Radiomics in Oesogastric Cancer: Staging and Prediction of Preoperative Treatment Response: A Narrative Review and the Results of Personal Experience
Abstract
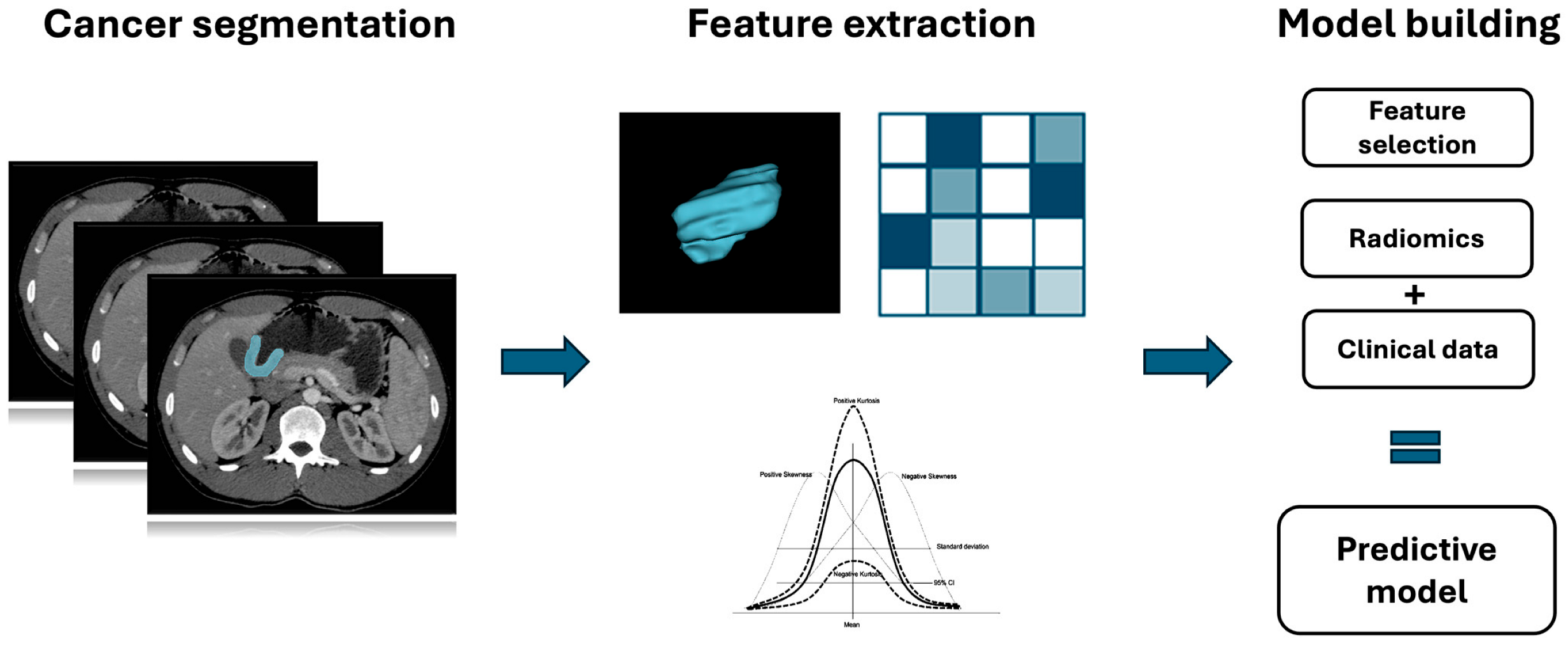
Simple Summary
Abstract
1. Introduction
2. Oesophageal Cancer
2.1. CT-Scan
2.2. MRI
2.3. 18F-FDG PET/CT
3. Gastroesophageal Junctional Cancer
4. Gastric Cancer
4.1. CT-Scan
4.2. MRI
4.3. 18F-FDG PET/CT
5. Results of Personal Experience
6. Limitations
7. Conclusions
8. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Obermannová, R.; Alsina, M.; Cervantes, A.; Leong, T.; Lordick, F.; Nilsson, M.; van Grieken, N.C.T.; Vogel, A.; Smyth, E.C. Oesophageal Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2022, 33, 992–1004. [Google Scholar] [CrossRef] [PubMed]
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E.C. Gastric Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Farjah, F.; Gerdes, H.; et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2023. JNCCN J. Natl. Compr. Cancer Netw. 2023, 21, 393–422. [Google Scholar] [CrossRef] [PubMed]
- van Hagen, P.; Hulshof, M.C.C.M.; van Lanschot, J.J.B.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; Richel, D.J.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative Chemotherapy with Fluorouracil plus Leucovorin, Oxaliplatin, and Docetaxel versus Fluorouracil or Capecitabine plus Cisplatin and Epirubicin for Locally Advanced, Resectable Gastric or Gastro-Oesophageal Junction Adenocarcinoma (FLOT4): A Ra. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Bass, A.J.; Thorsson, V.; Shmulevich, I.; Reynolds, S.M.; Miller, M.; Bernard, B.; Hinoue, T.; Laird, P.W.; Curtis, C.; Shen, H.; et al. Comprehensive Molecular Characterization of Gastric Adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef]
- Cristescu, R.; Lee, J.; Nebozhyn, M.; Kim, K.M.; Ting, J.C.; Wong, S.S.; Liu, J.; Yue, Y.G.; Wang, J.; Yu, K.; et al. Molecular Analysis of Gastric Cancer Identifies Subtypes Associated with Distinct Clinical Outcomes. Nat. Med. 2015, 21, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Wotherspoon, A.; Peckitt, C.; Gonzalez, D.; Hulkki-Wilson, S.; Eltahir, Z.; Fassan, M.; Rugge, M.; Valeri, N.; Okines, A.; et al. Mismatch Repair Deficiency, Microsatellite Instability, and Survival: An Exploratory Analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial. JAMA Oncol. 2017, 3, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Tourassi, G.D. Journey toward Computer-Aided Diagnosis: Role of Image Texture Analysis. Radiology 1999, 213, 317–320. [Google Scholar] [CrossRef] [PubMed]
- van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in Medical Imaging—“how-to” Guide and Critical Reflection. Insights Imaging 2020, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Wesdorp, N.J.; Hellingman, T.; Jansma, E.P.; van Waesberghe, J.H.T.M.; Boellaard, R.; Punt, C.J.A.; Huiskens, J.; Kazemier, G. Advanced Analytics and Artificial Intelligence in Gastrointestinal Cancer: A Systematic Review of Radiomics Predicting Response to Treatment. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1785. [Google Scholar] [CrossRef] [PubMed]
- Sah, B.R.; Owczarczyk, K.; Siddique, M.; Cook, G.J.R.; Goh, V. Radiomics in Esophageal and Gastric Cancer. Abdom. Radiol. 2019, 44, 2048–2058. [Google Scholar] [CrossRef]
- Guo, H.; Tang, H.T.; Hu, W.L.; Wang, J.J.; Liu, P.Z.; Yang, J.J.; Hou, S.L.; Zuo, Y.J.; Deng, Z.Q.; Zheng, X.Y.; et al. The Application of Radiomics in Esophageal Cancer: Predicting the Response after Neoadjuvant Therapy. Front. Oncol. 2023, 13, 1082960. [Google Scholar] [CrossRef]
- Menon, N.; Guidozzi, N.; Chidambaram, S.; Markar, S.R. Performance of Radiomics-Based Artificial Intelligence Systems in the Diagnosis and Prediction of Treatment Response and Survival in Esophageal Cancer: A Systematic Review and Meta-Analysis of Diagnostic Accuracy. Dis. Esophagus 2023, 36, doad034. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Fang, M.J.; Tang, L.; Shan, X.H.; Gao, J.B.; Giganti, F.; Wang, R.P.; Chen, X.; Wang, X.X.; Palumbo, D.; et al. Deep Learning Radiomic Nomogram Can Predict the Number of Lymph Node Metastasis in Locally Advanced Gastric Cancer: An International Multicenter Study. Ann. Oncol. 2020, 31, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.X.; Liu, C.; Qi, L.; Sun, S.W.; Song, Y.; Yang, G.; Zhang, Y.D.; Liu, X.S. An Intelligent Clinical Decision Support System for Preoperative Prediction of Lymph Node Metastasis in Gastric Cancer. J. Am. Coll. Radiol. 2019, 16, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Adili, D.; Mohetaer, A.; Zhang, W. Diagnostic Accuracy of Radiomics-Based Machine Learning for Neoadjuvant Chemotherapy Response and Survival Prediction in Gastric Cancer Patients: A Systematic Review and Meta-Analysis. Eur. J. Radiol. 2024, 173, 111249. [Google Scholar] [CrossRef] [PubMed]
- Beukinga, R.J.; Poelmann, F.B.; Kats-Ugurlu, G.; Viddeleer, A.R.; Boellaard, R.; de Haas, R.J.; Plukker, J.T.M.; Hulshoff, J.B. Prediction of Non-Response to Neoadjuvant Chemoradiotherapy in Esophageal Cancer Patients with 18F-FDG PET Radiomics Based Machine Learning Classification. Diagnostics 2022, 12, 1070. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Li, R.; Liu, Y.; Zhan, T.; Li, Y.; Zhang, J. Preoperative Prediction of Perineural Invasion and Prognosis in Gastric Cancer Based on Machine Learning through a Radiomics-Clinicopathological Nomogram. Cancers 2024, 16, 614. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.F. Esophageal Cancer: Staging System and Guidelines for Staging and Treatment. J. Thorac. Dis. 2014, 6 (Suppl. S3), S289–S297. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Chen, T.; Han, J.; Ye, P.; Hu, J. Staging Accuracy of Endoscopic Ultrasound for Esophageal Cancer after Neoadjuvant Chemotherapy: A Meta-Analysis and Systematic Review. Dis. Esophagus Off. J. Int. Soc. Dis. Esophagus 2015, 28, 757–771. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, T.D.; Kosinski, A.S.; Puri, V.; Burfeind, W.; Bharat, A.; Patterson, G.A.; Hofstetter, W.; Meyers, B.F. Evaluation of the Reliability of Clinical Staging of T2N0 Esophageal Cancer: A Review of the Society of Thoracic Surgeons Database. Ann. Thorac. Surg. 2013, 96, 382. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.L.; Yadav, P.; Starekova, J.; Christensen, L.; Chandereng, T.; Chappell, R.; Reeder, S.B.; Bassetti, M.F. Diagnostic Performance of MRI for Esophageal Carcinoma: A Systematic Review and Meta-Analysis. Radiology 2021, 299, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Leeflang, M.M.G. The Accuracy of MRI for Esophageal Cancer Staging. Radiology 2021, 299, 595–596. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Hu, P.; Li, M.; Ding, R.; Wang, Y.; Pan, S.; Kang, M.; Kong, W.; Du, D.; Wang, F. Computed Tomography-Based Radiomics in Predicting T Stage and Length of Esophageal Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 722961. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, D.; Murakami, Y.; Tani, S.; Nagata, Y. A Prediction Model for Degree of Differentiation for Resectable Locally Advanced Esophageal Squamous Cell Carcinoma Based on CT Images Using Radiomics and Machine-Learning. Br. J. Radiol. 2021, 94, 20210525. [Google Scholar] [CrossRef]
- Wang, Z.L.; Zhou, Z.G.; Chen, Y.; Li, X.T.; Sun, Y.S. Support Vector Machines Model of Computed Tomography for Assessing Lymph Node Metastasis in Esophageal Cancer with Neoadjuvant Chemotherapy. J. Comput. Assist. Tomogr. 2017, 41, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Ma, Z.; Yan, L.; Ye, W.; Liu, Z.; Liang, C. Radiomics Nomogram Outperforms Size Criteria in Discriminating Lymph Node Metastasis in Resectable Esophageal Squamous Cell Carcinoma. Eur. Radiol. 2019, 29, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Ren, W.; Li, S.; Liu, J.; Sun, Y.; Yan, J.; Wan, S. Radiomic Analysis in Contrast-Enhanced CT: Predict Treatment Response to Chemoradiotherapy in Esophageal Carcinoma. Oncotarget 2017, 8, 104444–104454. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xie, C.; Yang, H.; Ho, J.W.K.; Wen, J.; Han, L.; Lam, K.O.; Wong, I.Y.H.; Law, S.Y.K.; Chiu, K.W.H.; et al. Computed Tomography-Based Deep-Learning Prediction of Neoadjuvant Chemoradiotherapy Treatment Response in Esophageal Squamous Cell Carcinoma. Radiother. Oncol. 2021, 154, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xie, C.; Yang, H.; Ho, J.W.K.; Wen, J.; Han, L.; Chiu, K.W.H.; Fu, J.; Vardhanabhuti, V. Assessment of Intratumoral and Peritumoral Computed Tomography Radiomics for Predicting Pathological Complete Response to Neoadjuvant Chemoradiation in Patients With Esophageal Squamous Cell Carcinoma. JAMA Netw. Open 2020, 3, e2015927. [Google Scholar] [CrossRef] [PubMed]
- Rishi, A.; Zhang, G.G.; Yuan, Z.; Sim, A.J.; Song, E.Y.; Moros, E.G.; Tomaszewski, M.R.; Latifi, K.; Pimiento, J.M.; Fontaine, J.P.; et al. Pretreatment CT and 18 F-FDG PET-Based Radiomic Model Predicting Pathological Complete Response and Loco-Regional Control Following Neoadjuvant Chemoradiation in Oesophageal Cancer. J. Med. Imaging Radiat. Oncol. 2021, 65, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Riyahi, S.; Choi, W.; Liu, C.J.; Zhong, H.; Wu, A.J.; Mechalakos, J.G.; Lu, W. Quantifying Local Tumor Morphological Changes with Jacobian Map for Prediction of Pathologic Tumor Response to Chemo-Radiotherapy in Locally Advanced Esophageal Cancer. Phys. Med. Biol. 2018, 63, 145020. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Shen, C.; Qin, J.; Wang, Z.; Liu, Z.; Guo, J.; Zhang, H.; Gao, P.; Bei, T.; Wang, Y.; et al. The MR Radiomic Signature Can Predict Preoperative Lymph Node Metastasis in Patients with Esophageal Cancer. Eur. Radiol. 2019, 29, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-H.; Lu, P.; Gao, M.-C.; Wang, R.; Li, Y.-Y.; Guo, R.-Q.; Zhang, W.-S.; Song, J.-X. Nomogram Based on Multimodal Magnetic Resonance Combined with B7-H3mRNA for Preoperative Lymph Node Prediction in Esophagus Cancer. World J. Clin. Oncol. 2024, 15, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-H.; Lu, P.; Gao, M.-C.; Wang, R.; Li, Y.-Y.; Song, J.-X. Progress of Magnetic Resonance Imaging Radiomics in Preoperative Lymph Node Diagnosis of Esophageal Cancer. World J. Radiol. 2023, 15, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, C.; Liu, Y.; Chu, F.; Jia, Z.; Zhang, H.; Wang, Z.; Lu, Y.; Wang, S.; Yang, G.; et al. The MRI Radiomics Signature Can Predict the Pathologic Response to Neoadjuvant Chemotherapy in Locally Advanced Esophageal Squamous Cell Carcinoma. Eur. Radiol. 2024, 34, 485–494. [Google Scholar] [CrossRef]
- Qu, J.; Ma, L.; Lu, Y.; Wang, Z.; Guo, J.; Zhang, H.; Yan, X.; Liu, H.; Kamel, I.R.; Qin, J.; et al. DCE-MRI Radiomics Nomogram Can Predict Response to Neoadjuvant Chemotherapy in Esophageal Cancer. Discov. Oncol. 2022, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Li, S.; Ren, W.; Liu, J.; Yan, J.; Wan, S. Radiomic Analysis in T2W and SPAIR T2W MRI: Predict Treatment Response to Chemoradiotherapy in Esophageal Squamous Cell Carcinoma. J. Thorac. Dis. 2018, 10, 2256–2267. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Wang, X.; Xue, L.; Zhang, H.; Ma, Z.; Deng, H.; Yang, Z.; Sun, X.; Men, Y.; et al. MR Radiomics Predicts Pathological Complete Response of Esophageal Squamous Cell Carcinoma after Neoadjuvant Chemoradiotherapy: A Multicenter Study. Cancer Imaging 2024, 24, 16. [Google Scholar] [CrossRef] [PubMed]
- Heethuis, S.E.; van Rossum, P.S.N.; Lips, I.M.; Goense, L.; Voncken, F.E.; Reerink, O.; van Hillegersberg, R.; Ruurda, J.P.; Philippens, M.E.; van Vulpen, M.; et al. Dynamic Contrast-Enhanced MRI for Treatment Response Assessment in Patients with Oesophageal Cancer Receiving Neoadjuvant Chemoradiotherapy. Radiother. Oncol. 2016, 120, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Cao, Z.; Wu, Y.; Lin, J.; Zhang, Z.; Jin, J.; Ai, Y.; Zhang, J.; Du, D.; Tian, Z.; et al. Preoperative Prediction of Clinical and Pathological Stages for Patients with Esophageal Cancer Using PET/CT Radiomics. Insights Imaging 2023, 14, 174. [Google Scholar] [CrossRef] [PubMed]
- Beukinga, R.J.; Hulshoff, J.B.; Van Dijk, L.V.; Muijs, C.T.; Burgerhof, J.G.M.; Kats-Ugurlu, G.; Slart, R.H.J.A.; Slump, C.H.; Mul, V.E.M.; Plukker, J.T.M. Predicting Response to Neoadjuvant Chemoradiotherapy in Esophageal Cancer with Textural Features Derived from Pretreatment 18F-FDG PET/CT Imaging. J. Nucl. Med. 2017, 58, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Van Rossum, P.S.N.; Fried, D.V.; Zhang, L.; Hofstetter, W.L.; Van Vulpen, M.; Meijer, G.J.; Court, L.E.; Lin, S.H. The Incremental Value of Subjective and Quantitative Assessment of 18F-FDG PET for the Prediction of Pathologic Complete Response to Preoperative Chemoradiotherapy in Esophageal Cancer. J. Nucl. Med. 2016, 57, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Beukinga, R.J.; Wang, D.; Karrenbeld, A.; Dijksterhuis, W.P.M.; Faber, H.; Burgerhof, J.G.M.; Mul, V.E.M.; Slart, R.H.J.A.; Coppes, R.P.; Plukker, J.T.M. Addition of HER2 and CD44 to 18F-FDG PET-Based Clinico-Radiomic Models Enhances Prediction of Neoadjuvant Chemoradiotherapy Response in Esophageal Cancer. Eur. Radiol. 2021, 31, 3306–3314. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Palumbo, D.; De Cobelli, F.; Fiorino, C. Does Radiomics Play a Role in the Diagnosis, Staging and Re-Staging of Gastroesophageal Junction Adenocarcinoma? Updates Surg. 2023, 75, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, S.; Court, L.E.; Verma, V.; Koay, E.J.; Zhang, L.; Zhang, W.; Tang, C.; Lin, S.; Welsh, J.D.; et al. Radiomics Predicts Clinical Outcome in Primary Gastroesophageal Junction Adenocarcinoma Treated by Chemo/Radiotherapy and Surgery. Phys. Imaging Radiat. Oncol. 2017, 3, 37–42. [Google Scholar] [CrossRef]
- Chang, X.; Guo, X.; Li, X.; Han, X.; Li, X.; Liu, X.; Ren, J. Potential Value of Radiomics in the Identification of Stage T3 and T4a Esophagogastric Junction Adenocarcinoma Based on Contrast-Enhanced CT Images. Front. Oncol. 2021, 11, 627947. [Google Scholar] [CrossRef] [PubMed]
- Du, K.P.; Huang, W.P.; Liu, S.Y.; Chen, Y.J.; Li, L.M.; Liu, X.N.; Han, Y.J.; Zhou, Y.; Liu, C.C.; Gao, J.B. Application of Computed Tomography-Based Radiomics in Differential Diagnosis of Adenocarcinoma and Squamous Cell Carcinoma at the Esophagogastric Junction. World J. Gastroenterol. 2022, 28, 4363–4375. [Google Scholar] [CrossRef] [PubMed]
- Ba-Ssalamah, A.; Muin, D.; Schernthaner, R.; Kulinna-Cosentini, C.; Bastati, N.; Stift, J.; Gore, R.; Mayerhoefer, M.E. Texture-Based Classification of Different Gastric Tumors at Contrast-Enhanced CT. Eur. J. Radiol. 2013, 82, e537–e543. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Fang, M.; Huang, Y.; He, L.; Chen, X.; Liang, C.; Huang, X.; Cheng, Z.; Dong, D.; Liang, C.; et al. CT-Based Radiomics Signature for Differentiating Borrmann Type IV Gastric Cancer from Primary Gastric Lymphoma. Eur. J. Radiol. 2017, 91, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, S.; Ji, C.; Zheng, H.; Pan, X.; Zhang, Y.; Guan, W.; Chen, L.; Guan, Y.; Li, W.; et al. Application of CT Texture Analysis in Predicting Histopathological Characteristics of Gastric Cancers. Eur. Radiol. 2017, 27, 4951–4959. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Feng, Q.X.; Qi, L.; Liu, C.; Zhang, J.; Yang, G.; Zhang, Y.D.; Liu, X.S. Prognostic Aspects of Lymphovascular Invasion in Localized Gastric Cancer: New Insights into the Radiomics and Deep Transfer Learning from Contrast-Enhanced CT Imaging. Abdom. Radiol. 2022, 47, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Langer, R.; Reim, D.; Novotny, A.; Meyer Zum Buschenfelde, C.; Engel, J.; Friess, H.; Hofler, H. Significance of Histopathological Tumor Regression after Neoadjuvant Chemotherapy in Gastric Adenocarcinomas: A Summary of 480 Cases. Ann. Surg. 2011, 253, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Reim, D.; Novotny, A.; Friess, H.; Slotta-Huspenina, J.; Weichert, W.; Ott, K.; Dislich, B.; Lorenzen, S.; Becker, K.; Langer, R. Significance of Tumour Regression in Lymph Node Metastases of Gastric and Gastro-Oesophageal Junction Adenocarcinomas. J. Pathol. Clin. Res. 2020, 6, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhuang, Z.; Pen, L.; Xue, J.; Zhu, H.; Zhang, L.; Wang, D. Intratumoral and Peritumoral CT-Based Radiomics for Predicting the Microsatellite Instability in Gastric Cancer. Abdom. Radiol. 2024, 49, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, S.; Dong, D.; Gao, X.; Zhou, K.; Li, J.; Lv, B.; Li, H.; Wu, X.; Fang, M.; et al. Evaluation of Lymph Node Metastasis in Advanced Gastric Cancer Using Magnetic Resonance Imaging-Based Radiomics. Front. Oncol. 2019, 9, 1265. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yin, H.; Wang, Y.; Zhang, H.; Ma, F.; Li, H.; Qu, J. Multiparametric MRI-Based Radiomics Nomogram for Early Prediction of Pathological Response to Neoadjuvant Chemotherapy in Locally Advanced Gastric Cancer. Eur. Radiol. 2023, 33, 2746–2756. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.L.; Yin, H.K.; Zhang, H.K.; Wang, Y.; Xu, S.N.; Ma, F.; Gao, J.B.; Li, H.L.; Qu, J.R. Comparison of MRI and CT-Based Radiomics and Their Combination for Early Identification of Pathological Response to Neoadjuvant Chemotherapy in Locally Advanced Gastric Cancer. J. Magn. Reson. Imaging 2023, 58, 907–923. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A.; Ramos, M.F.K.P.; Cardili, L.; de Moraes, R.D.R.; Dias, A.R.; Szor, D.J.; Zilberstein, B.; Alves, V.A.F.; de Mello, E.S.; Ribeiro, U. Prognostic Implications of Tumor-Infiltrating Lymphocytes within the Tumor Microenvironment in Gastric Cancer. J. Gastrointest. Surg. 2024, 28, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, Z.; Xia, Y.; Zhao, Z.; Wang, D.; Jin, H.; Liu, F.; Yang, Y.; Shen, L.; Lu, Z. Association between Radiomics Features of DCE-MRI and CD8+ and CD4+ TILs in Advanced Gastric Cancer. Pathol. Oncol. Res. 2023, 29, 1611001. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, Z.; Wang, D.; Yang, Y.; Jin, H.; Lu, Z. Machine Learning Models Based on Quantitative Dynamic Contrast-Enhanced MRI Parameters Assess the Expression Levels of CD3+, CD4+, and CD8+ Tumor-Infiltrating Lymphocytes in Advanced Gastric Carcinoma. Front. Oncol. 2024, 14, 1365550. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, J.; Xin, B.; Sun, Y.; Feng, D.; Fulham, M.J.; Wang, X.; Song, S. 18F-FDG PET/CT Radiomics for Preoperative Prediction of Lymph Node Metastases and Nodal Staging in Gastric Cancer. Front. Oncol. 2021, 11, 723345. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.Q.; Yu, W.J.; Shao, X.L.; Wang, Y.T. Incremental Value of PET Primary Lesion-Based Radiomics Signature to Conventional Metabolic Parameters and Traditional Risk Factors for Preoperative Prediction of Lymph Node Metastases in Gastric Cancer. Abdom. Radiol. 2023, 48, 510–518. [Google Scholar] [CrossRef]
- Xue, X.Q.; Yu, W.J.; Shi, X.; Shao, X.L.; Wang, Y.T. 18F-FDG PET/CT-Based Radiomics Nomogram for the Preoperative Prediction of Lymph Node Metastasis in Gastric Cancer. Front. Oncol. 2022, 12, 911168. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.Q.; Yu, W.J.; Shao, X.L.; Li, X.F.; Niu, R.; Zhang, F.F.; Shi, Y.M.; Wang, Y.T. Radiomics Model Based on Preoperative 18F-Fluorodeoxyglucose PET Predicts N2-3b Lymph Node Metastasis in Gastric Cancer Patients. Nucl. Med. Commun. 2022, 43, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Calabrò, A.; Dondi, F.; Bagnasco, S.; Tucci, A.; Bertagna, F. The Role of Baseline 2-[18 F]-FDG-PET/CT Metrics and Radiomics Features in Predicting Primary Gastric Lymphoma Diagnosis. Hematol. Oncol. 2024, 42, e3266. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Xin, B.; Sun, Y.; Wang, X.; Song, S. Preoperative 18F-FDG PET/CT Radiomics Analysis for Predicting HER2 Expression and Prognosis in Gastric Cancer. Quant. Imaging Med. Surg. 2023, 13, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Li, T.; Wang, J.; Zhang, Z.; Chen, X.; Zhang, J.; Zhao, X. Noninvasive Assessment of HER2 Expression Status in Gastric Cancer Using 18F-FDG Positron Emission Tomography/Computed Tomography-Based Radiomics: A Pilot Study. Cancer Biother. Radiopharm. 2024, 39, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Chen, W.; Ye, Y.; Yi, H.; Pang, W.; Long, B.; Wang, Y.; Ye, T.; Li, L. Prediction of HER2 Expression in Gastric Adenocarcinoma Based On Preoperative Noninvasive Multimodal 18F-FDG PET/CT Imaging. Acad. Radiol. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Li, J.; Zhang, H.; Yin, H.; Zhang, R.; Zhang, J.; Chen, X. Machine Learning Analysis for the Noninvasive Prediction of Lymphovascular Invasion in Gastric Cancer Using PET/CT and Enhanced CT-Based Radiomics and Clinical Variables. Abdom. Radiol. 2022, 47, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chu, W.; Li, M.; Xu, P.; Wang, M.; Peng, M.; Wang, K.; Zhang, L. Radiomics in Gastric Cancer: First Clinical Investigation to Predict Lymph Vascular Invasion and Survival Outcome Using 18F-FDG PET/CT Images. Front. Oncol. 2022, 12, 836098. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, C.; Guo, H.; Li, S.; You, Y.; Zheng, P.; Zhang, H.; Wang, H.; Bai, J. Non-Invasive Measurement of Tumor Immune Microenvironment and Prediction of Survival and Chemotherapeutic Benefits from 18F Fluorodeoxyglucose PET/CT Images in Gastric Cancer. Front. Immunol. 2022, 13, 1019386. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Xue, B.; Bian, S.; Ji, X.; Lin, J.; Zheng, X.; Tang, K. A Radiomics Nomogram Based on 18 F-FDG PET/CT and Clinical Risk Factors for the Prediction of Peritoneal Metastasis in Gastric Cancer. Nucl. Med. Commun. 2023, 44, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Pullen, L.C.E.; Noortman, W.A.; Triemstra, L.; de Jongh, C.; Rademaker, F.J.; Spijkerman, R.; Kalisvaart, G.M.; Gertsen, E.C.; de Geus-Oei, L.F.; Tolboom, N.; et al. Prognostic Value of [18F]FDG PET Radiomics to Detect Peritoneal and Distant Metastases in Locally Advanced Gastric Cancer-A Side Study of the Prospective Multicentre PLASTIC Study. Cancers 2023, 15, 2874. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, G.M.; Zerunian, M.; Berardi, E.; Mainardi, F.; Pilozzi, E.; Polici, M.; Guido, G.; Rucci, C.; Polidori, T.; Tarallo, M.; et al. Perioperative Chemotherapy with FLOT Scheme in Resectable Gastric Adenocarcinoma: A Preliminary Correlation between TRG and Radiomics. Appl. Sci. 2021, 11, 9211. [Google Scholar] [CrossRef]
- Becker, K.; Mueller, J.D.; Schulmacher, C.; Ott, K.; Fink, U.; Busch, R.; Böttcher, K.; Siewert, J.R.; Höfler, H. Histomorphology and Grading of Regression in Gastric Carcinoma Treated with Neoadjuvant Chemotherapy. Cancer 2003, 98, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhong, J.; Wang, L.; Shi, X.; Lu, W.; Li, J.; Feng, J.; Xia, Y.; Chang, R.; Fan, J.; et al. Robustness of CT Radiomics Features: Consistency within and between Single-Energy CT and Dual-Energy CT. Eur. Radiol. 2022, 32, 5480–5490. [Google Scholar] [CrossRef] [PubMed]
- Leithner, D.; Nevin, R.B.; Gibbs, P.; Weber, M.; Otazo, R.; Vargas, H.A.; Mayerhoefer, M.E. ComBat Harmonization for MRI Radiomics: Impact on Nonbinary Tissue Classification by Machine Learning. Investig. Radiol. 2023, 58, 697–701. [Google Scholar] [CrossRef]

| PreCHT Radiomics Features | ROC Curve Analysis | |||||
|---|---|---|---|---|---|---|
| AUC | Sensibility | Specificity | 95%C.I. | p | ||
| Shape | LeastAxisLength | 0.815 | 88.89% | 66.67% | 0.53–0.96 | 0.011 |
| GLCM | Cluster Shade | 0.907 | 66.67% | 100% | 0.64–0.99 | <0.0001 |
| Autocorrelation | 0.907 | 88.89% | 83.33% | 0.64–0.99 | <0.0001 | |
| First order | Skewness | 0.889 | 88.89% | 83.33% | 0.62–0.99 | <0.0001 |
| NGTDM | Strength | 0.815 | 55.56% | 100% | 0.53–0.96 | 0.007 |
| ΔRadiomics Features | ||||||
| Shape | Mesh Volume | 0.889 | 66.67% | 100% | 0.62–0.99 | <0.0001 |
| LeastAxisLength | 0.833 | 77.78% | 100% | 0.55–0.97 | 0.0045 | |
| SurfaceVolume | 0.852 | 88.89% | 83.33% | 0.57–0.97 | 0.0021 | |
| GLRLM | LongRunEmphasis | 0.889 | 66.7% | 100% | 0.62–0.99 | <0.0001 |
| GLSZM | LargeAreaLowGrayLevelEmphasis | 0.833 | 100% | 66.67% | 0.55–0.97 | 0.007 |
| NGTDM | Contrast | 0.796 | 66.67% | 83.33% | 0.53–0.96 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garbarino, G.M.; Polici, M.; Caruso, D.; Laghi, A.; Mercantini, P.; Pilozzi, E.; van Berge Henegouwen, M.I.; Gisbertz, S.S.; van Grieken, N.C.T.; Berardi, E.; et al. Radiomics in Oesogastric Cancer: Staging and Prediction of Preoperative Treatment Response: A Narrative Review and the Results of Personal Experience. Cancers 2024, 16, 2664. https://doi.org/10.3390/cancers16152664
Garbarino GM, Polici M, Caruso D, Laghi A, Mercantini P, Pilozzi E, van Berge Henegouwen MI, Gisbertz SS, van Grieken NCT, Berardi E, et al. Radiomics in Oesogastric Cancer: Staging and Prediction of Preoperative Treatment Response: A Narrative Review and the Results of Personal Experience. Cancers. 2024; 16(15):2664. https://doi.org/10.3390/cancers16152664
Chicago/Turabian StyleGarbarino, Giovanni Maria, Michela Polici, Damiano Caruso, Andrea Laghi, Paolo Mercantini, Emanuela Pilozzi, Mark I. van Berge Henegouwen, Suzanne S. Gisbertz, Nicole C. T. van Grieken, Eva Berardi, and et al. 2024. "Radiomics in Oesogastric Cancer: Staging and Prediction of Preoperative Treatment Response: A Narrative Review and the Results of Personal Experience" Cancers 16, no. 15: 2664. https://doi.org/10.3390/cancers16152664
APA StyleGarbarino, G. M., Polici, M., Caruso, D., Laghi, A., Mercantini, P., Pilozzi, E., van Berge Henegouwen, M. I., Gisbertz, S. S., van Grieken, N. C. T., Berardi, E., & Costa, G. (2024). Radiomics in Oesogastric Cancer: Staging and Prediction of Preoperative Treatment Response: A Narrative Review and the Results of Personal Experience. Cancers, 16(15), 2664. https://doi.org/10.3390/cancers16152664











