Distance of Biopsy-Confirmed High-Risk Breast Lesion from Concurrently Identified Breast Malignancy Associated with Risk of Carcinoma at the High-Risk Lesion Site
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Intraductal Papilloma
1.2. Lobular Hyperplasia
1.3. Flat Epithelial Atypia and Complex Sclerosing Lesion
1.4. Shift towards De-Escalation
2. Materials and Methods
2.1. Study Approval
2.2. Data Extraction
2.3. Statistical Analysis
3. Results
3.1. Cohort Description
3.2. MRI Features of High-Risk Lesions
3.3. Variables Utilized in Univariate Regression Models
3.4. Logistic Regression Model for Lesion Upgrade or Downgrade
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leithner, D.; Kaltenbach, B.; Hoedl, P.; Moebus, V.; Brandenbusch, V.; Falk, S.; Park, C.; Vogl, T.J.; Mueller-Schimpfle, M. Intraductal Papilloma without Atypia on Image-Guided Breast Biopsy: Upgrade Rates to Carcinoma at Surgical Excision. Breast Care 2018, 13, 364–368. [Google Scholar] [CrossRef]
- Nakhlis, F.; Harrison, B.T.; King, T.A. Non-classic LCIS versus Classic LCIS versus Atypical Hyperplasia: Should Management be the Same? Curr. Surg. Rep. 2018, 6, 1–11. [Google Scholar] [CrossRef]
- Rajan, S.; Sharma, N.; Dall, B.J.G.; Shaaban, A.M. What is the significance of flat epithelial atypia and what are the management implications? J. Clin. Pathol. 2011, 64, 1001–1004. [Google Scholar] [CrossRef]
- Kraft, E.; Limberg, J.N.; Dodelzon, K.; Newman, L.A.; Simmons, R.; Swistel, A.; Ginter, P.S.; Marti, J.L. Radial Scars and Complex Sclerosing Lesions of the Breast: Prevalence of Malignancy and Natural History under Active Surveillance. Ann. Surg. Oncol. 2021, 28, 5149–5155. [Google Scholar] [CrossRef]
- American Society of Breast Surgeons. Consensus Guideline on Concordance Assessment of Image-Guided Breast Biopsies and Management of Borderline or High-Risk Lesions; The American Society of Breast Surgeons: Columbia, MD, USA, 2016. [Google Scholar]
- Tierney, S.N. Intraductal Papillomas. Surg. Clin. N. Am. 2022, 102, 965–972. [Google Scholar] [CrossRef]
- Gulati, M.; Singla, V.; Singh, T.; Bal, A.; Irrinki, N.S. Nipple Discharge: When is it Worrisome? Curr. Probl. Diagn. Radiol. 2023, 52, 560–569. [Google Scholar] [CrossRef]
- Renshaw, A.A.; Derhagopian, R.P.; Tizol-Blanco, D.M.; Gould, E.W. Papillomas and atypical papillomas in breast core needle biopsy specimens: Risk of carcinoma in subsequent excision. Am. J. Clin. Pathol. 2004, 122, 217–221. [Google Scholar] [CrossRef]
- Agoff, S.N.; Lawton, T.J. Papillary lesions of the breast with and without atypical ductal hyperplasia: Can we accurately predict benign behavior from core needle biopsy? Am. J. Clin. Pathol. 2004, 122, 440–443. [Google Scholar] [CrossRef]
- Sohn, V.; Keylock, J.; Arthurs, Z.; Wilson, A.; Herbert, G.; Perry, J.; Eckert, M.; Smith, D.; Groo, S.; Brown, T. Breast Papillomas in the Era of Percutaneous Needle Biopsy. Ann. Surg. Oncol. 2007, 14, 2979–2984. [Google Scholar] [CrossRef]
- Nakhlis, F.; Baker, G.M.; Pilewskie, M.; Gelman, R.; Calvillo, K.Z.; Ludwig, K.; McAuliffe, P.F.; Willey, S.; Rosenberger, L.H.; Parker, C.; et al. The Incidence of Adjacent Synchronous Invasive Carcinoma and/or Ductal Carcinoma In Situ in Patients with Intraductal Papilloma without Atypia on Core Biopsy: Results from a Prospective Multi-Institutional Registry (TBCRC 034). Ann. Surg. Oncol. 2021, 28, 2573–2578. [Google Scholar] [CrossRef]
- Lee, S.-J.; Wahab, R.A.; Sobel, L.D.; Zhang, B.; Brown, A.L.; Lewis, K.; Vijapura, C.; Mahoney, M.C. Analysis of 612 Benign Papillomas Diagnosed at Core Biopsy: Rate of Upgrade to Malignancy, Factors Associated with Upgrade, and a Proposal for Selective Surgical Excision. Am. J. Roentgenol. 2021, 217, 1299–1311. [Google Scholar] [CrossRef]
- Choi, H.Y.; Kim, S.M.; Jang, M.; La Yun, B.; Kang, E.; Kim, E.-K.; Park, S.Y.; Kim, B.; Cho, N.; Moon, W.K. Benign Breast Papilloma without Atypia: Outcomes of Surgical Excision versus US-guided Directional Vacuum-assisted Removal or US Follow-up. Radiology 2019, 293, 72–80. [Google Scholar] [CrossRef]
- Simpson, P.T.; Gale, T.; Fulford, L.G.; Reis-Filho, J.S.; Lakhani, S.R. The diagnosis and management of pre-invasive breast disease: Pathology of atypical lobular hyperplasia and lobular carcinoma in situ. Breast Cancer Res. 2003, 5, 258–262. [Google Scholar] [CrossRef]
- Bowman, K.; Munoz, A.; Mahvi, D.M.; Breslin, T.M. Lobular Neoplasia Diagnosed at Core Biopsy Does Not Mandate Surgical Excision. J. Surg. Res. 2007, 142, 275–280. [Google Scholar] [CrossRef]
- Rendi, M.H.; Dintzis, S.M.; Lehman, C.D.; Calhoun, K.E.; Allison, K.H. Lobular In-Situ Neoplasia on Breast Core Needle Biopsy: Imaging Indication and Pathologic Extent Can Identify Which Patients Require Excisional Biopsy. Ann. Surg. Oncol. 2012, 19, 914–921. [Google Scholar] [CrossRef]
- Middleton, L.P.; Sneige, N.; Coyne, R.; Shen, Y.; Dong, W.; Dempsey, P.; Bevers, T.B. Most lobular carcinoma in situ and atypical lobular hyperplasia diagnosed on core needle biopsy can be managed clinically with radiologic follow-up in a multidisciplinary setting. Cancer Med. 2014, 3, 492–499. [Google Scholar] [CrossRef]
- Nakhlis, F.; Gilmore, L.; Gelman, R.; Bedrosian, I.; Ludwig, K.; Hwang, E.S.; Willey, S.; Hudis, C.; Iglehart, J.D.; Lawler, E.; et al. Incidence of Adjacent Synchronous Invasive Carcinoma and/or Ductal Carcinoma In-Situ in Patients with Lobular Neoplasia on Core Biopsy: Results from a Prospective Multi-Institutional Registry (TBCRC 020). Ann. Surg. Oncol. 2016, 23, 722–728. [Google Scholar] [CrossRef]
- Morrow, M.; Schnitt, S.J.; Norton, L. Current management of lesions associated with an increased risk of breast cancer. Nat. Rev. Clin. Oncol. 2015, 12, 227–238. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Venables, B.; Ripley, B. Modern Applied Statistics with S; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Ciocca, R.M.; Li, T.; Freedman, G.M.; Morrow, M. Presence of Lobular Carcinoma In Situ Does Not Increase Local Recurrence in Patients Treated with Breast-Conserving Therapy. Ann. Surg. Oncol. 2008, 15, 2263–2271. [Google Scholar] [CrossRef][Green Version]
- Sadek, B.T.; Shenouda, M.N.; Raad, R.F.A.; Niemierko, A.; Keruakous, A.R.; Goldberg, S.I.; Taghian, A.G. Risk of Local Failure in Breast Cancer Patients with Lobular Carcinoma In Situ at the Final Surgical Margins: Is Re-excision Necessary? Int. J. Radiat. Oncol. 2013, 87, 726–730. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Yang, Y.; Zeng, Y.; Deng, H.; Jia, H.; Feng, H.; Li, Y.; Song, E.; Liu, Q.; et al. Impact of atypical hyperplasia at margins of breast-conserving surgery on the recurrence of breast cancer. J. Cancer Res. Clin. Oncol. 2014, 140, 599–605. [Google Scholar] [CrossRef]
- Greene, T.; Tartter, P.I.; Smith, S.R.; Estabrook, A. The signficance of surgical margins for patients with atypical ductal hyperplasia. Am. J. Surg. 2006, 192, 499–501. [Google Scholar] [CrossRef]
- Baker, J.L.; Hasteh, F.; Blair, S.L. Atypical Ductal Hyperplasia at the Margin of Lumpectomy Performed for Early Stage Breast Cancer: Is there Enough Evidence to Formulate Guidelines? Int. J. Surg. Oncol. 2012, 2012, 297832. [Google Scholar] [CrossRef]
- Wang, L.-J.; Wu, P.; Li, X.-X.; Luo, R.; Wang, D.-B.; Guan, W.-B. Magnetic resonance imaging features for differentiating breast papilloma with high-risk or malignant lesions from benign papilloma: A retrospective study on 158 patients. World J. Surg. Oncol. 2018, 16, 234. [Google Scholar] [CrossRef]
- Li, X.; Sun, K.; Chai, W.; Zhu, H.; Yan, F. Role of breast MRI in predicting histologic upgrade risks in high-risk breast lesions: A review. Eur. J. Radiol. 2021, 142, 109855. [Google Scholar] [CrossRef]
- Bargallo, X.; Ubeda, B.; Ganau, S.; Gonzalez, B.; Macedo, M.; Alonso, I.; Oses, G.; Vidal, M.; Santamaria, G. Magnetic Resonance Imaging Assessment of Radial Scars/complex Sclerosing Lesions of the Breast. Curr. Med. Imaging 2022, 18, 242–248. [Google Scholar] [CrossRef]
- Strigel, R.M.; Eby, P.R.; DeMartini, W.B.; Gutierrez, R.L.; Allison, K.H.; Peacock, S.; Lehman, C.D. Frequency, Upgrade Rates, and Characteristics of High-Risk Lesions Initially Identified With Breast MRI. Am. J. Roentgenol. 2010, 195, 792–798. [Google Scholar] [CrossRef]
- Heller, S.L.; Moy, L. Imaging Features and Management of High-Risk Lesions on Contrast-Enhanced Dynamic Breast MRI. Am. J. Roentgenol. 2012, 198, 249–255. [Google Scholar] [CrossRef]
- Han, S.-H.; Kim, M.; Chung, Y.R.; La Yun, B.; Jang, M.; Kim, S.M.; Kang, E.; Kim, E.-K.; Park, S.Y. Benign Intraductal Papilloma without Atypia on Core Needle Biopsy Has a Low Rate of Upgrading to Malignancy after Excision. J. Breast Cancer 2018, 21, 80–86. [Google Scholar] [CrossRef]
- Onesti, J.K.; Mangus, B.E.; Helmer, S.D.; Osland, J.S. Breast cancer tumor size: Correlation between magnetic resonance imaging and pathology measurements. Am. J. Surg. 2008, 196, 844–848; discussion 849–850. [Google Scholar] [CrossRef]
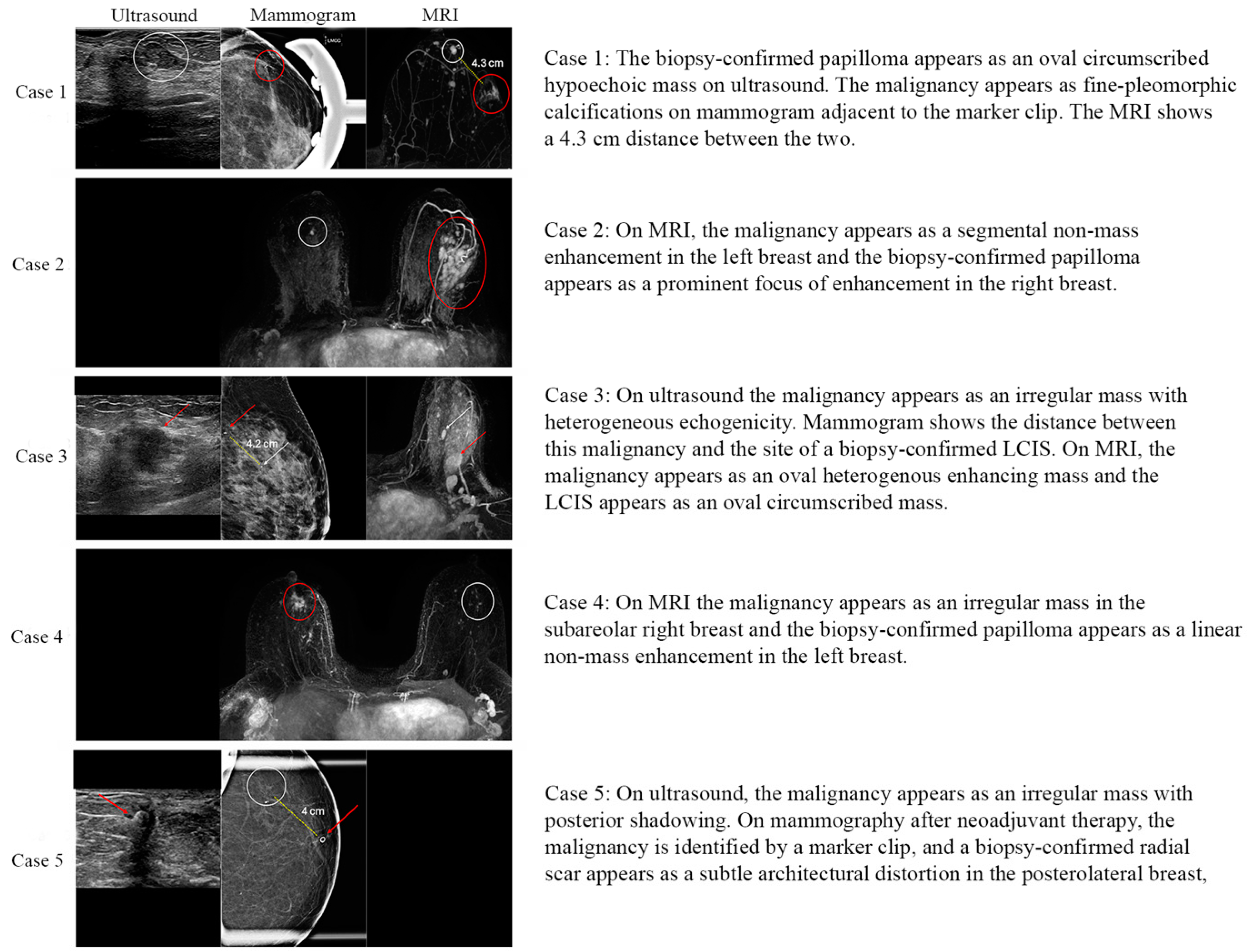
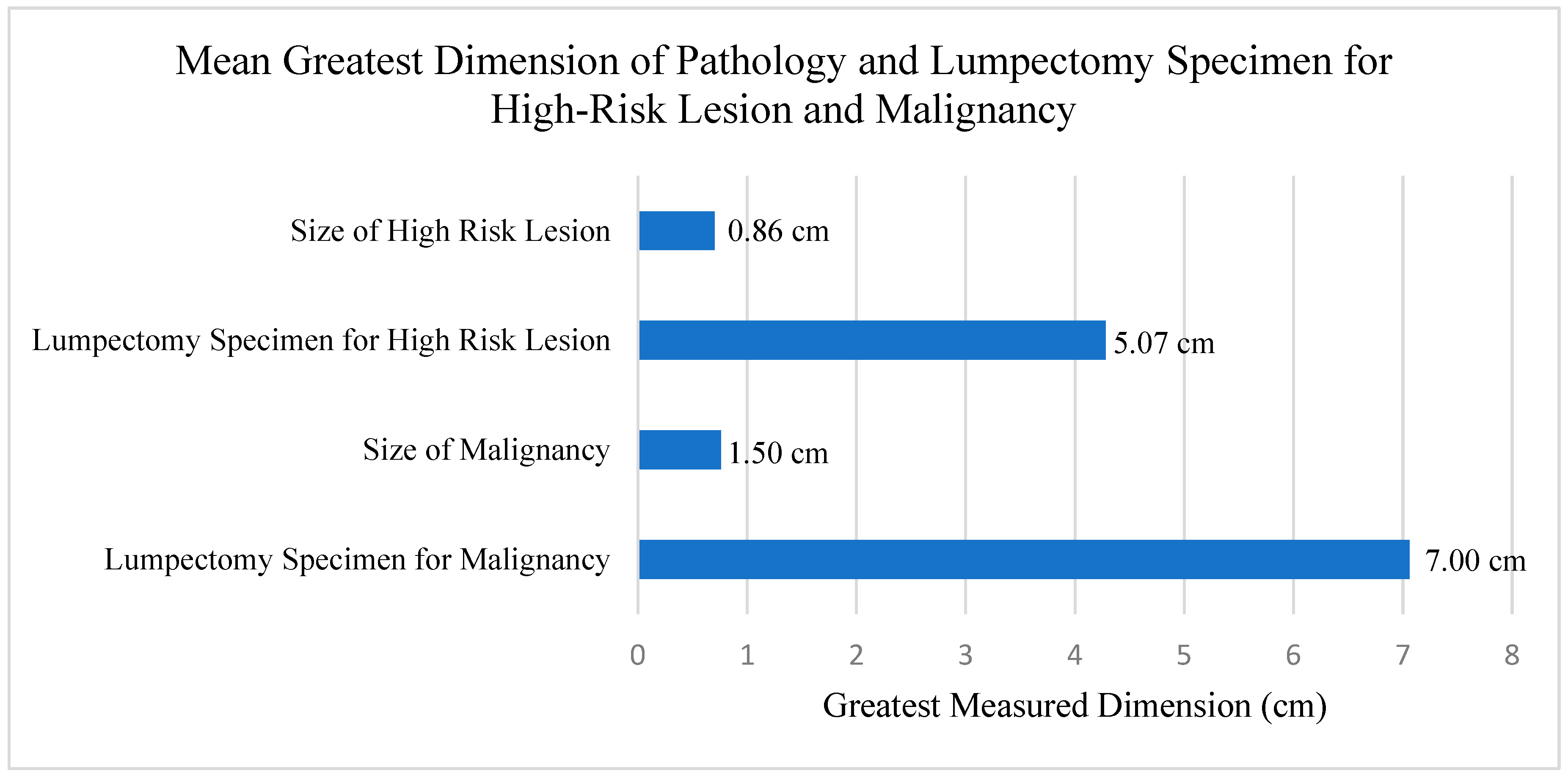
| Variable | Full (n = 65) | Upgrade (n = 5) | Downgrade (n = 18) | Persistent High-Risk Lesion (n = 42) | |
|---|---|---|---|---|---|
| Age (years) | ≤50 | 17 (26.2%) | 1 (20%) | 7 (38.9%) | 9 (21.4%) |
| 50–60 | 16 (24.6%) | 3 (60%) | 3 (16.7%) | 10 (23.8%) | |
| 60–65 | 15 (23.1%) | 1 (20%) | 4 (22.2%) | 10 (23.8%) | |
| >65 | 17 (26.2%) | 0 (0%) | 4 (22.2%) | 13 (31%) | |
| Race | Caucasian | 31 (47.7%) | 2 (40%) | 14 (77.8%) | 15 (35.7%) |
| African American | 3 (4.6%) | 0 (0%) | 0 (0%) | 3 (7.1%) | |
| Asian | 10 (15.4%) | 2 (40%) | 1 (5.6%) | 7 (16.7%) | |
| Hispanic | 14 (21.5%) | 1 (20%) | 2 (11.1%) | 11 (26.2%) | |
| Other | 7 (10.8%) | 0 (0%) | 1 (5.6%) | 6 (14.3%) | |
| Modality of carcinoma detection | Self-palpated | 12 (18.5%) | 1 (20%) | 5 (27.8%) | 6 (14.3%) |
| Mammogram | 44 (67.7%) | 4 (80%) | 9 (50%) | 31 (73.8%) | |
| Ultrasound | 7 (10.8%) | 0 (0%) | 3 (16.7%) | 4 (9.5%) | |
| MRI | 2 (3.1%) | 0 (0%) | 1 (5.6%) | 1 (2.4%) | |
| Imaging modality of high-risk lesion detection | MRI | 46 (70.8%) | 4 (80%) | 13 (72.2%) | 29 (69%) |
| Mammography | 9 (13.8%) | 1 (20%) | 1 (5.6%) | 7 (16.7%) | |
| Ultrasound | 10 (15.4%) | 0 (0%) | 4 (22.2%) | 6 (14.3%) | |
| Type of high-risk lesion | Papilloma | 38 (58.5%) | 3 (60%) | 8 (44.4%) | 27 (64.3%) |
| CSL | 17 (26.2%) | 1 (20%) | 7 (38.9%) | 9 (21.4%) | |
| LCIS, ALH or FEA | 10 (15.4%) | 1 (20%) | 3 (16.7%) | 6 (14.3%) | |
| Size of high-risk lesion on preoperative imaging (cm) | <0.75 | 25 (38.5%) | 0 (0%) | 9 (50%) | 16 (38.1%) |
| 0.75–1.5 | 20 (30.8%) | 2 (40%) | 5 (27.8%) | 13 (31%) | |
| >1.5 | 20 (30.8%) | 3 (60%) | 4 (22.2%) | 13 (31%) | |
| Quadrant location of high-risk lesion | Upper outer | 26 (40%) | 4 (80%) | 5 (27.8%) | 17 (40.5%) |
| Lower outer | 16 (24.6%) | 1 (20%) | 5 (27.8%) | 10 (23.8%) | |
| Central, subareolar or lower inner | 14 (21.5%) | 0 (0%) | 1 (5.6%) | 13 (31%) | |
| Upper inner | 9 (13.8%) | 0 (0%) | 7 (38.9%) | 2 (4.8%) | |
| Location of high-risk lesion relative to carcinoma | Contralateral or Ipsilateral >5 cm | 53 (81.5%) | 2 (40%) | 16 (88.9%) | 35 (83.3%) |
| Ipsilateral, ≤5 cm | 12 (18.5%) | 3 (60%) | 2 (11.1%) | 7 (16.7%) | |
| Carcinoma Type | Invasive ductal carcinoma | 42 (64.6%) | 3 (60%) | 14 (77.8%) | 25 (59.5%) |
| Ductal carcinoma in situ | 11 (16.9%) | 2 (40%) | 1 (5.6%) | 8 (19%) | |
| Invasive lobular carcinoma | 5 (7.7%) | 0 (0%) | 2 (11.1%) | 3 (7.1%) | |
| Mixed invasive ductal and lobular | 3 (4.6%) | 0 (0%) | 0 (0%) | 3 (7.1%) | |
| Other | 4 (6.2%) | 0 (0%) | 1 (5.6%) | 3 (7.1%) | |
| Estrogen receptor status | Positive | 50 (76.9%) | 4 (80%) | 13 (72.2%) | 33 (78.6%) |
| Negative | 15 (23.1%) | 1 (20%) | 5 (27.8%) | 9 (21.4%) | |
| Progesterone receptor status | Positive | 44 (67.7%) | 4 (80%) | 10 (55.6%) | 30 (71.4%) |
| Negative | 21 (32.3%) | 1 (20%) | 8 (44.4%) | 12 (28.6%) | |
| HER2 IHC status | Negative | 33 (50.8%) | 2 (40%) | 11 (61.1%) | 20 (47.6%) |
| Borderline | 12 (18.5%) | 0 (0%) | 5 (27.8%) | 7 (16.7%) | |
| Positive | 7 (10.8%) | 0 (0%) | 1 (5.6%) | 6 (14.3%) | |
| NA | 13 (20%) | 3 (60%) | 1 (5.6%) | 9 (21.4%) | |
| Clinical stage | Stage 0 | 11 (16.9%) | 2 (40%) | 1 (5.6%) | 8 (19%) |
| Stage 1 | 24 (36.9%) | 0 (0%) | 7 (38.9%) | 17 (40.5%) | |
| Stage 2 | 25 (38.5%) | 3 (60%) | 8 (44.4%) | 14 (33.3%) | |
| Stage 3 | 4 (6.2%) | 0 (0%) | 2 (11.1%) | 2 (4.8%) | |
| Stage 4 | 1 (1.5%) | 0 (0%) | 0 (0%) | 1 (2.4%) | |
| Pathologic stage | No residual invasive disease (ypT0N0) | 5 (7.7%) | 0 (0%) | 2 (11.1%) | 3 (7.1%) |
| ypTisN0 | 2 (3.1%) | 1 (20%) | 1 (5.6%) | 0 (0%) | |
| ypT+ or ypN+ | 13 (20%) | 2 (40%) | 5 (27.8%) | 6 (14.3%) | |
| Stage 0 | 9 (13.8%) | 1 (20%) | 1 (5.6%) | 7 (16.7%) | |
| Stage 1 | 29 (44.6%) | 1 (20%) | 9 (50%) | 19 (45.2%) | |
| Stage 2 | 4 (6.2%) | 0 (0%) | 0 (0%) | 4 (9.5%) | |
| Stage 3 | 2 (3.1%) | 0 (0%) | 0 (0%) | 2 (4.8%) | |
| Stage 4 | 1 (1.5%) | 0 (0%) | 0 (0%) | 1 (2.4%) | |
| Neoadjuvant chemotherapy received | No | 47 (72.3%) | 3 (60%) | 11 (61.1%) | 33 (78.6%) |
| Yes | 18 (27.7%) | 2 (40%) | 7 (38.9%) | 9 (21.4%) | |
| Neoadjuvant endocrine therapy received | No | 62 (95.4%) | 4 (80%) | 17 (94.4%) | 41 (97.6%) |
| Yes | 3 (4.6%) | 1 (20%) | 1 (5.6%) | 1 (2.4%) |
| No Disease | Upgrade to Carcinoma | |||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | |
| Distance from malignancy | ||||
| Contralateral or ipsilateral, >5 cm | Ref | - | Ref | - |
| Ipsilateral, ≤5 cm | 0.65 (0.10–4.46) | 0.66 | 12.7 (1.32–121.9) | 0.03 |
| Quadrant of high-risk lesion | ||||
| Upper outer | Ref | - | Ref | - |
| Upper inner | 12.2 (1.88–78.8) | 0.009 | N/A | - |
| Lower outer | 1.79 (0.41–7.94) | 0.44 | 0.23 (0.02–3.19) | 0.27 |
| Central, subareolar or lower inner | 0.27 (0.03–2.67) | 0.27 | N/A | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, J.; O’Keefe, T.J.; Khan, S.; Grossi, S.M.; Choi, H.Y.; Ojeda-Fournier, H.; Armani, A.; Wallace, A.M.; Blair, S.L. Distance of Biopsy-Confirmed High-Risk Breast Lesion from Concurrently Identified Breast Malignancy Associated with Risk of Carcinoma at the High-Risk Lesion Site. Cancers 2024, 16, 2268. https://doi.org/10.3390/cancers16122268
Le J, O’Keefe TJ, Khan S, Grossi SM, Choi HY, Ojeda-Fournier H, Armani A, Wallace AM, Blair SL. Distance of Biopsy-Confirmed High-Risk Breast Lesion from Concurrently Identified Breast Malignancy Associated with Risk of Carcinoma at the High-Risk Lesion Site. Cancers. 2024; 16(12):2268. https://doi.org/10.3390/cancers16122268
Chicago/Turabian StyleLe, Julie, Thomas J. O’Keefe, Sohini Khan, Sara M. Grossi, Hye Young Choi, Haydee Ojeda-Fournier, Ava Armani, Anne M. Wallace, and Sarah L. Blair. 2024. "Distance of Biopsy-Confirmed High-Risk Breast Lesion from Concurrently Identified Breast Malignancy Associated with Risk of Carcinoma at the High-Risk Lesion Site" Cancers 16, no. 12: 2268. https://doi.org/10.3390/cancers16122268
APA StyleLe, J., O’Keefe, T. J., Khan, S., Grossi, S. M., Choi, H. Y., Ojeda-Fournier, H., Armani, A., Wallace, A. M., & Blair, S. L. (2024). Distance of Biopsy-Confirmed High-Risk Breast Lesion from Concurrently Identified Breast Malignancy Associated with Risk of Carcinoma at the High-Risk Lesion Site. Cancers, 16(12), 2268. https://doi.org/10.3390/cancers16122268






