseweir, A.; Gay, D.M.; Mackay, G.; Malviya, G.; et al. Mannose impairs tumour growth and enhances chemotherapy. Nature 2018, 563, 719–723. [Google Scholar] [CrossRef]
- Saito, Y.; Kinoshita, M.; Yamada, A.; Kawano, S.; Liu, H.; Kamimura, S.; Nakagawa, M.; Nagasawa, S.; Taguchi, T.; Yamada, S.; et al. Mannose and phosphomannose isomerase regulate energy metabolism under glucose starvation in leukemia. Cancer Sci. 2021, 112, 4944–4956. [Google Scholar] [CrossRef]
- Dalirfardouei, R.; Karimi, G.; Jamialahmadi, K. Molecular mechanisms and biomedical applications of glucosamine as a potential multifunctional therapeutic agent. Life Sci. 2016, 152, 21–29. [Google Scholar] [CrossRef]
- Konopka, J.B. N-acetylglucosamine (GlcNAc) functions in cell signaling. Scientifica 2012, 2012, 489208. [Google Scholar] [CrossRef]
- Reginster, J.-Y.; Neuprez, A.; Lecart, M.-P.; Sarlet, N.; Bruyere, O. Role of glucosamine in the treatment for osteoarthritis. Rheumatol. Int. 2012, 32, 2959–2967. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, Z.; Lin, Z.; Wang, W.; Wan, R.; Liu, T. Association between glucosamine use and cancer mortality: A large prospective cohort study. Front. Nutr. 2022, 9, 947818. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Liu, Y.; Zhang, J.; Li, L.; Huang, X.; Thabane, L.; Lip, G.Y. Relationship between glucosamine use and the risk of lung cancer: Data from a nationwide prospective cohort study. Eur. Respir. J. 2022, 59, 2101399. [Google Scholar] [CrossRef]
- Quastel, J.H.; Cantero, A. Inhibition of tumour growth by D-glucosamine. Nature 1953, 171, 252–254. [Google Scholar] [CrossRef]
- Molnar, Z.; Bekesi, J.G. Cytotoxic effects of D-glucosamine on the ultrastructures of normal and neoplastic tissues in vivo. Cancer Res. 1972, 32, 756–765. [Google Scholar]
- Friedman, S.J.; Skehan, P. Membrane-active drugs potentiate the killing of tumor cells by D-glucosamine. Proc. Natl. Acad. Sci. USA 1980, 77, 1172–1176. [Google Scholar] [CrossRef]
- Oh, H.-J.; Lee, J.S.; Song, D.-K.; Shin, D.-H.; Jang, B.-C.; Suh, S.-I.; Park, J.-W.; Suh, M.-H.; Baek, W.-K. D-glucosamine inhibits proliferation of human cancer cells through inhibition of p70S6K. Biochem. Biophys. Res. Commun. 2007, 360, 840–845. [Google Scholar] [CrossRef]
- Wang, L.S.; Chen, S.J.; Zhang, J.F.; Liu, M.N.; Zheng, J.H.; Yao, X.D. Anti-proliferative potential of Glucosamine in renal cancer cells via inducing cell cycle arrest at G0/G1 phase. BMC Urol. 2017, 17, 38. [Google Scholar] [CrossRef]
- de Keizer, P.L.; Packer, L.M.; Szypowska, A.A.; Riedl-Polderman, P.E.; van den Broek, N.J.; de Bruin, A.; Dansen, T.B.; Marais, R.; Brenkman, A.B.; Burgering, B.M. Activation of forkhead box O transcription factors by oncogenic BRAF promotes p21cip1-dependent senescence. Cancer Res. 2010, 70, 8526–8536. [Google Scholar] [CrossRef]
- Huang, H.; Tindall, D.J. Dynamic FoxO transcription factors. J. Cell Sci. 2007, 120 Pt 15, 2479–2487. [Google Scholar] [CrossRef]
- Yu, Z.; Ju, Y.; Liu, H. Anti-lung cancer effect of glucosamine by suppressing the phosphorylation of FOXO. Mol. Med. Rep. 2017, 16, 3395–3400. [Google Scholar] [CrossRef]
- Yu, H.; Jove, R. The STATs of cancer--new molecular targets come of age. Nat. Rev. Cancer 2004, 4, 97–105. [Google Scholar] [CrossRef]
- Chesnokov, V.; Sun, C.; Itakura, K. Glucosamine suppresses proliferation of human prostate carcinoma DU145 cells through inhibition of STAT3 signaling. Cancer Cell Int. 2009, 9, 25. [Google Scholar] [CrossRef]
- Lin, J.H.; Walter, P.; Yen, T.S. Endoplasmic reticulum stress in disease pathogenesis. Annu. Rev. Pathol. 2008, 3, 399–425. [Google Scholar] [CrossRef]
- Morin, M.J.; Porter, C.W.; McKernan, P.; Bernacki, R.J. The biochemical and ultrastructural effects of tunicamycin and D-glucosamine in L1210 leukemic cells. J. Cell Physiol. 1983, 114, 162–172. [Google Scholar] [CrossRef]
- Werstuck, G.H.; Khan, M.I.; Femia, G.; Kim, A.J.; Tedesco, V.; Trigatti, B.; Shi, Y. Glucosamine-induced endoplasmic reticulum dysfunction is associated with accelerated atherosclerosis in a hyperglycemic mouse model. Diabetes 2006, 55, 93–101. [Google Scholar] [CrossRef]
- Hwang, M.-S.; Baek, W.-K. Glucosamine induces autophagic cell death through the stimulation of ER stress in human glioma cancer cells. Biochem. Biophys. Res. Commun. 2010, 399, 111–116. [Google Scholar] [CrossRef]
- Hientz, K.; Mohr, A.; Bhakta-Guha, D.; Efferth, T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget 2017, 8, 8921–8946. [Google Scholar] [CrossRef]
- Lefranc, F.; Facchini, V.; Kiss, R. Proautophagic drugs: A novel means to combat apoptosis-resistant cancers, with a special emphasis on glioblastomas. Oncologist 2007, 12, 1395–1403. [Google Scholar] [CrossRef]
- Newens, K.; Walton, J. A review of sugar consumption from nationally representative dietary surveys across the world. J. Hum. Nutr. Diet. 2016, 29, 225–240. [Google Scholar] [CrossRef]
- Holesh, J.E.; Aslam, S.; Martin, A. Physiology, Carbohydrates. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459280/ (accessed on 23 August 2023).
- Kilcoyne, M.; Joshi, L. Carbohydrates in therapeutics. Cardiovasc. Hematol. Agents Med. Chem. 2007, 5, 186–197. [Google Scholar] [CrossRef]
- Varki, A.; Sharon, N. Historical Background and Overview. In Essentials of Glycobiology, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2009; Chapter 1. [Google Scholar]
- Iannetti, E.F.; Smeitink, J.A.M.; Willems, P.H.G.M.; Beyrath, J.; Koopman, W.J.H. Rescue from galactose-induced death of Leigh Syndrome patient cells by pyruvate and NAD+. Cell Death Dis. 2018, 9, 1135. [Google Scholar] [CrossRef]
- King, M.W. Carbohydrates: Galactose Metabolism. In Integrative Medical Biochemistry Examination and Board Review; McGraw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Conte, F.; van Buuringen, N.; Voermans, N.C.; Lefeber, D.J. Galactose in human metabolism, glycosylation and congenital metabolic diseases: Time for a closer look. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129898. [Google Scholar] [CrossRef]
- Li, N.; He, Y.; Wang, L.; Mo, C.; Zhang, J.; Zhang, W.; Li, J.; Liao, Z.; Tang, X.; Xiao, H. D-galactose induces necroptotic cell death in neuroblastoma cell lines. J. Cell Biochem. 2011, 112, 3834–3844. [Google Scholar] [CrossRef]
- Shiratori, R.; Furuichi, K.; Yamaguchi, M.; Miyazaki, N.; Aoki, H.; Chibana, H.; Ito, K.; Aoki, S. Glycolytic suppression dramatically changes the intracellular metabolic profile of multiple cancer cell lines in a mitochondrial metabolism-dependent manner. Sci. Rep. 2019, 9, 18699. [Google Scholar] [CrossRef]
- Zheng, D.; Sussman, J.H.; Jeon, M.P.; Parrish, S.T.; MacMullan, M.A.; Delfarah, A.; Graham, N.A. AKT but not MYC promotes reactive oxygen species-mediated cell death in oxidative culture. J. Cell Sci. 2020, 133, jcs239277. [Google Scholar] [CrossRef]
- Elstrom, R.L.; Bauer, D.E.; Buzzai, M.; Karnauskas, R.; Harris, M.H.; Plas, D.R.; Zhuang, H.; Cinalli, R.M.; Alavi, A.; Rudin, C.M.; et al. Akt Stimulates Aerobic Glycolysis in Cancer Cells. Cancer Res. 2004, 64, 3892–3899. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Ishimoto, T.; Li, N.; Cicerchi, C.; Orlicky, D.J.; Ruzycki, P.; Rivard, C.; Inaba, S.; Roncal-Jimenez, C.A.; Bales, E.S.; et al. Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat. Commun. 2013, 4, 2434. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.S.; Slot, J.W.; Geuze, H.J.; James, D.E. Polarized distribution of glucose transporter isoforms in Caco-2 cells. Proc. Natl. Acad. Sci. USA 1992, 89, 7556–7560. [Google Scholar] [CrossRef] [PubMed]
- Mahraoui, L.; Rousset, M.; Dussaulx, E.; Darmoul, D.; Zweibaum, A.; Brot-Laroche, E.; Jones, H.F.; Butler, R.N.; Brooks, D.A.; Nicola, J.P.; et al. Expression and localization of GLUT-5 in Caco-2 cells, human small intestine, and colon. Am. J. Physiol. 1992, 263 Pt 1, G312–G318. [Google Scholar] [CrossRef] [PubMed]
- Zamora-León, S.P.; Golde, D.W.; I Concha, I.; I Rivas, C.; Delgado-López, F.; Baselga, J.; Nualart, F.; Vera, J.C. Expression of the fructose transporter GLUT5 in human breast cancer. Proc. Natl. Acad. Sci. USA 1996, 93, 1847–1852. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Lanaspa, M.A.; Millan, I.S.; Fini, M.; Rivard, C.J.; Sanchez-Lozada, L.G.; Andres-Hernando, A.; Tolan, D.R.; Johnson, R.J. Fructose contributes to the Warburg effect for cancer growth. Cancer Metab. 2020, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Tuttle, K.R.; A Short, R.; Johnson, R.J. Hypothesis: Fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat. Clin. Pract. Nephrol. 2005, 1, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, M.D.; Lu, C.; Tutnauer, J.; Hartman, T.E.; Hwang, S.-K.; Murphy, C.J.; Pauli, C.; Morris, R.; Taylor, S.; Bosch, K.; et al. High-fructose corn syrup enhances intestinal tumor growth in mice. Science 2019, 363, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Fan, X.; Bai, Y.; Wang, S.; Huang, H.; Yang, H.; Zhu, J.; Zhang, F. SLC2A5 promotes lung adenocarcinoma cell growth and metastasis by enhancing fructose utilization. Cell Death Discov. 2018, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Zwarts, I.; van Zutphen, T.; Kruit, J.K.; Liu, W.; Oosterveer, M.H.; Verkade, H.J.; Uhlenhaut, N.H.; Jonker, J.W. Identification of the fructose transporter GLUT5 (SLC2A5) as a novel target of nuclear receptor LXR. Sci. Rep. 2019, 9, 9299. [Google Scholar]
- Godoy, A.; Ulloa, V.; Rodríguez, F.; Reinicke, K.; Yañez, A.J.; García, M.D.L.A.; Medina, R.A.; Carrasco, M.; Barberis, S.; Castro, T.; et al. Differential subcellular distribution of glucose transporters GLUT1–6 and GLUT9 in human cancer: Ultrastructural localization of GLUT1 and GLUT5 in breast tumor tissues. J. Cell Physiol. 2006, 207, 614–627. [Google Scholar] [CrossRef]
- Karbassi, M.; Monzavi-Karbassi, B.; Hine, R.J.; Stanley, J.S.; Ramani, V.P.; Carcel-Trullols, J.; Whitehead, T.L.; Kelly, T.; Siegel, E.R.; Artaud, C.; et al. Fructose as a carbon source induces an aggressive phenotype in MDA-MB-468 breast tumor cells. Int. J. Oncol. 2010, 37, 615–622. [Google Scholar]
- Wang, X.; Taniguchi, N. Core Fucosylation of N-Linked Glycan for Fine-Tuning TGF b Receptor Function. Glycosci. Biol. Med. 2015, 2014, 991–997. [Google Scholar]
- Zhao, Y.; Takahashi, M.; Gu, J.; Miyoshi, E.; Matsumoto, A.; Kitazume, S.; Taniguchi, N. Functional roles of N-glycans in cell signaling and cell adhesion in cancer. Cancer Sci. 2008, 99, 1304–1310. [Google Scholar] [CrossRef]
- Listinsky, J.J.; Listinsky, C.M.; Alapati, V.; Siegal, G.P. Cell surface fucose ablation as a therapeutic strategy for malignant neoplasms. Adv. Anat. Pathol. 2001, 8, 330–337. [Google Scholar] [CrossRef]
- MacDougall, S.L.; Schwarting, G.A.; Parkinson, D.; Sullivan, A.K. Increased fucosylation of glycolipids in a human leukaemia cell line (K562-Clone I) with decreased sensitivity to NK-mediated lysis. Immunology 1987, 62, 75–80. [Google Scholar]
- Eccles, M.R.; Chatterjee, A.; Rodger, E.J. Identifying drivers of metastasis; towards a systematic approach. Transl. Cancer Res. 2017, 6, 1273–1276. [Google Scholar] [CrossRef]
- Vanhooren, P.T.; Vandamme, E.J. L-Fucose: Occurrence, physiological role, chemical, enzymatic and microbial synthesis. J. Chem. Technol. Biotechnol. 1999, 74, 479–497. [Google Scholar] [CrossRef]
- Gorelik, E.; Xu, F.; Henion, T.; Anaraki, F.; Galili, U. Reduction of metastatic properties of BL6 melanoma cells expressing terminal fucose(alpha)1-2-galactose after alpha1,2-fucosyltransferase cDNA transfection. Cancer Res. 1997, 57, 332–336. [Google Scholar]
- Hotta, H.; Hamamura, K.; Yamashita, K.; Shibuya, H.; Tokuda, N.; Hashimoto, N.; Furukawa, K.; Yamamoto, N.; Hattori, H.; Toyokuni, S.; et al. Lewis y antigen is expressed in oral squamous cell carcinoma cell lines and tissues, but disappears in the invasive regions leading to the enhanced malignant properties irrespective of sialyl-Lewis x. Glycoconj J. 2013, 30, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, S.; Prorok, M.; Benoliel, A.-M.; Uch, R.; Langlet, C.; Bongrand, P.; Gerolami, R.; El-Battari, A. Transgene Expression of α(1,2)-Fucosyltransferase-I (FUT1) in Tumor Cells Selectively Inhibits Sialyl-Lewis x Expression and Binding to E-Selectin without Affecting Synthesis of Sialyl-Lewis a or Binding to P-Selectin. Am. J. Pathol. 2004, 164, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Lester, D.K.; Burton, C.; Gardner, A.; Innamarato, P.; Kodumudi, K.; Liu, Q.; Adhikari, E.; Ming, Q.; Williamson, D.B.; Frederick, D.T.; et al. Fucosylation of HLA-DRB1 regulates CD4+ T cell-mediated anti-melanoma immunity and enhances immunotherapy efficacy. Nat. Cancer 2023, 4, 222–239. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Y.; Zhang, L.; Qian, W.; Hou, X.; Lin, R. Exogenous l-fucose protects the intestinal mucosal barrier depending on upregulation of FUT2-mediated fucosylation of intestinal epithelial cells. Faseb J. 2021, 35, e21699. [Google Scholar] [CrossRef]
- Mullen, J.L.; Rosato, F.E.; Allen, T.R.; Miller, E.E.; Roseman, J.; Rosato, E.F. Continuous intravenous fucose therapy in rat mammary cancer. II. J. Surg. Oncol. 1973, 5, 61–69. [Google Scholar] [CrossRef]
- Rosato, F.E.; Mullen, J.L.; Rosato, E.F.; Steiger, E.; Miller, E. Continuous intravenous fucose treatment of rat mammary tumor. J. Surg. Oncol. 1972, 4, 94–101. [Google Scholar] [CrossRef]
- Roseman, J.M.; Miller, E.; Seltzer, M.H.; Wolfe, D.; Rosato, F.E. The effect of L-fucose on rat mammary tumor growth. II. In vitro studies. J. Surg. Oncol. 1971, 3, 79–88. [Google Scholar] [CrossRef]
- Tomsik, P.; Soukup, T.; Cermakova, E.; Micuda, S.; Niang, M.; Sucha, L.; Rezacova, M. L-rhamnose and L-fucose suppress cancer growth in mice. Cent. Eur. J. Biol. 2011, 6, 1–9. [Google Scholar] [CrossRef]
- Yao, Y.; Qian, C.; Chang, Z.; Yang, J.; Chen, Y.; Wang, H.; Zhu, J.; Xiao, Y.; Li, Y.; Zhao, J.; et al. L-Fucose increases the fucosylation of colorectal cancer cells via promoting the accumulation of serine. Food Funct. 2023, 14, 4314–4326. [Google Scholar] [CrossRef]
- Christiansen, M.N.; Chik, J.; Lee, L.; Anugraham, M.; Abrahams, J.L.; Packer, N.H. Cell surface protein glycosylation in cancer. Proteomics 2014, 14, 525–546. [Google Scholar] [CrossRef]
- Kudelka, M.R.; Stowell, S.R.; Cummings, R.D.; Neish, A.S. Intestinal epithelial glycosylation in homeostasis and gut microbiota interactions in IBD. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 597–617. [Google Scholar] [CrossRef] [PubMed]
- Izumori, K. Bioproduction strategies for rare hexose sugars. Sci. Nat. 2002, 89, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Avery, A.; Ford, R.; Yang, Q.; Goux, A.; Mukherjee, I.; Neville, D.C.A.; Jethwa, P.H. Rare sugars: Metabolic impacts and mechanisms of action: A scoping review. Br. J. Nutr. 2021, 128, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Umai, D.; Kayalvizhi, R.; Kumar, V.; Jacob, S. Xylitol: Bioproduction and Applications—A Review. Front. Sustain. 2022, 3, 826190. [Google Scholar] [CrossRef]
- Chukwuma, C.I.; Islam, M.S. Effects of xylitol on carbohydrate digesting enzymes activity, intestinal glucose absorption and muscle glucose uptake: A multi-mode study. Food Funct. 2015, 6, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Chukwuma, C.; Islam, M. Xylitol: One Name, Numerous Benefits. In Sweetners: Reference Series in Phytochemistry; Springer: New York, NY, USA, 2016; pp. 1–27. [Google Scholar]
- Miyasawa-Hori, H.; Aizawa, S.; Takahashi, N. Difference in the xylitol sensitivity of acid production among Streptococcus mutans strains and the biochemical mechanism. Oral Microbiol. Immunol. 2006, 21, 201–205. [Google Scholar] [CrossRef]
- Sato, J.; Wang, Y.M.; van Eys, J. Metabolism of xylitol and glucose in rats bearing hepatocellular. Cancer Res. 1981, 41, 3192–3199. [Google Scholar]
- Ahuja, V.; Macho, M.; Ewe, D.; Singh, M.; Saha, S.; Saurav, K. Biological and Pharmacological Potential of Xylitol: A Molecular Insight of Unique Metabolism. Foods 2020, 9, 1592. [Google Scholar] [CrossRef]
- Park, E.; Park, M.H.; Na, H.S.; Chung, J. Xylitol induces cell death in lung cancer A549 cells by autophagy. Biotechnol. Lett. 2015, 37, 983–990. [Google Scholar] [CrossRef]
- Trachootham, D.; Chingsuwanrote, P.; Yoosadiang, P.; Mekkriangkrai, D.; Ratchawong, T.; Buraphacheep, N.; Kijanukul, S.; Saekhow, S.; Pongpitchayadej, O.; Vongvachvasin, K.; et al. Partial Substitution of Glucose with Xylitol Suppressed the Glycolysis and Selectively Inhibited the Proliferation of Oral Cancer Cells. Nutr. Cancer 2017, 69, 862–872. [Google Scholar] [CrossRef]
- Sahasakul, Y.; Angkhasirisap, W.; Lam-Ubol, A.; Aursalung, A.; Sano, D.; Takada, K.; Trachootham, D. Partial Substitution of Glucose with Xylitol Prolongs Survival and Suppresses Cell Proliferation and Glycolysis of Mice Bearing Orthotopic Xenograft of Oral Cancer. Nutrients 2022, 14, 2023. [Google Scholar] [CrossRef]
- Tomonobu, N.; Komalasari, N.L.G.Y.; Sumardika, I.W.; Jiang, F.; Chen, Y.; Yamamoto, K.I.; Kinoshita, R.; Murata, H.; Inoue, Y.; Sakaguchi, M. Xylitol acts as an anticancer monosaccharide to induce selective cancer death via regulation of the glutathione level. Chem. Biol. Interact. 2020, 324, 109085. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, D.; Fu, Q.; Hao, S.; Gu, Y.; Zhao, W.; Chen, S.; Sheng, F.; Xu, Y.; Chen, Z.; et al. CHAC1 as a Novel Contributor of Ferroptosis in Retinal Pigment Epithelial Cells with Oxidative Damage. Int. J. Mol. Sci. 2023, 24, 1582. [Google Scholar] [CrossRef] [PubMed]
- Iga, Y.; Nakamichi, K.; Shirai, Y.; Matsuo, T. Acute and sub-chronic toxicity of D-allose in rats. Biosci. Biotechnol. Biochem. 2010, 74, 1476–1478. [Google Scholar] [CrossRef] [PubMed]
- Weckwerth, W.; Loureiro, M.E.; Wenzel, K.; Fiehn, O. Differential metabolic networks unravel the effects of silent plant phenotypes. Proc. Natl. Acad. Sci. USA 2004, 101, 7809–7814. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.R.R.; Arumugam, R.; Anantharaman, P. Anantharaman, Chemical composition and antibacterial activity of Indian seagrasses against urinary tract pathogens. Food Chem. 2012, 135, 2470–2473. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, J.; Zhang, W.; Zhang, T.; Guang, C.; Mu, W. Recent research on the physiological functions, applications, and biotechnological production of d-allose. Appl. Microbiol. Biotechnol. 2018, 102, 4269–4278. [Google Scholar] [CrossRef] [PubMed]
- Sui, L.; Dong, Y.; Watanabe, Y.; Yamaguchi, F.; Hatano, N.; Tsukamoto, I.; Izumori, K.; Tokuda, M. The inhibitory effect and possible mechanisms of D-allose on cancer cell proliferation. Int. J. Oncol. 2005, 27, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Sui, L.; Dong, Y.; Watanabe, Y.; Yamaguchi, F.; Hatano, N.; Izumori, K.; Tokuda, M. Growth inhibitory effect of D-allose on human ovarian carcinoma cells in vitro. Anticancer Res. 2005, 25, 2639–2644. [Google Scholar] [PubMed]
- Jeong, R.U.; Lim, S.; Kim, M.O.; Moon, M.H. Effect of D-allose on prostate cancer cell lines: Phospholipid profiling by nanoflow liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2011, 401, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Saito, M.; Tsukamoto, I.; Yamaguchi, F.; Sui, L.; Kamitori, K.; Dong, Y.; Uehara, E.; Konishi, R.; Janjua, N.; et al. Analysis of the inhibitory mechanism of D-allose on MOLT-4F leukemia cell proliferation. J. Biosci. Bioeng. 2009, 107, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Jeon, J.H.; Ju, H.R.; Jung, U.; Kim, K.Y.; Yoo, H.S.; Lee, Y.H.; Song, K.S.; Hwang, H.M.; Na, Y.S.; et al. VDUP1 upregulated by TGF-β1 and 1,25-dihydorxyvitamin D3 inhibits tumor cell growth by blocking cell-cycle progression. Oncogene 2003, 22, 4035–4046. [Google Scholar] [CrossRef]
- Ohta, S.; Lai, E.W.; Pang, A.L.; Brouwers, F.M.; Chan, W.-Y.; Eisenhofer, G.; de Krijger, R.; Ksinantova, L.; Breza, J.; Blazicek, P.; et al. Downregulation of metastasis suppressor genes in malignant pheochromocytoma. Int. J. Cancer 2005, 114, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, F.; Takata, M.; Kamitori, K.; Nonaka, M.; Dong, Y.; Sui, L.; Tokuda, M. Rare sugar D-allose induces specific up-regulation of TXNIP and subsequent G1 cell cycle arrest in hepatocellular carcinoma cells by stabilization of p27kip1. Int. J. Oncol. 2008, 32, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Parikh, H.; Carlsson, E.; A Chutkow, W.; E Johansson, L.; Storgaard, H.; Poulsen, P.; Saxena, R.; Ladd, C.; Schulze, P.C.; Mazzini, M.J.; et al. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med. 2007, 4, e158. [Google Scholar] [CrossRef] [PubMed]
- Hoshikawa, H.; Mori, T.; Mori, N. In vitro and in vivo effects of D-allose: Up-regulation of thioredoxin-interacting protein in head and neck cancer cells. Ann. Otol. Rhinol. Laryngol. 2010, 119, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, C.; Kamitori, K.; Hossain, A.; Hoshikawa, H.; Katagi, A.; Dong, Y.; Sui, L.; Tokuda, M.; Yamaguchi, F. D-Allose Inhibits Cancer Cell Growth by Reducing GLUT1 Expression. Tohoku J. Exp. Med. 2016, 238, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Naha, N.; Lee, H.Y.; Jo, M.J.; Chung, B.C.; Kim, S.H.; Kim, M.O. Rare sugar D-allose induces programmed cell death in hormone refractory prostate cancer cells. Apoptosis 2008, 13, 1121–1134. [Google Scholar] [CrossRef]
- Torrealba, N.; Rodríguez-Berriguete, G.; Vera, R.; Fraile, B.; Olmedilla, G.; Martínez-Onsurbe, P.; Sánchez-Chapado, M.; Paniagua, R.; Royuela, M. Homeostasis: Apoptosis and cell cycle in normal and pathological prostate. Aging Male 2020, 23, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Kishida, K.; Iida, T.; Yamada, T.; Toyoda, Y. Intestinal absorption of D-fructose isomers, D-allulose, D-sorbose and D-tagatose, via glucose transporter type 5 (GLUT5) but not sodium-dependent glucose cotransporter 1 (SGLT1) in rats. Br. J. Nutr. 2023, 130, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Zebiri, I.; Balieu, S.; Guilleret, A.; Reynaud, R.; Haudrechy, A. The Chemistry of L-Sorbose. Eur. J. Org. Chem. 2011, 2011, 2905–2910. [Google Scholar] [CrossRef]
- Moore, D.; Stewart, G.R. Effects of 2-Deoxy-d-Glucose, d-Glucosamine, and l-Sorbose on the Growth of Coprinus lagopus hyphae. J. Gen. Microbiol. 1972, 71, 333–342. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Mishra, S.; Bisaria, V.S. Influence of L-Sorbose on Growth and Enzyme Synthesis of Trichoderma reesei C-5. Microbiology 1986, 132, 2761–2766. [Google Scholar] [CrossRef][Green Version]
- Noronha, J.C.; Braunstein, C.R.; Mejia, S.B.; Khan, T.A.; Kendall, C.W.C.; Wolever, T.M.S.; Leiter, L.A.; Sievenpiper, J.L. The Effect of Small Doses of Fructose and Its Epimers on Glycemic Control: A Systematic Review and Meta-Analysis of Controlled Feeding Trials. Nutrients 2018, 10, 1805. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-L.; Zhou, X.; Chen, S.; Xu, S.; Li, Z.; Nakanishi, H.; Gao, X.-D. Rare sugar l-sorbose exerts antitumor activity by impairing glucose metabolism. Commun. Biol. 2023, 6, 259. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Li, X.; Shao, F.; Lv, G.; Lv, H.; Lee, J.-H.; Qian, X.; Wang, Z.; Xia, Y.; Du, L.; et al. The protein kinase activity of fructokinase A specifies the antioxidant responses of tumor cells by phosphorylating p62. Sci. Adv. 2019, 5, eaav4570. [Google Scholar] [CrossRef] [PubMed]
- Giraud, M.-F.; Naismith, J.H. The rhamnose pathway. Curr. Opin. Struct. Biol. 2000, 10, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, R.T.C.; Hudon, J.; Hank, J.A.; Sondel, P.M.; Kiessling, L.L. Rhamnose glycoconjugates for the recruitment of endogenous anti-carbohydrate antibodies to tumor cells. Chembiochem 2014, 15, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Tomšík, P.; Stoklasová, A.; Mičuda, S.; Niang, M.; Šuba, P.; Knížek, J.; Řezáčová, M. Evaluation of the Antineoplastic Activity of L-rhamnose in vitro. A Comparison with 2-deoxyglucose. Acta Med. 2008, 51, 113–119. [Google Scholar] [CrossRef]
- Li, X.; Rao, X.; Cai, L.; Liu, X.; Wang, H.; Wu, W.; Zhu, C.; Chen, M.; Wang, P.G.; Yi, W. Targeting Tumor Cells by Natural Anti-Carbohydrate Antibodies Using Rhamnose-Functionalized Liposomes. ACS Chem. Biol. 2016, 11, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, F.; Li, Y.; Wang, L.; Li, H.; Gu, G.; Li, E. Rhamnose-Containing Compounds: Biosynthesis and Applications. Molecules 2022, 27, 5315. [Google Scholar] [CrossRef] [PubMed]
- Wijayasinghe, Y.S.; Bhansali, M.P.; Borkar, M.R.; Chaturbhuj, G.U.; Muntean, B.S.; Viola, R.E.; Bhansali, P.R. A Comprehensive Biological and Synthetic Perspective on 2-Deoxy-d-Glucose (2-DG), A Sweet Molecule with Therapeutic and Diagnostic Potentials. J. Med. Chem. 2022, 65, 3706–3728. [Google Scholar] [CrossRef]
- Singh, R.; Gupta, V.; Kumar, A.; Singh, K. 2-Deoxy-D-Glucose: A Novel Pharmacological Agent for Killing Hypoxic Tumor Cells, Oxygen Dependence-Lowering in COVID-19, and Other Pharmacological Activities. Adv. Pharmacol. Pharm. Sci. 2023, 2023, 9993386. [Google Scholar] [CrossRef]
- Laussel, C.; Léon, S. Cellular toxicity of the metabolic inhibitor 2-deoxyglucose and associated resistance mechanisms. Biochem. Pharmacol. 2020, 182, 114213. [Google Scholar] [CrossRef]
- Wick, A.N.; Drury, D.R.; Nakada, H.I.; Wolfe, J.B.; Britton, B.; Grabowski, R. Localization of the primary metabolic block produced by 2-deoxyglucose. J. Biol. Chem. 1957, 224, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gueron, M.J.B. The inhibition of bovine heart hexokinase by 2-deoxy-D-glucose-6-phosphate: Characterization by 31P NMR and metabolic implications. Biochimie 1992, 74, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Savaraj, N.; Priebe, W.; Lampidis, T.J. Hypoxia increases tumor cell sensitivity to glycolytic inhibitors: A strategy for solid tumor therapy (Model C). Biochem. Pharmacol. 2002, 64, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.W.; Lee, S.H. The Roles of Autophagy in Cancer. Int. J. Mol. Sci. 2018, 19, 3466. [Google Scholar] [CrossRef]
- Kurtoglu, M.; Gao, N.; Shang, J.; Maher, J.C.; Lehrman, M.A.; Wangpaichitr, M.; Savaraj, N.; Lane, A.N.; Lampidis, T.J. Under normoxia, 2-deoxy-D-glucose elicits cell death in select tumor types not by inhibition of glycolysis but by interfering with N-linked glycosylation. Mol. Cancer Ther. 2007, 6, 3049–3058. [Google Scholar] [CrossRef]
- Datema, R.; Schwarz, R.T.; Jankowski, A.W. Fluoroglucose-inhibition of protein glycosylation in vivo. Inhibition of mannose and glucose incorporation into lipid-linked oligosaccharides. Eur. J. Biochem. 1980, 109, 331–341. [Google Scholar] [CrossRef]
- Datema, R.; Schwarz, R.T. Interference with glycosylation of glycoproteins. Inhibition of formation of lipid-linked oligosaccharides in vivo. Biochem. J. 1979, 184, 113–123. [Google Scholar] [CrossRef]
- Schmidt, M.F.G.; Biely, P.; Kratky, Z.; Schwarz, R.T. Metabolism of 2-deoxy-2-fluoro-D-[3H]glucose and 2-deoxy-2-fluoro-D-[3H]mannose in yeast and chick-embryo cells. Eur. J. Biochem. 1978, 87, 55–68. [Google Scholar] [CrossRef]
- Schmidt, M.F.; Schwarz, R.T.; Scholtissek, C. Nucleoside-diphosphate derivatives of 2-deoxy-D-glucose in animal cells. Eur. J. Biochem. 1974, 49, 237–247. [Google Scholar] [CrossRef]
- Ramírez-Peinado, S.; Alcázar-Limones, F.; Lagares-Tena, L.; El Mjiyad, N.; Caro-Maldonado, A.; Tirado, O.M.; Muñoz-Pinedo, C. 2-deoxyglucose induces Noxa-dependent apoptosis in alveolar rhabdomyosarcoma. Cancer Res. 2011, 71, 6796–6806. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.; Lin, H.; Jeyamohan, C.; Dvorzhinski, D.; Gounder, M.; Bray, K.; Eddy, S.; Goodin, S.; White, E.; Dipaola, R.S. Targeting tumor metabolism with 2-deoxyglucose in patients with castrate-resistant prostate cancer and advanced malignancies. Prostate 2010, 70, 1388–1394. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, H.; Liu, D.X.; Niu, T.-K.; Ren, X.; Patel, R.; Hait, W.N.; Yang, J.-M. Silencing of elongation factor-2 kinase potentiates the effect of 2-deoxy-D-glucose against human glioma cells through blunting of autophagy. Cancer Res. 2009, 69, 2453–2460. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Barredo, J.C.; Merchan, J.R.; Lampidis, T.J. Endoplasmic reticulum stress induced by 2-deoxyglucose but not glucose starvation activates AMPK through CaMKKβ leading to autophagy. Biochem. Pharmacol. 2013, 85, 1463–1477. [Google Scholar] [CrossRef] [PubMed]
- Pradelli, L.A.; Bénéteau, M.; Chauvin, C.; A Jacquin, M.; Marchetti, S.; Muñoz-Pinedo, C.; Auberger, P.; Pende, M.; Ricci, J.-E. Glycolysis inhibition sensitizes tumor cells to death receptors-induced apoptosis by AMP kinase activation leading to Mcl-1 block in translation. Oncogene 2010, 29, 1641–1652. [Google Scholar] [CrossRef]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. mTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef]
- Tu, D.; Gao, Y.; Yang, R.; Guan, T.; Hong, J.-S.; Gao, H.-M. The pentose phosphate pathway regulates chronic neuroinflammation and dopaminergic neurodegeneration. J. Neuroinflamm. 2019, 16, 255. [Google Scholar] [CrossRef]
- Coleman, M.C.; Asbury, C.R.; Daniels, D.; Du, J.; Aykin-Burns, N.; Smith, B.J.; Li, L.; Spitz, D.R.; Cullen, J.J. 2-deoxy-D-glucose causes cytotoxicity, oxidative stress, and radiosensitization in pancreatic cancer. Free Radic. Biol. Med. 2008, 44, 322–331. [Google Scholar] [CrossRef]
- Xi, H.; Kurtoglu, M.; Lampidis, T.J. The wonders of 2-deoxy-d-glucose. IUBMB Life 2014, 66, 110–121. [Google Scholar] [CrossRef]
- Landau, B.R.; Laszlo, J.; Stengle, J.; Burk, D. Certain metabolic and pharmacologic effects in cancer patients given infusions of 2-deoxy-D-glucose. J. Natl. Cancer Inst. 1958, 21, 485–494. [Google Scholar] [PubMed]
- Fowler, J.S.; Ido, T. Initial and subsequent approach for the synthesis of 18FDG. Nucl. Med. Semin. 2002, 32, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Lampidis, T.J.; Kurtoglu, M.; Maher, J.C.; Liu, H.; Krishan, A.; Sheft, V.; Szymanski, S.; Fokt, I.; Rudnicki, W.R.; Ginalski, K.; et al. Efficacy of 2-halogen substituted d-glucose analogs in blocking glycolysis and killing “hypoxic tumor cells”. Cancer Chemother. Pharmacol. 2006, 58, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Priebe, W.; Zielinski, R.; Fokt, I.; Felix, E.; Radjendirane, V.; Arumugam, J.; Khuong, M.T.; Krasinski, M.; Skora, S. EXTH-07. Design and Evaluation of wp1122, an Inhibitor of Glycolysis with Increased Cns Uptake. Neuro-Oncol. 2018, 20 (Suppl. S6), vi86. [Google Scholar] [CrossRef][Green Version]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Park, K.-S.; Jeong, K.-C.; Lee, B.I.; Lee, C.-H.; Kim, S.-Y. Glucosamine is an effective chemo-sensitizer via transglutaminase 2 inhibition. Cancer Lett. 2009, 273, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Indo, K.; Hoshikawa, H.; Kamitori, K.; Yamaguchi, F.; Mori, T.; Tokuda, M.; Mori, N. Effects of D-allose in combination with docetaxel in human head and neck cancer cells. Int. J. Oncol. 2014, 45, 2044–2050. [Google Scholar] [CrossRef] [PubMed]
- Shenfield, G.M. Fixed Combination Drug Therapy. Drugs 1982, 23, 462–480. [Google Scholar] [CrossRef] [PubMed]
- Ekholm, F.S.; Berényi, Á.; Lagerquist, L.; Saloranta, T.; Zupkó, I.; Schneider, G.; Wölfling, J.; Leino, R. Cytotoxic activity of some glycoconjugates including saponins and anthracyclines. Carbohydr. Res. 2012, 356, 295–298. [Google Scholar] [CrossRef] [PubMed]
- La Ferla, B.; Airoldi, C.; Zona, C.; Orsato, A.; Cardona, F.; Merlo, S.; Sironi, E.; D’Orazio, G.; Nicotra, F. Natural glycoconjugates with antitumor activity. Nat. Prod. Rep. 2011, 28, 630–648. [Google Scholar] [CrossRef]
- Govindarajan, M. Amphiphilic glycoconjugates as potential anti-cancer chemotherapeutics. Eur. J. Med. Chem. 2018, 143, 1208–1253. [Google Scholar] [CrossRef] [PubMed]
- Molejon, M.I.; Weiz, G.; Breccia, J.D.; Vaccaro, M.I. Glycoconjugation: An approach to cancer therapeutics. World J. Clin. Oncol. 2020, 11, 110–120. [Google Scholar] [CrossRef]
- Pastuch-Gawołek, G.; Szreder, J.; Domińska, M.; Pielok, M.; Cichy, P.; Grymel, M. A Small Sugar Molecule with Huge Potential in Targeted Cancer Therapy. Pharmaceutics 2023, 15, 913. [Google Scholar] [CrossRef] [PubMed]
- Halmos, T.; Santarromana, M.; Antonakis, K.; Scherman, D. Synthesis of glucose-chlorambucil derivatives and their recognition by the human GLUT1 glucose transporter. Eur. J. Pharmacol. 1996, 318, 477–484. [Google Scholar] [CrossRef]
- Kumar, P.; Shustov, G.; Liang, H.; Khlebnikov, V.; Zheng, W.; Yang, X.H.; Cheeseman, C.; Wiebe, L.I. Design, Synthesis, and Preliminary Biological Evaluation of 6-O-Glucose–Azomycin Adducts for Diagnosis and Therapy of Hypoxic Tumors. J. Med. Chem. 2012, 55, 6033–6046. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, H.; Su, S.; Wang, T.; Zhang, C.; Fida, G.; Cui, S.; Zhao, J.; Gu, Y. Galactose as Broad Ligand for Multiple Tumor Imaging and Therapy. J. Cancer 2015, 6, 658–670. [Google Scholar] [CrossRef]
- Valieva, Y.; Ivanova, E.; Fayzullin, A.; Kurkov, A.; Igrunkova, A. Senescence-Associated β-Galactosidase Detection in Pathology. Diagnostics 2022, 12, 2309. [Google Scholar] [CrossRef]
- Meng, X.; Lian, X.; Li, X.; Ya, Q.; Li, T.; Zhang, Y.; Yang, Y.; Zhang, Y. Synthesis of 2′-paclitaxel 2-deoxy-2-fluoro-glucopyranosyl carbonate for specific targeted delivery to cancer cells. Carbohydr. Res. 2020, 493, 108034. [Google Scholar] [CrossRef] [PubMed]
- Najlah, M. Drug repurposing supported by nanotechnology: A promising strategy to fight cancer. Ther. Deliv. 2021, 12, 267–269. [Google Scholar] [CrossRef]


| Monosaccharide | Cancer Cells Tested In Vitro | Effective In Vitro Doses * | Cancer Cells Tested In Vivo | Effective In Vivo Doses | Mechanisms of Action | Refs |
|---|---|---|---|---|---|---|
| ᴅ-mannose | KP-4, U2OS, Saos-2, K562 | 10–25 mM | KP-4, K562 | 20–40% solution | Glycolytic suppression | [21,22] |
| ᴅ-glucosamine | DU145, MDA-MB-231, 786-O, Caki-1, A549, L1210, U87MG | 1–5 mM | - | - | Protein synthesis inhibition, cell cycle arrest, ER stress, and autophagy | [31,32,35,37,39,41] |
| ᴅ-galactose | N2a, SH-SY5Y, PC-3, HepG2, A549, HeLa, PANC-1 | 5–333 mM | - | - | Necroptosis, glycolytic suppression | [51,52] |
| ʟ-fucose | Rat mammary tumour cells, HCT-116 | 60–300 mM | Rat mammary tumour cells, Ehrlich carcinoma | 20% solution or 1–5 g/kg | Upregulation of fucosylation | [77,78,79,80,81] |
| Xylitol | A549, Caki-1, CAL-27, MeWo | 6.5–2100 mM | CAL-27, MeWo | 1–2 g/kg | Autophagy, glycolytic suppression, ER stress, apoptosis | [92,93,94,95] |
| ᴅ-allose | MOLT-4F, HSC-3, Ca9-22, DU145, PC-3 | 1–50 mM | HSC-3 | 2 g/kg | Upregulation of protein expression, cell cycle arrest, apoptosis | [104,107,109,111] |
| ʟ-sorbose | Huh-7, HepG2, A549, HeLa, MCF-7, K562 | 25 mM | Huh-7 | 20% solution | Apoptosis | [118] |
| ʟ-rhamnose | / | / | Ehlrich carcinoma | 1–5 g/kg | Necrosis | [80] |
| Combination | Cell Line | Refs |
|---|---|---|
| Mannose + doxorubicin | KP-4 | [21] |
| Glucosamine + doxorubicin | MCF-7/DOX | [151] |
| Xylitol + 5-fluorouracil | MeWo | [95] |
| Allose + docetaxel | HSC-3 | [152] |
| Sorbose + sorafenib | Huh7 | [118] |
| 2-DG + paclitaxel | A549 | [6] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCallum, N.; Najlah, M. The Anticancer Activity of Monosaccharides: Perspectives and Outlooks. Cancers 2024, 16, 2775. https://doi.org/10.3390/cancers16162775
McCallum N, Najlah M. The Anticancer Activity of Monosaccharides: Perspectives and Outlooks. Cancers. 2024; 16(16):2775. https://doi.org/10.3390/cancers16162775
Chicago/Turabian StyleMcCallum, Niamh, and Mohammad Najlah. 2024. "The Anticancer Activity of Monosaccharides: Perspectives and Outlooks" Cancers 16, no. 16: 2775. https://doi.org/10.3390/cancers16162775
APA StyleMcCallum, N., & Najlah, M. (2024). The Anticancer Activity of Monosaccharides: Perspectives and Outlooks. Cancers, 16(16), 2775. https://doi.org/10.3390/cancers16162775








