Real-World Efficacy and Safety of First-Line Nivolumab Plus Chemotherapy in Patients with Advanced Gastric, Gastroesophageal Junction, and Esophageal Adenocarcinoma: A Nationwide Observational Turkish Oncology Group (TOG) Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Efficacy Measures
2.4. Safety Endpoints
2.5. Data Analysis
3. Results
3.1. Patient Characteristics
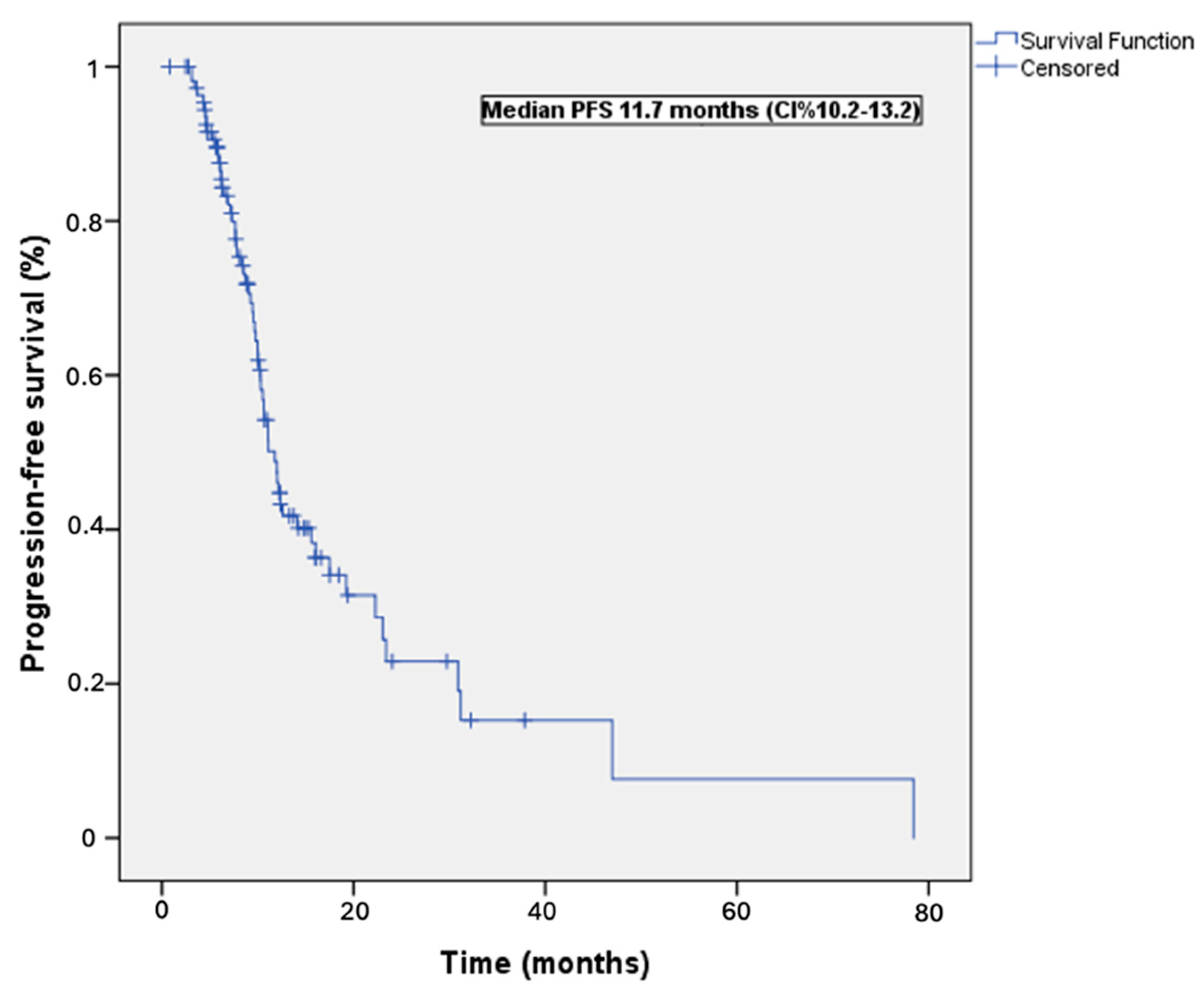
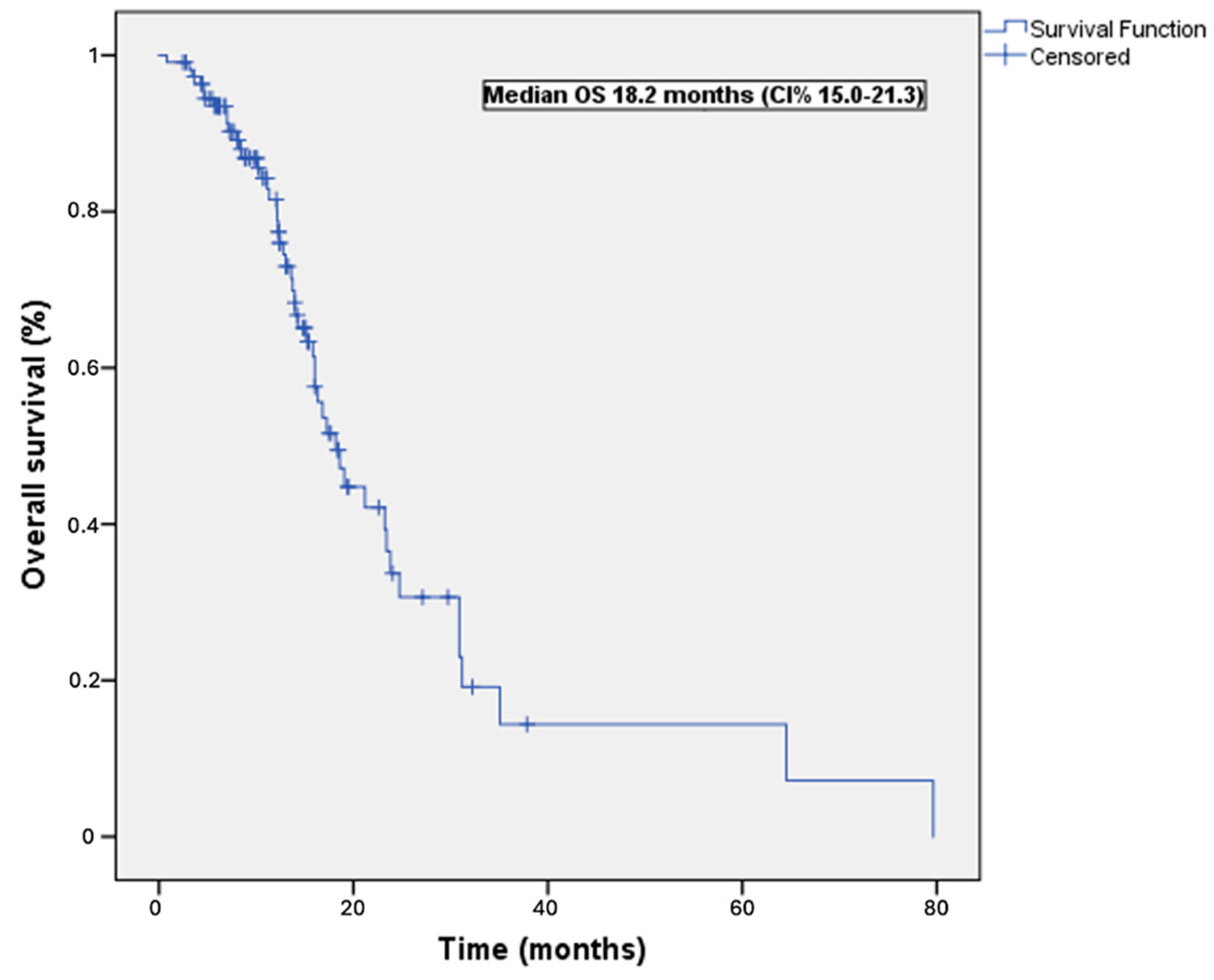
3.2. Survival Outcomes
3.3. Treatment Response
3.4. Treatment-Related Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moehler, M.; Xiao, H.; Blum, S.I.; Elimova, E.; Cella, D.; Shitara, K.; Ajani, J.A.; Janjigian, Y.Y.; Garrido, M.; Shen, L.; et al. Health-Related Quality of Life with Nivolumab Plus Chemotherapy versus Chemotherapy in Patients with Advanced Gastric/Gastroesophageal Junction Cancer or Esophageal Adenocarcinoma from CheckMate 649. J. Clin. Oncol. 2023, 41, 5388–5399. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Cha, C. Updates on esophageal and gastric cancers. World J. Gastroenterol. 2006, 12, 3237–3242. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; Lee, J.; Sano, T.; Janjigian, Y.Y.; Fan, D.; Song, S. Gastric adenocarcinoma. Nat. Rev. Dis. Primers 2017, 3, 17036. [Google Scholar] [CrossRef] [PubMed]
- Uhlenhopp, D.J.; Then, E.O.; Sunkara, T.; Gaduputi, V. Epidemiology of esophageal cancer: Update in global trends, etiology and risk factors. Clin. J. Gastroenterol. 2020, 13, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D. ESMO Guidelines Committee. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v38–v49. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Almhanna, K.; Bentrem, D.J.; Chao, J.; Das, P.; Denlinger, C.S.; Fanta, P.; Farjah, F.; Fuchs, C.S.; et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2016, 14, 1286–1312. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.D.; Syn, N.L.; Moehler, M.; Grothe, W.; Yong, W.P.; Tai, B.C.; Ho, J.; Unverzagt, S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst. Rev. 2017, 8, CD004064. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Hartmann, J.T.; Probst, S.; Schmalenberg, H.; Hollerbach, S.; Hofheinz, R.; Rethwisch, V.; Seipelt, G.; Homann, N.; Wilhelm, G.; et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: A study of the Arbeitsgemeinschaft Internistische Onkologie. J. Clin. Oncol. 2008, 26, 1435–1442. [Google Scholar] [CrossRef]
- Zaanan, A.; Samalin, E.; Aparicio, T.; Bouche, O.; Laurent-Puig, P.; Manfredi, S.; Michel, P.; Monterymard, C.; Moreau, M.; Rougier, P.; et al. Phase III randomized trial comparing 5-fluorouracil and oxaliplatin with or without docetaxel in first-line advanced gastric cancer chemotherapy (GASTFOX study). Dig. Liver Dis. 2018, 50, 408–410. [Google Scholar] [CrossRef]
- Kang, Y.K.; Kang, W.K.; Shin, D.B.; Chen, J.; Xiong, J.; Wang, J.; Lichinitser, M.; Guan, Z.; Khasanov, R.; Zheng, L.; et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: A randomised phase III noninferiority trial. Ann. Oncol. 2009, 20, 666–673. [Google Scholar] [CrossRef]
- Alsina, M.; Arrazubi, V.; Diez, M.; Tabernero, J. Current developments in gastric cancer: From molecular profiling to treatment strategy. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 155–170. [Google Scholar] [CrossRef]
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E.C. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Sohal, D. Current state of the art: Immunotherapy in esophageal cancer and gastroesophageal junction cancer. Cancer Immunol. Immunother. 2023, 72, 3939–3952. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar]
- Takei, S.; Kawazoe, A.; Shitara, K. The new era of immunotherapy in gastric cancer. Cancers 2022, 14, 1054. [Google Scholar] [CrossRef]
- Yoon, J.; Kim, T.Y.; Oh, D.Y. Recent progress in immunotherapy for gastric cancer. J. Gastric Cancer 2023, 23, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Ajani, J.A.; Moehler, M.; Shen, L.; Garrido, M.; Gallardo, C.; Wyrwicz, L.; Yamaguchi, K.; Cleary, J.M.; Elimova, E.; et al. First-line nivolumab plus chemotherapy for advanced gastric, gastroesophageal junction, and esophageal adenocarcinoma: 3-year follow-up of the phase III CheckMate 649 trial. J. Clin. Oncol. 2024, 42, JCO-23. [Google Scholar] [CrossRef]
- Tang, M.; Pearson, S.A.; Simes, R.J.; Chua, B.H. Harnessing real-world evidence to advance cancer research. Curr. Oncol. 2023, 30, 1844–1859. [Google Scholar] [CrossRef] [PubMed]
- Schad, F.; Thronicke, A. Real-World Evidence-Current Developments and Perspectives. Int. J. Environ. Res. Public Health 2022, 19, 10159. [Google Scholar] [CrossRef]
- Di Maio, M.; Perrone, F.; Conte, P. Real-world evidence in oncology: Opportunities and limitations. Oncologist 2020, 25, e746–e752. [Google Scholar] [CrossRef] [PubMed]
- Saesen, R.; Van Hemelrijck, M.; Bogaerts, J.; Booth, C.M.; Cornelissen, J.J.; Dekker, A.; Eisenhauer, E.A.; Freitas, A.; Gronchi, A.; Hernán, M.A. Defining the role of real-world data in cancer clinical research: The position of the European Organisation for Research and Treatment of Cancer. Eur. J. Cancer 2023, 186, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Azam, F.; Latif, M.F.; Farooq, A.; Tirmazy, S.H.; AlShahrani, S.; Bashir, S.; Bukhari, N. Performance status assessment by using ECOG (Eastern Cooperative Oncology Group) score for cancer patients by oncology healthcare professionals. Case Rep. Oncol. 2019, 12, 728–736. [Google Scholar] [CrossRef]
- Vranic, S.; Gatalica, Z. PD-L1 testing by immunohistochemistry in immuno-oncology. Biomol. Biomed. 2023, 23, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Freites-Martinez, A.; Santana, N.; Arias-Santiago, S.; Viera, A. Using the Common Terminology Criteria for Adverse Events (CTCAE—Version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr. 2021, 112, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Bai, Y.; Lin, X.; Li, W.; Wang, J.; Zhang, X.; Pan, H.; Bai, C.; Bai, L.; Cheng, Y.; et al. First-line nivolumab plus chemotherapy vs. chemotherapy in patients with advanced gastric, gastroesophageal junction and esophageal adenocarcinoma: CheckMate 649 Chinese subgroup analysis. Int. J. Cancer 2023, 152, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.N.; Xie, B.; Zhang, Y.; He, M.H.; Xing, Y.; Mu, D.M.; Wang, H.; Guo, R. Advances and key focus areas in gastric cancer immunotherapy: A comprehensive scientometric and clinical trial review (1999–2023). World J. Gastroenterol. 2023, 29, 5593–5617. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Ajani, J.A.; Moehler, M.; Garrido, M.; Gallardo, C.; Shen, L.; Yamaguchi, K.; Wyrwicz, L.; Skoczylas, T.; Bragagnoli, A.C.; et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature 2022, 603, 942–948. [Google Scholar] [CrossRef]
- Lin, D.; Nguyen, H.; Shah, R.; Qiao, Y.; Hartman, J.; Sugarman, R. Quality-adjusted time without symptoms or toxicity analysis of nivolumab plus chemotherapy versus chemotherapy alone for the management of previously untreated patients with advanced gastric cancer, gastroesophageal junction cancer, or esophageal adenocarcinoma. Gastric Cancer 2023, 26, 415–424. [Google Scholar] [CrossRef]
| Characteristic | n (%) |
|---|---|
| Sex | |
| Female | 54 (48.6) |
| Male | 57 (51.4) |
| Median age, years | 58 (31–82) |
| <65 | 79 (71.2) |
| >65 | 32 (28.8) |
| ECOG performance status | |
| 0 | 50 (45.1) |
| 1 | 49 (44.1) |
| 2 | 12 (10.8) |
| Primary tumor location | |
| Gastric adenocarcinoma | 88 (79.2) |
| GOJ adenocarcinoma | 21 (18.9) |
| Oesophageal adenocarcinoma | 2 (1.9) |
| Previous curative surgery | |
| Present | 27 (24.3) |
| Absent | 84 (75.7) |
| Initial disease stage | |
| Locally advanced | 24 (21.6) |
| Metastatic | 87 (78.4) |
| Signet ring cell carcinoma | |
| Present | 22 (19.9) |
| Absent | 89 (80.1) |
| Number of metastatic sites | |
| 1 | 59 (53.2) |
| ≥2 | 52 (46.8) |
| Site of metastasis | |
| Liver | 48 (43.2) |
| Lung | 19 (17.1) |
| Peritoneum | 44 (39.6) |
| Bone | 12 (10.8) |
| CNS | 1 (0.9) |
| Distant lymph nodes | 35 (31.5) |
| Other sites | 4 (3.6) |
| Chemotherapy regimen | |
| FOLFOX | 107 (96.4) |
| XELOX | 4 (3.6) |
| PD-L1 CPS | |
| <1 | 5 (4.5) |
| ≥1 | 106 (95.5) |
| ≥5 | 100 (90.1) |
| ≥10 | 76 (68.5) |
| Characteristic | n (%) | Median PFS (Months) | Univariable p Value | Multivariable p Value | HR (95 CI%) |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 54 (48.6) | 10.6 | 0.07 | ||
| Male | 57 (51.4) | 12.1 | |||
| Median age (interquartile range), years | 58 (31–82) | ||||
| <65 | 79 (71.2) | 11.0 | 0.75 | ||
| >65 | 32 (28.8) | 12.0 | |||
| ECOG performance status | |||||
| 0 | 50 (45.1) | 12.0 | 0.038 | ||
| 1 | 49 (44.1) | 12.1 | |||
| 2 | 12 (10.8) | 10.3 | 0.26 | 1.57 (0.70–3.54) | |
| Primary tumor location | |||||
| Gastric adenocarcinoma | 88 (79.2) | 11.5 | 0.44 | ||
| GOJ adenocarcinoma | 21 (18.9) | 12.1 | |||
| Esophageal adenocarcinoma | 2 (1.9) | 11.4 | |||
| Previous curative surgery | |||||
| Present | 27 (24.3) | 25.7 | <0.001 | 0.022 | 0.33 (0.13–0.85) |
| Absent | 84 (75.7) | 10.0 | |||
| Initial disease stage | |||||
| Locally advanced | 24 (21.6) | 23.0 | <0.001 | 0.12 | 2.00 (0.81–4.90) |
| Metastatic | 87 (78.4) | 10.1 | |||
| Signet ring cell carcinoma | |||||
| Present | 22 (19.9) | 10.1 | 0.82 | ||
| Absent | 89 (80.1) | 12.3 | |||
| Number of metastatic sites | |||||
| 1 | 59 (53.2) | 12.0 | 0.22 | ||
| ≥2 | 52 (46.8) | 11.0 | |||
| Site of metastasis | |||||
| Liver | 48 (43.2) | 10.6 | 0.65 | ||
| Lung | 19 (17.1) | 11.1 | |||
| Peritoneum | 44 (39.6) | 12.3 | |||
| Bone | 12 (10.8) | 10.5 | |||
| CNS | 1 (0.9) | NA | |||
| Distant lymph nodes | 35 (31.5) | 18.2 | |||
| Other sites | 4 (3.6) | 8.7 | |||
| Chemotherapy regimen | |||||
| FOLFOX | 107 (96.4) | 11.1 | 0.96 | ||
| XELOX | 4 (3.6) | 13.5 | |||
| PD-L1 CPS | |||||
| <5 | 20 (18.1) | 11.1 | 0.031 | 0.35 | 1.62 (0.58–4.48) |
| ≥5 | 91 (81.9) | 17.5 |
| Characteristic | n (%) | Median OS (Months) | Univariable p Value | Multivariable p Value | HR (95 CI%) |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 54 (48.6) | 17.2 | 0.19 | ||
| Male | 57 (51.4) | 19.0 | |||
| Median age (interquartile range), years | 58 (31–82) | ||||
| <65 | 79 (71.2) | 17.2 | 0.20 | ||
| >65 | 32 (28.8) | 23.2 | |||
| ECOG performance status | |||||
| 0 | 50 (45.1) | 21.2 | 0.032 | ||
| 1 | 49 (44.1) | 16.0 | |||
| 2 | 12 (10.8) | 11.3 | 0.021 | 3.34 (1.20–9.31) | |
| Primary tumor location | |||||
| Gastric adenocarcinoma | 88 (79.2) | 18.1 | 0.45 | ||
| GOJ adenocarcinoma | 21 (18.9) | 17.2 | |||
| Esophageal adenocarcinoma | 2 (1.9) | 16.8 | |||
| Previous curative surgery | |||||
| Present | 27 (24.3) | 16.0 | 0.005 | 0.026 | 0.52 (0.16–0.62) |
| Absent | 84 (75.7) | 23.8 | |||
| Initial disease stage | |||||
| Locally advanced | 24 (21.6) | 23.4 | 0.025 | 0.66 | 1.27 (0.42–3.82) |
| Metastatic | 87 (78.4) | 16.0 | |||
| Signet ring cell carcinoma | |||||
| Present | 22 (19.9) | 18.2 | 0.25 | ||
| Absent | 89 (80.1) | 16.8 | |||
| Number of metastatic sites | |||||
| 1 | 59 (53.2) | 21.2 | 0.22 | ||
| ≥2 | 52 (46.8) | 16.8 | |||
| Site of metastasis | |||||
| Liver | 48 (43.2) | 23.2 | 0.39 | ||
| Lung | 19 (17.1) | 13.7 | |||
| Peritoneum | 44 (39.6) | 18.2 | |||
| Bone | 12 (10.8) | 19.0 | |||
| CNS | 1 (0.9) | NA | |||
| Distant lymph nodes | 35 (31.5) | 19.7 | |||
| Other sites | 4 (3.6) | NA | |||
| Chemotherapy regimen | |||||
| FOLFOX | 107 (96.4) | 18.2 | 0.83 | ||
| XELOX | 4 (3.6) | 23.4 | |||
| PD-L1 CPS | |||||
| <5 | 20 (18.1) | 17.2 | 0.29 | 0.24 | 2.34 (0.55–9.85) |
| ≥5 | 91 (81.9) | 18.6 |
| Response | n | % |
|---|---|---|
| Complete response | 12 | 10.8 |
| Partial response | 66 | 59.5 |
| Stable disease | 18 | 16.2 |
| Progressive disease | 15 | 13.5 |
| Objective response rate | 78 | 70.3 |
| Disease control rate | 96 | 86.5 |
| Adverse Event | Grade 1 or 2, n (%) | Grade 3 or 4, n (%) |
|---|---|---|
| Nausea | 58 (52.2) | 2 (1.8) |
| Vomiting | 19 (6.8) | 2 (1.8) |
| Peripheral neuropathy | 32 (28.8) | 3 (2.7) |
| Diarrhea | 32 (28.8) | 1 (0.9) |
| Fatigue | 42 (37.8) | 5 (4.5) |
| Weight loss | 11 (9.9) | - |
| Decreased appetite | 14 (12.6) | 1 (0.9) |
| Stomatitis | 29 (26.1) | 2 (1.8) |
| Lipase increased | 7 (6.3) | - |
| Hypothyroidism | 7 (6.3) | 2 (1.8) |
| Neutropenia | 32 (28.8) | 8 (7.2) |
| Increased alanine aminotransferase | 18 (16.2) | 1 (0.9) |
| Increased aspartate aminotransferase | 21 (18.9) | 1 (0.9) |
| Rash | 7 (6.3) | - |
| Alopecia | 12 (10.8) | - |
| Anemia | 48 (43.2) | 11 (9.9) |
| Thrombocytopenia | 20 (18.0) | 5 (4.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutlu, Y.; Dae, S.A.; Yilmaz, F.; Erdem, D.; Sendur, M.A.N.; Akbas, S.; Senocak Tasci, E.; Bas, O.; Dane, F.; Sakin, A.; et al. Real-World Efficacy and Safety of First-Line Nivolumab Plus Chemotherapy in Patients with Advanced Gastric, Gastroesophageal Junction, and Esophageal Adenocarcinoma: A Nationwide Observational Turkish Oncology Group (TOG) Study. Cancers 2024, 16, 2251. https://doi.org/10.3390/cancers16122251
Kutlu Y, Dae SA, Yilmaz F, Erdem D, Sendur MAN, Akbas S, Senocak Tasci E, Bas O, Dane F, Sakin A, et al. Real-World Efficacy and Safety of First-Line Nivolumab Plus Chemotherapy in Patients with Advanced Gastric, Gastroesophageal Junction, and Esophageal Adenocarcinoma: A Nationwide Observational Turkish Oncology Group (TOG) Study. Cancers. 2024; 16(12):2251. https://doi.org/10.3390/cancers16122251
Chicago/Turabian StyleKutlu, Yasin, Shute Ailia Dae, Feride Yilmaz, Dilek Erdem, Mehmet Ali Nahit Sendur, Sinem Akbas, Elif Senocak Tasci, Onur Bas, Faysal Dane, Abdullah Sakin, and et al. 2024. "Real-World Efficacy and Safety of First-Line Nivolumab Plus Chemotherapy in Patients with Advanced Gastric, Gastroesophageal Junction, and Esophageal Adenocarcinoma: A Nationwide Observational Turkish Oncology Group (TOG) Study" Cancers 16, no. 12: 2251. https://doi.org/10.3390/cancers16122251
APA StyleKutlu, Y., Dae, S. A., Yilmaz, F., Erdem, D., Sendur, M. A. N., Akbas, S., Senocak Tasci, E., Bas, O., Dane, F., Sakin, A., Kaya, A. O., Aykan, M. B., Ergun, Y., Biter, S., Disel, U., Korkmaz, M., Selcukbiricik, F., Kose, F., Olmez, O. F., ... Yalcin, S. (2024). Real-World Efficacy and Safety of First-Line Nivolumab Plus Chemotherapy in Patients with Advanced Gastric, Gastroesophageal Junction, and Esophageal Adenocarcinoma: A Nationwide Observational Turkish Oncology Group (TOG) Study. Cancers, 16(12), 2251. https://doi.org/10.3390/cancers16122251






