Detection of Carcinoma-Associated Fibroblasts Derived from Mesothelial Cells via Mesothelial-to-Mesenchymal Transition in Primary Ovarian Carcinomas
Abstract
Simple Summary
Abstract
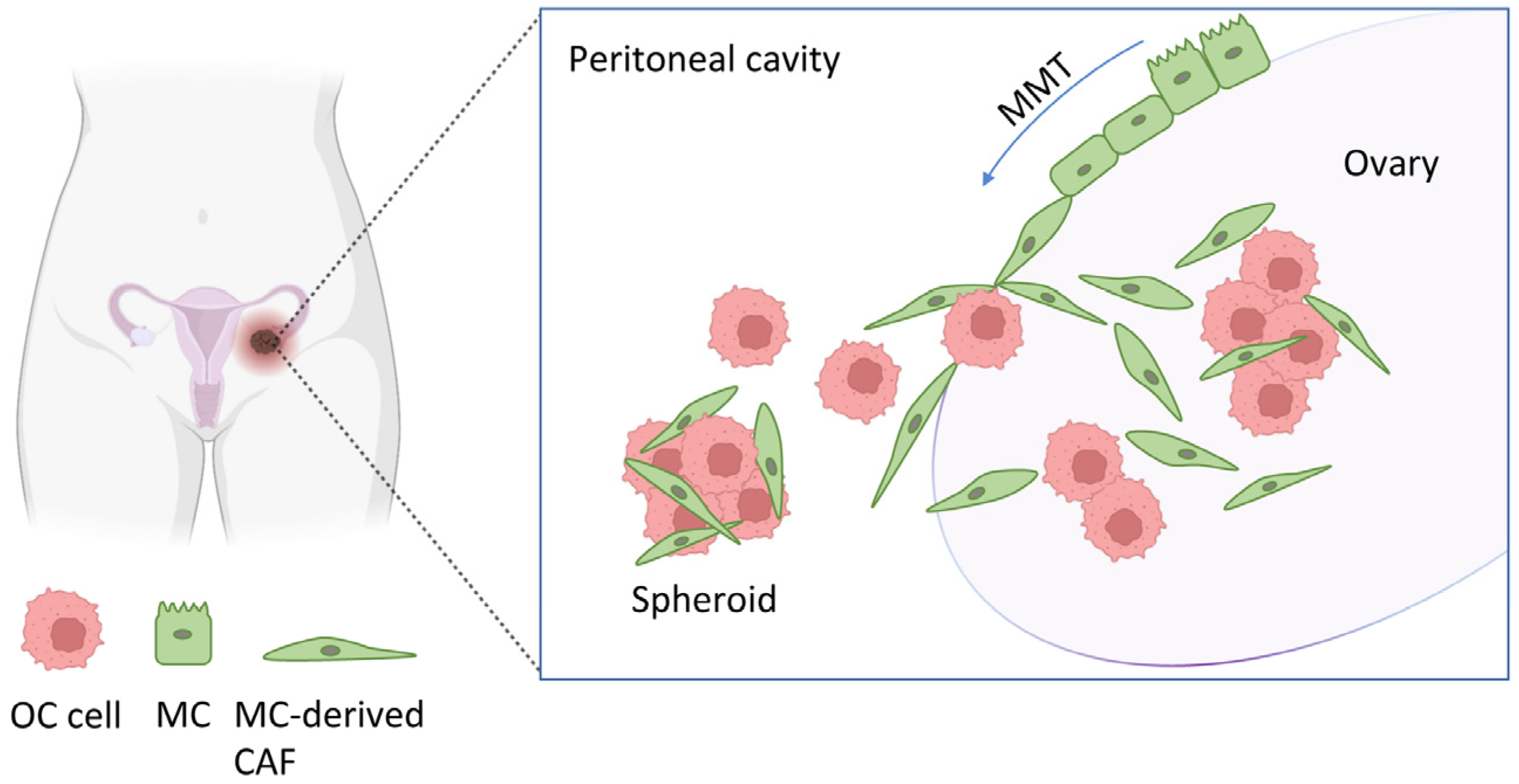
1. Introduction
2. Materials and Methods
2.1. Patient Samples and Cell Cultures
2.2. Immunofluorescence
2.3. Reverse Transcription—Quantitative PCR (RT-qPCR)
2.4. Immunoblotting
2.5. Immunohistochemistry
2.6. Statistical Analysis
3. Results
3.1. Mesothelial-to-Mesenchymal Transition Detected in CAFs Isolated from Primary Ovarian Tumors
3.2. CAFs Expressing Mesothelial Cell Markers Are Present in Primary Ovarian Carcinomas
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Okamura, H.; Katabuchi, H. Pathophysiological Dynamics of Human Ovarian Surface Epithelial Cells in Epithelial Ovarian Carcinogenesis. In International Review of Cytology; Elsevier: Amsterdam, The Netherlands, 2004; Volume 242, pp. 1–54. ISBN 978-0-12-364646-0. [Google Scholar]
- Salamanca, C.M.; Maines-Bandiera, S.L.; Leung, P.C.K.; Hu, Y.-L.; Auersperg, N. Effects of Epidermal Growth Factor/Hydrocortisone on the Growth and Differentiation of Human Ovarian Surface Epithelium. J. Soc. Gynecol. Investig. 2004, 11, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Auersperg, N.; Wong, A.S.T.; Choi, K.-C.; Kang, S.K.; Leung, P.C.K. Ovarian Surface Epithelium: Biology, Endocrinology, and Pathology*. Endocr. Rev. 2001, 22, 255–288. [Google Scholar] [CrossRef] [PubMed]
- Ritch, S.J.; Telleria, C.M. The Transcoelomic Ecosystem and Epithelial Ovarian Cancer Dissemination. Front. Endocrinol. 2022, 13, 886533. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Thompson, E.W.; Quinn, M.A. Epithelial–Mesenchymal Interconversions in Normal Ovarian Surface Epithelium and Ovarian Carcinomas: An Exception to the Norm. J. Cell. Physiol. 2007, 213, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zheng, T.; Hong, W.; Ye, H.; Hu, C.; Zheng, Y. Mechanism for the Decision of Ovarian Surface Epithelial Stem Cells to Undergo Neo-Oogenesis or Ovarian Tumorigenesis. Cell. Physiol. Biochem. 2018, 50, 214–232. [Google Scholar] [CrossRef] [PubMed]
- Schoutrop, E.; Moyano-Galceran, L.; Lheureux, S.; Mattsson, J.; Lehti, K.; Dahlstrand, H.; Magalhaes, I. Molecular, Cellular and Systemic Aspects of Epithelial Ovarian Cancer and Its Tumor Microenvironment. Semin. Cancer Biol. 2022, 86, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Sundfeldt, K. Cell–Cell Adhesion in the Normal Ovary and Ovarian Tumors of Epithelial Origin; an Exception to the Rule. Mol. Cell. Endocrinol. 2003, 202, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, N.; Sacchi, G. Atlas of Peritoneal Histology. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2000, 20 (Suppl. S3), S5–S96. [Google Scholar]
- Gordillo, C.H.; Sandoval, P.; Muñoz-Hernández, P.; Pascual-Antón, L.; López-Cabrera, M.; Jiménez-Heffernan, J.A. Mesothelial-to-Mesenchymal Transition Contributes to the Generation of Carcinoma-Associated Fibroblasts in Locally Advanced Primary Colorectal Carcinomas. Cancers 2020, 12, 499. [Google Scholar] [CrossRef]
- Pascual-Antón, L.; Cardeñes, B.; Sainz De La Cuesta, R.; González-Cortijo, L.; López-Cabrera, M.; Cabañas, C.; Sandoval, P. Mesothelial-to-Mesenchymal Transition and Exosomes in Peritoneal Metastasis of Ovarian Cancer. Int. J. Mol. Sci. 2021, 22, 11496. [Google Scholar] [CrossRef]
- Rynne-Vidal, A.; Jiménez-Heffernan, J.; Fernández-Chacón, C.; López-Cabrera, M.; Sandoval, P. The Mesothelial Origin of Carcinoma Associated-Fibroblasts in Peritoneal Metastasis. Cancers 2015, 7, 1994–2011. [Google Scholar] [CrossRef]
- Sandoval, P.; Jiménez-Heffernan, J.A.; Rynne-Vidal, Á.; Pérez-Lozano, M.L.; Gilsanz, Á.; Ruiz-Carpio, V.; Reyes, R.; García-Bordas, J.; Stamatakis, K.; Dotor, J.; et al. Carcinoma-associated Fibroblasts Derive from Mesothelial Cells via Mesothelial-to-mesenchymal Transition in Peritoneal Metastasis. J. Pathol. 2013, 231, 517–531. [Google Scholar] [CrossRef]
- Bajwa, P.; Kordylewicz, K.; Bilecz, A.; Lastra, R.R.; Wroblewski, K.; Rinkevich, Y.; Lengyel, E.; Kenny, H.A. Cancer-Associated Mesothelial Cell–Derived ANGPTL4 and STC1 Promote the Early Steps of Ovarian Cancer Metastasis. JCI Insight 2023, 8, e163019. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.; Wei, Y.; Zhao, Y.; Zhang, T.; Ma, X. The Role of Cancer-Associated Mesothelial Cells in the Progression and Therapy of Ovarian Cancer. Front. Immunol. 2022, 13, 1013506. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, J.; Shen, X.; Li, M.; Yue, Y.; Cheng, X.; Lu, W.; Wang, X.; Xie, X. LncRNA SPOCD1-AS from Ovarian Cancer Extracellular Vesicles Remodels Mesothelial Cells to Promote Peritoneal Metastasis via Interacting with G3BP1. J. Exp. Clin. Cancer Res. 2021, 40, 101. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Ramírez-Huesca, M.; Jiménez-Heffernan, J.A.; Sánchez-Tomero, J.A.; Álvarez, V.; Cirujeda, A.; Sánchez-Madrid, F. Peritoneal Dialysis and Epithelial-to-Mesenchymal Transition of Mesothelial Cells. N. Engl. J. Med. 2003, 348, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.-K.; Wilgenbus, P.; Dahl, U.; Semb, H.; Christofori, G. A Causal Role for E-Cadherin in the Transition from Adenoma to Carcinoma. Nature 1998, 392, 190–193. [Google Scholar] [CrossRef]
- Takeichi, M. Morphogenetic Roles of Classic Cadherins. Curr. Opin. Cell Biol. 1995, 7, 619–627. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular Mechanisms of Epithelial–Mesenchymal Transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Orimo, A.; Weinberg, R.A. Stromal Fibroblasts in Cancer: A Novel Tumor-Promoting Cell Type. Cell Cycle 2006, 5, 1597–1601. [Google Scholar] [CrossRef]
- Desmouliere, A.; Guyot, C.; Gabbiani, G. The Stroma Reaction Myofibroblast: A Key Player in the Control of Tumor Cell Behavior. Int. J. Dev. Biol. 2004, 48, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, R.G.; Korman, B.; Varga, J. Adipocytic Progenitor Cells Give Rise to Pathogenic Myofibroblasts: Adipocyte-to-Mesenchymal Transition and Its Emerging Role in Fibrosis in Multiple Organs. Curr. Rheumatol. Rep. 2020, 22, 79. [Google Scholar] [CrossRef] [PubMed]
- Sangai, T.; Ishii, G.; Kodama, K.; Miyamoto, S.; Aoyagi, Y.; Ito, T.; Magae, J.; Sasaki, H.; Nagashima, T.; Miyazaki, M.; et al. Effect of Differences in Cancer Cells and Tumor Growth Sites on Recruiting Bone Marrow-Derived Endothelial Cells and Myofibroblasts in Cancer-Induced Stroma. Int. J. Cancer 2005, 115, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Ishii, G.; Sangai, T.; Ito, T.; Hasebe, T.; Endoh, Y.; Sasaki, H.; Harigaya, K.; Ochiai, A. In Vivo Andin Vitro Characterization of Human Fibroblasts Recruited Selectively into Human Cancer Stroma. Int. J. Cancer 2005, 117, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, E.M.; Potenta, S.; Xie, L.; Zeisberg, M.; Kalluri, R. Discovery of Endothelial to Mesenchymal Transition as a Source for Carcinoma-Associated Fibroblasts. Cancer Res. 2007, 67, 10123–10128. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, P.; Loureiro, J.; González-Mateo, G.; Pérez-Lozano, M.L.; Maldonado-Rodríguez, A.; Sánchez-Tomero, J.A.; Mendoza, L.; Santamaría, B.; Ortiz, A.; Ruíz-Ortega, M.; et al. PPAR-γ Agonist Rosiglitazone Protects Peritoneal Membrane from Dialysis Fluid-Induced Damage. Lab. Investig. 2010, 90, 1517–1532. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, J.; Aguilera, A.; Selgas, R.; Sandoval, P.; Albar-Vizcaíno, P.; Pérez-Lozano, M.L.; Ruiz-Carpio, V.; Majano, P.L.; Lamas, S.; Rodríguez-Pascual, F.; et al. Blocking TGF-Β1 Protects the Peritoneal Membrane from Dialysate-Induced Damage. J. Am. Soc. Nephrol. 2011, 22, 1682–1695. [Google Scholar] [CrossRef] [PubMed]
- Strippoli, R.; Benedicto, I.; Perez Lozano, M.L.; Pellinen, T.; Sandoval, P.; Lopez-Cabrera, M.; Del Pozo, M.A. Inhibition of Transforming Growth Factor-Activated Kinase 1 (TAK1) Blocks and Reverses Epithelial to Mesenchymal Transition of Mesothelial Cells. PLoS ONE 2012, 7, e31492. [Google Scholar] [CrossRef]
- Ohashi, K.; Li, T.-S.; Miura, S.; Hasegawa, Y.; Miura, K. Biological Differences Between Ovarian Cancer-Associated Fibroblasts and Contralateral Normal Ovary-Derived Mesenchymal Stem Cells. Anticancer Res. 2022, 42, 1729–1737. [Google Scholar] [CrossRef]
- Rynne-Vidal, A.; Au-Yeung, C.L.; Jiménez-Heffernan, J.A.; Pérez-Lozano, M.L.; Cremades-Jimeno, L.; Bárcena, C.; Cristóbal-García, I.; Fernández-Chacón, C.; Yeung, T.L.; Mok, S.C.; et al. Mesothelial-to-Mesenchymal Transition as a Possible Therapeutic Target in Peritoneal Metastasis of Ovarian Cancer: Mesothelial-to-Mesenchymal Transition and Peritoneal Metastatic Niche. J. Pathol. 2017, 242, 140–151. [Google Scholar] [CrossRef]
- Pascual-Antón, L.; Sandoval, P.; González-Mateo, G.T.; Kopytina, V.; Tomero-Sanz, H.; Arriero-País, E.M.; Jiménez-Heffernan, J.A.; Fabre, M.; Egaña, I.; Ferrer, C.; et al. Targeting Carcinoma-associated Mesothelial Cells with Antibody–Drug Conjugates in Ovarian Carcinomatosis. J. Pathol. 2023, 261, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Lozano, M.L.; Sandoval, P.; Rynne-Vidal, Á.; Aguilera, A.; Jiménez-Heffernan, J.A.; Albar-Vizcaíno, P.; Majano, P.L.; Sánchez-Tomero, J.A.; Selgas, R.; López-Cabrera, M. Functional Relevance of the Switch of VEGF Receptors/Co-Receptors during Peritoneal Dialysis-Induced Mesothelial to Mesenchymal Transition. PLoS ONE 2013, 8, e60776. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.I.; Millien, G.; Hinds, A.; Cao, Y.; Seldin, D.C.; Williams, M.C. T1α, a Lung Type I Cell Differentiation Gene, Is Required for Normal Lung Cell Proliferation and Alveolus Formation at Birth. Dev. Biol. 2003, 256, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Mahtab, E.A.F.; Wijffels, M.C.E.F.; Van Den Akker, N.M.S.; Hahurij, N.D.; Lie-Venema, H.; Wisse, L.J.; DeRuiter, M.C.; Uhrin, P.; Zaujec, J.; Binder, B.R.; et al. Cardiac Malformations and Myocardial Abnormalities in Podoplanin Knockout Mouse Embryos: Correlation with Abnormal Epicardial Development. Dev. Dyn. 2008, 237, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Schacht, V.; Dadras, S.S.; Johnson, L.A.; Jackson, D.G.; Hong, Y.-K.; Detmar, M. Up-Regulation of the Lymphatic Marker Podoplanin, a Mucin-Type Transmembrane Glycoprotein, in Human Squamous Cell Carcinomas and Germ Cell Tumors. Am. J. Pathol. 2005, 166, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Schacht, V. T1 /Podoplanin Deficiency Disrupts Normal Lymphatic Vasculature Formation and Causes Lymphedema. EMBO J. 2003, 22, 3546–3556. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Zhong, K.; Chen, W.; Wang, S.; Huang, L. Podoplanin-Positive Cancer-Associated Fibroblasts Predict Poor Prognosis in Lung Cancer Patients. OncoTargets Ther. 2018, 11, 5607–5619. [Google Scholar] [CrossRef]
- Doglioni, C.; Dei, A.P.; Laurino, L.; Iuzzolino, P.; Chiarelli, C.; Celio, M.R.; Viale, G. Calretinin: A Novel Immunocytochemical Marker for Mesothelioma. Am. J. Surg. Pathol. 1996, 20, 1037–1046. [Google Scholar] [CrossRef]
- Gotzos, V.; Vogt, P.; Celio, M.R. The Calcium Binding Protein Calretinin Is a Selective Marker for Malignant Pleural Mesotheliomas of the Epithelial Type. Pathol.—Res. Pract. 1996, 192, 137–147. [Google Scholar] [CrossRef]
- Lee, E.S.; Leong, A.S.-Y.; Kim, Y.-S.; Lee, J.-H.; Kim, I.; Ahn, G.H.; Kim, H.S.; Chun, Y.K. Calretinin, CD34, and α-Smooth Muscle Actin in the Identification of Peritoneal Invasive Implants of Serous Borderline Tumors of the Ovary. Mod. Pathol. 2006, 19, 364–372. [Google Scholar] [CrossRef]
- Okamoto, S.; Okamoto, A.; Nikaido, T.; Saito, M.; Takao, M.; Yanaihara, N.; Takakura, S.; Ochiai, K.; Tanaka, T. Mesenchymal to Epithelial Transition in the Human Ovarian Surface Epithelium Focusing on Inclusion Cysts. Oncol. Rep. 2009, 21, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Bast, R.C.; Klug, T.L.; John, E.S.; Jenison, E.; Niloff, J.M.; Lazarus, H.; Berkowitz, R.S.; Leavitt, T.; Griffiths, C.T.; Parker, L.; et al. A Radioimmunoassay Using a Monoclonal Antibody to Monitor the Course of Epithelial Ovarian Cancer. N. Engl. J. Med. 1983, 309, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Assidi, M. High N-Cadherin Protein Expression in Ovarian Cancer Predicts Poor Survival and Triggers Cell Invasion. Front. Oncol. 2022, 12, 870820. [Google Scholar] [CrossRef]
- Duggan, M.A.; Robertson, D.I. The Cytokeratin Profiles of Ovarian Common “Epithelial” Tumors. Eur. J. Gynaecol. Oncol. 1989, 10, 73–79. [Google Scholar] [PubMed]
- Hassan, R.; Kreitman, R.J.; Pastan, I.; Willingham, M.C. Localization of Mesothelin in Epithelial Ovarian Cancer. Appl. Immunohistochem. Mol. Morphol. 2005, 13, 243–247. [Google Scholar] [CrossRef]
- Jiang, H.; Xi, Q.; Wang, F.; Sun, Z.; Huang, Z.; Qi, L. Increased Expression of Neuropilin 1 Is Associated with Epithelial Ovarian Carcinoma. Mol. Med. Rep. 2015, 12, 2114–2120. [Google Scholar] [CrossRef][Green Version]
- Vimercati, L.; Cavone, D.; Delfino, M.C.; Bruni, B.; De Maria, L.; Caputi, A.; Sponselli, S.; Rossi, R.; Resta, L.; Fortarezza, F.; et al. Primary Ovarian Mesothelioma: A Case Series with Electron Microscopy Examination and Review of the Literature. Cancers 2021, 13, 2278. [Google Scholar] [CrossRef]
- Gao, Q.; Yang, Z.; Xu, S.; Li, X.; Yang, X.; Jin, P.; Liu, Y.; Zhou, X.; Zhang, T.; Gong, C.; et al. Heterotypic CAF-Tumor Spheroids Promote Early Peritoneal Metastasis of Ovarian Cancer. J. Exp. Med. 2019, 216, 688–703. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomero-Sanz, H.; Jiménez-Heffernan, J.A.; Fernández-Chacón, M.C.; Cristóbal-García, I.; Sainz de la Cuesta, R.; González-Cortijo, L.; López-Cabrera, M.; Sandoval, P. Detection of Carcinoma-Associated Fibroblasts Derived from Mesothelial Cells via Mesothelial-to-Mesenchymal Transition in Primary Ovarian Carcinomas. Cancers 2024, 16, 2697. https://doi.org/10.3390/cancers16152697
Tomero-Sanz H, Jiménez-Heffernan JA, Fernández-Chacón MC, Cristóbal-García I, Sainz de la Cuesta R, González-Cortijo L, López-Cabrera M, Sandoval P. Detection of Carcinoma-Associated Fibroblasts Derived from Mesothelial Cells via Mesothelial-to-Mesenchymal Transition in Primary Ovarian Carcinomas. Cancers. 2024; 16(15):2697. https://doi.org/10.3390/cancers16152697
Chicago/Turabian StyleTomero-Sanz, Henar, José Antonio Jiménez-Heffernan, María Concepción Fernández-Chacón, Ignacio Cristóbal-García, Ricardo Sainz de la Cuesta, Lucía González-Cortijo, Manuel López-Cabrera, and Pilar Sandoval. 2024. "Detection of Carcinoma-Associated Fibroblasts Derived from Mesothelial Cells via Mesothelial-to-Mesenchymal Transition in Primary Ovarian Carcinomas" Cancers 16, no. 15: 2697. https://doi.org/10.3390/cancers16152697
APA StyleTomero-Sanz, H., Jiménez-Heffernan, J. A., Fernández-Chacón, M. C., Cristóbal-García, I., Sainz de la Cuesta, R., González-Cortijo, L., López-Cabrera, M., & Sandoval, P. (2024). Detection of Carcinoma-Associated Fibroblasts Derived from Mesothelial Cells via Mesothelial-to-Mesenchymal Transition in Primary Ovarian Carcinomas. Cancers, 16(15), 2697. https://doi.org/10.3390/cancers16152697






