Serum NF-κB in Epstein–Barr Virus-Related Oropharyngeal Carcinoma Diagnostic Usability
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Study Groups
Principles of Selecting Patients for the Study Group
2.2. Description of the Methods Used
2.3. Statistical Analysis
2.4. Ethics
3. Results
3.1. Assessment of the Incidence of NF-κB in Patients with Oropharyngeal Cancer Compared to the Control Group
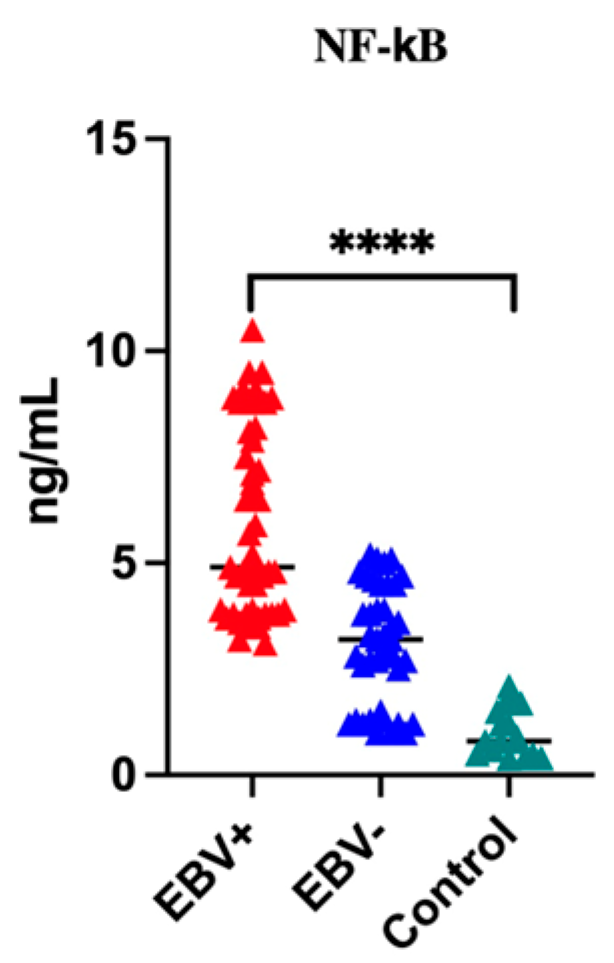
3.2. Assessment of Serum Level of NF-κB in Patients with Oropharyngeal Cancer Compared to the Control Group
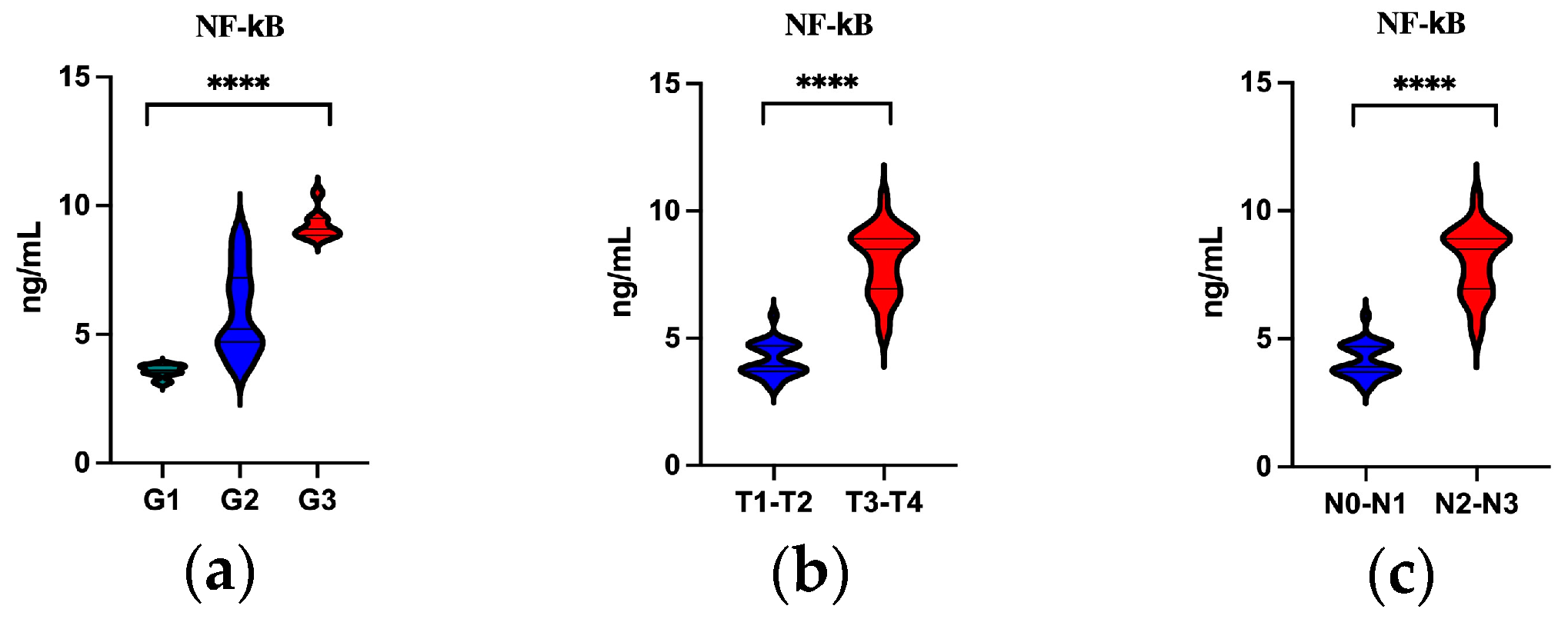
3.3. The Frequencies of NF-κB in OPSCC Patients by Grading (G)
3.4. The Frequencies of NF-κB by TNM Classification
3.5. Serum Level of NF-κB by Grading (G1–G3) and TN Classification
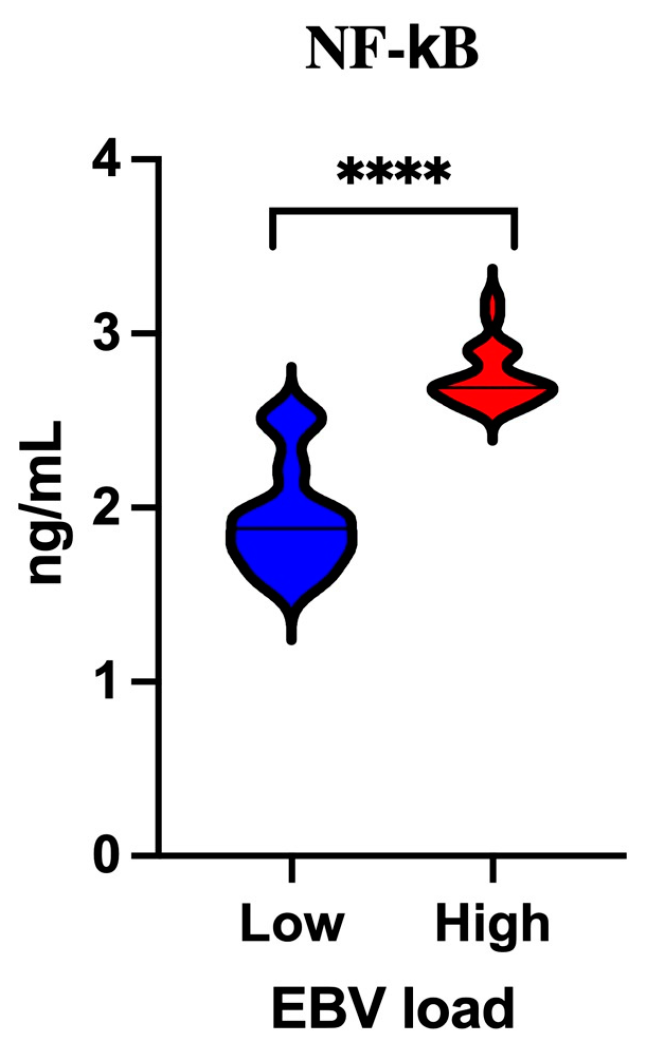
3.6. Analysis of the Serum Concentration of NF-κB Depending on the EBV Load
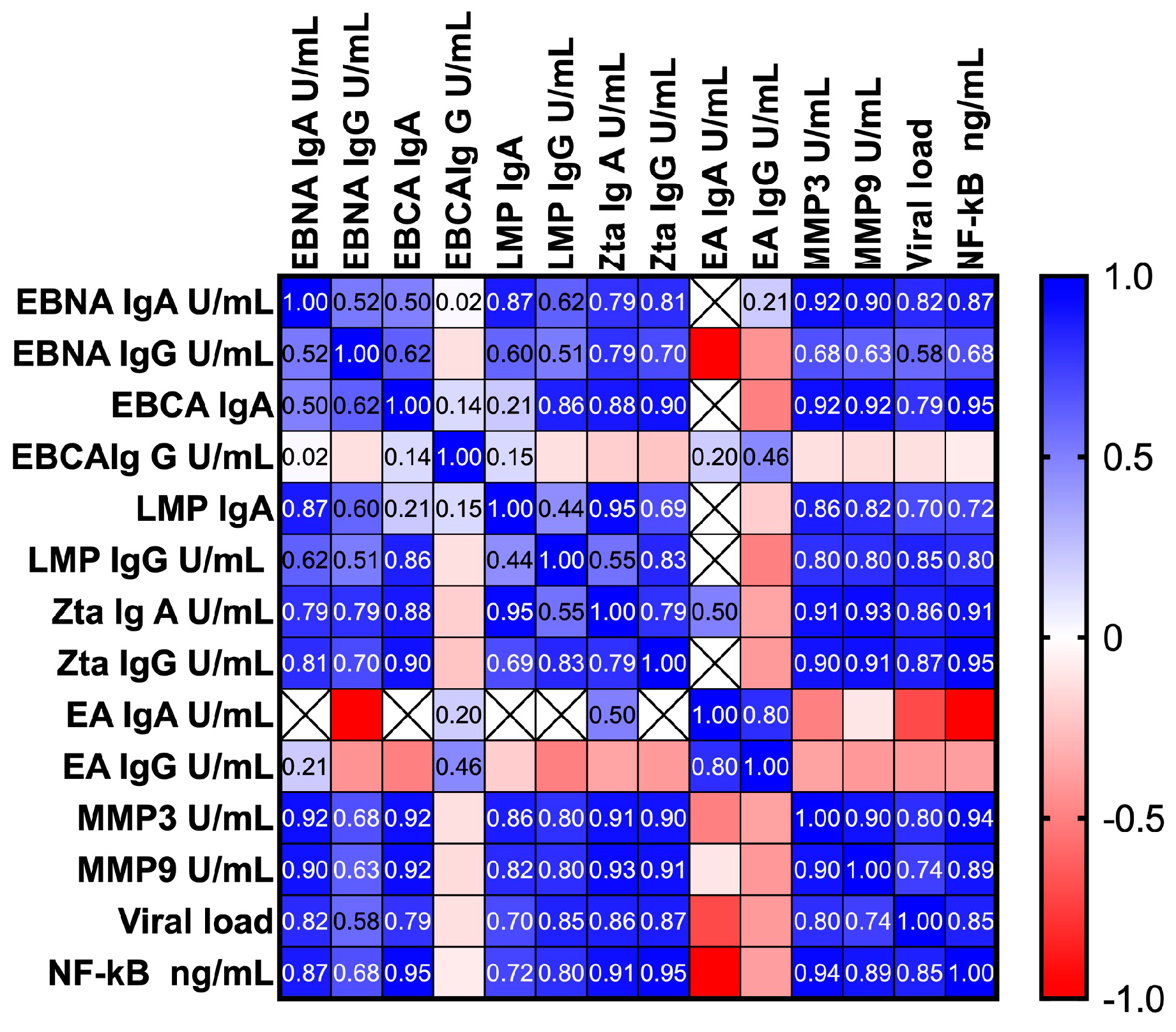
3.7. Correlation between the Serum Level of NF-κB, EBV Antibodies, MMP3, MMP9 and Viral Load
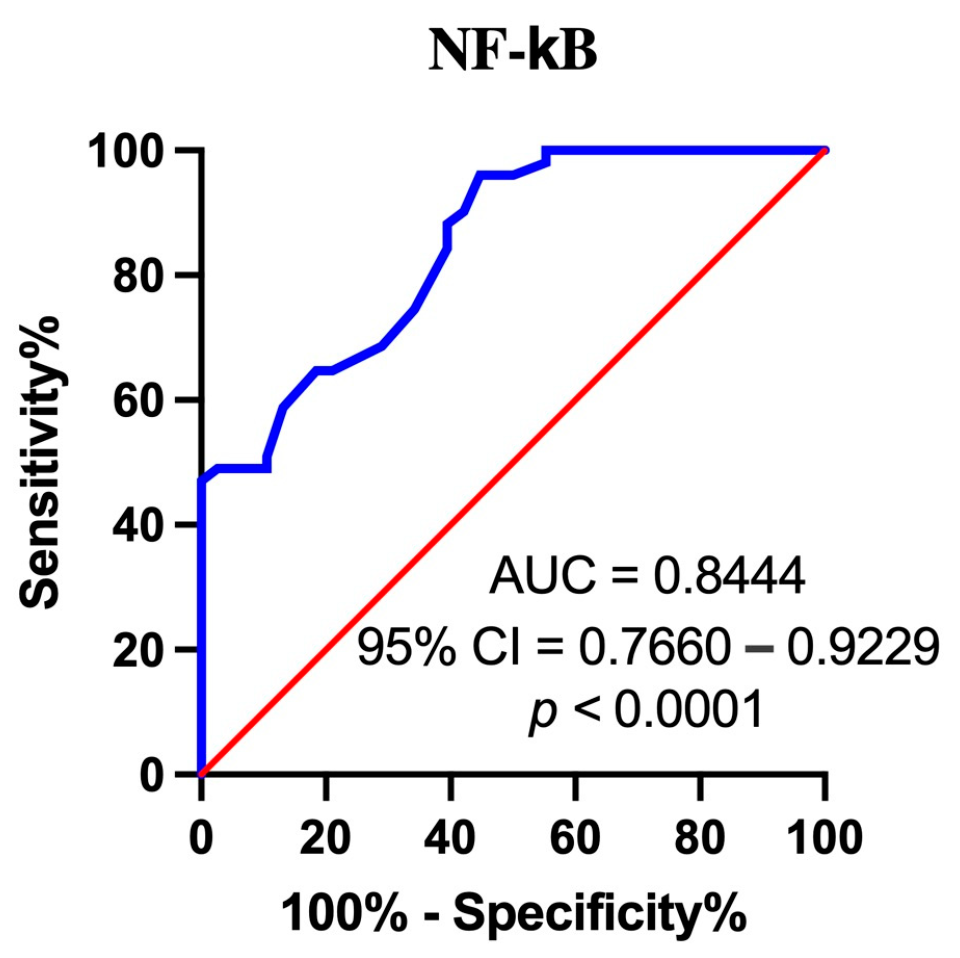
3.8. Assessment of the Diagnostic Accuracy of Serum NF-κB Measurements Using ROC Analysis (Receiver Operating Characteristic)
4. Discussion
5. Limitations of Own Research
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wojciechowska, U.; Didkowska, J. Polish National Cancer Registry. Available online: http://onkologia.org.pl/nowotwory-narzadow-glowy-i-szyi/ (accessed on 8 February 2024).
- Chen, C.J.; Hsu, W.L.; Yang, H.I.; Lee, M.H.; Chen, H.C.; Chien, Y.C.; You, S.L. Epidemiology of virus infection and human cancer. Recent Results Cancer Res. 2014, 193, 11–32. [Google Scholar] [PubMed]
- Carpén, T.; Syrjanen, S.; Jouhi, L.; Randen-Brady, R.; Haglund, C.; Mäkitie, A.; Mattila, P.S.; Hagström, J. Epstein-Barr Virus (EBV) and Polyomaviruses Are Detectable in Oropharyngeal Cancer and EBV May Have Prognostic Impact. Cancer Immunol. Immunother. 2020, 69, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M.A.; Achong, B.G.; Barr, Y.M. Virus particles in cultured lymphoblasts from Burkitt’s lymphoma. Lancet 1964, 1, 702–703. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, I.; Cohen, M.D. Epstein-Barr Virus Infection. N. Engl. J. Med. 2000, 343, 481–492. [Google Scholar]
- zur Hausen, H.; Schulte-Holthausen, H.; Klein, G.; Henle, W.; Clifford, P.; Santesson, L. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature 1970, 228, 1056–1058. [Google Scholar] [CrossRef] [PubMed]
- Current ICTV Taxonomy Release. Available online: https://ictv.global/taxonomy (accessed on 27 February 2024).
- Rivailler, P.; Cho, Y.G.; Wang, F. Complete genomic sequence of an Epstein-Barr virus-related herpesvirus naturally infecting a new world primate: A defining point in the evolution of oncogenic lymphocryptoviruses. J. Virol. 2002, 76, 12055–12068. [Google Scholar] [CrossRef]
- Damania, B.; Kenney, S.C.; Raab-Traub, N. Epstein-Barr virus: Biology and clinical disease. Cell 2022, 185, 3652–3670. [Google Scholar] [CrossRef] [PubMed]
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. A Review of Human Carcinogens. In Biological Agents; International Agency for Research on Cancer: Lyon, France, 2012; pp. 49–92.
- Shannon-Lowe, C.; Rickinson, A. The Global Landscape of EBV-Associated Tumors. Front. Oncol. 2019, 9, 713. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, Z.; Han, L.; Liu, S.; Luo, B. Epstein-Barr Virus Infection in Gastric Remnant Carcinoma and Recurrent Gastric Carcinoma in Qingdao of Northern China. PLoS ONE 2016, 11, e0148342. [Google Scholar] [CrossRef]
- Chen, X.Z.; Che, H.; Castro, F.H.; Hu, J.; Brenner, H. Epstein-Barr virus infection and gastric cancer: A systematic review. Medicine 2015, 94, e792. [Google Scholar] [CrossRef]
- Young, L.S.; Dawson, C.W. Epstein-Barr virus and nasopharyngeal carcinoma. Chin. J. Cancer 2014, 33, 581–590. [Google Scholar] [CrossRef]
- Tsao, S.-W.; Tsang, C.M.; To, K.-F.; Lo, K.-W. The role of Epstein-Barr virus in epithelial malignancies. J. Pathol. 2014, 235, 323–333. [Google Scholar] [CrossRef]
- Young, L.S.; Yap, L.F.; Murray, P.G. Epstein–Barr virus: More than 50 years old and still providing surprises. Nat. Rev. Cancer 2016, 16, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, S.; Yan, B.; Wang, C.; Wang, L.; Quaid, J.T.; Lin, C.F.; Briggs, S.D.; Majumder, J.; Canaria, D.A.; Chauss, D.; et al. Integrated Pan-Cancer Map of EBV-Associated Neoplasms Reveals Functional Host–Virus Interactions. Cancer Res. 2019, 79, 6010–6023. [Google Scholar] [CrossRef]
- He, Y.Q.; Xue, W.Q.; Xu, F.H.; Xu, Y.F.; Zhang, J.B.; Yu, H.L.; Feng, Q.S.; Chen, L.Z.; Cao, S.M.; Liu, Q.; et al. The Relationship Between Environmental Factors and the Profile of Epstein-Barr Virus Antibodies in the Lytic and Latent Infection Periods in Healthy Populations from Endemic and Non-Endemic Nasopharyngeal Carcinoma Areas in China. EBioMedicine 2018, 30, 184–191. [Google Scholar] [CrossRef]
- Globocan 2020. IARC WHO. Available online: https://gco.iarc.fr/today/data/factsheets/populations/616-poland-fact-sheets.pdf (accessed on 17 February 2024).
- Münz, C. Latency and lytic replication in Epstein–Barr virus-associated oncogenesis. Nat. Rev. Microbiol. 2019, 17, 691–700. [Google Scholar] [CrossRef]
- Kikuchi, K.; Noguchi, Y.; de Rivera, M.W.; Hoshino, M.; Sakashita, H.; Yamada, T.; Inoue, H.; Miyazaki, Y.; Nozaki, T.; González-López, B.S.; et al. Detection of Epstein-Barr virus genome and latent infection gene expression in normal epithelia, epithelial dysplasia, and squamous cell carcinoma of the oral cavity. Tumour Biol. 2016, 37, 3389–3404. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, T.; Sato, Y.; Ito, J.; Takaki, M.; Okuno, Y.; Yaguchi, M.; Al Masud, H.M.A.; Watanabe, T.; Sato, K.; Iwami, S.; et al. Direct Evidence of Abortive Lytic Infection-Mediated Establishment of Epstein-Barr Virus Latency During B-Cell Infection. Front. Microbiol. 2020, 11, 575255. [Google Scholar]
- Amon, W.; Farrell, P.J. Reactivation of Epstein-Barr virus from latency. Rev. Med. Virol. 2005, 15, 149–156. [Google Scholar] [CrossRef]
- Wang, L.; Ning, S. New Look of EBV LMP1 Signaling Landscape. Cancers 2021, 13, 5451. [Google Scholar] [CrossRef]
- Chen, Y.P.; Chan, A.T.C.; Le, Q.T.; Blanchard, P.; Sun, Y.; Ma, J. Nasopharyngeal carcinoma. Lancet 2019, 394, 64–80. [Google Scholar] [CrossRef]
- Cao, Y.; Xie, L.; Shi, F.; Tang, M.; Li, Y.; Zhao, L.; Yu, X.; Luo, X.; Liao, W.; Bode, A.M. Targeting the signaling in Epstein–Barr virus-associated diseases: Mechanism, regulation, and clinical study. Sig. Transduct. Target. Ther. 2021, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Abdulamir, A.S.; Hafidh, R.R.; Abu Bakar, F.; Abbas, K. Novel Epstein-Barr virus immunoglobulin G-based approach for the specific detection of nasopharyngeal carcinoma. Am. J. Otolaryngol. 2010, 31, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Linde, A. Diagnosis of Epstein-Barr virus-related diseases. Scand. J. Infect. Dis. 1996, 100, 83–88. [Google Scholar]
- Tay, J.K.; Chan, S.H.; Lim, C.M.; Siow, C.H.; Goh, H.L.; Loh, K.S. The role of Epstein-Barr virus DNA load and serology as screening tools for nasopharyngeal carcinoma. WJOHNS 2016, 155, 274–280. [Google Scholar] [CrossRef] [PubMed]
- De Paschale, M.; Clerici, P. Serological diagnosis of Epstein-Barr virus infection: Problems and solutions. World J. Virol. 2012, 1, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.P.; Hsu, C.L.; Chang, Y.L.; Tsang, N.M.; Chen, C.K.; Lee, T.J.; Tsao, K.C.; Huang, C.G.; Chang, Y.S.; Yu, J.S.; et al. Complementary serum test of antibodies to Epstein-Barr virus nuclear antigen-1 and early antigen: A possible alternative for primary screening of nasopharyngeal carcinoma. Oral. Oncol. 2008, 44, 784–792. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, L.G.; Wu, Y.C.; Huang, Y.S.; Huang, N.Q.; Li, J.Y.; Wang, Y.B.; Jiang, M.K.; Fang, Z.; Meng, N.N. Prospective studies on nasopharyngeal carcinoma in Epstein-Barr virus IgA/VCA antibody-positive persons in Wuzhou City. China. Int. J. Cancer 1985, 36, 545–547. [Google Scholar] [CrossRef]
- Rowe, M.; Rowe, D.T.; Gregory, C.D.; Young, L.S.; Farrell, P.J.; Rupani, H.; Rickinson, A.B. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt’s lymphoma cells. EMBO J. 1987, 6, 2743–2751. [Google Scholar] [CrossRef]
- Sen, R.; Baltimore, D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell 1986, 46, 705–716. [Google Scholar] [CrossRef]
- Baltimore, D. Discovering NF-kappaB. Cold Spring Harb. Perspect. Biol. 2009, 1, a000026. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Gosh, S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, 000034. [Google Scholar] [CrossRef]
- Zinatizadeh, M.R.; Schock, B.; Chalbatani, G.M.; Zarandi, P.K.; Jalali, S.A.; Miri, S.R. The Nuclear Factor Kappa B (NF-κB) signaling in cancer development and immune diseases. Genes Dis. 2021, 8, 287–297. [Google Scholar] [CrossRef]
- Baeuerle, P.A.; Baltimore, D. A 65-kD subunit of active NF-κB is required for inhibition of NF-κB by IκB. Genes Dev. 1989, 3, 1689–1698. [Google Scholar] [CrossRef]
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. Thirty years of NF-κB: A blossoming of relevance to human pathobiology. Cell 2017, 168, 37–57. [Google Scholar] [CrossRef]
- Hinz, M.; Scheidereit, C. The IκB kinase complex in NF-κB regulation and beyond. EMBO Rep. 2013, 15, 46–61. [Google Scholar] [CrossRef]
- Giuliani, C.; Bucci, I.; Napolitano, G. The Role of the Transcription Factor Nuclear Factor-kappa B in Thyroid Autoimmunity and Cancer. Front. Endocrinol. 2018, 9, 471. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Sig. Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, C.; Zhang, Z.; Zhang, H.; Hu, H. NF-κB signaling in inflammation and cancer. Med Commun. 2021, 2, 618–653. [Google Scholar] [CrossRef]
- Brücher, B.L.; Lang, F.; Jamall, I.S. NF-κB signaling and crosstalk during carcinogenesis. 4open 2019, 2, 13. [Google Scholar] [CrossRef]
- Charostad, J.; Nakhaie, M.; Dehghani, A.; Faghihloo, E. The interplay between EBV and KSHV viral products and NF-κB pathway in oncogenesis. Infect. Agents Cancer 2020, 15, 62. [Google Scholar] [CrossRef]
- Lo, A.K.; Lo, K.W.; Tsao, S.W.; Wong, H.L.; Hui, J.W.; To, K.F.; Hayward, D.S.; Chui, Y.L.; Lau, Y.L.; Takada, K.; et al. Epstein-Barr virus infection alters cellular signal cascades in human nasopharyngeal epithelial cells. Neoplasia 2006, 8, 173–180. [Google Scholar]
- Shi, W.; Bastianutto, C.; Li, A.; Perez-Ordonez, B.; Ng, R.; Chow, K.Y.; Zhang, W.; Jurisica, I.; Lo, K.W.; Bayley, A.; et al. Multiple dysregulated pathways in nasopharyngeal carcinoma revealed by gene expression profiling. Int. J. Cancer 2006, 119, 2467–2475. [Google Scholar] [CrossRef]
- Polz, A.; Morshed, K.; Drop, B.; Drop, A.; Polz-Dacewicz, M. Serum Anti-Zta and Anti-LMP1 Antibodies in Oropharyngeal Cancer Related to Epstein–Barr Virus—Diagnostic Usefulness. Cancers 2024, 16, 341. [Google Scholar] [CrossRef]
- Polz, A.; Morshed, K.; Drop, B.; Polz-Dacewicz, M. Could MMP3 and MMP9 Serve as Biomarkers in EBV-Related Oropharyngeal Cancer. Int. J. Mol. Sci. 2024, 25, 2561. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Machczyński, P.; Majchrzak, E.; Niewinski, P.; Marchlewska, J.; Golusiński, W. A review of the 8th edition of the AJCC staging system for oropharyngeal cancer according to HPV status. Eur. Arch. Otorhinolaryngol. 2020, 277, 2407–2412. [Google Scholar] [CrossRef]
- WHO Classification of Tumors Editorial Board. WHO Classification of Tumors Editorial Board. WHO classification of tumors series. In Head and Neck Tumors, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2022; Volume 9. [Google Scholar]
- Su, Z.Y.; Siak, P.Y.; Leong, C.O.; Cheah, S.C. The role of Epstein-Barr virus in nasopharyngeal carcinoma. Front. Microbiol. 2023, 14, 1116143. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.; Meehan, M.T.; Burrows, S.R.; Doolan, D.L.; Miles, J.J. Estimating the global burden of Epstein–Barr virus-related cancers. J. Cancer Res. Clin. Oncol. 2022, 148, 31–46. [Google Scholar] [CrossRef] [PubMed]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [PubMed]
- Meisner, A.; Parikh, C.R.; Kerr, K.F. Biomarker combinations for diagnosis and prognosis in multicenter studies: Principles and methods. Stat. Methods Med. Res. 2019, 28, 969–985. [Google Scholar] [CrossRef]
- Kandalai, S.; Li, H.; Zhang, N.; Peng, H.; Zheng, Q. The human microbiome and cancer: A diagnostic and therapeutic perspective. Cancer Biol. Ther. 2023, 24, 2240084. [Google Scholar] [CrossRef]
- Yang, L.; Li, A.; Wang, Y.; Zhang, Y. Intratumoral microbiota: Roles in cancer initiation, development and therapeutic efficacy. Signal. Transduct. Target. Ther. 2023, 8, 35. [Google Scholar] [CrossRef]
- Mundo, L.; Leoncini, L.; Accardi-Gheit, R. Epstein–Barr Virus Infection in Cancer. Cancers 2023, 15, 4659. [Google Scholar] [CrossRef]
- Sausen, D.G.; Bhutta, M.S.; Gallo, E.S.; Dahari, H.; Borenstein, R. Stress-Induced Epstein-Barr Virus Reactivation. Biomolecules 2021, 11, 1380. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhao, G.; Zhou, X.; Xuan, R.; Chen, Y.; Wang, Y. Clinicopathological analysis and prognostic significance of NF-κB p65 and IKKβ protein and mRNA expression in nasopharyngeal carcinoma. J. Inter. Med. Res. 2022, 50, 03000605211069195. [Google Scholar] [CrossRef]
- Sinha, S.; Dickey, B.L.; Coghill, A.E. Utility of Epstein-Barr virus (EBV) antibodies as screening markers for nasopharyngeal carcinoma: A narrative review. Ann. Nasopharynx Cancer 2022, 6, 6. [Google Scholar] [CrossRef]
- Liu, W.; Chen, G.; Gong, X.; Wang, Y.; Zheng, Y.; Liao, X.; Liao, W.; Song, L.; Xu, J.; Zhang, X. The diagnostic value of EBV-DNA and EBV-related antibodies detection for nasopharyngeal carcinoma: A meta-analysis. Cancer Cell Int. 2021, 21, 164. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Hu, J.; Luo, X.; Li, N.M.; Bode, A.; Cao, Y. Epstein-Barr virus lytic reactivation regulation and its pathogenic role in carcinogenesis. Int. J. Biol. Sci. 2016, 12, 1309–1318. [Google Scholar] [CrossRef]
- Morrison, T.E.; Kenney, S.C. BZLF1, an Epstein-Barr virus immediate-early protein, induces p65 nuclear translocation while inhibiting p65 transcriptional function. Virology 2004, 328, 219–232. [Google Scholar] [CrossRef]
- Wang, D.; Liebowitz, D.; Kieff, E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell 1985, 43, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.F.; Wang, H.; Lin, X.C.; Xiang, H.; Deng, X.Y.; Li, W.; Tang, M.; Cao, Y. NF-kappa B inhibitors induce lytic cytotoxicity in Epstein-Barr virus-positive nasopharyngeal carcinoma cells. Cell Biol. Int. 2008, 32, 1006–1013. [Google Scholar] [CrossRef]
- Walker, C.; Mojares, E.; del Río Hernández, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.C. Nasopharyngeal carcinoma: Molecular biomarker discovery and progress. Mol. Cancer. 2007, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Nagase, H. Progress in matrix metalloproteinase research. Mol. Asp. Med. 2008, 29, 290–308. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Baishya, N.; Sarma, A.; Kataki, A.C.; Rai, A.K.; Kalita, C.R. Assessment and clinicopathological correlation of matrix metalloproteinase 9 expression in nasopharyngeal carcinoma. J. Carcinog. 2019, 18, 1. [Google Scholar] [PubMed]
- Song, Z.; Wang, J.; Su, Q.; Luan, M.; Chen, X.; Xu, X. The role of MMP-2 and MMP-9 in the metastasis and development of hypopharyngeal carcinoma. Braz. J. Otorhinolaryngol. 2021, 87, 521–528. [Google Scholar]
- Liu, Z.; Li, L.; Yang, Z.; Luo, W.; Li, X.; Yang, H.; Yao, K.; Wu, B.; Fang, W. Increased expression of MMP9 is correlated with poor prognosis of nasopharyngeal carcinoma. BMC Cancer 2010, 10, 270. [Google Scholar] [CrossRef] [PubMed]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Chung, G.T.; Lui, V.W.; To, K.F.; Ma, B.B.; Chow, C.; Woo, J.K.; Yip, K.Y.; Seo, J.; Hui, E.P.; et al. Exome and genome sequencing of nasopharynx cancer identifies NF-κB pathway activating mutations. Nat. Commun. 2017, 8, 14121. [Google Scholar] [CrossRef]
- Bruce, J.P.; To, K.F.; Lui, V.W.Y.; Chung, G.T.Y.; Chan, Y.Y.; Tsang, C.M.; Yip, K.Y.; Ma, B.B.Y.; Woo, J.K.S.; Hui, E.P.; et al. Whole-genome profiling of nasopharyngeal carcinoma reveals viral-host co-operation in inflammatory NF-κB activation and immune escape. Nat. Commun. 2021, 12, 4193. [Google Scholar] [CrossRef] [PubMed]
- Chow, Y.P.; Tan, L.P.; Chai, S.J.; Abdul Aziz, N.; Choo, S.W.; Lim, P.V.H.; Pathmanathan, R.; Mohd Kornain, N.K.; Lum, C.L.; Pua, K.C.; et al. Exome Sequencing Identifies Potentially Druggable Mutations in Nasopharyngeal Carcinoma. Sci. Rep. 2017, 7, 42980. [Google Scholar] [CrossRef]
- Richardo, T.; Prattapong, P.; Ngernsombat, C.; Wisetyaningsih, N.; Iizasa, H.; Yoshiyama, H.; Janvilisri, T. Epstein-Barr Virus Mediated Signaling in Nasopharyngeal Carcinoma Carcinogenesis. Cancers 2020, 12, 2441. [Google Scholar] [CrossRef] [PubMed]
- Suryani, L.; Lee, H.P.Y.; Teo, W.K.; Chin, Z.K.; Loh, K.S.; Tay, J.K. Precision Medicine for Nasopharyngeal Cancer—A Review of Current Prognostic Strategies. Cancers 2024, 16, 918. [Google Scholar] [CrossRef] [PubMed]

| EBV | p | Total Patients | Control Group | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | ||||||||||
| N | % | N | % | N = 110 | % | N = 40 | % | ||||
| Sex | Female | 8 | 13.8 | 7 | 13.5 | 0.9999 | 15 | 13.8 | 6 | 15.0 | 0.7957 |
| Male | 50 | 86.2 | 45 | 86.5 | 95 | 86.2 | 34 | 85.0 | |||
| Age | 50–59 | 27 | 46.6 | 24 | 46.2 | 0.1116 | 59 | 53.4 | 21 | 52.5 | 0.9999 |
| 60–79 | 31 | 53.4 | 28 | 53.8 | 51 | 46.6 | 19 | 47.5 | |||
| Place of residence | Urban | 41 | 70.7 | 36 | 69.2 | 0.1667 | 77 | 70.7 | 28 | 70.0 | 0.9999 |
| Rural | 17 | 29.3 | 16 | 30.8 | 33 | 29.3 | 12 | 30.0 | |||
| Smoking | ≤10 >10 | 28 10 | 48.3 17.2 | 25 10 | 48.1 19.2 | 0.8427 | 53 20 | 48.3 18.2 | 16 10 | 40.0 25.0 | 0.9999 |
| No | 20 | 34.5 | 17 | 32.7 | 37 | 34.5 | 14 | 35.0 | |||
| Alcohol abuse | ≤10 >10 | 18 10 | 31.1 17.2 | 15 10 | 28.8 19.3 | 0.9834 | 53 | 48.3 | 19 | 47.5 | 0.9999 |
| No | 30 | 51.7 | 27 | 51.9 | 57 | 51.7 | 21 | 52.5 | |||
| G | G1 | 19 | 32.8 | 17 | 32.7 | 0.9997 | |||||
| G2 | 30 | 51.7 | 27 | 51.9 | |||||||
| G3 | 9 | 15.5 | 8 | 15.4 | |||||||
| T | T1 | 7 | 12.1 | 8 | 15.4 | 0.9505 | |||||
| T2 | 27 | 46.6 | 22 | 42.3 | |||||||
| T3 | 16 | 27.6 | 15 | 28.8 | |||||||
| T4 | 8 | 13.7 | 7 | 12.1 | |||||||
| N0 | 23 | 39.7 | 22 | 42.3 | |||||||
| N | N1 | 11 | 19.0 | 10 | 19.2 | 0.9844 | |||||
| N2 | 14 | 24.1 | 11 | 21.2 | |||||||
| N3 | 10 | 17.2 | 9 | 17.3 | |||||||
| M | M0 | 58 | 100.0 | 52 | 100.0 | ||||||
| EBV+ N = 58 N (%) | p-Value | EBV− N = 52 N (%) | Control Group N = 40 N (%) | p-Value | |
|---|---|---|---|---|---|
| NF-κB | 51 (87.9) | <0.0001 * | 28 (53.8) | 15 (37.5) | <0.0001 * |
| Group | Mean | Minimum | Maximum | SD | p-Value |
|---|---|---|---|---|---|
| EBV+ EBV− Control | 5.9 3.1 1.01 | 3.1 1.0 0.4 | 10.5 5.2 2.1 | 2.2 1.4 0.6 | <0.0001 * |
| Parameter (%) | G1 N = 19 N (%) | G2 N = 30 N (%) | G3 N = 9 N (%) | p-Value |
|---|---|---|---|---|
| NF-κB | 12 (63.2) | 30 (100.0) | 9 (100.0) | 0.0003 * |
| Parameter | T1–T2 N = 34 N (%) | T3–T4 N = 24 N (%) | p-Value |
| NF-κB | 27 (79.4) | 24 (100.0) | 0.0343 * |
| Parameter | N0–N1 N = 34 N (%) | N2–N3 N = 24 N (%) | p-Value |
| NF-κB | 31 (91.2) | 24 (100.0) | 0.2595 |
| Parameter | 95% CI of rs | p-Value |
|---|---|---|
| EBNA IgA U/mL | 0.6974–0.9509 | <0.0001 * |
| EBNA IgG U/mL | 0.4711–0.8150 | <0.0001 * |
| EBVCA IgA | 0.8671–0.9813 | 0.0001 * |
| EBVCA IgG U/mL | −0.3771–0.2295 | 0.5997 |
| LMP1 IgA U/mL | 0.3656–0.8907 | 0.0007 * |
| LMP1 IgG U/mL | 0.6133–0.8983 | <0.0001 * |
| Zta IgA U/mL | 0.8153–0.9603 | <0.0001 * |
| Zta IgG U/mL | 0.8957–0.9783 | <0.0001 * |
| EA IgA U/mL | 0.7982–0.1806 | 0.2333 |
| EA IgG U/mL | −0.7318–0.1449 | 0.1388 |
| MMP3 U/mL | 0.9018–0.9683 | <0.0001 * |
| MMP9 U/mL | 0.8128–0.9376 | <0.0001 * |
| Viral load | 0.7482–0.9140 | <0.0001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polz, A.; Morshed, K.; Drop, B.; Polz-Dacewicz, M. Serum NF-κB in Epstein–Barr Virus-Related Oropharyngeal Carcinoma Diagnostic Usability. Cancers 2024, 16, 2328. https://doi.org/10.3390/cancers16132328
Polz A, Morshed K, Drop B, Polz-Dacewicz M. Serum NF-κB in Epstein–Barr Virus-Related Oropharyngeal Carcinoma Diagnostic Usability. Cancers. 2024; 16(13):2328. https://doi.org/10.3390/cancers16132328
Chicago/Turabian StylePolz, Anna, Kamal Morshed, Bartłomiej Drop, and Małgorzata Polz-Dacewicz. 2024. "Serum NF-κB in Epstein–Barr Virus-Related Oropharyngeal Carcinoma Diagnostic Usability" Cancers 16, no. 13: 2328. https://doi.org/10.3390/cancers16132328
APA StylePolz, A., Morshed, K., Drop, B., & Polz-Dacewicz, M. (2024). Serum NF-κB in Epstein–Barr Virus-Related Oropharyngeal Carcinoma Diagnostic Usability. Cancers, 16(13), 2328. https://doi.org/10.3390/cancers16132328







