MRI Radiomics Data Analysis for Differentiation between Malignant Mixed Müllerian Tumors and Endometrial Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. MRI Techniques
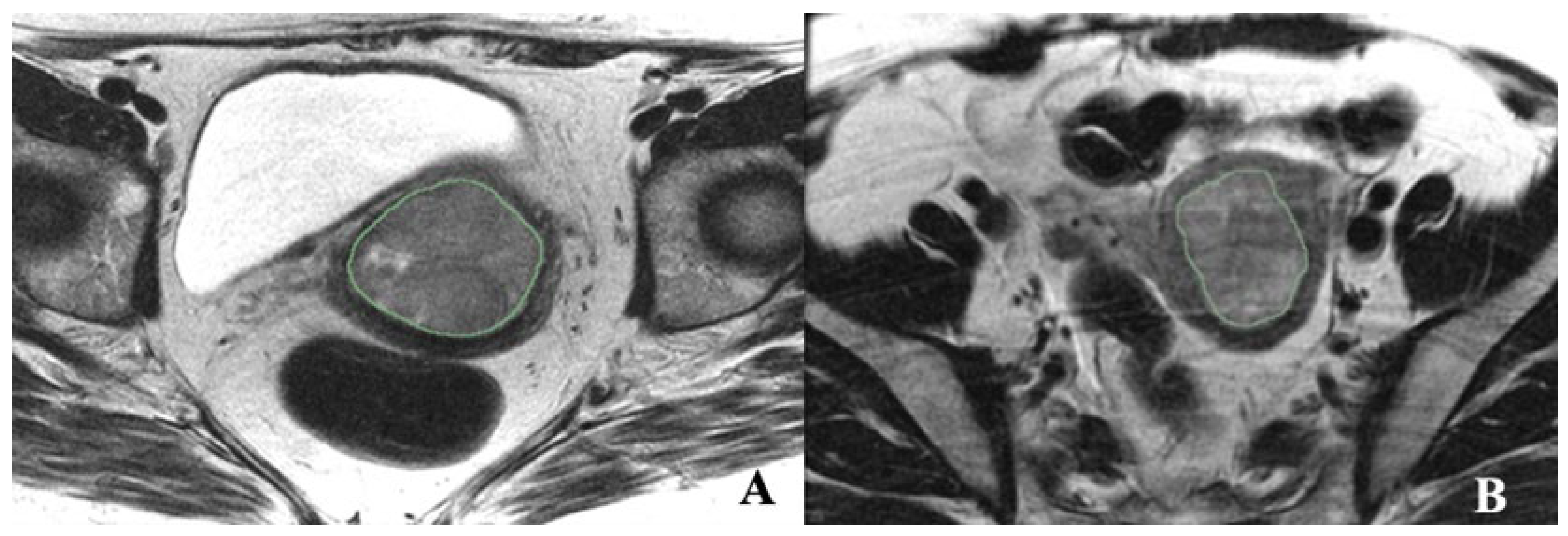
2.3. Tumor Segmentation and Radiomic Feature Extraction
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Descriptive Statistical Analysis
3.3. Univariate Analysis of AUROC for First-Order, Volume, and GLCM Features
3.4. Multivariate Analysis for First-Order, Volume, and GLCM Features at Baseline
- Skewness: A one-unit increase in skewness corresponds to a roughly 52.89% decrease in the odds of the outcome, indicating a link between lower skewness values and the outcome.
- Various features, such as the range of sum average, range of a sum of squares/variance, and range of sum entropy (8 bins), are associated with odds decreases of around 73.40%, 75.77%, and 79.07%, respectively.
- A higher volume leads to an approximately 82.37% decrease in the odds of the outcome.
- Angular variance of homogeneity (256 bins) is linked to an odds reduction of about 86.12% and angular variance of entropy (256 bins) to a decrease of about 91.08%.
- A higher average of homogeneity (256 bins) and an average of information measure of correlation (32 bins) are associated with odds decreases of approximately 92.78% and 95.49%, respectively.
- Similar odds reductions are seen for other features, such as the range of sum of squares/variance (16 bins) and average of cluster prominence (8 bins).
- Higher values of the average cluster shade (64 bins) and angular variance of the sum average (8 and 16 bins) correspond to significant odds decreases of up to 99.97%.
3.5. Overall Survival Prognosis
3.6. Multivariate Cox Regression
4. Discussion
5. Limitations
6. Future Directions and Clinical Implications
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makker, V.; MacKay, H.; Ray-Coquard, I.; Levine, D.A.; Westin, S.N.; Aoki, D.; Oaknin, A. Endometrial cancer. Nat. Rev. Dis. Primers 2021, 7, 88. [Google Scholar] [CrossRef]
- Mccluggage, W.G. Uterine carcinosarcomas (malignant mixed Mullerian tumors) are metaplastic carcinomas. Int. J. Gynecol. Cancer 2002, 12, 687. [Google Scholar] [CrossRef]
- Felix, A.S.; Stone, R.A.; Bowser, R.; Chivukula, M.; Edwards, R.P.; Weissfeld, J.L.; Linkov, F. Comparison of survival outcomes between patients with malignant mixed mullerian tumors and high-grade endometrioid, clear cell, and papillary serous endometrial cancers. Int. J. Gynecol. Cancer 2011, 21, 877–884. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garza, A.; Elsherif, S.B.; Faria, S.C.; Sagebiel, T.; Sun, J.; Ma, J.; Bhosale, P.R. Staging MRI of uterine malignant mixed Müllerian tumors versus endometrial carcinomas with emphasis on dynamic enhancement characteristics. Abdom. Radiol. 2020, 45, 1141–1154. [Google Scholar] [CrossRef]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef]
- Coppola, F.; Mottola, M.; Lo Monaco, S.; Cattabriga, A.; Cocozza, M.A.; Yuan, J.C.; De Benedittis, C.; Cuicchi, D.; Guido, A.; Rojas Llimpe, F.L.; et al. The Heterogeneity of Skewness in T2W-Based Radiomics Predicts the Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Diagnostics 2021, 11, 795. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Danala, G.; Thai, T.; Gunderson, C.C.; Moxley, K.M.; Moore, K.; Mannel, R.S.; Liu, H.; Zheng, B.; Qiu, Y. Applying Quantitative CT Image Feature Analysis to Predict Response of Ovarian Cancer Patients to Chemotherapy. Acad. Radiol. 2017, 24, 1233–1239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schick, U.; Lucia, F.; Dissaux, G.; Visvikis, D.; Badic, B.; Masson, I.; Pradier, O.; Bourbonne, V.; Hatt, M. MRI-derived radiomics: Methodology and clinical applications in the field of pelvic oncology. Br. J. Radiol. 2019, 92, 20190105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saida, T.; Mori, K.; Hoshiai, S.; Sakai, M.; Urushibara, A.; Ishiguro, T.; Satoh, T.; Nakajima, T. Differentiation of carcinosarcoma from endometrial carcinoma on magnetic resonance imaging using deep learning. Pol. J. Radiol. 2022, 87, 521–529. [Google Scholar] [CrossRef]
- Chen, T.; Li, Y.; Lu, S.S.; Zhang, Y.D.; Wang, X.N.; Luo, C.Y.; Shi, H.B. Quantitative evaluation of diffusion-kurtosis imaging for grading endometrial carcinoma: A comparative study with diffusion-weighted imaging. Clin. Radiol. 2017, 72, 995.e11–995.e20. [Google Scholar] [CrossRef]
- Kierans, A.S.; Doshi, A.M.; Dunst, D.; Popiolek, D.; Blank, S.V.; Rosenkrantz, A.B. Retrospective Assessment of Histogram-Based Diffusion Metrics for Differentiating Benign and Malignant Endometrial Lesions. J. Comput. Assist. Tomogr. 2016, 40, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Jia, H.; Zhang, Z.; Wei, C.; Wang, C.; Dong, J. The feasibility of combining ADC value with texture analysis of T2WI, DWI and CE-T1WI to preoperatively predict the expression levels of ki-67 and p53 of endometrial carcinoma. Front. Oncol. 2022, 11, 805545. [Google Scholar] [CrossRef] [PubMed]
- Song, J.C.; Lu, S.S.; Zhang, J.; Liu, X.S.; Luo, C.Y.; Chen, T. Quantitative assessment of diffusion kurtosis imaging depicting deep myometrial invasion: A comparative analysis with diffusion-weighted imaging. Diagn. Interv. Radiol. 2020, 26, 74–81. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamada, I.; Miyasaka, N.; Kobayashi, D.; Wakana, K.; Oshima, N.; Wakabayashi, A.; Sakamoto, J.; Saida, Y.; Tateishi, U.; Eishi, Y. Endometrial carcinoma: Texture analysis of apparent diffusion coefficient maps and its correlation with histopathologic findings and prognosis. Radiol. Imaging Cancer 2019, 1, e190054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, X.; Zhang, X.; Chen, S.; Song, Y.; Xie, L.; Chen, Y.; Ouyang, H. Whole-lesion apparent diffusion coefficient (ADC) histogram as a quantitative biomarker to preoperatively differentiate stage IA endometrial carcinoma from benign endometrial lesions. BMC Med. Imaging 2022, 22, 139. [Google Scholar] [CrossRef] [PubMed]
- Juan, M.W.; Yu, J.; Peng, G.X.; Jun, L.J.; Feng, S.P.; Fang, L.P. Correlation between DCE-MRI radiomics features and Ki-67 expression in invasive breast cancer. Oncol. Lett. 2018, 16, 5084–5090. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Ren, J.; Liu, A.; Gao, Y.; Hao, F.; Zhao, L.; Wu, H.; Niu, G. MR imaging of epithelial ovarian cancer: A combined model to predict histologic subtypes. Eur. Radiol. 2020, 30, 5815–5825. [Google Scholar] [CrossRef] [PubMed]
- Jajodia, A.; Gupta, A.; Prosch, H.; Mayerhoefer, M.; Mitra, S.; Pasricha, S.; Mehta, A.; Puri, S.; Chaturvedi, A. Combination of radiomics and machine learning with diffusion-weighted MR imaging for clinical outcome prognostication in cervical cancer. Tomography 2021, 7, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Yang, L.; Du, J.; Dong, Y.; Wu, S.; Shi, Q.; Wang, X.; Liu, L. Combination Analysis of a Radiomics-Based Predictive Model with Clinical Indicators for the Preoperative Assessment of Histological Grade in Endometrial Carcinoma. Front. Oncol. 2021, 11, 582495. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fasmer, K.E.; Hodneland, E.; Dybvik, J.A.; Wagner-Larsen, K.; Trovik, J.; Salvesen, Ø.; Krakstad, C.; Haldorsen, I.H. Whole-volume tumor MRI radiomics for prognostic modeling in endometrial cancer. J. Magn. Reson. Imaging 2021, 53, 928–937. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Wang, T.; Song, Y.; Yu, X.; Xie, L.; Chen, Y.; Ouyang, H. Multimodal MRI-Based Radiomics-Clinical Model for Preoperatively Differentiating Concurrent Endometrial Carcinoma from Atypical Endometrial Hyperplasia. Front. Oncol. 2022, 12, 887546. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kakkar, C.; Gupta, K.; Jain, K.; Narang, V.; Singh, A.; Saggar, K.; Bansal, N.; Cioni, D.; Neri, E. Diagnostic Accuracy of Calculated Tumor Volumes and Apparent Diffusion Coefficient Values in Predicting Endometrial Cancer Grade. Int. J. Appl. Basic. Med. Res. 2022, 12, 37–42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nougaret, S.; Reinhold, C.; Alsharif, S.S.; Addley, H.; Arceneau, J.; Molinari, N.; Guiu, B.; Sala, E. Endometrial Cancer: Combined MR Volumetry and Diffusion-Weighted Imaging for Assessment of Myometrial and Lymphovascular Invasion and Tumor Grade. Radiology 2015, 276, 797–808. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jin, X.; Shen, C.; Yang, X.; Yu, Y.; Wang, J.; Che, X. Association of Tumor Size with Myometrial Invasion, Lymphovascular Space Invasion, Lymph Node Metastasis, and Recurrence in Endometrial Cancer: A Meta-Analysis of 40 Studies with 53,276 Patients. Front. Oncol. 2022, 12, 881850. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Nakayama, K.; Ishikawa, N.; Minamoto, T.; Ishibashi, T.; Ohnishi, K.; Yamashita, H.; Ono, R.; Sasamori, H.; Razia, S.; et al. Preoperative tumor size is associated with deep myometrial invasion and lymph node metastases and is a negative prognostic indicator for patients with endometrial carcinoma. Oncotarget 2018, 9, 23164–23172. [Google Scholar] [CrossRef] [PubMed]
- López-González, E.; Rodriguez-Jiménez, A.; Gómez-Salgado, J.; Daza-Manzano, C.; Rojas-Luna, J.A.; Alvarez, R.M. Role of tumor volume in endometrial cancer: An imaging analysis and prognosis significance. Int. J. Gynaecol. Obs. 2023, 163, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, D.K.; Xi, Y.; Kapur, P.; Madhuranthakam, A.J.; Lewis, M.A.; Udayakumar, D.; Rasmussen, R.; Yuan, Q.; Bagrodia, A.; Margulis, V.; et al. Magnetic Resonance Imaging Radiomics Analyses for Prediction of High-Grade Histology and Necrosis in Clear Cell Renal Cell Carcinoma: Preliminary Experience. Clin. Genitourin. Cancer 2021, 19, 12–21.e1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, J.; Jain, R.; Khalil, K.; Griffith, B.; Bosca, R.; Rao, G.; Rao, A. Texture feature ratios from relative CBV maps of perfusion MRI are associated with patient survival in glioblastoma. Am. J. Neuroradiol. 2016, 37, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Gong, X.J.; Ge, Y.Q.; Zhao, H.; Wang, L.S.; Yu, H.Z.; Liu, B. Use of Texture Analysis on Noncontrast MRI in Classification of Early Stage of Liver Fibrosis. Can. J. Gastroenterol. Hepatol. 2021, 2021, 6677821. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, C.; Cigarroa, N.; Surabhi, V.; Ganeshan, B.; Pillai, A.K. Retrospective CT/MRI Texture Analysis of Rapidly Progressive Hepatocellular Carcinoma. J. Pers. Med. 2020, 10, 136. [Google Scholar] [CrossRef]
- Bhatnagar, G.; Makanyanga, J.; Ganeshan, B.; Groves, A.; Rodriguez-Justo, M.; Halligan, S.; Taylor, S.A. MRI texture analysis parameters of contrast-enhanced T1-weighted images of Crohn’s disease differ according to the presence or absence of histological markers of hypoxia and angiogenesis. Abdom. Radiol. 2016, 41, 1261–1269. [Google Scholar] [CrossRef] [PubMed]

| EC (N = 36) | MMMT (N = 25) | Total (N = 61) | p Value | |
|---|---|---|---|---|
| Minimum | 0.348 | |||
| N | 36 | 25 | 61 | |
| Mean (SD) | 131.14 (167.63) | 79.90 (64.03) | 110.14 (136.66) | |
| Median (Range) | 69.00 (4.00, 872.00) | 64.00 (9.00, 246.00) | 67.00 (4.00, 872.00) | |
| Maximum | 0.628 | |||
| N | 36 | 25 | 61 | |
| Mean (SD) | 411.81 (283.91) | 402.52 (276.26) | 408.00 (278.51) | |
| Median (Range) | 313.50 (115.00, 1496.00) | 291.00 (112.00, 1077.00) | 311.00 (112.00, 1496.00) | |
| Mean | 0.918 | |||
| N | 36 | 25 | 61 | |
| Mean (SD) | 244.21 (206.65) | 225.47 (152.38) | 236.53 (185.16) | |
| Median (Range) | 167.33 (58.99, 1075.43) | 179.69 (55.22, 579.68) | 173.40 (55.22, 1075.43) | |
| Standard Deviation | 0.587 | |||
| N | 36 | 25 | 61 | |
| Mean (SD) | 45.14 (30.75) | 46.46 (38.07) | 45.68 (33.64) | |
| Median (Range) | 35.93 (10.66, 159.46) | 27.84 (12.94, 129.67) | 35.40 (10.66, 159.46) | |
| Percentile 1 | 0.730 | |||
| N | 36 | 25 | 61 | |
| Mean (SD) | 159.11 (171.05) | 125.02 (84.76) | 145.14 (142.22) | |
| Median (Range) | 96.38 (30.24, 915.58) | 109.12 (30.00, 306.55) | 106.00 (30.00, 915.58) | |
| Percentile 5 | 0.849 | |||
| N | 36 | 25 | 61 | |
| Mean (SD) | 178.66 (174.55) | 152.41 (100.83) | 167.90 (148.35) | |
| Median (Range) | 124.50 (42.00, 938.00) | 128.00 (36.00, 374.92) | 126.00 (36.00, 938.00) | |
| Percentile 95 | 0.730 | |||
| N | 36 | 25 | 61 | |
| Mean (SD) | 323.19 (247.80) | 304.04 (212.38) | 315.34 (232.27) | |
| Median (Range) | 253.40 (77.00, 1251.20) | 226.25 (78.00, 771.86) | 253.00 (77.00, 1251.20) | |
| Percentile 99 | 0.603 | |||
| N | 36 | 25 | 61 | |
| Mean (SD) | 364.11 (265.18) | 341.80 (241.91) | 354.97 (254.07) | |
| Median (Range) | 280.74 (87.00, 1342.26) | 242.25 (90.00, 916.29) | 277.40 (87.00, 1342.26) | |
| Skewness | 0.045 | |||
| N | 36 | 25 | 61 | |
| Mean (SD) | 0.49 (0.57) | 0.22 (0.47) | 0.38 (0.55) | |
| Median (Range) | 0.55 (−1.07, 2.18) | 0.27 (−1.00, 0.88) | 0.45 (−1.07, 2.18) | |
| Kurtosis | 0.557 | |||
| N | 36 | 25 | 61 | |
| Mean (SD) | 3.81 (1.41) | 3.55 (0.95) | 3.71 (1.24) | |
| Median (Range) | 3.58 (1.90, 9.24) | 3.42 (2.30, 6.92) | 3.45 (1.90, 9.24) | |
| Volume | 0.007 | |||
| N | 36 | 25 | 61 | |
| Mean (SD) | 5541.92 (6532.11) | 13,646.22 (16,182.72) | 8863.35 (12,074.47) | |
| Median (Range) | 3215.29 (280.96, 31,591.33) | 7011.87 (691.41, 64,373.78) | 5603.25 (280.96, 64,373.78) |
| Feature | Coefficient |
|---|---|
| Skewness | 0.47 |
| 8 Range of Sum average | 0.27 |
| 8 Range of Sum of squares Variance | 0.24 |
| 8 Range of Sum entropy | 0.21 |
| Volume | 0.18 |
| 16 Range of Sum average | 0.16 |
| 256 Angular Variance of Homogeneity | 0.14 |
| 256 Angular Variance of Entropy | 0.089 |
| 256 Average of Homogeneity | 0.072 |
| 32 Average of Information measure of correlation 1 | 0.045 |
| 16 Range of Sum of squares Variance | 0.040 |
| 8 Average of Cluster Prominence | 0.0055 |
| 8 Angular Variance of Sum average | 0.0033 |
| 64 Average of Cluster Shade | 0.00080 |
| 16 Angular Variance of Sum average | 0.00030 |
| Time (Years) | EC | MMMT | ||||
|---|---|---|---|---|---|---|
| Survival (%) | L CI (%) | U CI (%) | Survival (%) | L CI (%) | U CI (%) | |
| 2 | 94.3% | 86.9% | 100% | 70.1% | 53.7% | 91.5% |
| 5 | 73.9% | 58.1% | 93.9% | 45.6% | 27.5% | 75.6% |
| 7 | 65.6% | 47.1% | 91.6% | 36.5% | 18.7% | 71.2% |
| HR | CI Lower HR | CI Upper HR | p Value | |
|---|---|---|---|---|
| 256 Angular Variance of Energy | 1.081 | 1.025 | 1.140 | 0.004 |
| Group (MMMT as reference) | 2.297 | 0.925 | 5.702 | 0.073 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virarkar, M.; Daoud, T.; Sun, J.; Montanarella, M.; Menendez-Santos, M.; Mahmoud, H.; Saleh, M.; Bhosale, P. MRI Radiomics Data Analysis for Differentiation between Malignant Mixed Müllerian Tumors and Endometrial Carcinoma. Cancers 2024, 16, 2647. https://doi.org/10.3390/cancers16152647
Virarkar M, Daoud T, Sun J, Montanarella M, Menendez-Santos M, Mahmoud H, Saleh M, Bhosale P. MRI Radiomics Data Analysis for Differentiation between Malignant Mixed Müllerian Tumors and Endometrial Carcinoma. Cancers. 2024; 16(15):2647. https://doi.org/10.3390/cancers16152647
Chicago/Turabian StyleVirarkar, Mayur, Taher Daoud, Jia Sun, Matthew Montanarella, Manuel Menendez-Santos, Hagar Mahmoud, Mohammed Saleh, and Priya Bhosale. 2024. "MRI Radiomics Data Analysis for Differentiation between Malignant Mixed Müllerian Tumors and Endometrial Carcinoma" Cancers 16, no. 15: 2647. https://doi.org/10.3390/cancers16152647
APA StyleVirarkar, M., Daoud, T., Sun, J., Montanarella, M., Menendez-Santos, M., Mahmoud, H., Saleh, M., & Bhosale, P. (2024). MRI Radiomics Data Analysis for Differentiation between Malignant Mixed Müllerian Tumors and Endometrial Carcinoma. Cancers, 16(15), 2647. https://doi.org/10.3390/cancers16152647






