Long-Term Survival and Factors Associated with Increased Mortality in Patients with Ocular Adnexal Lymphomas
Abstract
Simple Summary
Abstract
1. Introduction
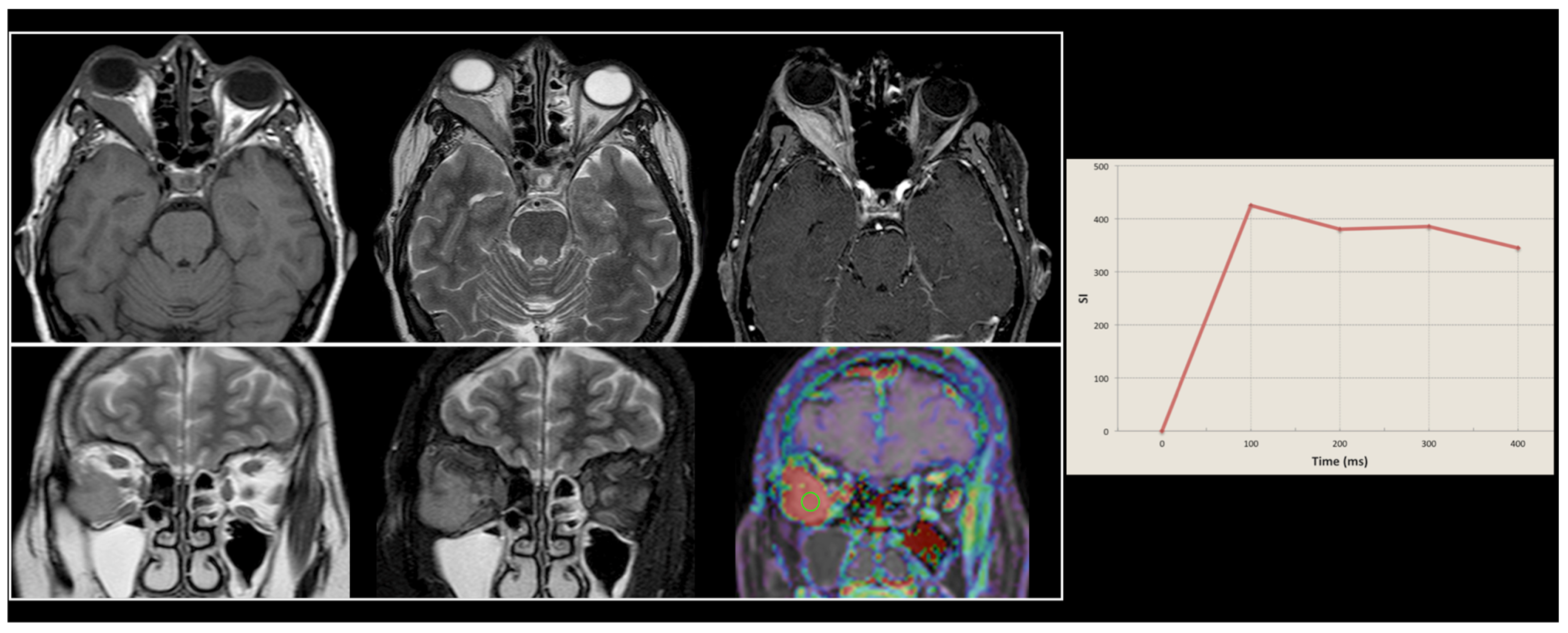
2. Materials and Methods
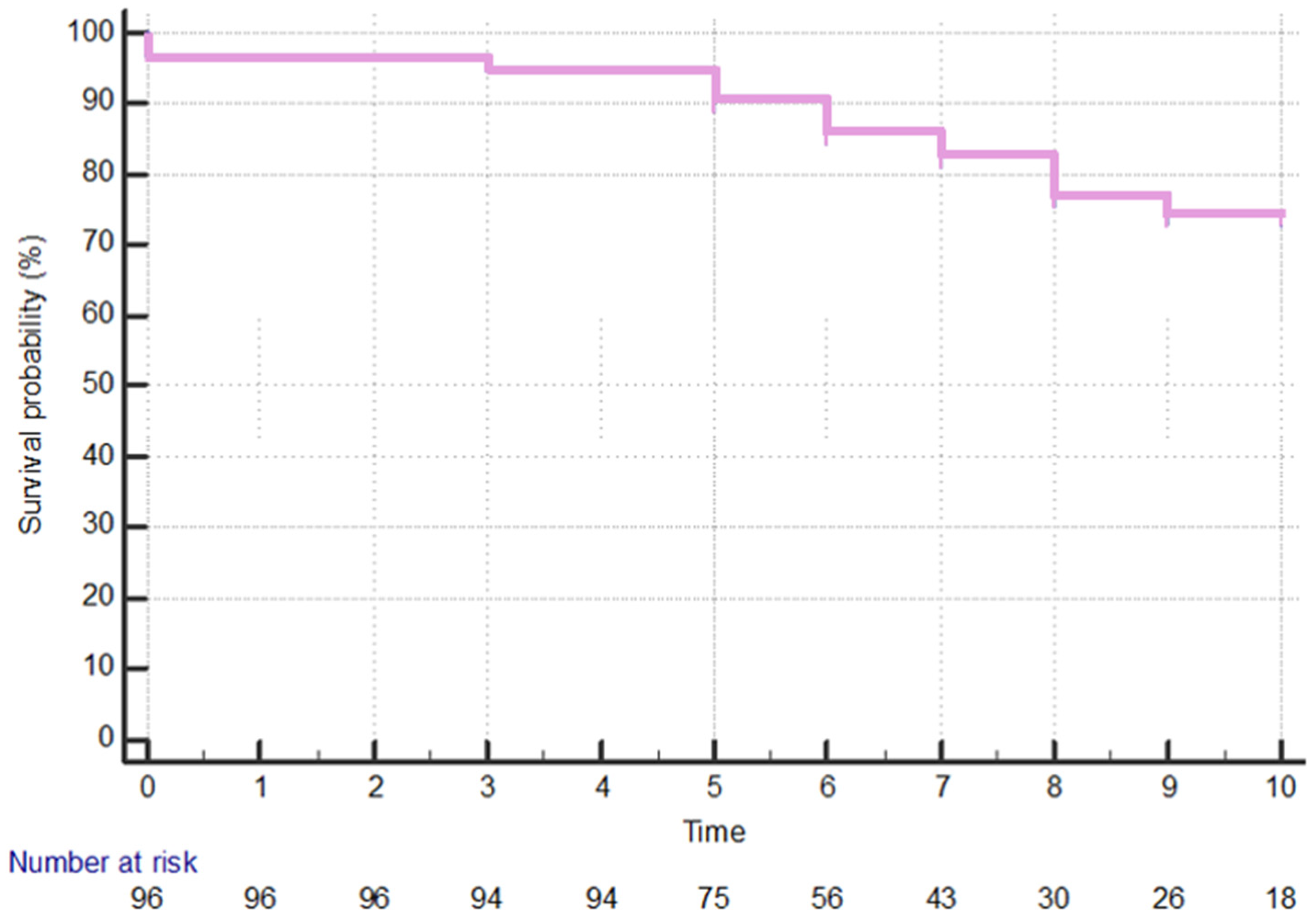
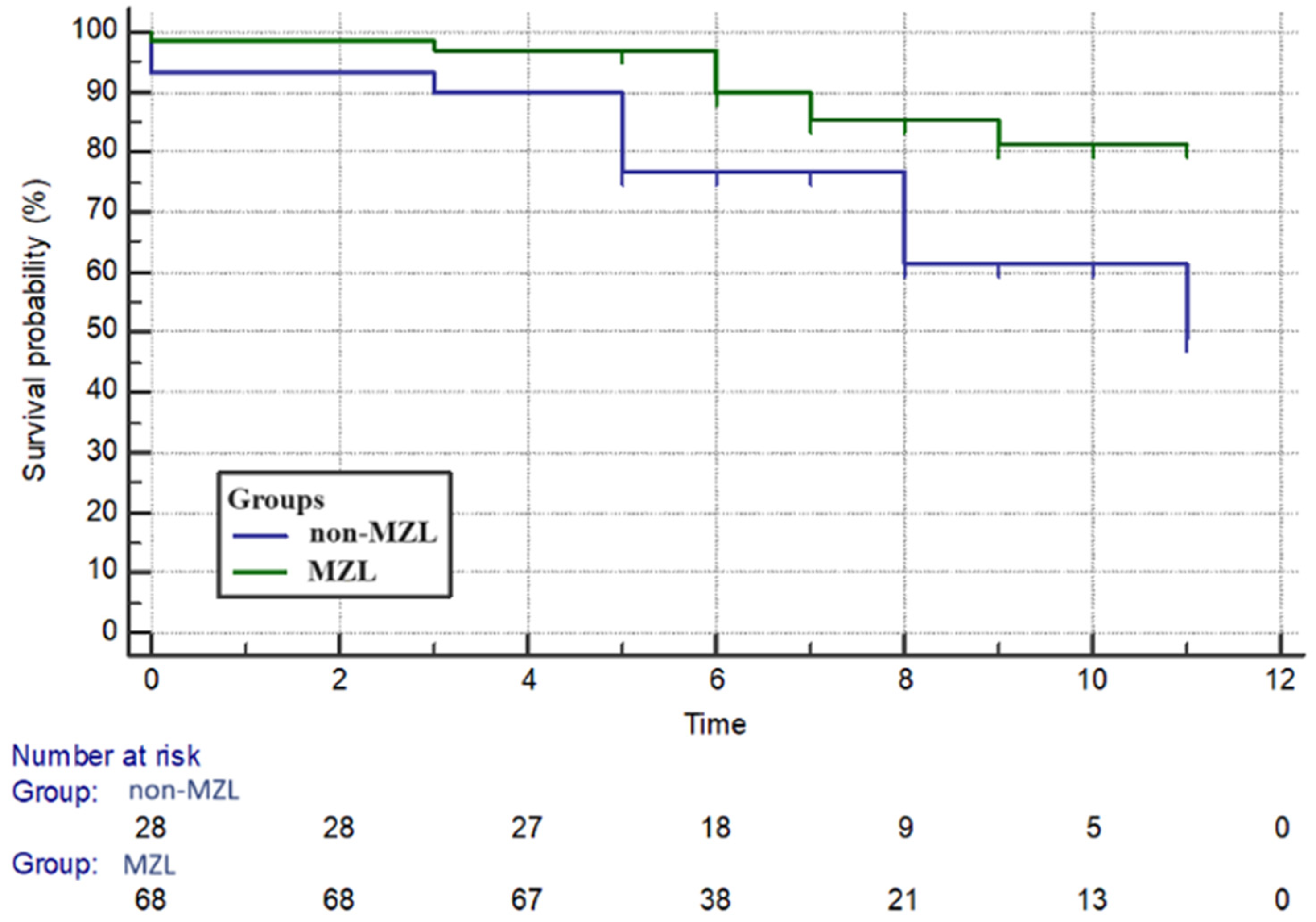
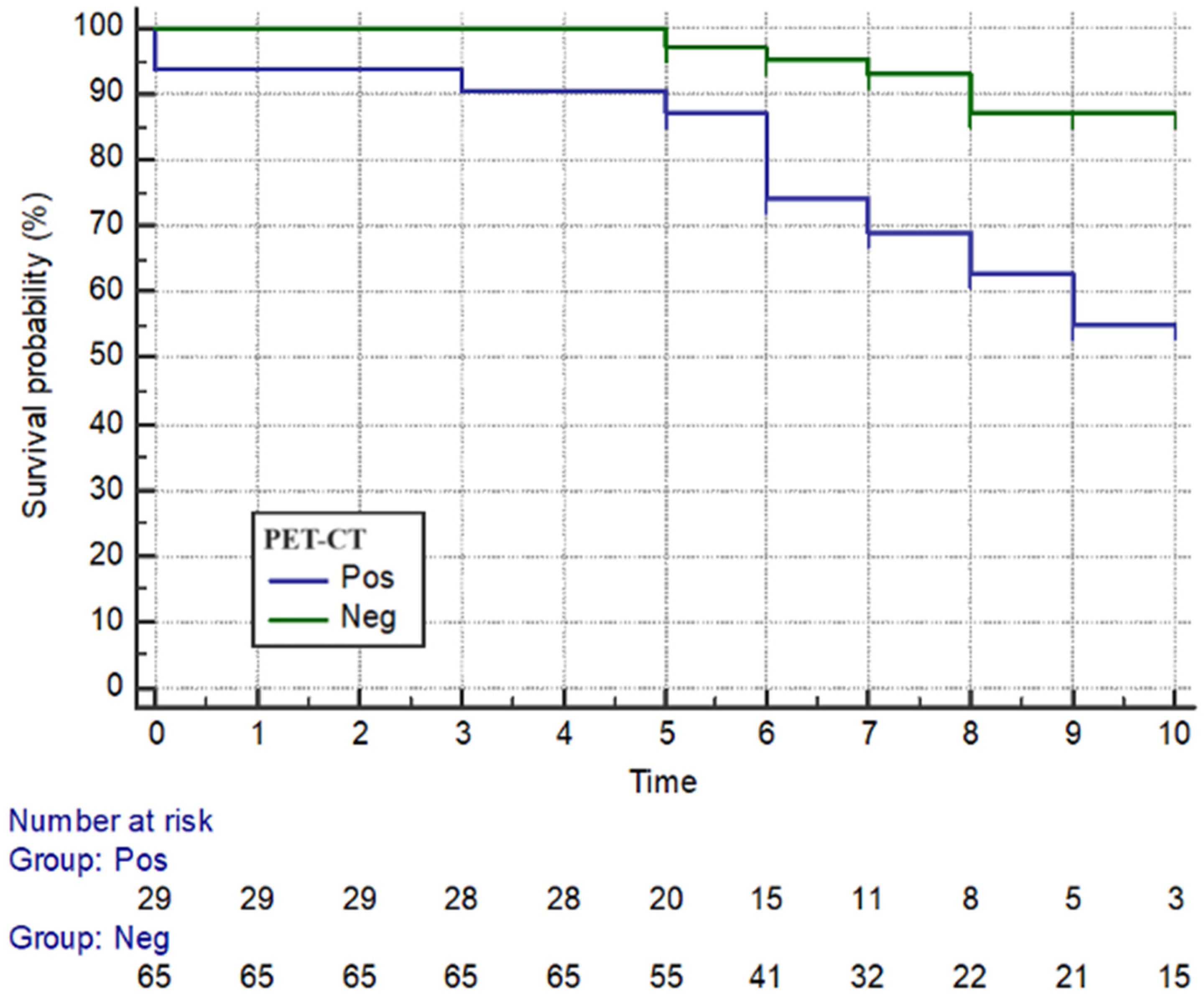
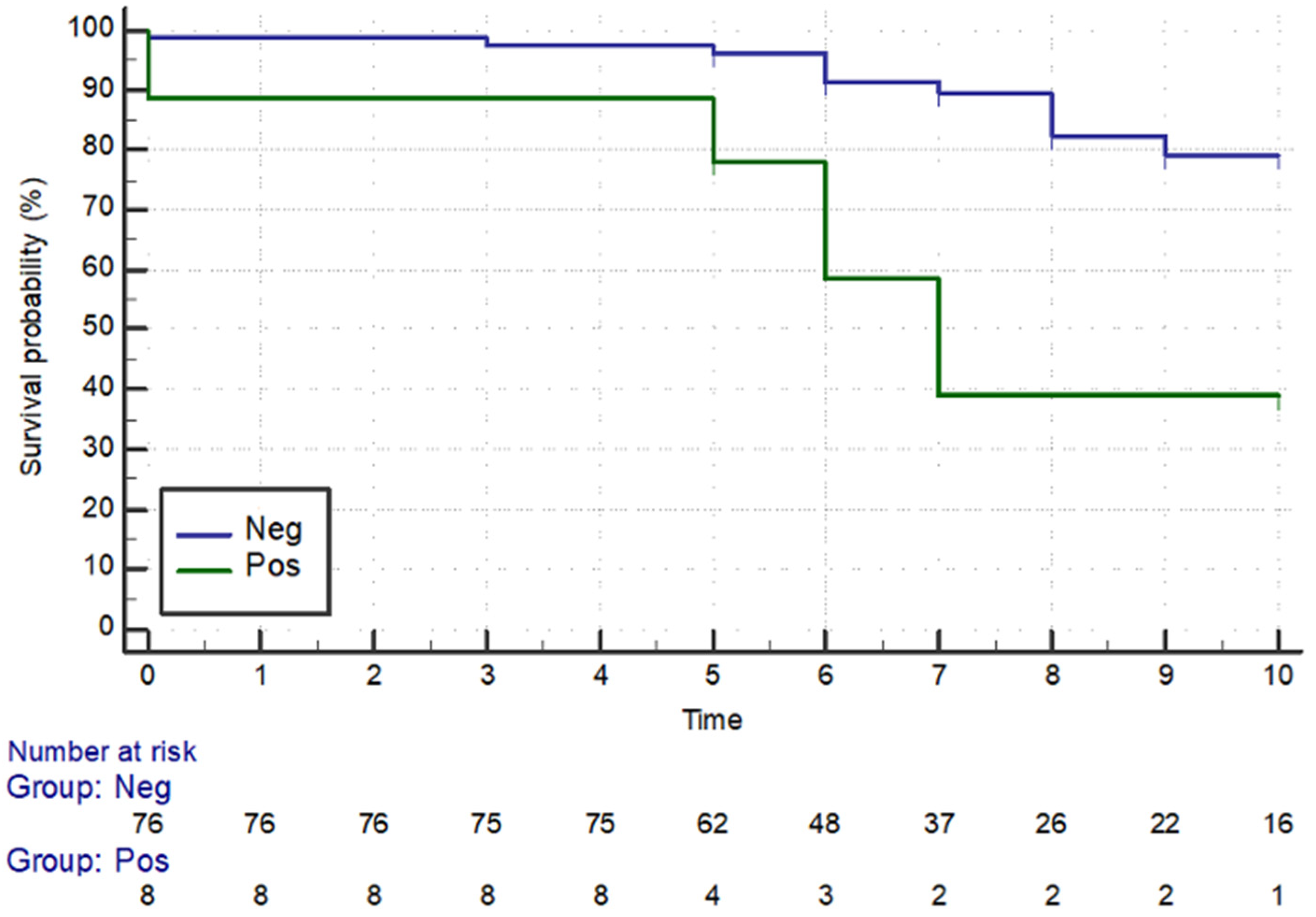
3. Results
3.1. Clinical Features
3.2. Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J. (Eds.) WHO classification of tumours of haematopoietic and lymphoid tissues. In WHO Classification of Tumours, 4th ed.; WHO: Geneva, Switzerland, 2017; Volume 2, ISBN 9789283244943. [Google Scholar]
- Bonavolontà, G.; Strianese, D.; Grassi, P.; Comune, C.; Tranfa, F.; Uccello, G.; Iuliano, A. An analysis of 2,480 space-occupying lesions of the orbit from 1976 to 2011. Ophthalmic Plast Reconstr. Surg. 2013, 29, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Margo, C.E.; Mulla, Z.D. Malignant tumors of the orbit. Analysis of the Florida Cancer Registry. Ophthalmology 1998, 105, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, F.H.; Rasmussen, P.K.; Coupland, S.E.; Esmaeli, B.; Finger, P.T.; Graue, G.F.; Grossniklaus, H.E.; Honavar, S.G.; Khong, J.J.; McKelvie, P.A.; et al. Lymphoma of the Eyelid—An International Multicenter Retrospective Study. Am. J. Ophthalmol. 2017, 177, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Lanni, V.; Iuliano, A.; Fossataro, F.; Russo, C.; Uccello, G.; Tranfa, F.; Strianese, D.; Vallone, G. The role of ultrasonography in differential diagnosis of orbital lesions. J. Ultrasound. 2021, 24, 5–40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olsen, T.G.; Heegaard, S. Orbital lymphoma. Surv. Ophthalmol. 2019, 64, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Wierda, W.G.; Brown, J.; Abramson, J.S.; Awan, F.; Bilgrami, S.F.; Bociek, G.; Brander, D.; Chanan-Khan, A.A.; Coutre, S.E.; Davis, R.S.; et al. NCCN Guidelines® Insights: Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma, Version 3.2022. J. Natl. Compr. Cancer Netw. 2022, 20, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Strianese, D.; Tranfa, F.; Finelli, M.; De Renzo, A.; Staibano, S.; Schiemer, R.; Cardone, D.; Pacelli, R.; Perna, F.; Mascolo, M.; et al. Hepatitis C virus infection in ocular adnexal lymphomas. Arch. Ophthalmol. 2010, 128, 1295–1299. [Google Scholar] [CrossRef] [PubMed]
- Collina, F.; De Chiara, A.; De Renzo, A.; De Rosa, G.; Botti, G.; Franco, R. Chlamydia psittaci in ocular adnexa MALT lymphoma: A possible role in lymphomagenesis and a different geographical distribution. Infect. Agent. Cancer. 2012, 7, 8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chanudet, E.; Zhou, Y.; Bacon, C.M.; Wotherspoon, A.C.; Müller-Hermelink, H.K.; Adam, P.; Dong, H.Y.; de Jong, D.; Li, Y.; Wei, R.; et al. Chlamydia psittaci is variably associated with ocular adnexal MALT lymphoma in different geographical regions. J. Pathol. 2006, 209, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Sjö, L.D.; Ralfkiaer, E.; Prause, J.U.; Petersen, J.H.; Madsen, J.; Pedersen, N.T.; Heegaard, S. Increasing incidence of ophthalmic lymphoma in Denmark from 1980 to 2005. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3283–3288. [Google Scholar] [CrossRef] [PubMed]
- Coupland, S.E.; White, V.A.; Rootman, J.; Damato, B.; Finger, P.T. A TNM-based clinical staging system of ocular adnexal lymphomas. Arch. Pathol. Lab. Med. 2009, 133, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ye, L.; Li, X. Construction and validation of a novel web-based nomogram for primary ocular adnexal lymphoma: A real-world analysis based on the Surveillance, Epidemiology, and End Results database. Transl Cancer Res. 2024, 13, 864–878. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abalo-Lojo, J.M.; Baleato-Gonzalez, S.; Abdulkader, I.; Gonzalez, F. Extraocular muscle involvement in MALT lymphomas. Orbit 2011, 30, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, A.; Fossataro, F.; Laezza, M.P.; Lanni, V.; Mascolo, M.; Varricchio, S.; Uccello, G.; Tranfa, F.; Strianese, D. Primary cutaneous anaplastic large-cell lymphoma of the eyelid: Report of two cases and review of the literature. Orbit 2021, 40, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Aldave, A.P.; Jaiswal, S.; Davidson, S.L. Marginal zone mucosa associated lymphoid tissue diffuse large B cell lymphoma. N. Am. J. Med. Sci. 2014, 6, 422–424. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmed, A.H.; Foster, C.S.; Shields, C.L. Association of Disease Location and Treatment With Survival in Diffuse Large B-Cell Lymphoma of the Eye and Ocular Adnexal Region. JAMA Ophthalmol. 2017, 135, 1062–1068. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amin, S.; Ramsay, A.; Marafioti, T. Diagnostic Pitfalls in “Low-Grade Lymphoma” of the Orbit and Lacrimal Gland. Orbit 2015, 34, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Strianese, D.; Elefante, A.; Matarazzo, F.; Panico, A.; Ferrara, M.; Tranfa, F. Orbital lymphoma mimicking lacrimal gland pleomorphic adenoma. Case Rep. Ophthalmol. 2013, 4, 109–113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- AlSemari, M.A.; Maktabi, A.; AlSamnan, M.S.; Alrajeh, M.S.; Strianese, D. Conjunctival Pediatric Follicular Lymphoma: Case Report and Literature Review. Ophthalmic Plast. Reconstr. Surg. 2020, 36, e14–e15. [Google Scholar] [CrossRef] [PubMed]
- Ferry, J.A.; Fung, C.Y.; Zukerberg, L.; Lucarelli, M.J.; Hasserjian, R.P.; Preffer, F.I.; Harris, N.L. Lymphoma of the ocular adnexa: A study of 353 cases. Am. J. Surg. Pathol. 2007, 31, 170–184. [Google Scholar] [CrossRef] [PubMed]
- A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood 1997, 89, 3909–3918. [Google Scholar] [PubMed]
- English, J.F.; Sullivan, T.J. The Role of FDG-PET in the Diagnosis and Staging of Ocular Adnexal Lymphoproliferative Disease. Orbit 2015, 34, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Alavi, A.; Hess, S.; Werner, T.J.; Høilund-Carlsen, P.F. An update on the unparalleled impact of FDG-PET imaging on the day-to-day practice of medicine with emphasis on management of infectious/inflammatory disorders. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Kung, B.T.; Seraj, S.M.; Zadeh, M.Z.; Rojulpote, C.; Kothekar, E.; Ayubcha, C.; Ng, K.S.; Ng, K.K.; Au-Yong, T.K.; Werner, T.J.; et al. An update on the role of 18F-FDG-PET/CT in major infectious and inflammatory diseases. Am. J. Nucl. Med. Mol. Imaging 2019, 9, 255–273. [Google Scholar] [PubMed] [PubMed Central]
- Cherry, S.R.; Jones, T.; Karp, J.S.; Qi, J.; Moses, W.W.; Badawi, R.D. Total-Body PET: Maximizing Sensitivity to Create New Opportunities for Clinical Research and Patient Care. J. Nucl. Med. 2018, 59, 3–12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sjö, L.D. Ophthalmic lymphoma: Epidemiology and pathogenesis. Acta Ophthalmol. 2009, 87, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Di Salle, F.; Esposito, F.; Elefante, A.; Scarabino, T.; Volpicelli, A.; Cirillo, S.; Elefante, R.; Seifritz, E. High field functional MRI. Eur. J. Radiol. 2003, 48, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Striano, P.; Tortora, F.; Evoli, A.; Palmieri, G.; Elefante, A.; Zara, F.; Tarr, P.E.; Striano, S. Periodic myoclonus due to cytomegalovirus encephalitis in a patient with good syndrome. Arch. Neurol. 2007, 64, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Vishwanath, V.; Jafarieh, S.; Rembielak, A. The role of imaging in head and neck cancer: An overview of different imaging modalities in primary diagnosis and staging of the disease. J. Contemp. Brachytherapy 2020, 12, 512–518. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saleh, A.; Schroeter, M.; Jonkmanns, C.; Hartung, H.P.; Mödder, U.; Jander, S. In vivo MRI of brain inflammation in human ischaemic stroke. Brain 2004, 127, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Vemuri, P.; Jack, C.R., Jr. Role of structural MRI in Alzheimer’s disease. Alzheimers Res. Ther. 2010, 2, 23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cocozza, S.; Russo, C.; Pontillo, G.; Ugga, L.; Macera, A.; Cervo, A.; De Liso, M.; Di Paolo, N.; Ginocchio, M.I.; Giordano, F.; et al. Is advanced neuroimaging for neuroradiologists? A systematic review of the scientific literature of the last decade. Neuroradiology 2016, 58, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Heegaard, S.; Chevez-Barrios, P.; White, V.A.; Coupland, S.E.; Finger, P.T. Ophthalmic sites: Ocular adnexal lymphoma. In AJCC Cancer Staging Manual, 8th ed.; Amin, M.B., Edge, S., Greene, F., Byrd, D.R., Brookland, R.K., Washington, M.K., Gershenwald, J.E., Compton, C.C., Hess, K.R., Sullivan, D.C., et al., Eds.; Springer: New York, NY, USA, 2017; pp. 849–854. [Google Scholar]
- De Cicco, L.; Cella, L.; Liuzzi, R.; Solla, R.; Farella, A.; Punzo, G.; Tranfa, F.; Strianese, D.; Conson, M.; Bonavolontà, G.; et al. Radiation therapy in primary orbital lymphoma: A single institution retrospective analysis. Radiat. Oncol. 2009, 4, 60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flanagan, J.P.M.; Ng, M.; Kibrom, A.Z.; Filshie, R.J.A.; Stawell, R.J.; O’Day, R.F. Ultra-low dose external beam radiotherapy for presumed choroidal lymphoma: A case report. J. Ophthalmic Inflamm. Infect. 2022, 12, 10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cerrone, M.; Collina, F.; De Chiara, A.; Corazzelli, G.; Curcio, M.P.; De Renzo, A.; Russo, F.; Cantile, M.; Staibano, S.; Strianese, D.; et al. BCL10 expression and localization in ocular adnexa MALT lymphomas: A comparative cytogenetic and immunohistochemical study. Histol. Histopathol. 2014, 29, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Paik, J.S.; Cho, W.K.; Choi, B.O.; Lee, S.N.; Jung, S.E.; Park, K.S.; Kang, C.S.; Kim, S.H.; Yang, S.W.; et al. Feasibility of the TNM-based staging system of ocular adnexal extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). Am. J. Hematol. 2011, 86, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Tranfa, F.; Di Matteo, G.; Strianese, D.; Forte, R.; Bonavolontà, G. Primary orbital lymphoma. Orbit 2001, 20, 119–124. [Google Scholar] [CrossRef] [PubMed]
- McKelvie, P.A.; McNab, A.; Francis, I.C.; Fox, R.; O’Day, J. Ocular adnexal lymphoproliferative disease: A series of 73 cases. Clin. Exp. Ophthalmol. 2001, 29, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, T.J.; Whitehead, K.; Williamson, R.; Grimes, D.; Schlect, D.; Brown, I.; Dickie, G. Lymphoproliferative disease of the ocular adnexa: A clinical and pathologic study with statistical analysis of 69 patients. Ophthalmic Plast. Reconstr. Surg. 2005, 21, 177–188. [Google Scholar] [CrossRef] [PubMed]
| EMZL | SLL | DLBCL | MCL | FL | LL | |
|---|---|---|---|---|---|---|
| No. of patients | 69 | 11 | 10 | 6 | 2 | 1 |
| Gender (M:F) | 36:33 | 5:6 | 7:3 | 5:1 | 0:2 | 0:1 |
| Median Age | 66 | 66 | 64.5 | 73 | 75.5 | 57 |
| Location | ||||||
| -orbit | 45 | 10 | 8 | 2 | 2 | 1 |
| -lacrimal gland | 17 | 0 | 2 | 3 | 0 | 0 |
| -eyelid | 2 | 0 | 0 | 0 | 0 | 0 |
| -conjunctiva | 5 | 1 | 0 | 1 | 0 | 0 |
| Laterality (Unilateral:Bilateral) | 66:3 | 10:1 | 10:0 | 4:2 | 2:0 | 0:1 |
| Clinical features at diagnosis | ||||||
| -mass | 19 | 3 | 4 | 1 | 1 | 1 |
| -swelling | 5 | 3 | 2 | 2 | 0 | 0 |
| -ptosis | 19 | 1 | 2 | 3 | 1 | 0 |
| -epiphora | 3 | 3 | 1 | 0 | 1 | 0 |
| -exophtalmos | 22 | 6 | 2 | 2 | 2 | 0 |
| -diplopia | 4 | 2 | 1 | 0 | 0 | 0 |
| -conjunctival chemosis | 5 | 2 | 0 | 1 | 0 | 0 |
| -trigeminal paraesthesia | 0 | 0 | 1 | 0 | 0 | 0 |
| RTx | CTx | RTx + CTx | No Treatment | CTx + Rituximab | |
|---|---|---|---|---|---|
| EMZL | 44 | 7 | 12 | 1 | 5 |
| SLL | 7 | 0 | 3 | 1 | 0 |
| DLBCL | 4 | 1 | 3 | 2 | 0 |
| MCL | 3 | 2 | 1 | 0 | 0 |
| FL | 0 | 2 | 0 | 0 | 0 |
| LL | 0 | 0 | 0 | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strianese, D.; Laezza, M.P.; Tortora, F.; Fusco, G.; de Divitiis, O.; D’Aponte, A.; Briganti, F.; Elefante, A. Long-Term Survival and Factors Associated with Increased Mortality in Patients with Ocular Adnexal Lymphomas. Cancers 2024, 16, 2252. https://doi.org/10.3390/cancers16122252
Strianese D, Laezza MP, Tortora F, Fusco G, de Divitiis O, D’Aponte A, Briganti F, Elefante A. Long-Term Survival and Factors Associated with Increased Mortality in Patients with Ocular Adnexal Lymphomas. Cancers. 2024; 16(12):2252. https://doi.org/10.3390/cancers16122252
Chicago/Turabian StyleStrianese, Diego, Maria Paola Laezza, Fabio Tortora, Giancarlo Fusco, Oreste de Divitiis, Antonella D’Aponte, Francesco Briganti, and Andrea Elefante. 2024. "Long-Term Survival and Factors Associated with Increased Mortality in Patients with Ocular Adnexal Lymphomas" Cancers 16, no. 12: 2252. https://doi.org/10.3390/cancers16122252
APA StyleStrianese, D., Laezza, M. P., Tortora, F., Fusco, G., de Divitiis, O., D’Aponte, A., Briganti, F., & Elefante, A. (2024). Long-Term Survival and Factors Associated with Increased Mortality in Patients with Ocular Adnexal Lymphomas. Cancers, 16(12), 2252. https://doi.org/10.3390/cancers16122252







