Genetic Testing Uptake among Ovarian Cancer Survivors in the Genetic Risk Analysis in Ovarian Cancer (GRACE) Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Study Population
2.3. Chart Review
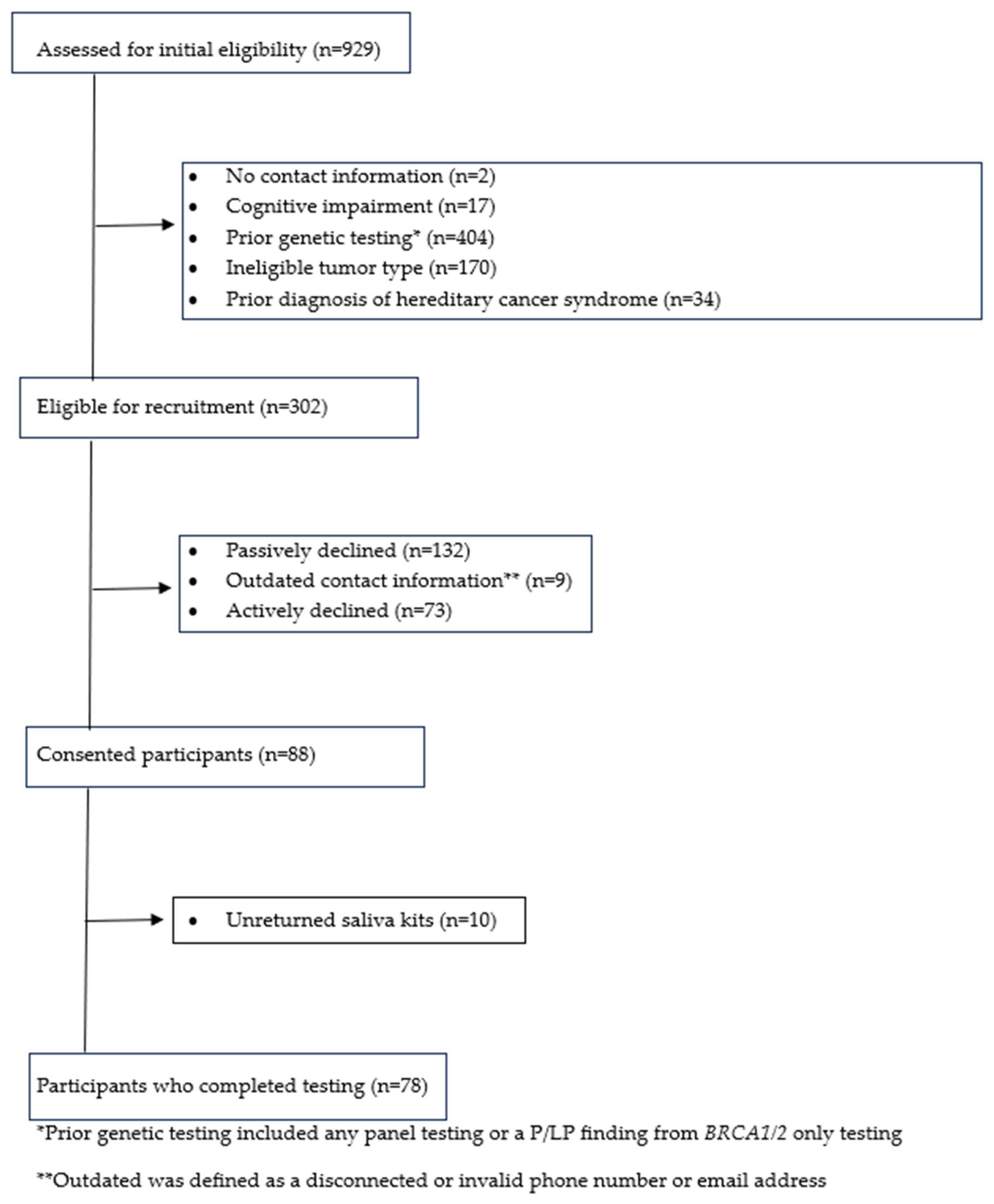
2.4. Recruitment Procedure
2.5. Genetic Testing
2.6. Results Disclosure
2.7. Data Analysis
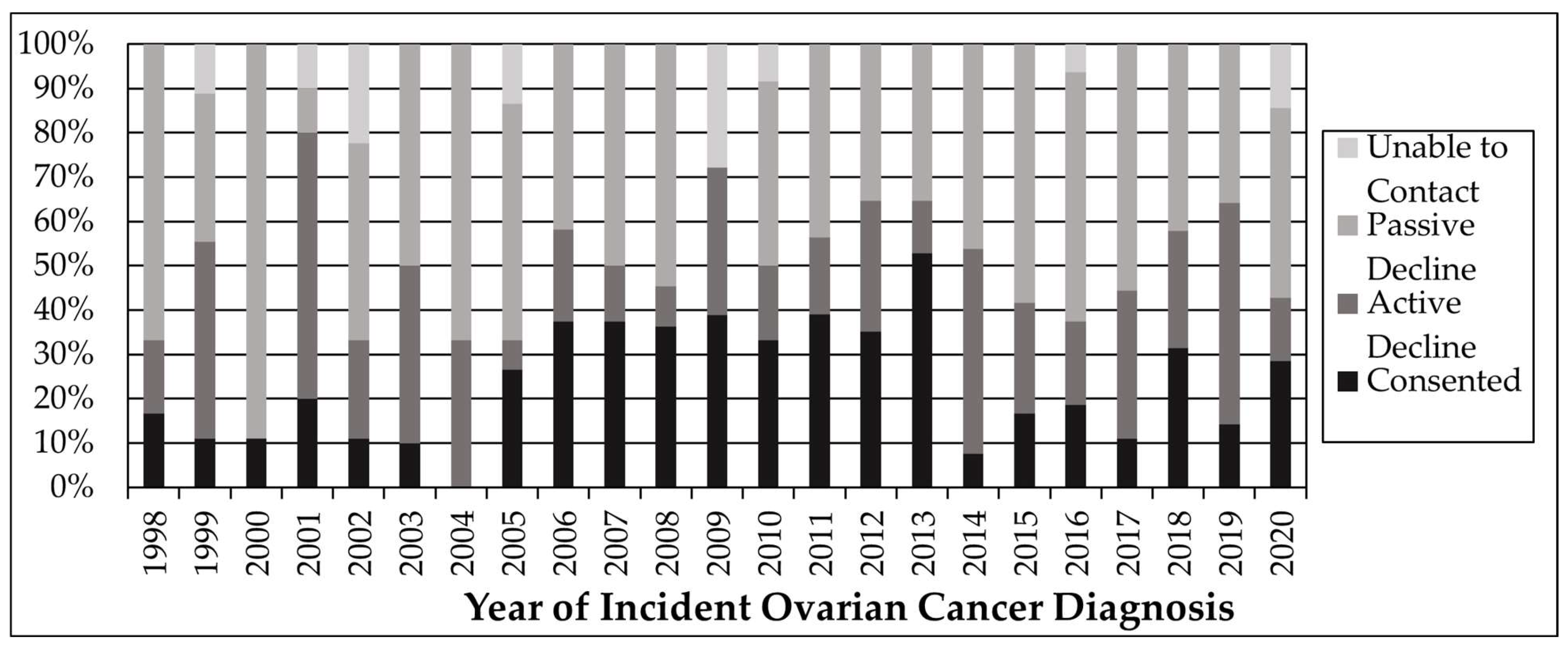
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Cancer Institute. Ovarian, Fallopian Tube, and Primary Peritoneal Cancer—Health Professional Version. Available online: https://www.cancer.gov/types/ovarian/hp (accessed on 15 May 2024).
- Zhang, S.; Royer, R.; Li, S.; McLaughlin, J.R.; Rosen, B.; Risch, H.A.; Fan, I.; Bradley, L.; Shaw, P.A.; Narod, S.A. Frequencies of BRCA1 and BRCA2 Mutations among 1342 Unselected Patients with Invasive Ovarian Cancer. Gynecol. Oncol. 2011, 121, 353–357. [Google Scholar] [CrossRef]
- American Cancer Society. Key Statistics for Ovarian Cancer. Available online: https://www.cancer.org/cancer/types/ovarian-cancer/about/key-statistics.html (accessed on 15 May 2024).
- Toss, A.; Tomasello, C.; Razzaboni, E.; Contu, G.; Grandi, G.; Cagnacci, A.; Schilder, R.J.; Cortesi, L. Hereditary ovarian cancer: Not only BRCA 1 and 2 genes. BioMed Res. Int. 2015, 2015, 341723. [Google Scholar] [CrossRef]
- National Cancer Institute. SEER Cancer Stat Facts: Ovarian Cancer. Available online: https://seer.cancer.gov/statfacts/html/ovary.html (accessed on 15 May 2024).
- Prat, J. Ovarian carcinomas: Five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012, 460, 237–249. [Google Scholar] [CrossRef]
- Doubeni, C.A.; Doubeni, A.R.; Myers, A.E. Diagnosis and Management of Ovarian Cancer. Am. Fam. Physician 2016, 93, 937–944. [Google Scholar]
- Fantone, S.; Piani, F.; Olivieri, F.; Rippo, M.R.; Sirico, A.; Di Simone, N.; Marzioni, D.; Tossetta, G. Role of SLC7A11/xCT in Ovarian Cancer. Int. J. Mol. Sci. 2024, 25, 587. [Google Scholar] [CrossRef]
- Samimi, G.; Bernardini, M.Q.; Brody, L.C.; Caga-Anan, C.F.; Campbell, I.G.; Chenevix-Trench, G.; Couch, F.J.; Dean, M.; de Hullu, J.A.; Domchek, S.M.; et al. Traceback: A Proposed Framework to Increase Identification and Genetic Counseling of BRCA1 and BRCA2 Mutation Carriers through Family-Based Outreach. J. Clin. Oncol. 2017, 35, 2329–2337. [Google Scholar] [CrossRef]
- Moss, H.A.; Samimi, G.; Havrilesky, L.J.; Sherman, M.E.; Myers, E.R. Estimating the Number of Potential Family Members Eligible for BRCA1 and BRCA2 Mutation Testing in a “Traceback” Approach. Genet. Epidemiol. 2018, 42, 117–122. [Google Scholar] [CrossRef]
- Kurian, A.W.; Ward, K.C.; Howlader, N.; Deapen, D.; Hamilton, A.S.; Mariotto, A.; Miller, D.; Penberthy, L.S.; Katz, S.J. Genetic Testing and Results in a Population-Based Cohort of Breast Cancer Patients and Ovarian Cancer Patients. J. Clin. Oncol. 2019, 37, 1305–1315. [Google Scholar] [CrossRef]
- Meyer, L.A.; Anderson, M.E.; Lacour, R.A.; Suri, A.; Daniels, M.S.; Urbauer, D.L.; Nogueras-Gonzalez, G.M.; Schmeler, K.M.; Gershenson, D.M.; Lu, K.H. Evaluating women with ovarian cancer for BRCA1 and BRCA2 mutations: Missed opportunities. Obstet. Gynecol. 2010, 115, 945–952. [Google Scholar] [CrossRef]
- Febbraro, T.; Robison, K.; Wilbur, J.S.; Laprise, J.; Bregar, A.; Lopes, V.; Legare, R.; Stuckey, A. Adherence patterns to National Comprehensive Cancer Network (NCCN) guidelines for referral to cancer genetic professionals. Gynecol. Oncol. 2015, 138, 109–114. [Google Scholar] [CrossRef]
- Daly, M.B.; Pilarski, R.; Yurgelun, M.B.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Garber, J.E.; et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 1.2020. J. Natl. Compr. Cancer Netw. JNCCN 2020, 18, 380–391. [Google Scholar] [CrossRef]
- Daly, M.B.; Pal, T.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Goggins, M.; Hutton, M.L.; et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2021, 19, 77–102. [Google Scholar] [CrossRef]
- Temkin, S.M.; Bergstrom, J.; Samimi, G.; Minasian, L. Ovarian Cancer Prevention in High-risk Women. Clin. Obstet. Gynecol. 2017, 60, 738–757. [Google Scholar] [CrossRef]
- Kauffman, T.L.; Prado, Y.K.; Reyes, A.A.; Zepp, J.M.; Sawyer, J.; White, L.L.; Martucci, J.; Salas, S.B.; Vertrees, S.; Rope, A.F.; et al. Feasibility of a Traceback Approach for Using Pathology Specimens to Facilitate Genetic Testing in the Genetic Risk Analysis in Ovarian Cancer (GRACE) Study Protocol. J. Pers. Med. 2021, 11, 1194. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Lawrence, C.E.; Dunkel, L.; McEver, M.; Israel, T.; Taylor, R.; Chiriboga, G.; Goins, K.V.; Rahn, E.J.; Mudano, A.S.; Roberson, E.D.; et al. A REDCap-based model for electronic consent (eConsent): Moving toward a more personalized consent. J. Clin. Transl. Sci. 2020, 4, 345–353. [Google Scholar] [CrossRef]
- Romagnoli, K.M.; Kulchak Rahm, A.; Jonas, M.C.; Schwiter, R.; Klinger, T.; Ladd, I.; Salvati, Z.; DiNucci, A.; Blasi, P.R.; Sheridan, L.; et al. Human-Centered Design Study to Inform Traceback Cascade Genetic Testing Programs at Three Integrated Health Systems. Public Health Genom. 2023, 26, 45–57. [Google Scholar] [CrossRef]
- McGee, J.; Peart, T.M.; Foley, N.; Bertrand, M.; Prefontaine, M.; Sugimoto, A.; Ettler, H.; Welch, S.; Panabaker, K. Direct Genetics Referral Pathway for High-Grade Serous Ovarian Cancer Patients: The “Opt-Out” Process. J. Oncol. 2019, 2019, 6029097. [Google Scholar] [CrossRef]
- Petzel, S.V.; Vogel, R.I.; McNiel, J.; Leininger, A.; Argenta, P.A.; Geller, M.A. Improving referral for genetic risk assessment in ovarian cancer using an electronic medical record system. Int. J. Gynecol. Cancer 2014, 24, 1003–1009. [Google Scholar] [CrossRef]
- Norquist, B.M.; Harrell, M.I.; Brady, M.F.; Walsh, T.; Lee, M.K.; Gulsuner, S.; Bernards, S.S.; Casadei, S.; Yi, Q.; Burger, R.A.; et al. Inherited Mutations in Women With Ovarian Carcinoma. JAMA Oncol. 2016, 2, 482–490. [Google Scholar] [CrossRef]
- Alsop, K.; Fereday, S.; Meldrum, C.; deFazio, A.; Emmanuel, C.; George, J.; Dobrovic, A.; Birrer, M.J.; Webb, P.M.; Stewart, C.; et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: A report from the Australian Ovarian Cancer Study Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 2654–2663. [Google Scholar] [CrossRef]
- Augustinsson, A.; Nilsson, M.P.; Ellberg, C.; Kristoffersson, U.; Olsson, H.; Ehrencrona, H. Genetic testing in women with early-onset breast cancer: A Traceback pilot study. Breast Cancer Res. Treat. 2021, 190, 307–315. [Google Scholar] [CrossRef]
- Randall, T.C.; Armstrong, K. Health care disparities in hereditary ovarian cancer: Are we reaching the underserved population? Curr. Treat. Options Oncol. 2016, 17, 39. [Google Scholar] [CrossRef]
- Sekine, M.; Isobe, M.; Nishino, K.; Adachi, S.; Suda, K.; Yoshihara, K. Challenges for clinical application of “TRACEBACK” study: Testing of historical Tubo-Ovarian cancer patients for hereditary risk genes. Ann. Transl. Med. 2023, 11, 295. [Google Scholar] [CrossRef]
| Gene List | |||||
|---|---|---|---|---|---|
| APC | CDH1 | KIT | NBN | PTCH1 | SDHD |
| ATM | CDK4 | MAX | NF1 | PTEN | SMAD4 |
| AXIN2 | CDKN2A | MEN1 | NF2 | RAD51C | SMARCA4 |
| BAP1 | CHEK2 | MET | NTHL1 | RAD51D | SMARCB1 |
| BARD1 | DICER1 | MITF | PALB2 | RB1 | STK11 |
| BMPR1A | EPCAM | MLH1 | PDGFRA | RET | TEME127 |
| BRCA1 | FH | MSH2 | PMS2 | SDHA | TP53 |
| BRCA2 | FLCN | MSH3 | POLD1 | SDHAF2 | TSC1 |
| BRIP1 | GREM1 | MSH6 | POLE | SDHB | TSC2 |
| CDC73 | HOXB13 | MUTYH | PRKAR1A | SDHC | VHL |
| Characteristic | Overall (n = 929) N (%) | Eligible (n = 302) N (%) | Consented (n = 88) N (%) | p * |
|---|---|---|---|---|
| Age at diagnosis | 0.43 | |||
| <18 | 10 (1%) | 1 (<1%) | 0 (0%) | |
| 18–44 | 216 (23%) | 60 (20%) | 20 (23%) | |
| 45–64 | 481 (52%) | 184 (61%) | 48 (54%) | |
| ≥65 | 222 (24%) | 57 (19%) | 20 (23%) | |
| Age at recruitment | 0.12 | |||
| <18 | 0 (0%) | 0 (0%) | 0 (0%) | |
| 18–44 | 87 (9%) | 19 (6%) | 9 (10%) | |
| 45–64 | 357 (39%) | 113 (38%) | 28 (32%) | |
| ≥65 | 485 (52%) | 170 (56%) | 51 (58%) | |
| Race | 0.69 | |||
| American Indian/Alaskan Native | 2 (<1%) | 1 (<1%) | 0 (0%) | |
| Native Hawaiian/Other Pacific Islander | 1 (<1%) | 0 (0%) | 0 (0%) | |
| Asian | 34 (4%) | 11 (4%) | 3 (3%) | |
| Black/African American | 25 (3%) | 14 (5%) | 4 (5%) | |
| White | 764 (82%) | 239 (79%) | 75 (85%) | |
| Multiple Races | 32 (3%) | 5 (2%) | 0 (0%) | |
| Other Race | 20 (2%) | 7 (2%) | 1 (1%) | |
| Unknown/Not Reported | 51 (6%) | 25 (8%) | 5 (6%) | |
| Hispanic/Latino | 0.58 | |||
| Yes | 89 (10%) | 29 (10%) | 6 (7%) | |
| No | 821 (88%) | 262 (87%) | 79 (90%) | |
| Unknown/Not Reported | 19 (2%) | 11 (3%) | 3 (3%) | |
| Stage at diagnosis | <0.0001 | |||
| 0 | 2 (<1%) | 0 (0%) | 0 (0%) | |
| 1 | 374 (40%) | 157 (52%) | 59 (67%) | |
| 2 | 83 (9%) | 37 (12%) | 5 (6%) | |
| 3 | 190 (20%) | 58 (19%) | 13 (15%) | |
| 4 | 71 (8%) | 11 (4%) | 0 (0%) | |
| Unknown/Unstaged | 209 (23%) | 39 (13%) | 11 (12%) |
| Contact Method * | Consented (Paper) | Consented (Electronic) | Did Not Consent | Total |
|---|---|---|---|---|
| Letter (row %) | 3 (10%) | 1 (3%) | 27 (87%) | 31 |
| Email (row %) | 0 (0%) | 12 (23%) | 40 (77%) | 52 |
| Phone (row %) | 0 (0%) | 0 (0%) | 1 (100%) | 1 |
| Letter + Email (row %) | 8 (4%) | 64 (29%) | 146 (67%) | 218 |
| Total (row %) | 11 (4%) | 77 (25%) | 214 (71%) | 302 |
| Gene | Variant | Reference Sequence c | Diagnosis Year | Diagnosis Age |
|---|---|---|---|---|
| ATM | p.M1? (c.3G>A) | NM_000051.4 | 2006 | 65 |
| APC a,b | p.I1307K (c.3920T>A) | NM_000038.6 | 2018 | 34 |
| BARD1 b | c.2300_2301delTG | NM_000465.2 | 2013 | 61 |
| BRCA1 | c.3756_3759delGTCT | NM_007294.4 | 2013 | 54 |
| BRCA1 | p.V1736A (c.5207T>C) | NM_007294.4 | 2006 | 48 |
| BRCA2 | c.5073dupA | NM_000059.4 | 2010 | 57 |
| BRCA2 | c.3847_3848delGT | NM_000059.4 | 2011 | 60 |
| CHEK2 | p.H143Y (c.427C>T) | NM_007194.4 | 2007 | 55 |
| DICER1 | p.R688* (c.2062C>T) | NM_177438.3 | 2013 | 23 |
| HOXB13 a,b | p.G84E (c.251G>A) | NM_006361.6 | 2007 | 47 |
| MITF a,b | p.E318K (c.952G>A) | NM_000248.3 | 2007 | 51 |
| MUTYH a,b | p.G396D (c.1187G>A) | NM_001128425.1 | 2006 | 49 |
| MUTYH a,b | p.G396D (c.1187G>A) | NM_001128425.1 | 2013 | 68 |
| PMS2 | c.1040delA | NM_000535.7 | 2013 | 52 |
| RAD51D | c.896_*505del761insT | NM_002878.4 | 2006 | 83 |
| SDHB a,b | p.L111V (c.331C>G) | NM_003000.3 | 2009 | 62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
White, L.L.; Sawyer, J.K.; Zepp, J.M.; Prado, Y.K.; Reyes, A.A.; Maiyani, M.; Shuster, E.; Zucker, R.; Henrikson, N.B.; Rope, A.F.; et al. Genetic Testing Uptake among Ovarian Cancer Survivors in the Genetic Risk Analysis in Ovarian Cancer (GRACE) Study. Cancers 2024, 16, 2563. https://doi.org/10.3390/cancers16142563
White LL, Sawyer JK, Zepp JM, Prado YK, Reyes AA, Maiyani M, Shuster E, Zucker R, Henrikson NB, Rope AF, et al. Genetic Testing Uptake among Ovarian Cancer Survivors in the Genetic Risk Analysis in Ovarian Cancer (GRACE) Study. Cancers. 2024; 16(14):2563. https://doi.org/10.3390/cancers16142563
Chicago/Turabian StyleWhite, Larissa L., Jennifer K. Sawyer, Jamilyn M. Zepp, Yolanda K. Prado, Ana A. Reyes, Mahesh Maiyani, Elizabeth Shuster, Rachel Zucker, Nora B. Henrikson, Alan F. Rope, and et al. 2024. "Genetic Testing Uptake among Ovarian Cancer Survivors in the Genetic Risk Analysis in Ovarian Cancer (GRACE) Study" Cancers 16, no. 14: 2563. https://doi.org/10.3390/cancers16142563
APA StyleWhite, L. L., Sawyer, J. K., Zepp, J. M., Prado, Y. K., Reyes, A. A., Maiyani, M., Shuster, E., Zucker, R., Henrikson, N. B., Rope, A. F., Weinmann, S., Feigelson, H. S., & Ezzell Hunter, J. (2024). Genetic Testing Uptake among Ovarian Cancer Survivors in the Genetic Risk Analysis in Ovarian Cancer (GRACE) Study. Cancers, 16(14), 2563. https://doi.org/10.3390/cancers16142563








