Fluorescence-Guided Surgical Techniques in Adult Diffuse Low-Grade Gliomas: State-of-the-Art and Emerging Techniques: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
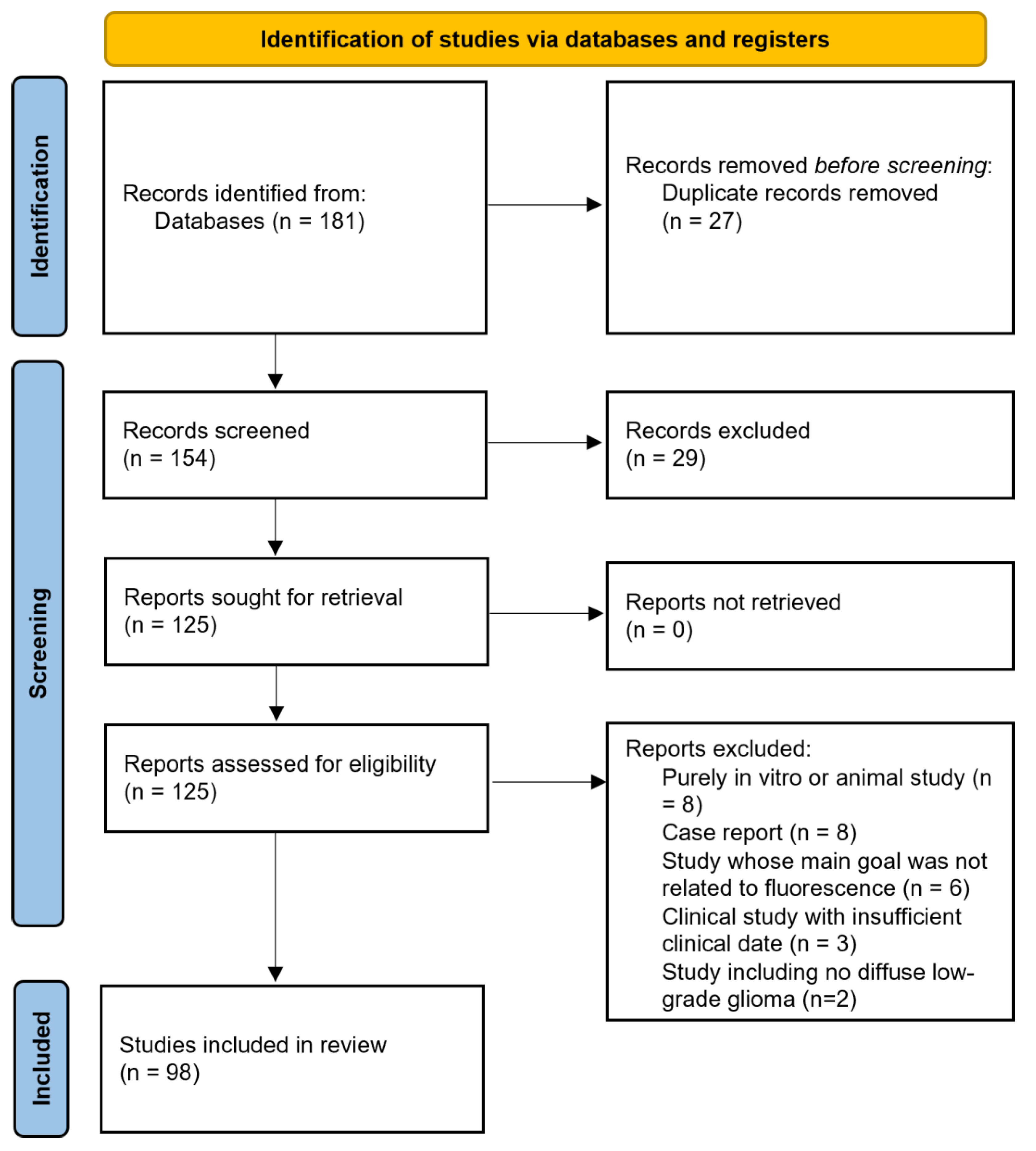
2. Materials and Methods
3. Principles of Fluorescence
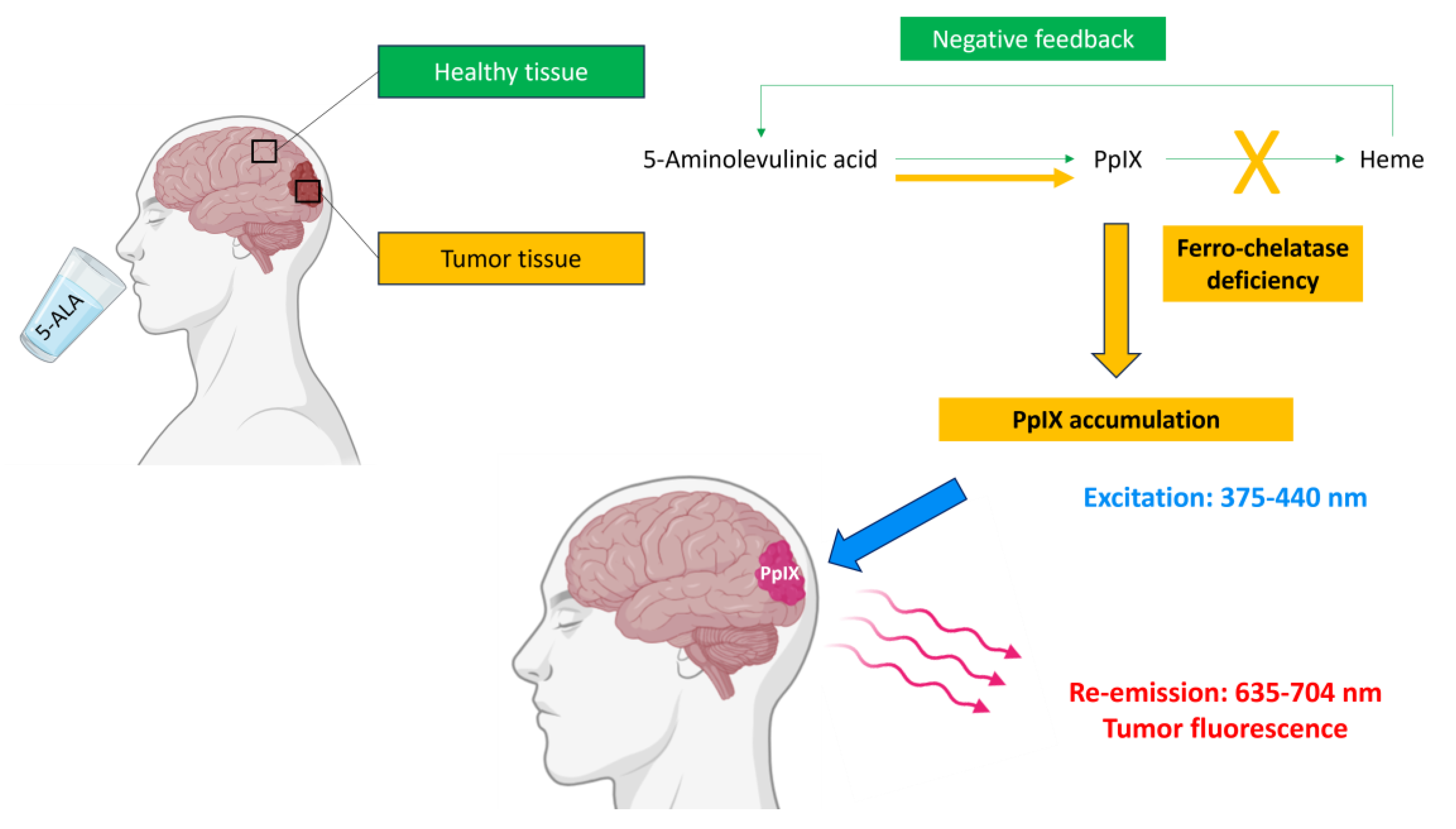
3.1. 5-Amino-Levulinic Acid
3.2. Fluorescein Sodium
4. Results Provided by Macroscopic Fluorescence-Guided Techniques Applied to DLGGs
4.1. Fluorescence-Guided Resection of DLGGs
4.1.1. Assessment of the Rate of Positive 5-ALA Fluorescence in DLGGs
| Study | Design | Effectives | Positive 5-ALA Fluorescence |
|---|---|---|---|
| Tsugu, 2011 [56] | Retrospective, monocentric (WHO 2007) | 33 gliomas including 6 (18.2%) DLGGs | 0/6 (0%) of DLGGs. |
| Marbacher, 2014 [54] | Retrospective, monocentric (WHO 2007) | 376 tumors including 17 (4.5%) DLGGs | 8/17 (47.1%) DLGGs. |
| Chan, 2017 [9] | Retrospective, monocentric (WHO 2016) | 16 gliomas with heterogenous CE, finally including 3 (18.8%) DLGGs | All (100%) DLGGs. |
| Ji, 2019 [53] | Retrospective, monocentric (WHO 2016) | 827 presumed HGGs, finally including 70 (8.5%) DLGGs | 11/70 (15.7%) DLGGs
|
| Goryaynov, 2019 [39] | Retrospective, multicentric (WHO 2016) | 653 tumors, including 52 (7.9%) DLGGs | 22/52 (42.3%) DLGGs. All pleomorphic xanthoastrocytomas (n = 2, 100%). |
| Takeda, 2023 [55] | Prospective, Monocentric (WHO 2021) | 30 deep-seated tumors, including 3 (10%) DLGGs | 1/3 (33.3%) DLGGs Weak fluorescence in a gemistocytic astrocytoma. |
4.1.2. Assessment of the Diagnostic Performances of 5-ALA Fluorescence for the Identification of Anaplastic Foci in Diffuse Gliomas
4.1.3. Identification of the Predictors of 5-ALA Fluorescence Positivity in DLGGs
4.1.4. Assessment of the Prognostic Value of Intraoperative 5-ALA Fluorescence Positivity in DLGGs
4.1.5. Assessment of the Usefulness of FS for the Resection of DLGGs
4.2. Fluorescence-Guided Biopsy of DLGGs
4.2.1. 5-ALA-Guided Biopsies
4.2.2. FS-Guided Biopsies
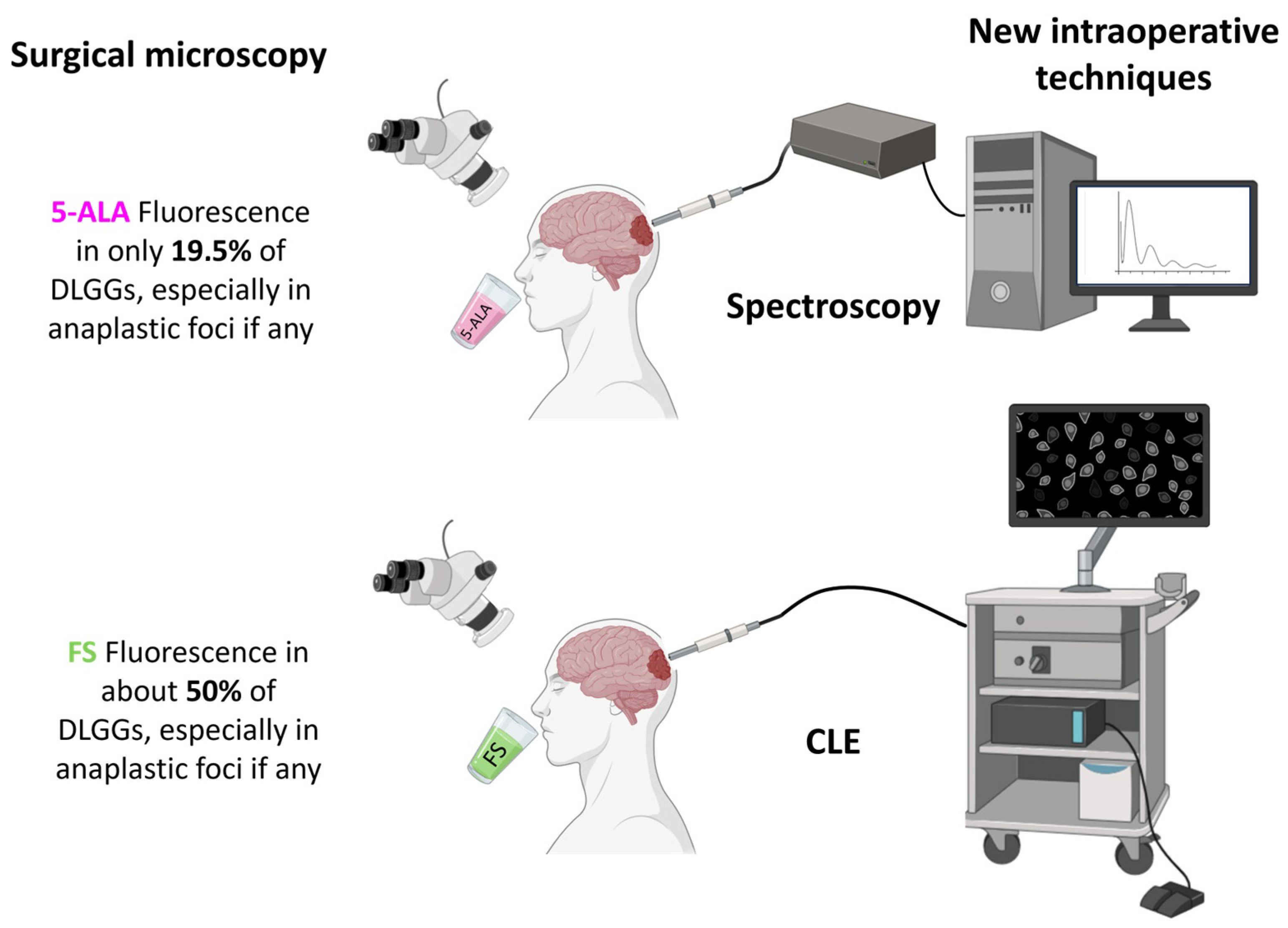
4.3. Main Limitations
5. Emerging Techniques
5.1. Laser Spectroscopic Detection of Autofluorescence
5.1.1. Proof of Concept
5.1.2. Spectroscopic Signature of DLGGs
5.2. Spectroscopic Detection of 5-ALA-Induced Fluorescence
5.2.1. Proof of Concept
5.2.2. Quantification of PpIX Concentration within Glioma Tissue
5.2.3. Spectroscopic Signature of DLGGs
5.2.4. Refinements and Optimization of 5-ALA-Induced Fluorescence Spectroscopic Detection
5.3. Confocal Laser Endomicroscopy
| Study | Design | Effectives | Main Conclusions |
|---|---|---|---|
| 5-ALA | |||
| Sanai, 2011 [142] | Prospective, monocentric in vivo + ex vivo | 10 gliomas, including 2 (20%) DLGGs | No macroscopic fluorescence. 100% in vivo and ex vivo fluorescence for superficial and tumor core samples. Perfect concordance with histopathological analysis for margin samples. |
| FLUORESCEIN SODIUM | |||
| Sanai, 2011 [153] | Prospective, monocentric in vivo (WHO 2007) | 33 tumors, including 13 (39.4%) DLGGs | Feasibility study. Increase in surgical duration of 15–20 min. Correct identification of tumor margin. Morphological aspect consistent with histopathological sections. |
| Eschbacher, 2012 [145] | Prospective, monocentric In vivo (WHO 2007) | 50 tumors, including 8 (16%) DLGGs | Cell density and atypia well correlated with histological sections. Astrocytoma cells more elongated and atypical than oligodendroglioma cells. Blinded analysis: 4/4 (100%) accurate diagnosis for gliomas. |
| Martirosyan, 2016 [146] | Prospective, monocentric in vivo + ex vivo (WHO 2007) | 74 tumors, including 21 gliomas (8 DLGGs) | Mean duration of 5.8 min per patient. Performances for detection of gliomas (all grade): Sensitivity = 91%, Specificity = 94%. Precise estimation of the grade not possible in all cases. |
| Pavlov, 2016 [147] | Prospective, monocentric in vivo (WHO 2007) | 9 tumors, including 2 (22.2%) DLGGs Resection or biopsy | Tumor detected in all cases but impossible to precisely estimate the grade, as expected criteria were not clearly identified (mitosis, endothelial proliferation, and necrosis). |
| Belykh, 2020 [148] | Prospective, monocentric Ex vivo (WHO 2016) | 47 tumors, including 32 gliomas (3 DLGGs) | Performances for detection of gliomas, independently of the grade: Sensitivity = 66%, Specificity = 94%. Fluorescein Sodium re-injection: more pictures with accurate diagnosis (67% to 93%) and fewer non-diagnostic pictures (26% to 13%) |
| Höhne, 2021 [149] | Retrospective, monocentric in vivo (WHO 2016) | 12 tumors, including 1 (8.3%) grade 2 oligodendroglioma | Macroscopic fluorescence visible at the tumor center and borders but not in the perilesional zone. Confirmation of abnormal aspects in these areas compared to adjacent brain. Timing of dye injection: no impact on picture quality. |
| Xu, 2022 [150] | Re-analysis of 2 monocentric prospective series in vivo + ex vivo (WHO 2016) | 73 tumors, including 42 gliomas | Compared to ex vivo pictures, in vivo pictures have significantly higher brightness and contrast values and better diagnostic performances. For ex vivo pictures: negative correlation between contrast and time from dye injection. |
| Xu, 2024 [151] | Retrospective, bicentric in vivo (WHO 2021) | 28 gliomas, including 2 (7.1%) DLGGs | Review of CLE pictures from resection margins Concordance of CLE and histological sections: 61.6%. CLE: Sensitivity = 79%, Specificity = 37% PPV = 65%, PNV = 53%. |
| Wagner, 2024 [152] | Prospective, tricentric in vivo (WHO 2021) | 203 tumors, including 9 (4.4%) DLGGs and 77 (37.9%) HGGs | DLGGs Sensitivity: 56% (CLE) vs. 78% (frozen sections). Specificity: 99% (CLE) vs. 99% (frozen sections). HGGs Sensitivity: 86% (CLE) vs. 94% (frozen sections). Specificity: 95% (CLE) vs. 100% (frozen sections). Median assessment 3 min (CLE) vs. 27 min (frozen sections). |
6. Conclusions
7. Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albert, F.K.; Forsting, M.; Sartor, K.; Adams, H.P.; Kunze, S. Early Postoperative Magnetic Resonance Imaging after Resection of Malignant Glioma: Objective Evaluation of Residual Tumor and Its Influence on Regrowth and Prognosis. Neurosurgery 1994, 34, 45–60; discussion 60–61. [Google Scholar] [CrossRef]
- Hadjipanayis, C.G.; Widhalm, G.; Stummer, W. What Is the Surgical Benefit of Utilizing 5-Aminolevulinic Acid for Fluorescence-Guided Surgery of Malignant Gliomas? Neurosurgery 2015, 77, 663–673. [Google Scholar] [CrossRef]
- Bonosi, L.; Marrone, S.; Benigno, U.E.; Buscemi, F.; Musso, S.; Porzio, M.; Silven, M.P.; Torregrossa, F.; Grasso, G. Maximal Safe Resection in Glioblastoma Surgery: A Systematic Review of Advanced Intraoperative Image-Guided Techniques. Brain Sci. 2023, 13, 216. [Google Scholar] [CrossRef]
- Picart, T.; Berhouma, M.; Dumot, C.; Pallud, J.; Metellus, P.; Armoiry, X.; Guyotat, J. Optimization of High-Grade Glioma Resection Using 5-ALA Fluorescence-Guided Surgery: A Literature Review and Practical Recommendations from the Neuro-Oncology Club of the French Society of Neurosurgery. Neurochirurgie 2019, 65, 164–177. [Google Scholar] [CrossRef]
- Gautheron, A.; Bernstock, J.D.; Picart, T.; Guyotat, J.; Valdés, P.A.; Montcel, B. 5-ALA Induced PpIX Fluorescence Spectroscopy in Neurosurgery: A Review. Front. Neurosci. 2024, 18, 1310282. [Google Scholar] [CrossRef]
- Panciani, P.P.; Fontanella, M.; Garbossa, D.; Agnoletti, A.; Ducati, A.; Lanotte, M. 5-Aminolevulinic Acid and Neuronavigation in High-Grade Glioma Surgery: Results of a Combined Approach. Neurocirugia 2012, 23, 23–28. [Google Scholar] [CrossRef]
- Kim, S.K.; Choi, S.H.; Kim, Y.H.; Park, C.-K. Impact of Fluorescence-Guided Surgery on the Improvement of Clinical Outcomes in Glioblastoma Patients. Neurooncol. Pract. 2014, 1, 81–85. [Google Scholar] [CrossRef]
- Díez Valle, R.; Tejada Solis, S.; Idoate Gastearena, M.A.; García de Eulate, R.; Domínguez Echávarri, P.; Aristu Mendiroz, J. Surgery Guided by 5-Aminolevulinic Fluorescence in Glioblastoma: Volumetric Analysis of Extent of Resection in Single-Center Experience. J. Neurooncol. 2011, 102, 105–113. [Google Scholar] [CrossRef]
- Chan, D.T.M.; Yi-Pin Sonia, H.; Poon, W.S. 5-Aminolevulinic Acid Fluorescence Guided Resection of Malignant Glioma: Hong Kong Experience. Asian J. Surg. 2017, 41, 467–472. [Google Scholar] [CrossRef]
- Lakomkin, N.; Hadjipanayis, C.G. Fluorescence-Guided Surgery for High-Grade Gliomas. J. Surg. Oncol. 2018, 118, 356–361. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J.; ALA-Glioma Study Group. Fluorescence-Guided Surgery with 5-Aminolevulinic Acid for Resection of Malignant Glioma: A Randomised Controlled Multicentre Phase III Trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Picart, T.; Pallud, J.; Berthiller, J.; Dumot, C.; Berhouma, M.; Ducray, F.; Armoiry, X.; Margier, J.; Guerre, P.; Varlet, P.; et al. Use of 5-ALA Fluorescence-Guided Surgery versus White-Light Conventional Microsurgery for the Resection of Newly Diagnosed Glioblastomas (RESECT Study): A French Multicenter Randomized Phase III Study. J. Neurosurg. 2023, 140, 987–1000. [Google Scholar] [CrossRef]
- Acerbi, F.; Broggi, M.; Schebesch, K.-M.; Höhne, J.; Cavallo, C.; De Laurentis, C.; Eoli, M.; Anghileri, E.; Servida, M.; Boffano, C.; et al. Fluorescein-Guided Surgery for Resection of High-Grade Gliomas: A Multicentric Prospective Phase II Study (FLUOGLIO). Clin. Cancer Res. 2018, 24, 52–61. [Google Scholar] [CrossRef]
- Hong, J.; Chen, B.; Yao, X.; Yang, Y. Outcome Comparisons of High-Grade Glioma Resection with or without Fluorescein Sodium-Guidance. Curr. Probl. Cancer 2019, 43, 236–244. [Google Scholar] [CrossRef]
- Luzzi, S.; Giotta Lucifero, A.; Martinelli, A.; Maestro, M.D.; Savioli, G.; Simoncelli, A.; Lafe, E.; Preda, L.; Galzio, R. Supratentorial High-Grade Gliomas: Maximal Safe Anatomical Resection Guided by Augmented Reality High-Definition Fiber Tractography and Fluorescein. Neurosurg. Focus 2021, 51, E5. [Google Scholar] [CrossRef]
- Neira, J.A.; Ung, T.H.; Sims, J.S.; Malone, H.R.; Chow, D.S.; Samanamud, J.L.; Zanazzi, G.J.; Guo, X.; Bowden, S.G.; Zhao, B.; et al. Aggressive Resection at the Infiltrative Margins of Glioblastoma Facilitated by Intraoperative Fluorescein Guidance. J. Neurosurg. 2017, 127, 111–122. [Google Scholar] [CrossRef]
- Mellinghoff, I.K.; van den Bent, M.J.; Blumenthal, D.T.; Touat, M.; Peters, K.B.; Clarke, J.; Mendez, J.; Yust-Katz, S.; Welsh, L.; Mason, W.P.; et al. Vorasidenib in IDH1- or IDH2-Mutant Low-Grade Glioma. N. Engl. J. Med. 2023, 389, 589–601. [Google Scholar] [CrossRef]
- Hervey-Jumper, S.L.; Zhang, Y.; Phillips, J.J.; Morshed, R.A.; Young, J.S.; McCoy, L.; Lafontaine, M.; Luks, T.; Ammanuel, S.; Kakaizada, S.; et al. Interactive Effects of Molecular, Therapeutic, and Patient Factors on Outcome of Diffuse Low-Grade Glioma. J. Clin. Oncol. 2023, 41, 2029–2042. [Google Scholar] [CrossRef]
- De Witt Hamer, P.C.; Robles, S.G.; Zwinderman, A.H.; Duffau, H.; Berger, M.S. Impact of Intraoperative Stimulation Brain Mapping on Glioma Surgery Outcome: A Meta-Analysis. J. Clin. Oncol. 2012, 30, 2559–2565. [Google Scholar] [CrossRef]
- Hendricks, B.K.; Sanai, N.; Stummer, W. Fluorescence-Guided Surgery with Aminolevulinic Acid for Low-Grade Gliomas. J. Neurooncol. 2019, 141, 13–18. [Google Scholar] [CrossRef]
- Almekkawi, A.K.; El Ahmadieh, T.Y.; Wu, E.M.; Abunimer, A.M.; Abi-Aad, K.R.; Aoun, S.G.; Plitt, A.R.; El Tecle, N.E.; Patel, T.; Stummer, W.; et al. The Use of 5-Aminolevulinic Acid in Low-Grade Glioma Resection: A Systematic Review. Oper. Neurosurg. 2020, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, A.; Bonada, M.; Zeppa, P.; Colonna, S.; Tartara, F.; Melcarne, A.; Garbossa, D.; Cofano, F. How Reliable Is Fluorescence-Guided Surgery in Low-Grade Gliomas? A Systematic Review Concerning Different Fluorophores. Cancers 2023, 15, 4130. [Google Scholar] [CrossRef]
- Novotny, A.; Xiang, J.; Stummer, W.; Teuscher, N.S.; Smith, D.E.; Keep, R.F. Mechanisms of 5-Aminolevulinic Acid Uptake at the Choroid Plexus. J. Neurochem. 2000, 75, 321–328. [Google Scholar] [CrossRef]
- Stummer, W.; Stocker, S.; Novotny, A.; Heimann, A.; Sauer, O.; Kempski, O.; Plesnila, N.; Wietzorrek, J.; Reulen, H.J. In Vitro and in Vivo Porphyrin Accumulation by C6 Glioma Cells after Exposure to 5-Aminolevulinic Acid. J. Photochem. Photobiol. B Biol. 1998, 45, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Collaud, S.; Juzeniene, A.; Moan, J.; Lange, N. On the Selectivity of 5-Aminolevulinic Acid-Induced Protoporphyrin IX Formation. Curr. Med. Chem. Anticancer Agents 2004, 4, 301–316. [Google Scholar] [CrossRef]
- Ennis, S.R.; Novotny, A.; Xiang, J.; Shakui, P.; Masada, T.; Stummer, W.; Smith, D.E.; Keep, R.F. Transport of 5-Aminolevulinic Acid between Blood and Brain. Brain Res. 2003, 959, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Novotny, A.; Stepp, H.; Goetz, C.; Bise, K.; Reulen, H.J. Fluorescence-Guided Resection of Glioblastoma Multiforme by Using 5-Aminolevulinic Acid-Induced Porphyrins: A Prospective Study in 52 Consecutive Patients. J. Neurosurg. 2000, 93, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Stepp, H.; Möller, G.; Ehrhardt, A.; Leonhard, M.; Reulen, H.J. Technical Principles for Protoporphyrin-IX-Fluorescence Guided Microsurgical Resection of Malignant Glioma Tissue. Acta Neurochir. 1998, 140, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Stepp, H.; Beck, T.; Pongratz, T.; Meinel, T.; Kreth, F.-W.; Tonn, J.C.; Stummer, W. ALA and Malignant Glioma: Fluorescence-Guided Resection and Photodynamic Treatment. J. Environ. Pathol. Toxicol. Oncol. 2007, 26, 157–164. [Google Scholar] [CrossRef]
- Zhao, S.-G.; Chen, X.-F.; Wang, L.-G.; Yang, G.; Han, D.-Y.; Teng, L.; Yang, M.-C.; Wang, D.-Y.; Shi, C.; Liu, Y.-H.; et al. Increased Expression of ABCB6 Enhances Protoporphyrin IX Accumulation and Photodynamic Effect in Human Glioma. Ann. Surg. Oncol. 2013, 20, 4379–4388. [Google Scholar] [CrossRef]
- Lau, D.; Hervey-Jumper, S.L.; Chang, S.; Molinaro, A.M.; McDermott, M.W.; Phillips, J.J.; Berger, M.S. A Prospective Phase II Clinical Trial of 5-Aminolevulinic Acid to Assess the Correlation of Intraoperative Fluorescence Intensity and Degree of Histologic Cellularity during Resection of High-Grade Gliomas. J. Neurosurg. 2016, 124, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Guyotat, J.; Pallud, J.; Armoiry, X.; Pavlov, V.; Metellus, P. 5-Aminolevulinic Acid-Protoporphyrin IX Fluorescence-Guided Surgery of High-Grade Gliomas: A Systematic Review. Adv. Tech. Stand. Neurosurg. 2016, 43, 61–90. [Google Scholar] [CrossRef]
- Georgakoudi, I.; Keng, P.C.; Foster, T.H. Hypoxia Significantly Reduces Aminolaevulinic Acid-Induced Protoporphyrin IX Synthesis in EMT6 Cells. Br. J. Cancer 1999, 79, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Wyld, L.; Reed, M.W.; Brown, N.J. The Influence of Hypoxia and pH on Aminolaevulinic Acid-Induced Photodynamic Therapy in Bladder Cancer Cells in Vitro. Br. J. Cancer 1998, 77, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Wyld, L.; Tomlinson, M.; Reed, M.W.R.; Brown, N.J. Aminolaevulinic Acid-Induced Photodynamic Therapy: Cellular Responses to Glucose Starvation. Br. J. Cancer 2002, 86, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Reulen, H.J.; Novotny, A.; Stepp, H.; Tonn, J.C. Fluorescence-Guided Resections of Malignant Gliomas--an Overview. Acta Neurochir. Suppl. 2003, 88, 9–12. [Google Scholar] [PubMed]
- Markwardt, N.A.; Haj-Hosseini, N.; Hollnburger, B.; Stepp, H.; Zelenkov, P.; Rühm, A. 405 Nm versus 633 Nm for Protoporphyrin IX Excitation in Fluorescence-Guided Stereotactic Biopsy of Brain Tumors. J. Biophotonics. 2016, 9, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Tonn, J.-C.; Stummer, W. Fluorescence-Guided Resection of Malignant Gliomas Using 5-Aminolevulinic Acid: Practical Use, Risks, and Pitfalls. Clin. Neurosurg. 2008, 55, 20–26. [Google Scholar] [PubMed]
- Goryaynov, S.A.; Okhlopkov, V.A.; Golbin, D.A.; Chernyshov, K.A.; Svistov, D.V.; Martynov, B.V.; Kim, A.V.; Byvaltsev, V.A.; Pavlova, G.V.; Batalov, A.; et al. Fluorescence Diagnosis in Neurooncology: Retrospective Analysis of 653 Cases. Front. Oncol. 2019, 9, 830. [Google Scholar] [CrossRef]
- Cordova, J.S.; Gurbani, S.S.; Holder, C.A.; Olson, J.J.; Schreibmann, E.; Shi, R.; Guo, Y.; Shu, H.-K.G.; Shim, H.; Hadjipanayis, C.G. Semi-Automated Volumetric and Morphological Assessment of Glioblastoma Resection with Fluorescence-Guided Surgery. Mol. Imaging Biol. 2016, 18, 454–462. [Google Scholar] [CrossRef]
- Yamada, S.; Muragaki, Y.; Maruyama, T.; Komori, T.; Okada, Y. Role of Neurochemical Navigation with 5-Aminolevulinic Acid during Intraoperative MRI-Guided Resection of Intracranial Malignant Gliomas. Clin. Neurol. Neurosurg. 2015, 130, 134–139. [Google Scholar] [CrossRef]
- Teixidor, P.; Arráez, M.Á.; Villalba, G.; Garcia, R.; Tardáguila, M.; González, J.J.; Rimbau, J.; Vidal, X.; Montané, E. Safety and Efficacy of 5-Aminolevulinic Acid for High Grade Glioma in Usual Clinical Practice: A Prospective Cohort Study. PLoS ONE 2016, 11, e0149244. [Google Scholar] [CrossRef]
- Stummer, W.; Rodrigues, F.; Schucht, P.; Preuss, M.; Wiewrodt, D.; Nestler, U.; Stein, M.; Artero, J.M.C.; Platania, N.; Skjøth-Rasmussen, J.; et al. Predicting the “Usefulness” of 5-ALA-Derived Tumor Fluorescence for Fluorescence-Guided Resections in Pediatric Brain Tumors: A European Survey. Acta Neurochir. 2014, 156, 2315–2324. [Google Scholar] [CrossRef]
- Moore, G.E.; Peyton, W.T. The Clinical Use of Sodium Fluorescein and Radioactive Diiodofluorescein in the Localization of Tumors of the Central Nervous System. Minn. Med. 1948, 31, 1073–1076. [Google Scholar]
- Kozler, P.; Pokorný, J. Altered Blood-Brain Barrier Permeability and Its Effect on the Distribution of Evans Blue and Sodium Fluorescein in the Rat Brain Applied by Intracarotid Injection. Physiol. Res. 2003, 52, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.J.; Dios, R.R.; Hattab, E.M.; Burrell, K.; Rakopoulos, P.; Sabha, N.; Hawkins, C.; Zadeh, G.; Rutka, J.T.; Cohen-Gadol, A.A. Study of the Biodistribution of Fluorescein in Glioma-Infiltrated Mouse Brain and Histopathological Correlation of Intraoperative Findings in High-Grade Gliomas Resected under Fluorescein Fluorescence Guidance. J. Neurosurg. 2015, 122, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Yoshioka, H.; Kato, A. Fluorescence-Guided Surgery for Glioblastoma Multiforme Using High-Dose Fluorescein Sodium with Excitation and Barrier Filters. J. Clin. Neurosci. 2012, 19, 1719–1722. [Google Scholar] [CrossRef]
- Hawkins, B.T.; Egleton, R.D. Fluorescence Imaging of Blood–Brain Barrier Disruption. J. Neurosci. Methods 2006, 151, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Khan, K.A.; Singh, A.K.; Kaif, M.; Yadav, K.; Kumar Singh, R.; Ahmad, F. Fluorescein Sodium Fluorescence: Role in Stereotactic Brain Biopsy. Br. J. Neurosurg. 2023, 37, 82–85. [Google Scholar] [CrossRef]
- Lynagh, R.; Ishak, M.; Georges, J.; Lopez, D.; Osman, H.; Kakareka, M.; Boyer, B.; Goldman, H.W.; Eschbacher, J.; Preul, M.C.; et al. Fluorescence-Guided Stereotactic Biopsy: A Proof-of-Concept Study. J. Neurosurg. 2019, 132, 530–536. [Google Scholar] [CrossRef]
- Rey-Dios, R.; Hattab, E.M.; Cohen-Gadol, A.A. Use of Intraoperative Fluorescein Sodium Fluorescence to Improve the Accuracy of Tissue Diagnosis during Stereotactic Needle Biopsy of High-Grade Gliomas. Acta Neurochir. 2014, 156, 1071–1075; discussion 1075. [Google Scholar] [CrossRef] [PubMed]
- Nevzati, E.; Chatain, G.P.; Hoffman, J.; Kleinschmidt-DeMasters, B.K.; Lillehei, K.O.; Ormond, D.R. Reliability of Fluorescein-Assisted Stereotactic Brain Biopsies in Predicting Conclusive Tissue Diagnosis. Acta Neurochir. 2020, 162, 1941–1947. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.Y.; Kim, J.W.; Park, C.K. Experience Profiling of Fluorescence-Guided Surgery I: Gliomas. Brain Tumor Res. Treat. 2019, 7, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Marbacher, S.; Klinger, E.; Schwyzer, L.; Fischer, I.; Nevzati, E.; Diepers, M.; Roelcke, U.; Fathi, A.-R.; Coluccia, D.; Fandino, J. Use of Fluorescence to Guide Resection or Biopsy of Primary Brain Tumors and Brain Metastases. Neurosurg. Focus 2014, 36, E10. [Google Scholar] [CrossRef] [PubMed]
- Takeda, J.; Nonaka, M.; Li, Y.; Isozaki, H.; Kamei, T.; Hashiba, T.; Asai, A. 5-Aminolevulinic acid fluorescence-guided endoscopic surgery for intraventricular tumors. Surg. Neurol. Int. 2022, 13, 302. [Google Scholar] [CrossRef]
- Tsugu, A.; Ishizaka, H.; Mizokami, Y.; Osada, T.; Baba, T.; Yoshiyama, M.; Nishiyama, J.; Matsumae, M. Impact of the Combination of 5-Aminolevulinic Acid-Induced Fluorescence with Intraoperative Magnetic Resonance Imaging-Guided Surgery for Glioma. World Neurosurg. 2011, 76, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Widhalm, G.; Wolfsberger, S.; Minchev, G.; Woehrer, A.; Krssak, M.; Czech, T.; Prayer, D.; Asenbaum, S.; Hainfellner, J.A.; Knosp, E. 5-Aminolevulinic Acid Is a Promising Marker for Detection of Anaplastic Foci in Diffusely Infiltrating Gliomas with Nonsignificant Contrast Enhancement. Cancer 2010, 116, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Ewelt, C.; Floeth, F.W.; Felsberg, J.; Steiger, H.J.; Sabel, M.; Langen, K.-J.; Stoffels, G.; Stummer, W. Finding the Anaplastic Focus in Diffuse Gliomas: The Value of Gd-DTPA Enhanced MRI, FET-PET, and Intraoperative, ALA-Derived Tissue Fluorescence. Clin. Neurol. Neurosurg. 2011, 113, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Widhalm, G.; Kiesel, B.; Woehrer, A.; Traub-Weidinger, T.; Preusser, M.; Marosi, C.; Prayer, D.; Hainfellner, J.A.; Knosp, E.; Wolfsberger, S. 5-Aminolevulinic Acid Induced Fluorescence Is a Powerful Intraoperative Marker for Precise Histopathological Grading of Gliomas with Non-Significant Contrast-Enhancement. PLoS ONE 2013, 8, e76988. [Google Scholar] [CrossRef]
- Floeth, F.W.; Sabel, M.; Ewelt, C.; Stummer, W.; Felsberg, J.; Reifenberger, G.; Steiger, H.J.; Stoffels, G.; Coenen, H.H.; Langen, K.-J. Comparison of (18)F-FET PET and 5-ALA Fluorescence in Cerebral Gliomas. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 731–741. [Google Scholar] [CrossRef]
- Watts, C.; Dayimu, A.; Matys, T.; Ashkan, K.; Price, S.; Jenkinson, M.D.; Doughton, G.; Mather, C.; Young, G.; Qian, W.; et al. Refining the Intraoperative Identification of Suspected High-Grade Glioma Using a Surgical Fluorescence Biomarker: GALA BIDD Study Report. J. Pers. Med. 2023, 13, 514. [Google Scholar] [CrossRef] [PubMed]
- Arita, H.; Kinoshita, M.; Kagawa, N.; Fujimoto, Y.; Kishima, H.; Hashimoto, N.; Yoshimine, T. 11C-Methionine Uptake and Intraoperative 5-Aminolevulinic Acid-Induced Fluorescence as Separate Index Markers of Cell Density in Glioma: A Stereotactic Image-Histological Analysis. Cancer 2012, 118, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Hirai, T.; Takeshima, H.; Kadota, Y.; Yamashita, S.; Ivanova, A.; Yokogami, K. Genetic Factors Affecting Intraoperative 5-Aminolevulinic Acid-Induced Fluorescence of Diffuse Gliomas. Radiol. Oncol. 2017, 51, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Goryaynov, S.A.; Widhalm, G.; Goldberg, M.F.; Chelushkin, D.; Spallone, A.; Chernyshov, K.A.; Ryzhova, M.; Pavlova, G.; Revischin, A.; Shishkina, L.; et al. The Role of 5-ALA in Low-Grade Gliomas and the Influence of Antiepileptic Drugs on Intraoperative Fluorescence. Front. Oncol. 2019, 9, 423. [Google Scholar] [CrossRef]
- Wadiura, L.I.; Mischkulnig, M.; Hosmann, A.; Borkovec, M.; Kiesel, B.; Rötzer, T.; Mercea, P.A.; Furtner, J.; Hervey-Jumper, S.; Rössler, K.; et al. Influence of Corticosteroids and Antiepileptic Drugs on Visible 5-Aminolevulinic Acid Fluorescence in a Series of Initially Suspected Low-Grade Gliomas Including World Health Organization Grade II, III, and IV Gliomas. World Neurosurg. 2020, 137, e437–e446. [Google Scholar] [CrossRef]
- Widhalm, G.; Olson, J.; Weller, J.; Bravo, J.; Han, S.J.; Phillips, J.; Hervey-Jumper, S.L.; Chang, S.M.; Roberts, D.W.; Berger, M.S. The Value of Visible 5-ALA Fluorescence and Quantitative Protoporphyrin IX Analysis for Improved Surgery of Suspected Low-Grade Gliomas. J. Neurosurg. 2019, 133, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Batalov, A.I.; Goryaynov, S.A.; Zakharova, N.E.; Solozhentseva, K.D.; Kosyrkova, A.V.; Potapov, A.A.; Pronin, I.N. Prediction of Intraoperative Fluorescence of Brain Gliomas: Correlation between Tumor Blood Flow and the Fluorescence. J. Clin. Med. 2021, 10, 2387. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Suero Molina, E.; Sporns, P.; Schipmann, S.; Black, D.; Stummer, W. Fluorescence Real-Time Kinetics of Protoporphyrin IX after 5-ALA Administration in Low-Grade Glioma. J. Neurosurg. 2022, 136, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Müther, M.; Jaber, M.; Johnson, T.D.; Orringer, D.A.; Stummer, W. A Data-Driven Approach to Predicting 5-Aminolevulinic Acid-Induced Fluorescence and World Health Organization Grade in Newly Diagnosed Diffuse Gliomas. Neurosurgery 2022, 90, 800–806. [Google Scholar] [CrossRef]
- Jaber, M.; Wölfer, J.; Ewelt, C.; Holling, M.; Hasselblatt, M.; Niederstadt, T.; Zoubi, T.; Weckesser, M.; Stummer, W. The Value of 5-Aminolevulinic Acid in Low-Grade Gliomas and High-Grade Gliomas Lacking Glioblastoma Imaging Features: An Analysis Based on Fluorescence, Magnetic Resonance Imaging, 18F-Fluoroethyl Tyrosine Positron Emission Tomography, and Tumor Molecular Factors. Neurosurgery 2016, 78, 401–411; discussion 411. [Google Scholar] [CrossRef]
- Jaber, M.; Ewelt, C.; Wölfer, J.; Brokinkel, B.; Thomas, C.; Hasselblatt, M.; Grauer, O.; Stummer, W. Is Visible Aminolevulinic Acid-Induced Fluorescence an Independent Biomarker for Prognosis in Histologically Confirmed (World Health Organization 2016) Low-Grade Gliomas? Neurosurgery 2018, 84, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Hosmann, A.; Millesi, M.; Wadiura, L.I.; Kiesel, B.; Mercea, P.A.; Mischkulnig, M.; Borkovec, M.; Furtner, J.; Roetzer, T.; Wolfsberger, S.; et al. 5-ALA Fluorescence Is a Powerful Prognostic Marker during Surgery of Low-Grade Gliomas (WHO Grade II)-Experience at Two Specialized Centers. Cancers 2021, 13, 2540. [Google Scholar] [CrossRef] [PubMed]
- Hosmann, A.; Jaber, M.; Roetzer-Pejrimovsky, T.; Timelthaler, G.; Borkovec, M.; Kiesel, B.; Wadiura, L.I.; Millesi, M.; Mercea, P.A.; Phillips, J.; et al. CD34 Microvascularity in Low-Grade Glioma: Correlation with 5-Aminolevulinic Acid Fluorescence and Patient Prognosis in a Multicenter Study at Three Specialized Centers. J. Neurosurg. 2023, 138, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.E.; Steele, C.J.; Rovin, R.A.; Belton, R.J.; Winn, R.J. Dexamethasone Alone and in Combination with Desipramine, Phenytoin, Valproic Acid or Levetiracetam Interferes with 5-ALA-Mediated PpIX Production and Cellular Retention in Glioblastoma Cells. J. Neurooncol. 2016, 127, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Novotny, A.; Stummer, W. 5-Aminolevulinic Acid and the Blood-Brain Barrier—A Review. Med. Laser Appl. 2003, 18, 36–40. [Google Scholar] [CrossRef]
- Mischkulnig, M.; Sperl, V.; Erhart, F.; Kiesel, B.; Lang, A.; Hosmann, A.; Roetzer, T.; Makolli, J.; Traxler, D.; Borkovec, M.; et al. Analysis of Corticosteroid and Antiepileptic Drug Treatment Effects on Heme Biosynthesis mRNA Expression in Lower-Grade Gliomas: Potential Implications for 5-ALA Metabolization. Photodiagn. Photodyn. Ther. 2022, 38, 102755. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Coburger, J.; Scheuerle, A.; Pala, A.; Thal, D.; Wirtz, C.R.; König, R. Histopathological Insights on Imaging Results of Intraoperative Magnetic Resonance Imaging, 5-Aminolevulinic Acid, and Intraoperative Ultrasound in Glioblastoma Surgery. Neurosurgery 2017, 81, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, H.; Ge, P.; Zhao, J.; Li, W.; Gu, H.; Wang, G.; Luo, Y.; Chen, D. Gross Total Resection of Glioma with the Intraoperative Fluorescence-Guidance of Fluorescein Sodium. Int. J. Med. Sci. 2012, 9, 708–714. [Google Scholar] [CrossRef]
- Schebesch, K.-M.; Brawanski, A.; Doenitz, C.; Rosengarth, K.; Proescholdt, M.; Riemenschneider, M.J.; Grosse, J.; Hellwig, D.; Höhne, J. Fluorescence-Guidance in Non-Gadolinium Enhancing, but FET-PET Positive Gliomas. Clin. Neurol. Neurosurg. 2018, 172, 177–182. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhu, X.-P.; Zhao, J.-N.; Huang, G.-H.; Tang, J.-H.; Chen, H.-R.; Du, L.; Zhang, D.; Tang, X.-F.; Yang, H.; et al. Blood-Brain Barrier Disruption, Sodium Fluorescein, And Fluorescence-Guided Surgery of Gliomas. Br. J. Neurosurg. 2018, 32, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Schebesch, K.-M.; Proescholdt, M.; Höhne, J.; Hohenberger, C.; Hansen, E.; Riemenschneider, M.J.; Ullrich, W.; Doenitz, C.; Schlaier, J.; Lange, M.; et al. Sodium Fluorescein-Guided Resection under the YELLOW 560 Nm Surgical Microscope Filter in Malignant Brain Tumor Surgery--a Feasibility Study. Acta Neurochir. 2013, 155, 693–699. [Google Scholar] [CrossRef]
- Yamaguchi, F.; Takahashi, H.; Teramoto, A. Photodiagnosis for Frameless Stereotactic Biopsy of Brain Tumor. Photodiagn. Photodyn. Ther. 2007, 4, 71–75. [Google Scholar] [CrossRef]
- Æbelø, A.M.; Noer, V.R.; Schulz, M.K.; Kristensen, B.W.; Pedersen, C.B.; Poulsen, F.R. Frameless Stereotactic Neuronavigated Biopsy: A Retrospective Study of Morbidity, Diagnostic Yield, and the Potential of Fluorescence: A Single-Center Clinical Investigation. Clin. Neurol. Neurosurg. 2019, 181, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Akshulakov, S.K.; Kerimbayev, T.T.; Biryuchkov, M.Y.; Urunbayev, Y.A.; Farhadi, D.S.; Byvaltsev, V.A. Current Trends for Improving Safety of Stereotactic Brain Biopsies: Advanced Optical Methods for Vessel Avoidance and Tumor Detection. Front. Oncol. 2019, 9, 947. [Google Scholar] [CrossRef] [PubMed]
- Widhalm, G.; Minchev, G.; Woehrer, A.; Preusser, M.; Kiesel, B.; Furtner, J.; Mert, A.; Di Ieva, A.; Tomanek, B.; Prayer, D.; et al. Strong 5-Aminolevulinic Acid-Induced Fluorescence Is a Novel Intraoperative Marker for Representative Tissue Samples in Stereotactic Brain Tumor Biopsies. Neurosurg. Rev. 2012, 35, 381–391; discussion 391. [Google Scholar] [CrossRef]
- Xu, R.; Rösler, J.; Teich, W.; Radke, J.; Früh, A.; Scherschinski, L.; Onken, J.; Vajkoczy, P.; Misch, M.; Faust, K. Correlation of Tumor Pathology with Fluorescein Uptake and MRI Contrast-Enhancement in Stereotactic Biopsies. J. Clin. Med. 2022, 11, 3330. [Google Scholar] [CrossRef]
- von Campe, G.; Moschopulos, M.; Hefti, M. 5-Aminolevulinic Acid-Induced Protoporphyrin IX Fluorescence as Immediate Intraoperative Indicator to Improve the Safety of Malignant or High-Grade Brain Tumor Diagnosis in Frameless Stereotactic Biopsies. Acta Neurochir. 2012, 154, 585–588; discussion 588. [Google Scholar] [CrossRef] [PubMed]
- Shofty, B.; Richetta, C.; Haim, O.; Kashanian, A.; Gurevich, A.; Grossman, R. 5-ALA-Assisted Stereotactic Brain Tumor Biopsy Improve Diagnostic Yield. Eur. J. Surg. Oncol. 2019, 45, 2375–2378. [Google Scholar] [CrossRef]
- Millesi, M.; Kiesel, B.; Wöhrer, A.; Mercea, P.A.; Bissolo, M.; Roetzer, T.; Wolfsberger, S.; Furtner, J.; Knosp, E.; Widhalm, G. Is Intraoperative Pathology Needed If 5-Aminolevulinic-Acid-Induced Tissue Fluorescence Is Found in Stereotactic Brain Tumor Biopsy? Neurosurgery 2020, 86, 366–373. [Google Scholar] [CrossRef]
- Malinova, V.; von Eckardstein, K.; Mielke, D.; Rohde, V. Diagnostic Yield of Fluorescence-Assisted Frame-Based Stereotactic Biopsies of Intracerebral Lesions in Comparison with Frozen-Section Analysis. J. Neurooncol. 2020, 149, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Thien, A.; Han, J.X.; Kumar, K.; Ng, Y.P.; Rao, J.P.; Ng, W.H.; King, N.K.K. Investigation of the Usefulness of Fluorescein Sodium Fluorescence in Stereotactic Brain Biopsy. Acta Neurochir. 2018, 160, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.W.; Valdés, P.A.; Harris, B.T.; Fontaine, K.M.; Hartov, A.; Fan, X.; Ji, S.; Lollis, S.S.; Pogue, B.W.; Leblond, F.; et al. Coregistered Fluorescence-Enhanced Tumor Resection of Malignant Glioma: Relationships between δ-Aminolevulinic Acid-Induced Protoporphyrin IX Fluorescence, Magnetic Resonance Imaging Enhancement, and Neuropathological Parameters. Clinical Article. J. Neurosurg. 2011, 114, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Idoate, M.A.; Díez Valle, R.; Echeveste, J.; Tejada, S. Pathological Characterization of the Glioblastoma Border as Shown during Surgery Using 5-Aminolevulinic Acid-Induced Fluorescence. Neuropathology 2011, 31, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Coburger, J.; Engelke, J.; Scheuerle, A.; Thal, D.R.; Hlavac, M.; Wirtz, C.R.; König, R. Tumor Detection with 5-Aminolevulinic Acid Fluorescence and Gd-DTPA-Enhanced Intraoperative MRI at the Border of Contrast-Enhancing Lesions: A Prospective Study Based on Histopathological Assessment. Neurosurg. Focus 2014, 36, E3. [Google Scholar] [CrossRef] [PubMed]
- Kiesel, B.; Mischkulnig, M.; Woehrer, A.; Martinez-Moreno, M.; Millesi, M.; Mallouhi, A.; Czech, T.; Preusser, M.; Hainfellner, J.A.; Wolfsberger, S.; et al. Systematic Histopathological Analysis of Different 5-Aminolevulinic Acid-Induced Fluorescence Levels in Newly Diagnosed Glioblastomas. J. Neurosurg. 2018, 129, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, T.; Nonoguchi, N.; Ikeda, N.; Yagi, R.; Kawabata, S.; Furuse, M.; Hirose, Y.; Kuwabara, H.; Tamura, Y.; Kajimoto, Y.; et al. Spectral Radiance of Protoporphyrin IX Fluorescence and Its Histopathological Implications in 5-Aminolevulinic Acid-Guided Surgery for Glioblastoma. Photomed. Laser Surg. 2018, 36, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.W.; Valdés, P.A.; Harris, B.T.; Hartov, A.; Fan, X.; Ji, S.; Leblond, F.; Tosteson, T.D.; Wilson, B.C.; Paulsen, K.D. Glioblastoma Multiforme Treatment with Clinical Trials for Surgical Resection (Aminolevulinic Acid). Neurosurg. Clin. N. Am. 2012, 23, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Pacioni, S.; D’Alessandris, Q.G.; Giannetti, S.; Della Pepa, G.M.; Offi, M.; Giordano, M.; Caccavella, V.M.; Falchetti, M.L.; Lauretti, L.; Pallini, R. 5-Aminolevulinic Acid (5-ALA)-Induced Protoporphyrin IX Fluorescence by Glioma Cells-A Fluorescence Microscopy Clinical Study. Cancers 2022, 14, 2844. [Google Scholar] [CrossRef]
- Kim, J.E.; Cho, H.R.; Xu, W.J.; Kim, J.Y.; Kim, S.K.; Kim, S.-K.; Park, S.-H.; Kim, H.; Lee, S.-H.; Choi, S.H.; et al. Mechanism for Enhanced 5-Aminolevulinic Acid Fluorescence in Isocitrate Dehydrogenase 1 Mutant Malignant Gliomas. Oncotarget 2015, 6, 20266–20277. [Google Scholar] [CrossRef]
- Zimmermann, M.; Stan, A.C. PepT2 Transporter Protein Expression in Human Neoplastic Glial Cells and Mediation of Fluorescently Tagged Dipeptide Derivative Beta-Ala-Lys-Nepsilon-7-Amino-4-Methyl-Coumarin-3-Acetic Acid Accumulation. J. Neurosurg. 2010, 112, 1005–1014. [Google Scholar] [CrossRef]
- Hou, C.; Yamaguchi, S.; Ishi, Y.; Terasaka, S.; Kobayashi, H.; Motegi, H.; Hatanaka, K.C.; Houkin, K. Identification of PEPT2 as an Important Candidate Molecule in 5-ALA-Mediated Fluorescence-Guided Surgery in WHO Grade II/III Gliomas. J. Neurooncol. 2019, 143, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Coburger, J.; Nabavi, A.; König, R.; Wirtz, C.R.; Pala, A. Contemporary Use of Intraoperative Imaging in Glioma Surgery: A Survey among EANS Members. Clin. Neurol. Neurosurg. 2017, 163, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Suero Molina, E.; Black, D.; Kaneko, S.; Müther, M.; Stummer, W. Double Dose of 5-Aminolevulinic Acid and Its Effect on Protoporphyrin IX Accumulation in Low-Grade Glioma. J. Neurosurg. 2022, 137, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Bottiroli, G.; Croce, A.C.; Locatelli, D.; Nano, R.; Giombelli, E.; Messina, A.; Benericetti, E. Brain Tissue Autofluorescence: An Aid for Intraoperative Delineation of Tumor Resection Margins. Cancer Detect. Prev. 1998, 22, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.G.; Schwartz, J.A.; Gardner, C.M.; Sawaya, R.E.; Jacques, S.L. Diagnostic Potential of Laser-Induced Autofluorescence Emission in Brain Tissue. J. Korean Med. Sci. 1997, 12, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Toms, S.A.; Motamedi, M.; Jansen, E.D.; Mahadevan-Jansen, A. Brain Tumor Demarcation Using Optical Spectroscopy; an in Vitro Study. J. Biomed. Opt. 2000, 5, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Toms, S.A.; Johnson, M.; Jansen, E.D.; Mahadevan-Jansen, A. In Vivo Brain Tumor Demarcation Using Optical Spectroscopy. Photochem. Photobiol. 2001, 73, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.K.; Gebhart, S.; Johnson, M.D.; Thompson, R.; Lin, W.-C.; Mahadevan-Jansen, A. A Probability-Based Spectroscopic Diagnostic Algorithm for Simultaneous Discrimination of Brain Tumor and Tumor Margins from Normal Brain Tissue. Appl. Spectrosc. 2007, 61, 548–557. [Google Scholar] [CrossRef]
- Toms, S.A.; Lin, W.-C.; Weil, R.J.; Johnson, M.D.; Jansen, E.D.; Mahadevan-Jansen, A. Intraoperative Optical Spectroscopy Identifies Infiltrating Glioma Margins with High Sensitivity. Neurosurgery 2005, 57, 382–391; discussion 382–391. [Google Scholar] [CrossRef]
- Valdés, P.A.; Leblond, F.; Jacobs, V.L.; Wilson, B.C.; Paulsen, K.D.; Roberts, D.W. Quantitative, Spectrally-Resolved Intraoperative Fluorescence Imaging. Sci. Rep. 2012, 2, 798. [Google Scholar] [CrossRef]
- Bravo, J.J.; Olson, J.D.; Davis, S.C.; Roberts, D.W.; Paulsen, K.D.; Kanick, S.C. Hyperspectral Data Processing Improves PpIX Contrast during Fluorescence Guided Surgery of Human Brain Tumors. Sci. Rep. 2017, 7, 9455. [Google Scholar] [CrossRef]
- Yong, W.H.; Butte, P.V.; Pikul, B.K.; Jo, J.A.; Fang, Q.; Papaioannou, T.; Black, K.; Marcu, L. Distinction of Brain Tissue, Low Grade and High Grade Glioma with Time-Resolved Fluorescence Spectroscopy. Front. Biosci. 2006, 11, 1255–1263. [Google Scholar] [CrossRef]
- Butte, P.V.; Fang, Q.; Jo, J.A.; Yong, W.H.; Pikul, B.K.; Black, K.L.; Marcu, L. Intraoperative Delineation of Primary Brain Tumors Using Time-Resolved Fluorescence Spectroscopy. J. Biomed. Opt. 2010, 15, 027008. [Google Scholar] [CrossRef]
- Butte, P.V.; Mamelak, A.N.; Nuno, M.; Bannykh, S.I.; Black, K.L.; Marcu, L. Fluorescence Lifetime Spectroscopy for Guided Therapy of Brain Tumors. Neuroimage 2011, 54 (Suppl. S1), S125–S135. [Google Scholar] [CrossRef]
- Poulon, F.; Mehidine, H.; Juchaux, M.; Varlet, P.; Devaux, B.; Pallud, J.; Abi Haidar, D. Optical Properties, Spectral, and Lifetime Measurements of Central Nervous System Tumors in Humans. Sci. Rep. 2017, 7, 13995. [Google Scholar] [CrossRef]
- Mehidine, H.; Chalumeau, A.; Poulon, F.; Jamme, F.; Varlet, P.; Devaux, B.; Refregiers, M.; Abi Haidar, D. Optical Signatures Derived from Deep UV to NIR Excitation Discriminates Healthy Samples from Low and High Grades Glioma. Sci. Rep. 2019, 9, 8786. [Google Scholar] [CrossRef]
- Utsuki, S.; Oka, H.; Sato, S.; Suzuki, S.; Shimizu, S.; Tanaka, S.; Fujii, K. Possibility of Using Laser Spectroscopy for the Intraoperative Detection of Nonfluorescing Brain Tumors and the Boundaries of Brain Tumor Infiltrates. Technical Note. J. Neurosurg. 2006, 104, 618–620. [Google Scholar] [CrossRef]
- Ishihara, R.; Katayama, Y.; Watanabe, T.; Yoshino, A.; Fukushima, T.; Sakatani, K. Quantitative Spectroscopic Analysis of 5-Aminolevulinic Acid-Induced Protoporphyrin IX Fluorescence Intensity in Diffusely Infiltrating Astrocytomas. Neurol. Med. Chir. 2007, 47, 53–57; discussion 57. [Google Scholar] [CrossRef]
- Ando, T.; Kobayashi, E.; Liao, H.; Maruyama, T.; Muragaki, Y.; Iseki, H.; Kubo, O.; Sakuma, I. Precise Comparison of Protoporphyrin IX Fluorescence Spectra with Pathological Results for Brain Tumor Tissue Identification. Brain Tumor. Pathol. 2011, 28, 43–51. [Google Scholar] [CrossRef]
- Johansson, A.; Palte, G.; Schnell, O.; Tonn, J.-C.; Herms, J.; Stepp, H. 5-Aminolevulinic Acid-Induced Protoporphyrin IX Levels in Tissue of Human Malignant Brain Tumors. Photochem. Photobiol. 2010, 86, 1373–1378. [Google Scholar] [CrossRef]
- Kröger, S.; Niehoff, A.-C.; Jeibmann, A.; Sperling, M.; Paulus, W.; Stummer, W.; Karst, U. Complementary Molecular and Elemental Mass-Spectrometric Imaging of Human Brain Tumors Resected by Fluorescence-Guided Surgery. Anal. Chem. 2018, 90, 12253–12260. [Google Scholar] [CrossRef]
- Valdés, P.A.; Leblond, F.; Kim, A.; Harris, B.T.; Wilson, B.C.; Fan, X.; Tosteson, T.D.; Hartov, A.; Ji, S.; Erkmen, K.; et al. Quantitative Fluorescence in Intracranial Tumor: Implications for ALA-Induced PpIX as an Intraoperative Biomarker. J. Neurosurg. 2011, 115, 11–17. [Google Scholar] [CrossRef]
- Valdés, P.A.; Kim, A.; Brantsch, M.; Niu, C.; Moses, Z.B.; Tosteson, T.D.; Wilson, B.C.; Paulsen, K.D.; Roberts, D.W.; Harris, B.T. δ-Aminolevulinic Acid-Induced Protoporphyrin IX Concentration Correlates with Histopathologic Markers of Malignancy in Human Gliomas: The Need for Quantitative Fluorescence-Guided Resection to Identify Regions of Increasing Malignancy. Neuro-Oncol. 2011, 13, 846–856. [Google Scholar] [CrossRef]
- Martínez-Moreno, M.; Kiesel, B.; Woehrer, A.; Mischkulnig, M.; Furtner, J.; Timelthaler, G.; Berger, W.; Knosp, E.; Hainfellner, J.A.; Wolfsberger, S.; et al. Ex-Vivo Analysis of Quantitative 5-ALA Fluorescence Intensity in Diffusely Infiltrating Gliomas Using a Handheld Spectroscopic Probe: Correlation with Histopathology, Proliferation and Microvascular Density. Photodiagn. Photodyn. Ther. 2019, 27, 354–361. [Google Scholar] [CrossRef]
- Valdés, P.A.; Moses, Z.B.; Kim, A.; Belden, C.J.; Wilson, B.C.; Paulsen, K.D.; Roberts, D.W.; Harris, B.T. Gadolinium- and 5-Aminolevulinic Acid-Induced Protoporphyrin IX Levels in Human Gliomas: An Ex Vivo Quantitative Study to Correlate Protoporphyrin IX Levels and Blood-Brain Barrier Breakdown. J. Neuropathol. Exp. Neurol. 2012, 71, 806–813. [Google Scholar] [CrossRef]
- Valdés, P.A.; Jacobs, V.; Harris, B.T.; Wilson, B.C.; Leblond, F.; Paulsen, K.D.; Roberts, D.W. Quantitative Fluorescence Using 5-Aminolevulinic Acid-Induced Protoporphyrin IX Biomarker as a Surgical Adjunct in Low-Grade Glioma Surgery. J. Neurosurg. 2015, 123, 771–780. [Google Scholar] [CrossRef]
- Valdés, P.A.; Kim, A.; Leblond, F.; Conde, O.M.; Harris, B.T.; Paulsen, K.D.; Wilson, B.C.; Roberts, D.W. Combined Fluorescence and Reflectance Spectroscopy for in Vivo Quantification of Cancer Biomarkers in Low- and High-Grade Glioma Surgery. J. Biomed. Opt. 2011, 16, 116007. [Google Scholar] [CrossRef]
- Melø, T.B.; Reisaeter, G. The Physicochemical State of Protoporphyrin IX in Aqueous Solution Investigated by Fluorescence and Light Scattering. Biophys. Chem. 1986, 25, 99–104. [Google Scholar] [CrossRef]
- Alston, L.; Rousseau, D.; Hebert, M.; Mahieu-Williame, L.; Montcel, B. Nonlinear Relation between Concentration and Fluorescence Emission of Protoporphyrin IX in Calibrated Phantoms. J. Biomed. Opt. 2018, 23, 097002. [Google Scholar] [CrossRef]
- Montcel, B.; Mahieu-Williame, L.; Armoiry, X.; Meyronet, D.; Guyotat, J. Two-Peaked 5-ALA-Induced PpIX Fluorescence Emission Spectrum Distinguishes Glioblastomas from Low Grade Gliomas and Infiltrative Component of Glioblastomas. Biomed. Opt. Express 2013, 4, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Alston, L.; Mahieu-Williame, L.; Hebert, M.; Kantapareddy, P.; Meyronet, D.; Rousseau, D.; Guyotat, J.; Montcel, B. Spectral Complexity of 5-ALA Induced PpIX Fluorescence in Guided Surgery: A Clinical Study towards the Discrimination of Healthy Tissue and Margin Boundaries in High and Low Grade Gliomas. Biomed. Opt. Express 2019, 10, 2478–2492. [Google Scholar] [CrossRef] [PubMed]
- Black, D.; Kaneko, S.; Walke, A.; König, S.; Stummer, W.; Suero Molina, E. Characterization of Autofluorescence and Quantitative Protoporphyrin IX Biomarkers for Optical Spectroscopy-Guided Glioma Surgery. Sci. Rep. 2021, 11, 20009. [Google Scholar] [CrossRef]
- Suero Molina, E.; Black, D.; Walke, A.; Azemi, G.; D’Alessandro, F.; König, S.; Stummer, W. Unraveling the Blue Shift in Porphyrin Fluorescence in Glioma: The 620 Nm Peak and Its Potential Significance in Tumor Biology. Front. Neurosci. 2023, 17, 1261679. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, P.; Ray, C.; Mahieu-Williame, L.; Alston, L.; Frindel, C.; Brevet, P.-F.; Meyronet, D.; Guyotat, J.; Montcel, B.; Rousseau, D. Machine Learning-Based Prediction of Glioma Margin from 5-ALA Induced PpIX Fluorescence Spectroscopy. Sci. Rep. 2020, 10, 1462. [Google Scholar] [CrossRef] [PubMed]
- Reichert, D.; Erkkilä, M.T.; Holst, G.; Hecker-Denschlag, N.; Wilzbach, M.; Hauger, C.; Drexler, W.; Gesperger, J.; Kiesel, B.; Roetzer, T.; et al. Towards Real-Time Wide-Field Fluorescence Lifetime Imaging of 5-ALA Labeled Brain Tumors with Multi-Tap CMOS Cameras. Biomed. Opt. Express 2020, 11, 1598–1616. [Google Scholar] [CrossRef] [PubMed]
- Erkkilä, M.T.; Reichert, D.; Hecker-Denschlag, N.; Wilzbach, M.; Hauger, C.; Leitgeb, R.A.; Gesperger, J.; Kiesel, B.; Roetzer, T.; Widhalm, G.; et al. Surgical Microscope with Integrated Fluorescence Lifetime Imaging for 5-Aminolevulinic Acid Fluorescence-Guided Neurosurgery. J. Biomed. Opt. 2020, 25, 071202. [Google Scholar] [CrossRef]
- Erkkilä, M.T.; Reichert, D.; Gesperger, J.; Kiesel, B.; Roetzer, T.; Mercea, P.A.; Drexler, W.; Unterhuber, A.; Leitgeb, R.A.; Woehrer, A.; et al. Macroscopic Fluorescence-Lifetime Imaging of NADH and Protoporphyrin IX Improves the Detection and Grading of 5-Aminolevulinic Acid-Stained Brain Tumors. Sci. Rep. 2020, 10, 20492. [Google Scholar] [CrossRef] [PubMed]
- Reichert, D.; Erkkilae, M.T.; Gesperger, J.; Wadiura, L.I.; Lang, A.; Roetzer, T.; Woehrer, A.; Andreana, M.; Unterhuber, A.; Wilzbach, M.; et al. Fluorescence Lifetime Imaging and Spectroscopic Co-Validation for Protoporphyrin IX-Guided Tumor Visualization in Neurosurgery. Front. Oncol. 2021, 11, 741303. [Google Scholar] [CrossRef]
- Xie, Y.; Thom, M.; Ebner, M.; Wykes, V.; Desjardins, A.; Miserocchi, A.; Ourselin, S.; McEvoy, A.W.; Vercauteren, T. Wide-Field Spectrally Resolved Quantitative Fluorescence Imaging System: Toward Neurosurgical Guidance in Glioma Resection. J. Biomed. Opt. 2017, 22, 116006. [Google Scholar] [CrossRef]
- Sankar, T.; Delaney, P.M.; Ryan, R.W.; Eschbacher, J.; Abdelwahab, M.; Nakaji, P.; Coons, S.W.; Scheck, A.C.; Smith, K.A.; Spetzler, R.F.; et al. Miniaturized Handheld Confocal Microscopy for Neurosurgery: Results in an Experimental Glioblastoma Model. Neurosurgery 2010, 66, 410–417; discussion 417–418. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Snyder, L.A.; Honea, N.J.; Coons, S.W.; Eschbacher, J.M.; Smith, K.A.; Spetzler, R.F. Intraoperative Confocal Microscopy in the Visualization of 5-Aminolevulinic Acid Fluorescence in Low-Grade Gliomas. J. Neurosurg. 2011, 115, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, T.; Watanabe, T.; Tamai, S.; Miyashita, K.; Nakada, M. Bright Spot Analysis for Photodynamic Diagnosis of Brain Tumors Using Confocal Microscopy. Photodiagn. Photodyn. Ther. 2019, 25, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Chen, Y.; Yin, C.; Borwege, S.; Sanai, N.; Liu, J.T.C. Optical-Sectioning Microscopy of Protoporphyrin IX Fluorescence in Human Gliomas: Standardization and Quantitative Comparison with Histology. J. Biomed. Opt. 2017, 22, 46005. [Google Scholar] [CrossRef] [PubMed]
- Eschbacher, J.; Martirosyan, N.L.; Nakaji, P.; Sanai, N.; Preul, M.C.; Smith, K.A.; Coons, S.W.; Spetzler, R.F. In Vivo Intraoperative Confocal Microscopy for Real-Time Histopathological Imaging of Brain Tumors. J. Neurosurg. 2012, 116, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Martirosyan, N.L.; Eschbacher, J.M.; Kalani, M.Y.S.; Turner, J.D.; Belykh, E.; Spetzler, R.F.; Nakaji, P.; Preul, M.C. Prospective Evaluation of the Utility of Intraoperative Confocal Laser Endomicroscopy in Patients with Brain Neoplasms Using Fluorescein Sodium: Experience with 74 Cases. Neurosurg Focus 2016, 40, E11. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, V.; Meyronet, D.; Meyer-Bisch, V.; Armoiry, X.; Pikul, B.; Dumot, C.; Beuriat, P.-A.; Signorelli, F.; Guyotat, J. Intraoperative Probe-Based Confocal Laser Endomicroscopy in Surgery and Stereotactic Biopsy of Low-Grade and High-Grade Gliomas: A Feasibility Study in Humans. Neurosurgery 2016, 79, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Belykh, E.; Zhao, X.; Ngo, B.; Farhadi, D.S.; Byvaltsev, V.A.; Eschbacher, J.M.; Nakaji, P.; Preul, M.C. Intraoperative Confocal Laser Endomicroscopy Ex Vivo Examination of Tissue Microstructure During Fluorescence-Guided Brain Tumor Surgery. Front. Oncol. 2020, 10, 599250. [Google Scholar] [CrossRef] [PubMed]
- Höhne, J.; Schebesch, K.-M.; Zoubaa, S.; Proescholdt, M.; Riemenschneider, M.J.; Schmidt, N.O. Intraoperative Imaging of Brain Tumors with Fluorescein: Confocal Laser Endomicroscopy in Neurosurgery. Clinical and User Experience. Neurosurg. Focus 2021, 50, E19. [Google Scholar] [CrossRef]
- Xu, Y.; Abramov, I.; Belykh, E.; Mignucci-Jiménez, G.; Park, M.T.; Eschbacher, J.M.; Preul, M.C. Characterization of Ex Vivo and in Vivo Intraoperative Neurosurgical Confocal Laser Endomicroscopy Imaging. Front. Oncol. 2022, 12, 979748. [Google Scholar] [CrossRef]
- Xu, Y.; Mathis, A.M.; Pollo, B.; Schlegel, J.; Maragkou, T.; Seidel, K.; Schucht, P.; Smith, K.A.; Porter, R.W.; Raabe, A.; et al. Intraoperative in Vivo Confocal Laser Endomicroscopy Imaging at Glioma Margins: Can We Detect Tumor Infiltration? J. Neurosurg. 2024, 140, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Brielmaier, M.C.; Kampf, C.; Baumgart, L.; Aftahy, A.K.; Meyer, H.S.; Kehl, V.; Höhne, J.; Schebesch, K.-M.; Schmidt, N.O.; et al. Fluorescein-Stained Confocal Laser Endomicroscopy versus Conventional Frozen Section for Intraoperative Histopathological Assessment of Intracranial Tumors. Neuro Oncol. 2024, 26, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Eschbacher, J.; Hattendorf, G.; Coons, S.W.; Preul, M.C.; Smith, K.A.; Nakaji, P.; Spetzler, R.F. Intraoperative Confocal Microscopy for Brain Tumors: A Feasibility Analysis in Humans. Neurosurgery 2011, 68, 282–290; discussion 290. [Google Scholar] [CrossRef] [PubMed]
- Forest, F.; Cinotti, E.; Yvorel, V.; Habougit, C.; Vassal, F.; Nuti, C.; Perrot, J.-L.; Labeille, B.; Péoc’h, M. Ex Vivo Confocal Microscopy Imaging to Identify Tumor Tissue on Freshly Removed Brain Sample. J. Neurooncol. 2015, 124, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Charalampaki, P.; Javed, M.; Daali, S.; Heiroth, H.-J.; Igressa, A.; Weber, F. Confocal Laser Endomicroscopy for Real-Time Histomorphological Diagnosis: Our Clinical Experience With 150 Brain and Spinal Tumor Cases. Neurosurgery 2015, 62 (Suppl. S1), 171–176. [Google Scholar] [CrossRef]
- Snuderl, M.; Wirth, D.; Sheth, S.A.; Bourne, S.K.; Kwon, C.-S.; Ancukiewicz, M.; Curry, W.T.; Frosch, M.P.; Yaroslavsky, A.N. Dye-Enhanced Multimodal Confocal Imaging as a Novel Approach to Intraoperative Diagnosis of Brain Tumors. Brain Pathol. 2013, 23, 73–81. [Google Scholar] [CrossRef]
- Martirosyan, N.L.; Georges, J.; Eschbacher, J.M.; Belykh, E.; Carotenuto, A.; Spetzler, R.F.; Nakaji, P.; Preul, M.C. Confocal Scanning Microscopy Provides Rapid, Detailed Intraoperative Histological Assessment of Brain Neoplasms: Experience with 106 Cases. Clin. Neurol. Neurosurg. 2018, 169, 21–28. [Google Scholar] [CrossRef]
- Abramov, I.; Dru, A.B.; Belykh, E.; Park, M.T.; Bardonova, L.; Preul, M.C. Redosing of Fluorescein Sodium Improves Image Interpretation During Intraoperative Ex Vivo Confocal Laser Endomicroscopy of Brain Tumors. Front. Oncol. 2021, 11, 668661. [Google Scholar] [CrossRef]
- Giannoni, L.; Bonaudo, C.; Marradi, M.; Puppa, A.D.; Pavone, F.S. Optical Characterisation and Study of Ex Vivo Glioma Tissue for Hyperspectral Imaging during Neurosurgery. In Proceedings of the Diffuse Optical Spectroscopy and Imaging IX, Munich, Germany, 25–29 June 2023; SPIE: Bellingham, DC, USA; Volume 12628, pp. 153–159. [Google Scholar]
- Giannoni, L.; Marradi, M.; Marchetti, M.; Degl’Innocenti, D.R.; Ezhov, I.; Caredda, C.; Gautheron, A.; Fort, F.; Schneider, F.; Berhouma, M.; et al. HyperProbe Consortium: Innovate Tumour Neurosurgery with Innovative Photonic Solutions. In Proceedings of the Diffuse Optical Spectroscopy and Imaging IX, Munich, Germany, 25–29 June 2023; SPIE: Bellingham, DC, USA, 2023; Volume 12628, pp. 80–87. [Google Scholar]
- Göbel, W.; Brucker, D.; Kienast, Y.; Johansson, A.; Kniebühler, G.; Rühm, A.; Eigenbrod, S.; Fischer, S.; Goetz, M.; Kreth, F.-W.; et al. Optical Needle Endoscope for Safe and Precise Stereotactically Guided Biopsy Sampling in Neurosurgery. Opt. Express 2012, 20, 26117–26126. [Google Scholar] [CrossRef]
- Richter, J.; Haj-Hosseini, N.; Milos, P.; Hallbeck, M.; Wårdell, K. Optical Brain Biopsy with a Fluorescence and Vessel Tracing Probe. Oper. Neurosurg. 2021, 21, 217–224. [Google Scholar] [CrossRef]
- Radtke, K.; Schulz-Schaeffer, W.J.; Oertel, J. Confocal Laser Endomicroscopy in Glial Tumors-a Histomorphological Analysis. Neurosurg. Rev. 2024, 47, 65. [Google Scholar] [CrossRef]
- Breuskin, D.; Szczygielski, J.; Urbschat, S.; Kim, Y.-J.; Oertel, J. Confocal Laser Endomicroscopy in Neurosurgery-An Alternative to Instantaneous Sections? World Neurosurg. 2017, 100, 180–185. [Google Scholar] [CrossRef]
- Izadyyazdanabadi, M.; Belykh, E.; Mooney, M.A.; Eschbacher, J.M.; Nakaji, P.; Yang, Y.; Preul, M.C. Prospects for Theranostics in Neurosurgical Imaging: Empowering Confocal Laser Endomicroscopy Diagnostics via Deep Learning. Front. Oncol. 2018, 8, 240. [Google Scholar] [CrossRef]
- Haglund, M.M.; Berger, M.S.; Hochman, D.W. Enhanced Optical Imaging of Human Gliomas and Tumor Margins. Neurosurgery 1996, 38, 308–317. [Google Scholar] [CrossRef]
- Cho, S.S.; Salinas, R.; Lee, J.Y.K. Indocyanine-Green for Fluorescence-Guided Surgery of Brain Tumors: Evidence, Techniques, and Practical Experience. Front. Surg. 2019, 6, 11. [Google Scholar] [CrossRef]
- Li, C.; Sullivan, P.Z.; Cho, S.; Nasrallah, M.P.; Buch, L.; Isaac Chen, H.-C.; Lee, J.Y.K. Intraoperative Molecular Imaging with Second Window Indocyanine Green Facilitates Confirmation of Contrast-Enhancing Tissue During Intracranial Stereotactic Needle Biopsy: A Case Series. World Neurosurg. 2019, 126, e1211–e1218. [Google Scholar] [CrossRef]
- Senders, J.T.; Muskens, I.S.; Schnoor, R.; Karhade, A.V.; Cote, D.J.; Smith, T.R.; Broekman, M.L.D. Agents for Fluorescence-Guided Glioma Surgery: A Systematic Review of Preclinical and Clinical Results. Acta Neurochir. 2017, 159, 151–167. [Google Scholar] [CrossRef]
- Martirosyan, N.L.; Cavalcanti, D.D.; Eschbacher, J.M.; Delaney, P.M.; Scheck, A.C.; Abdelwahab, M.G.; Nakaji, P.; Spetzler, R.F.; Preul, M.C. Use of in Vivo Near-Infrared Laser Confocal Endomicroscopy with Indocyanine Green to Detect the Boundary of Infiltrative Tumor. J. Neurosurg. 2011, 115, 1131–1138. [Google Scholar] [CrossRef]
- Lee, J.Y.-K.; Thawani, J.P.; Pierce, J.; Zeh, R.; Martinez-Lage, M.; Chanin, M.; Venegas, O.; Nims, S.; Learned, K.; Keating, J.; et al. Intraoperative Near-Infrared Optical Imaging Can Localize Gadolinium-Enhancing Gliomas During Surgery. Neurosurgery 2016, 79, 856–871. [Google Scholar] [CrossRef] [PubMed]
- Ferroli, P.; Acerbi, F.; Albanese, E.; Tringali, G.; Broggi, M.; Franzini, A.; Broggi, G. Application of Intraoperative Indocyanine Green Angiography for CNS Tumors: Results on the First 100 Cases. Acta Neurochir. Suppl. 2011, 109, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Patil, C.G.; Walker, D.G.; Miller, D.M.; Butte, P.; Morrison, B.; Kittle, D.S.; Hansen, S.J.; Nufer, K.L.; Byrnes-Blake, K.A.; Yamada, M.; et al. Phase 1 Safety, Pharmacokinetics, and Fluorescence Imaging Study of Tozuleristide (BLZ-100) in Adults with Newly Diagnosed or Recurrent Gliomas. Neurosurgery 2019, 85, E641–E649. [Google Scholar] [CrossRef]
| Study | Design | Effectives | Main Conclusions |
|---|---|---|---|
| Widhalm, 2010 [57] | Prospective, monocentric 11C Methionine PET (WHO 2007) | 17 gliomas without significant CE: 8 (47.1%) grade 2 9 (52.9%) grade 3 | Performance of negative fluorescence for low-grade detection Sensibility = 100%. PPV = 89%. Performance of fluorescence for high-grade detection Sensibility = 89%. PPV = 100%. |
| Floeth, 2010 [60] | Prospective, multicentric 18F FET PET (WHO 2007) | 38 gliomas with CE in 13 (43.3%) cases: 17 (44.7%) grade 2 19 (50%) grade 3 2 (5.3%) grade 4 | Performance of negative fluorescence for low-grade detection Sensibility = 94%. Performance of fluorescence for high-grade detection Sensibility = 57%. Hypermetabolism in 18F-FET 7/17 (41%) grade 2 gliomas. 18/21 (86%) grade 3–4 gliomas. |
| Ewelt, 2011 [58] | Prospective, monocentric 18F FET PET (WHO 2007) | 30 gliomas with CE in 12 (40%) cases: 13 (43.3%) grade 2 15 (50%) grade 3 2 (6.7%) grade 4 | Performance of negative fluorescence for low-grade detection Sensibility = 92.3%. Performance of fluorescence for high-grade detection Sensitivity = 70.6%. Specificity = 92.3%. Negative fluorescence and positive FET uptake 6/13 (46.2%) grade 2 gliomas. Positive fluorescence and FET uptake 1/13 (7.7%) grade 2 gliomas. |
| Widhalm, 2013 [59] | Prospective, monocentric 18F FET or 11C Methionine PET (WHO 2007) | 59 gliomas without significant CE 33 (55.9%) grade 2 26 (44.1%) grade 3 | Performance of negative fluorescence for low-grade detection Sensibility = 91%. Performance of fluorescence for high-grade detection Sensitivity = 88%. Specificity = 89%. PPV = 85%. |
| Watts, 2023 [61] | Prospective, tricentric (WHO 2021) | 89 suspected HGGs, including finally 1 (1.1%) grade 1 8 (9.0%) grade 2 3 (3.4%) grade 3 77 (86.5%) grade 4 | Performance of negative fluorescence for low-grade detection Sensibility = 87.5%. Performance of fluorescence for high-grade detection Sensitivity = 100%. Specificity = 88.9%. PPV = 98.8%. NPV = 100%. |
| Study | Design | Effectives | Predictors of Intraoperative Fluorescence |
|---|---|---|---|
| Arita, 2012 [62] | Prospective, monocentric 11C Methionine PET (WHO 2007) | 11 gliomas, including 2 (18.2%) grade 2 (peripheric samples) | Fluorescence and 11C-methionine uptake are independently associated with cell density. Fluorescence is associated with proliferation index and cell density. |
| Jaber, 2016 [70] | Retrospective analysis of a prospectively collected database, monocentric (WHO 2007) | 166 gliomas lacking the typical presentation of glioblastoma 82 (49.4%) grade 2 76 (45.8%) grade 3 8 (4.8%) grade 4 | Age, tumor grade, tumor volume, contrast enhancement and 18F-FET uptake. |
| Saito, 2017 [63] | Retrospective, Monocentric (WHO 2007) | 60 gliomas 8 (13.3%) grade 2 17 (28.3%) grade 3 35 (58.3%) grade 4 | In univariate analysis: IDH1wt, no 1p19 codeletion, proliferation index, tumor margin heterogeneity, contrast enhancement. In multivariate analysis: IDH1wt. |
| Jaber, 2018 [71] | Retrospective analysis of a prospectively collected database, monocentric (WHO 2016) | 74 DLGGs 12 (16.2%) oligodendrogliomas 62 (13.8%) astrocytomas | In univariate and multivariate analysis: FET uptake and preoperative contrast enhancement. |
| Goryaynov, 2019bis [64] | Retrospective, Monocentric (WHO 2016) | 27 gliomas including 22 (81.5%) grade 2 | Cell density, proliferation index, anti-epileptic drug intake. |
| Widhalm, 2019 [66] | Prospective, monocentric (WHO 2016) | 22 suspected DLGGs 8 (36%) grade 2 11 (50%) grade 3 3 (14%) grade 4 | Contrast enhancement, increased cell density. |
| Wadiura, 2020 [65] | Retrospective, Bicentric (WHO 2016) | 110 suspected DLGGs 65 (59%) grade 2 38 (35%) grade 3 7 (6%) grade 4 | Dexamethasone/anti-epileptic drugs intake were not independent predictors. |
| Batalov, 2021 [67] | Retrospective, Monocentric (WHO 2016) | 75 gliomas with CE in 57 (76%) cases: 16 (21.3%) grade 2 13 (17.3%) grade 3 46 (61.4%) grade 4 | Increased Tumor Blood Flow (assessed by Arterial Spin Labelling) is a predictor of positive fluorescence, both in gliomas with and without contrast enhancement. |
| Kaneko, 2021 [68] | Retrospective, Monocentric (WHO 2016) | 25 DLGGs initially suspected to be high-grade (24% CE) 8 (32%) oligodendrogliomas 15 (60%) IDHmut and 2 (8%) IDHwt astrocytomas | In univariate analysis: gadolinium enhancement, proliferation index, 18F-FET PET uptake ratio and ADC-based tumor cellularity In multivariate analysis: proliferation index and 18F FET PET uptake ratio. |
| Hosmann, 2021 [72] | Retrospective, bicentric (WHO 2016) | 59 DLGGs 29 (49%) IDH1mut and 3 (5%) IDH1wt astrocytomas 23 (39%) Oligodendrogliomas 4 (7%) Not otherwise specified | IDH1wt status significantly more frequent in fluorescent tumors than in non-fluorescent tumors. |
| Müther, 2022 [69] | Retrospective, Monocentric (WHO 2016) | 179 gliomas 113 (63.1%) grade 2 66 (36.9%) grade 3 | Contrast enhancement on the MRI, proliferation index. |
| Hosmann, 2023 [73] | Retrospective, 3 centers (WHO 2016) | 86 DLGGs with CE for 23 (26.7%) cases 56 (65.1%) astocytomas 30 (34.9%) oligodendroliomas | Contrast enhancement and CD34 expression correlated with fluorescence positivity. |
| TOTAL | Total: Table 1, Table 2 and Table 3 | Positive 5-ALA fluorescence | |
| 659 DLGGs | 19.4% (128/659) DLGGs | ||
| 231 grade 3 (all) | 74.5% (172/231) grade 3 (all) and | ||
| and grade 4 (without CE) gliomas | Grade 4 (without CE) gliomas | ||
| Study | Design | Effectives | Main Conclusions |
|---|---|---|---|
| STUDIES ASSESSING RESECTION GUIDED BY FLUORESCEIN SODIUM | |||
| Chen, 2012 [79] | Prospective, monocentric Dose: 15–20 mg/kg (WHO 2007) | 10 gliomas, including 4 (40%) grade 2 | FS fluorescence positivity 3/4 (75%) grade 2. Contrast enhancement in all fluorescent DLGGs but not in the remaining one. |
| Schebesch, 2013 [82] | Retrospective, monocentric Dose: 3–4 mg/kg (WHO 2007) | 26 gliomas, including 3 (11.5%) grade 2 | FS fluorescence Helpful in 2/3 DLGGs. |
| Schebesch, 2018 [80] | Retrospective, monocentric, Dose: 5 mg/kg (WHO 2016) | 5 gliomas without contrast enhancement but with 18F FET uptake, including 1 (20%) grade 2 3 (60%) grade 3 | FS fluorescence positivity 100% cases (diffuse or focal). |
| Xiang, 2018 [81] | Retrospective, monocentric Dose: 5 mg/kg (WHO 2016) | 28 gliomas 5 (17.9%) grade 2 6 (21.4%) grade 3 17 (60.7%) grade 4 | FS fluorescence positivity 0/5 (0%) grade 2. Significant decrease in Claudin-5 expression by endothelial cells in fluorescent gliomas. |
| TOTAL | Positive FS fluorescence | ||
| 13 DLGGs | 46% (6/13) DLGGs | ||
| 5 grade 3 gliomas without CE | 60% (3/5) grade 3 gliomas without CE | ||
| Study | Design | Effectives | Ex Vivo Positive 5-ALA Fluorescence at Target |
|---|---|---|---|
| 5-ALA-GUIDED BIOPSIES | |||
| Von Campe, 2012 [88] | Prospective, monocentric Frameless biopsies (WHO 2007) | 14 tumors, including 2 (14.3%) DLGGs | In 0/2 (0%) DLGGs. |
| Widhalm, 2012 [86] | Prospective, monocentric Frameless biopsies (WHO 2007) | 50 tumors, including 6 (12%) DLGGs | In 0/19 (0%) samples from DLGGs. |
| Marbacher, 2014 [54] | Retrospective, monocentric Frameless biopsies (WHO 2007) | 82 tumors, including 12 (14.6%) DLGGs | In 3/12 (25%) DLGGs. |
| Shofty, 2019 [89] | Retrospective, monocentric Frameless biopsies (WHO 2007) | 34 tumors, including 3 (8.8%) DLGGs | In 2/3 (66%) DLGGs. |
| Millesi, 2020 [90] | Prospective, monocentric Frameless biopsies (WHO 2007 & 2016) | 79 tumors, including 6 (7.6%) DLGGs | In 2/6 (33.3%) DLGGs (vague). |
| Malinova, 2020 [91] | Retrospective, monocentric Frame-based biopsies (WHO 2016) | 39 tumors, including 3 (7.7%) DLGGs | In 0/3 (0%) DLGGs. |
| TOTAL | 32 DLGGs | Positive 5-ALA fluorescence 21.9% (7/32) DLGGs | |
| FS-GUIDED BIOPSIES | |||
| Thien, 2018 [92] | Prospective, monocentric Frameless biopsies Dose: 5 mg/kg (WHO 2016) | 18 tumors with CE, including 3 (16.7%) DLGGs | In 3/3 (100%) samples from DLGGs. |
| Nevzati, 2020 [52] | Prospective, monocentric Frameless biopsies Dose: 3 mg/kg (WHO 2016) | 17 tumors, including 1 (5.9%) DLGG | In all (100%) DLGGs. |
| Xu, 2022 [87] | Retrospective, monocentric Frameless and frame-based biopsies Dose: 5 mg/kg (WHO 2016) | 44 tumors, including 3 (6.8%) DLGGs (one astrocytoma, 2 oligodendrogliomas) | 0% of samples from grade 2 astrocytoma 36% of samples from grade 2 oligodendrogliomas, but 9% of samples were not glioma-infiltrated |
| TOTAL * | 7 DLGGs | Positive FS fluorescence 57.1% (4/7) DLGGs | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picart, T.; Gautheron, A.; Caredda, C.; Ray, C.; Mahieu-Williame, L.; Montcel, B.; Guyotat, J. Fluorescence-Guided Surgical Techniques in Adult Diffuse Low-Grade Gliomas: State-of-the-Art and Emerging Techniques: A Systematic Review. Cancers 2024, 16, 2698. https://doi.org/10.3390/cancers16152698
Picart T, Gautheron A, Caredda C, Ray C, Mahieu-Williame L, Montcel B, Guyotat J. Fluorescence-Guided Surgical Techniques in Adult Diffuse Low-Grade Gliomas: State-of-the-Art and Emerging Techniques: A Systematic Review. Cancers. 2024; 16(15):2698. https://doi.org/10.3390/cancers16152698
Chicago/Turabian StylePicart, Thiebaud, Arthur Gautheron, Charly Caredda, Cédric Ray, Laurent Mahieu-Williame, Bruno Montcel, and Jacques Guyotat. 2024. "Fluorescence-Guided Surgical Techniques in Adult Diffuse Low-Grade Gliomas: State-of-the-Art and Emerging Techniques: A Systematic Review" Cancers 16, no. 15: 2698. https://doi.org/10.3390/cancers16152698
APA StylePicart, T., Gautheron, A., Caredda, C., Ray, C., Mahieu-Williame, L., Montcel, B., & Guyotat, J. (2024). Fluorescence-Guided Surgical Techniques in Adult Diffuse Low-Grade Gliomas: State-of-the-Art and Emerging Techniques: A Systematic Review. Cancers, 16(15), 2698. https://doi.org/10.3390/cancers16152698






