Safety and Efficacy of Peptide Receptor Radionuclide Therapy (PRRT) Following Bland Embolization for Metastatic Neuroendocrine Tumors
Abstract
Simple Summary
Abstract
1. Introduction
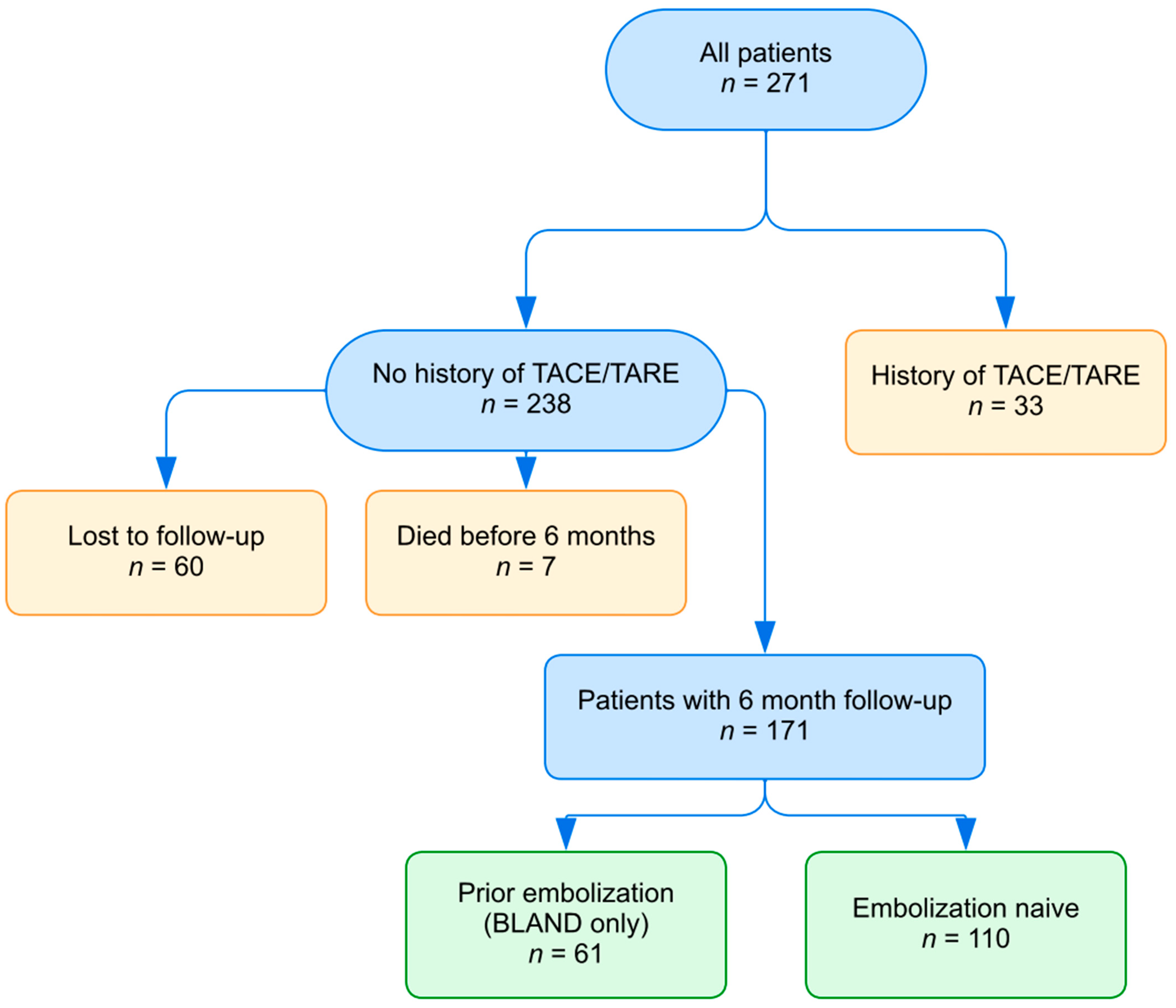
2. Materials and Methods
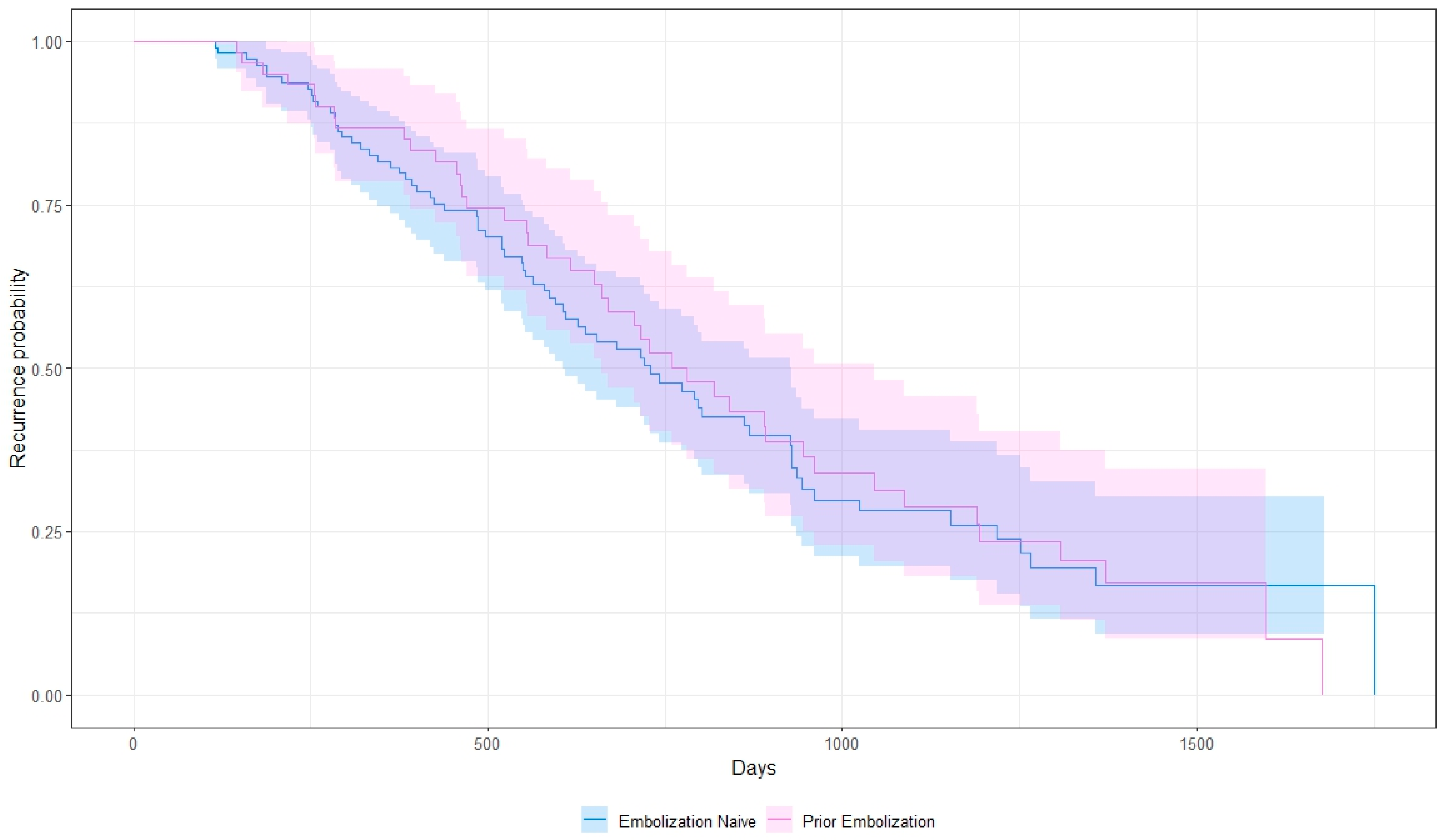
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Weber, J.M.; Feldman, M.; Coppola, D.; Meredith, K.; Kvols, L.K. Prognostic validity of the American Joint Committee on Cancer staging classification for midgut neuroendocrine tumors. J. Clin. Oncol. 2013, 31, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; de Herder, W.W. ENETS Consensus Guidelines for the Standard of Care in Neuroendocrine Tumors. Neuroendocrinology 2017, 105, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.H.; Goldner, W.S.; Benson, A.B.; Bergsland, E.; Blaszkowsky, L.S.; Brock, P.; Chan, J.; Das, S.; Dickson, P.V.; Fanta, P.; et al. Neuroendocrine and Adrenal Tumors, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2021, 19, 839–868. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Brabander, T.; van der Zwan, W.A.; Teunissen, J.J.M.; Kam, B.L.R.; Feelders, R.A.; de Herder, W.W.; van Eijck, C.H.J.; Franssen, G.J.H.; Krenning, E.P.; Kwekkeboom, D.J. Long-Term Efficacy, Survival, and Safety of [177Lu-DOTA0,Tyr3]octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin. Cancer. Res. 2017, 23, 4617–4624. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.; Bester, L.; Salem, R.; Sharma, R.A.; Parks, R.W.; Ruszniewski, P.; Conference, N.E.-L.-M.C. Role of hepatic intra-arterial therapies in metastatic neuroendocrine tumours (NET): Guidelines from the NET-Liver-Metastases Consensus Conference. HPB (Oxford) 2015, 17, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; de Vries, E.G.; et al. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 514–523. [Google Scholar] [CrossRef]
- Kunz, P.L.; Graham, N.T.; Catalano, P.J.; Nimeiri, H.S.; Fisher, G.A.; Longacre, T.A.; Suarez, C.J.; Martin, B.A.; Yao, J.C.; Kulke, M.H.; et al. A Randomized Study of Temozolomide or Temozolomide and Capecitabine in Patients with Advanced Pancreatic Neuroendocrine Tumors (ECOG-ACRIN E2211). J. Clin. Oncol. 2022, 41, 7. [Google Scholar] [CrossRef]
- Faivre, S.; Niccoli, P.; Castellano, D.; Valle, J.W.; Hammel, P.; Raoul, J.L.; Vinik, A.; Van Cutsem, E.; Bang, Y.J.; Lee, S.H.; et al. Sunitinib in pancreatic neuroendocrine tumors: Updated progression-free survival and final overall survival from a phase III randomized study. Ann. Oncol. 2017, 28, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Kwekkeboom, D.J.; de Herder, W.W.; Kam, B.L.; van Eijck, C.H.; van Essen, M.; Kooij, P.P.; Feelders, R.A.; van Aken, M.O.; Krenning, E.P. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate: Toxicity, efficacy, and survival. J. Clin. Oncol. 2008, 26, 2124–2130. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Q.; Wang, G.; Sui, H.; Wang, R.; Wang, J.; Zhang, J.; Zhu, Z.; Chen, X. Safety and efficacy of peptide receptor radionuclide therapy with (177)Lu-DOTA-EB-TATE in patients with metastatic neuroendocrine tumors. Theranostics 2022, 12, 6437–6445. [Google Scholar] [CrossRef]
- Hamiditabar, M.; Ali, M.; Bolek, L.; Vahdati, G.; Tworowska, I.; Delpassand, E.S. Safety and Effectiveness of 177Lu-DOTATATE Peptide Receptor Radionuclide Therapy After Regional Hepatic Embolization in Patients With Somatostatin-Expressing Neuroendocrine Tumors. Clin. Nucl. Med. 2017, 42, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Braat, A.; Bruijnen, R.C.G.; van Rooij, R.; Braat, M.; Wessels, F.J.; van Leeuwaarde, R.S.; van Treijen, M.J.C.; de Herder, W.W.; Hofland, J.; Tesselaar, M.E.T.; et al. Additional holmium-166 radioembolisation after lutetium-177-dotatate in patients with neuroendocrine tumour liver metastases (HEPAR PLuS): A single-centre, single-arm, open-label, phase 2 study. Lancet Oncol. 2020, 21, 561–570. [Google Scholar] [CrossRef]
- Braat, A.; Ahmadzadehfar, H.; Kappadath, S.C.; Stothers, C.L.; Frilling, A.; Deroose, C.M.; Flamen, P.; Brown, D.B.; Sze, D.Y.; Mahvash, A.; et al. Radioembolization with (90)Y Resin Microspheres of Neuroendocrine Liver Metastases After Initial Peptide Receptor Radionuclide Therapy. Cardiovasc. Intervent. Radiol. 2020, 43, 246–253. [Google Scholar] [CrossRef]

| Prior Embolization (n = 61) | Embolization-Naïve (n = 110) | |
|---|---|---|
| Gender | ||
| Male | 34 (56%) | 61 (56%) |
| Female | 27 (44%) | 49 (44%) |
| Age at PRRT | ||
| Mean (SD) | 64.2 (10) | 64.4 (8.9) |
| Follow-up Time (months) | ||
| Mean (SD) | 22.4 (11.4) | 20.9 (10.4) |
| Years From Diagnosis to PRRT | ||
| Mean (SD) | 7.2 (5.5) | 6.4 (5.8) |
| Primary Tumor Location | ||
| Foregut | 3 (5%) | 6 (5%) |
| Midgut | 43 (71%) | 57 (52%) |
| Hindgut | 2 (3%) | 7 (6%) |
| Pancreas | 11 (18%) | 33 (30%) |
| Other/Unknown | 2 (3%) | 7 (7%) |
| Tumor Grade | ||
| 1 | 19 (31%) | 42 (38%) |
| 2 | 29 (48%) | 52 (47%) |
| 3 | 8 (13%) | 6 (6%) |
| Uncertain | 5 (8%) | 10 (9%) |
| Tumor Burden | ||
| <25% | 40 (66%) | 90 (82%) |
| 25–50% | 16 (26%) | 15 (14%) |
| >50% | 5 (8%) | 5 (5%) |
| PRRT Cycles | ||
| 1 | 0 (0%) | 3 (3%) |
| 2 | 5 (8%) | 4 (4%) |
| 3 | 6 (10%) | 10 (9%) |
| 4 | 50 (82%) | 93 (84%) |
| Toxicity Metric | Prior Embolization | Embolization-Naïve | p-Value |
|---|---|---|---|
| Patients with grade 3 hepatic adverse events | 6 | 7 | 0.548 |
| Patients with grade 4 hepatic adverse events | 1 | 2 | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alayli, A.; Ngo, H.; Sikaria, D.; Ahmed, A.; Salloum, E.; Strosberg, J.R.; Al-Toubah, T.E.; Kis, B.; Haider, M.; El-Haddad, G. Safety and Efficacy of Peptide Receptor Radionuclide Therapy (PRRT) Following Bland Embolization for Metastatic Neuroendocrine Tumors. Cancers 2024, 16, 2703. https://doi.org/10.3390/cancers16152703
Alayli A, Ngo H, Sikaria D, Ahmed A, Salloum E, Strosberg JR, Al-Toubah TE, Kis B, Haider M, El-Haddad G. Safety and Efficacy of Peptide Receptor Radionuclide Therapy (PRRT) Following Bland Embolization for Metastatic Neuroendocrine Tumors. Cancers. 2024; 16(15):2703. https://doi.org/10.3390/cancers16152703
Chicago/Turabian StyleAlayli, Adam, Hoang Ngo, Dhiraj Sikaria, Altan Ahmed, Elias Salloum, Jonathan R. Strosberg, Taymeyah E. Al-Toubah, Bela Kis, Mintallah Haider, and Ghassan El-Haddad. 2024. "Safety and Efficacy of Peptide Receptor Radionuclide Therapy (PRRT) Following Bland Embolization for Metastatic Neuroendocrine Tumors" Cancers 16, no. 15: 2703. https://doi.org/10.3390/cancers16152703
APA StyleAlayli, A., Ngo, H., Sikaria, D., Ahmed, A., Salloum, E., Strosberg, J. R., Al-Toubah, T. E., Kis, B., Haider, M., & El-Haddad, G. (2024). Safety and Efficacy of Peptide Receptor Radionuclide Therapy (PRRT) Following Bland Embolization for Metastatic Neuroendocrine Tumors. Cancers, 16(15), 2703. https://doi.org/10.3390/cancers16152703








