Mixtures of Three Mortaparibs with Enhanced Anticancer, Anti-Migration, and Antistress Activities: Molecular Characterization in p53-Null Cancer Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Reagents
2.2. Cell Viability Assay
2.3. Colony Formation Assay
2.4. Cell-Cycle Analysis
2.5. Western Blot Analysis
2.6. Immunocytochemistry
2.7. RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction (RTqPCR)
2.8. Trapping Assay
2.9. Wound Healing Assay
2.10. Invasion Assay
2.11. Reactive Oxygen Species (ROS) Assay
2.12. Mitochondrial Membrane Potential [ΔΨm] Assay
2.13. Statistical Analysis
3. Results
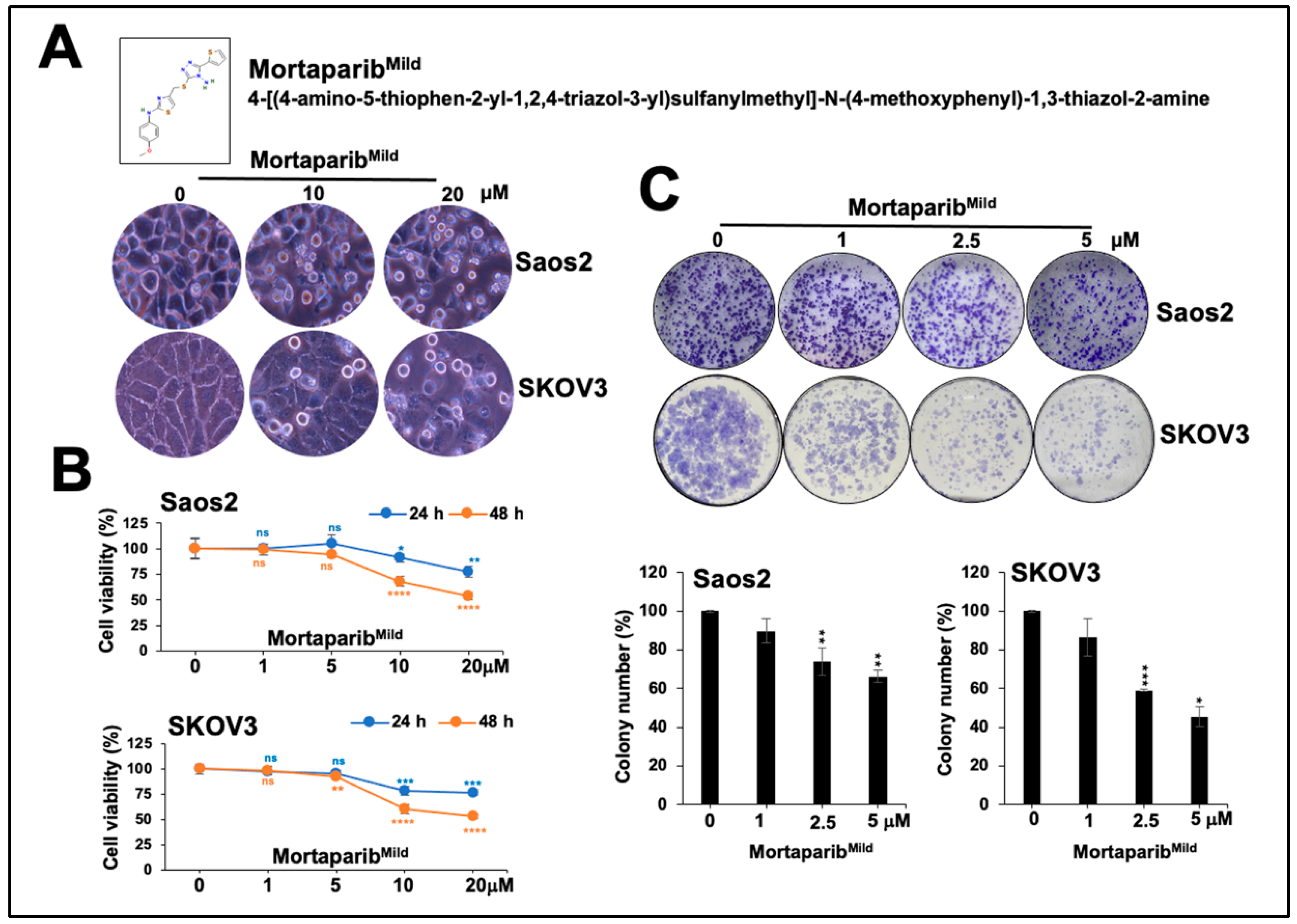
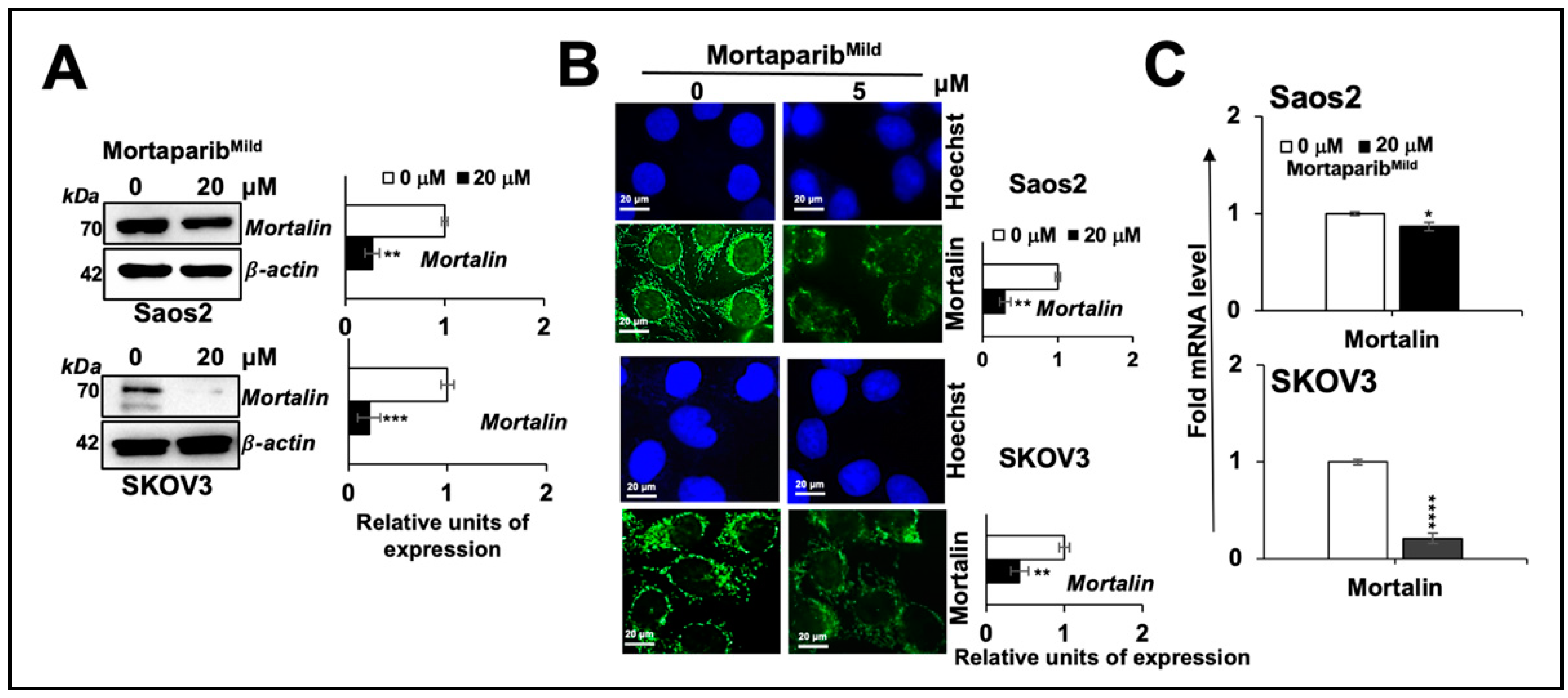
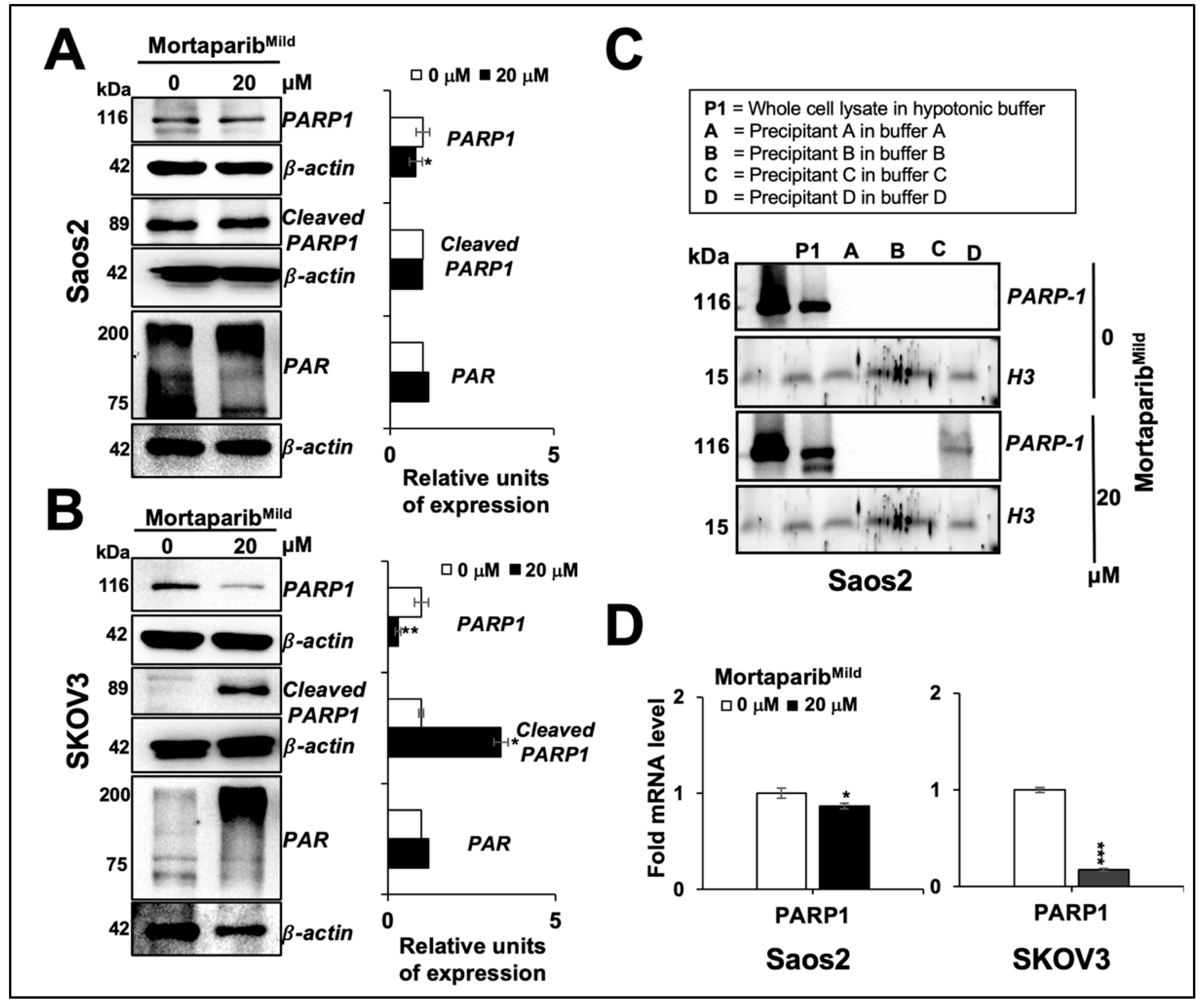
3.1. MortaparibMild Caused Growth Arrest of p53-Null Cancer Cells
3.2. MortaparibMild Caused Inhibition of Metastatic Properties of p53-Null Cancer Cells
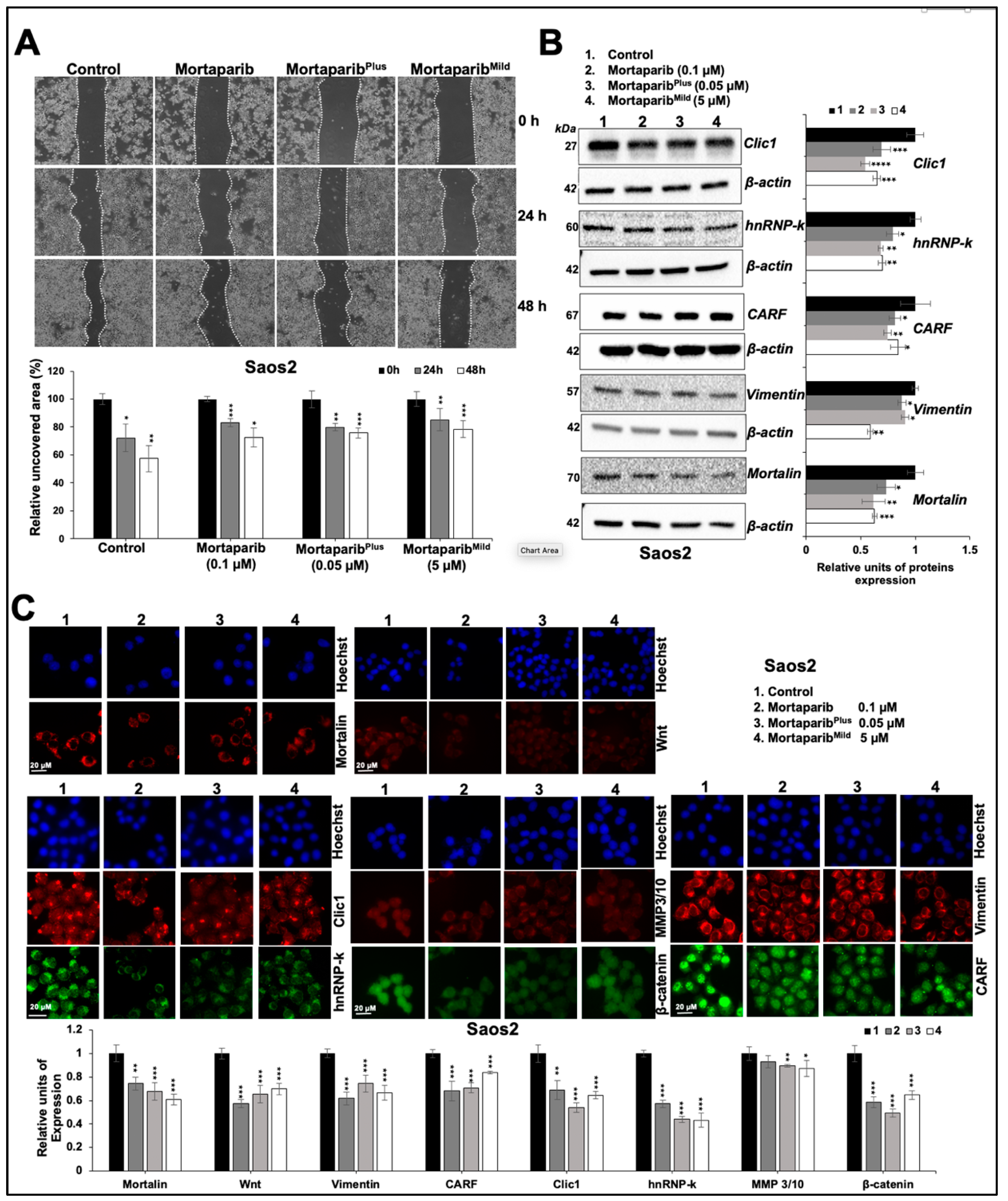
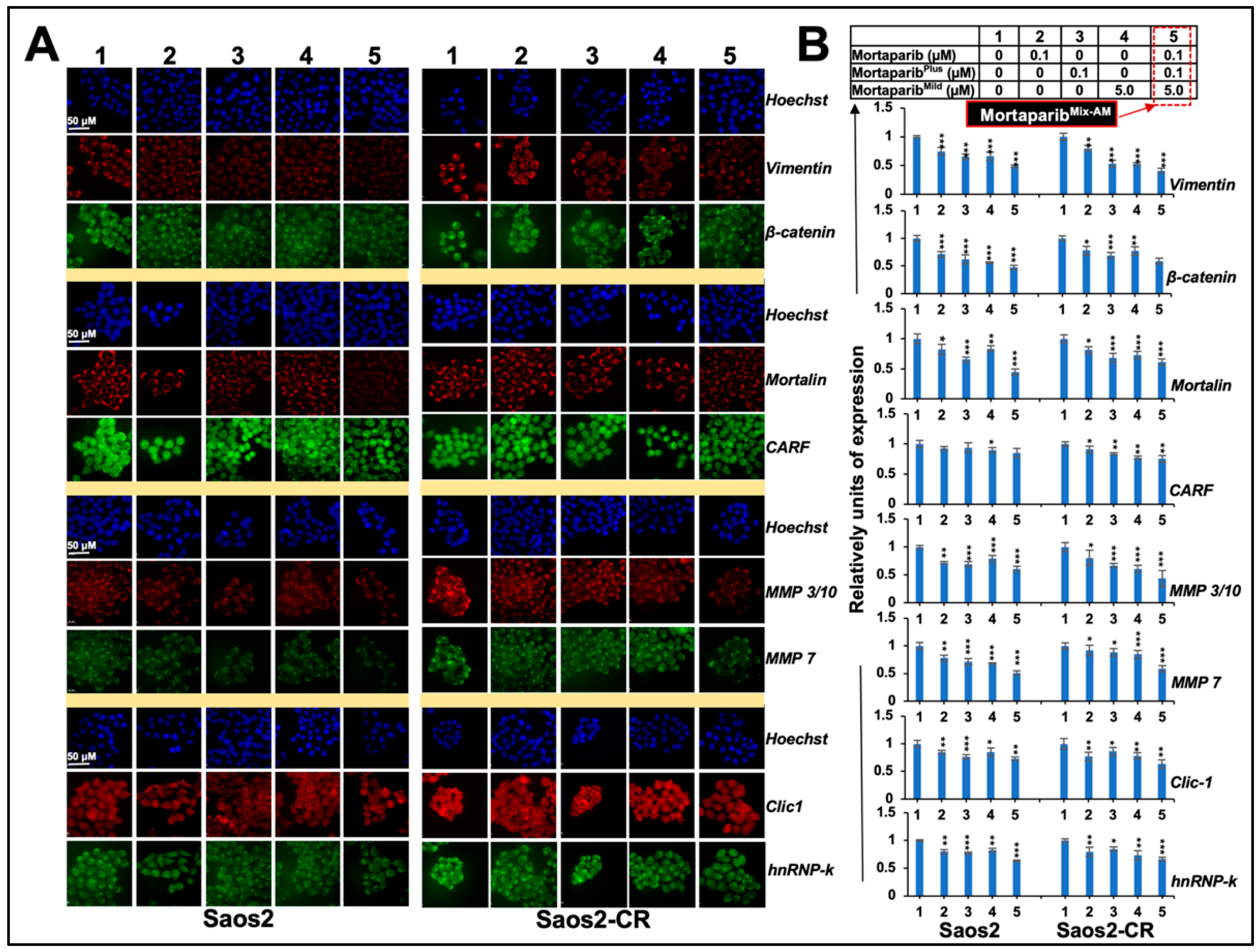
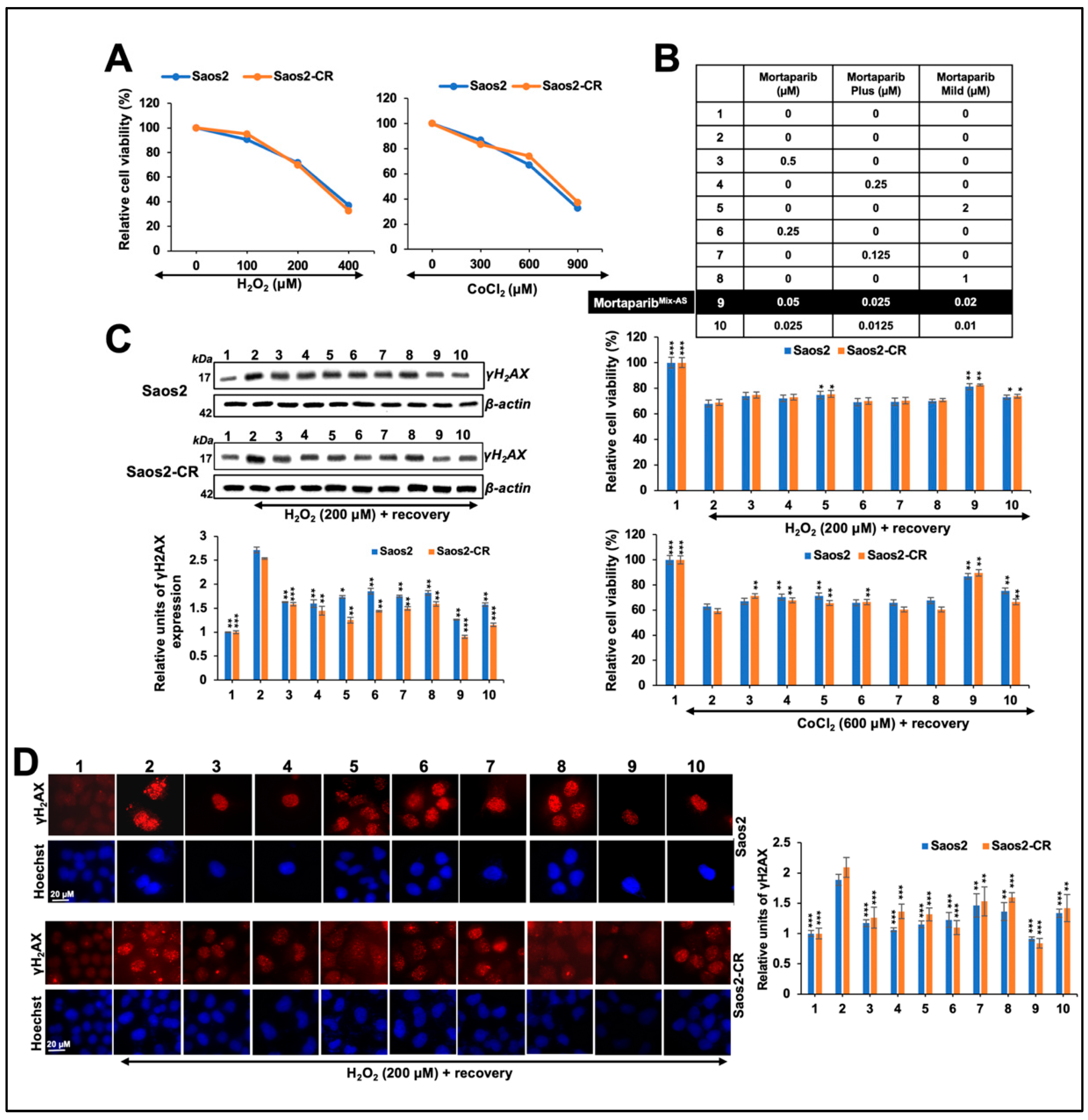
3.3. Combinations of Three Mortaparibs Offered Better Anticancer, Anti-Metastasis, and Antistress Activities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shin, D.; Lee, J.; Roh, J.L. Pioneering the future of cancer therapy: Deciphering the p53-ferroptosis nexus for precision medicine. Cancer Lett. 2024, 585, 216645. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Yang, P.; Zhang, S. Cell fate regulation governed by p53: Friends or reversible foes in cancer therapy. Cancer Commun. 2024, 44, 297–360. [Google Scholar] [CrossRef] [PubMed]
- Maphutha, J.; Twilley, D.; Lall, N. The Role of the PTEN Tumor Suppressor Gene and Its Anti-Angiogenic Activity in Melanoma and Other Cancers. Molecules 2024, 29, 721. [Google Scholar] [CrossRef] [PubMed]
- Papavassiliou, K.A.; Sofianidi, A.A.; Gogou, V.A.; Anagnostopoulos, N.; Papavassiliou, A.G. P53 and Rb Aberrations in Small Cell Lung Cancer (SCLC): From Molecular Mechanisms to Therapeutic Modulation. Int. J. Mol. Sci. 2024, 25, 2479. [Google Scholar] [CrossRef]
- Khamidullina, A.I.; Abramenko, Y.E.; Bruter, A.V.; Tatarskiy, V.V. Key Proteins of Replication Stress Response and Cell Cycle Control as Cancer Therapy Targets. Int. J. Mol. Sci. 2024, 25, 1263. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.J.; Kabeer, A.; Abbas, Z.; Siddiqui, H.A.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. Interplay of oxidative stress, cellular communication and signaling pathways in cancer. Cell Commun. Signal. 2024, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Shi, X.; Wan, J.; Zhong, X. Role of sestrins in metabolic and aging-related diseases. Biogerontology 2024, 25, 9–22. [Google Scholar] [CrossRef]
- Guan, X.; Ruan, Y.; Che, X.; Feng, W. Dual role of PRDX1 in redox-regulation and tumorigenesis: Past and future. Free Radic. Biol. Med. 2024, 210, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Somu, P.; Mohanty, S.; Basavegowda, N.; Yadav, A.K.; Paul, S.; Baek, K.H. The Interplay between Heat Shock Proteins and Cancer Pathogenesis: A Novel Strategy for Cancer Therapeutics. Cancers 2024, 16, 638. [Google Scholar] [CrossRef]
- Hu, B.; Liu, G.; Zhao, K.; Zhang, G. Diversity of extracellular HSP70 in cancer: Advancing from a molecular biomarker to a novel therapeutic target. Front. Oncol. 2024, 14, 1388999. [Google Scholar] [CrossRef]
- Yoon, A.R.; Wadhwa, R.; Kaul, S.C.; Yun, C.O. Why is Mortalin a Potential Therapeutic Target for Cancer? Front. Cell Dev. Biol. 2022, 10, 914540. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Luk, J.M.; Lee, N.P.; Peng, J.; Leng, X.; Guan, X.Y.; Lau, G.K.; Beretta, L.; Fan, S.T. Association of mortalin (HSPA9) with liver cancer metastasis and prediction for early tumor recurrence. Mol. Cell Proteom. 2008, 7, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Dundas, S.R.; Lawrie, L.C.; Rooney, P.H.; Murray, G.I. Mortalin is over-expressed by colorectal adenocarcinomas and correlates with poor survival. J. Pathol. 2005, 205, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Benbrook, D.M. SHetA2 Attack on Mortalin and Colleagues in Cancer Therapy and Prevention. Front. Cell Dev. Biol. 2022, 10, 848682. [Google Scholar] [CrossRef]
- Esfahanian, N.; Knoblich, C.D.; Bowman, G.A.; Rezvani, K. Mortalin: Protein partners, biological impacts, pathological roles, and therapeutic opportunities. Front. Cell Dev. Biol. 2023, 11, 1028519. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.; Dunmire, M.; Choi, J.; Szalai, G.; Johnson, A.; Lei, W.; Chen, X.; Liu, L.; Li, W.; Walter, M.J.; et al. HSPA9/mortalin inhibition disrupts erythroid maturation through a TP53-dependent mechanism in human CD34+ hematopoietic progenitor cells. Cell Stress Chaperones 2024, 29, 300–311. [Google Scholar] [CrossRef]
- Ao, K.; Yin, M.; Lyu, X.; Xiao, Y.; Chen, X.; Zhong, S.; Wen, X.; Yuan, J.; Ye, M.; Zhang, J.; et al. METTL3-mediated HSPA9 m6A modification promotes malignant transformation and inhibits cellular senescence by regulating exosomal mortalin protein in cervical cancer. Cancer Lett. 2024, 587, 216658. [Google Scholar] [CrossRef] [PubMed]
- Sari, A.N.; Bhargava, P.; Dhanjal, J.K.; Putri, J.F.; Radhakrishnan, N.; Shefrin, S.; Ishida, Y.; Terao, K.; Sundar, D.; Kaul, S.C.; et al. Combination of Withaferin-A and CAPE Provides Superior Anticancer Potency: Bioinformatics and Experimental Evidence to Their Molecular Targets and Mechanism of Action. Cancers 2020, 12, 1160. [Google Scholar] [CrossRef]
- Elwakeel, A. Abrogating the Interaction Between p53 and Mortalin (Grp75/HSPA9/mtHsp70) for Cancer Therapy: The Story so far. Front. Cell Dev. Biol. 2022, 10, 879632. [Google Scholar] [CrossRef]
- Na, Y.; Kaul, S.C.; Ryu, J.; Lee, J.S.; Ahn, H.M.; Kaul, Z.; Kalra, R.S.; Li, L.; Widodo, N.; Yun, C.O.; et al. Stress chaperone mortalin contributes to epithelial-mesenchymal transition and cancer metastasis. Cancer Res. 2016, 76, 2754–2765. [Google Scholar] [CrossRef]
- Wei, B.; Cao, J.; Tian, J.H.; Yu, C.Y.; Huang, Q.; Yu, J.J.; Ma, R.; Wang, J.; Xu, F.; Wang, L.B. Mortalin maintains breast cancer stem cells stemness via activation of Wnt/GSK3beta/beta-catenin signaling pathway. Am. J. Cancer Res. 2021, 11, 2696–2716. [Google Scholar] [PubMed]
- Xu, M.; Jin, T.; Chen, L.; Zhang, X.; Zhu, G.; Wang, Q.; Lin, Z. Mortalin is a distinct bio-marker and prognostic factor in serous ovarian carcinoma. Gene 2019, 696, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.K.; Hong, S.K.; Park, J.I. Steady-State Levels of Phosphorylated Mitogen-Activated Protein Kinase Kinase 1/2 Determined by Mortalin/HSPA9 and Protein Phosphatase 1 Alpha in KRAS and BRAF Tumor Cells. Mol. Cell Biol. 2017, 37, e00061-17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Meng, Z.; Wu, X.; Zhang, M.; Zhang, S.; Jin, T. Mortalin promotes breast cancer malignancy. Exp. Mol. Pathol. 2021, 118, 104593. [Google Scholar] [CrossRef] [PubMed]
- Putri, J.F.; Bhargava, P.; Dhanjal, J.K.; Yaguchi, T.; Sundar, D.; Kaul, S.C.; Wadhwa, R. Mortaparib, a novel dual inhibitor of mortalin and PARP1, is a potential drug candidate for ovarian and cervical cancers. J. Exp. Clin. Cancer Res. 2019, 38, 499. [Google Scholar] [CrossRef] [PubMed]
- Sari, A.N.; Elwakeel, A.; Dhanjal, J.K.; Kumar, V.; Sundar, D.; Kaul, S.C.; Wadhwa, R. Identification and Characterization of Mortaparib(Plus)-A Novel Triazole Derivative That Targets Mortalin-p53 Interaction and Inhibits Cancer-Cell Proliferation by Wild-Type p53-Dependent and -Independent Mechanisms. Cancers 2021, 13, 835. [Google Scholar] [CrossRef] [PubMed]
- Elwakeel, A.; Sari, A.N.; Dhanjal, J.K.; Meidinna, H.N.; Sundar, D.; Kaul, S.C.; Wadhwa, R. Mutant p53(L194F) Harboring Luminal-A Breast Cancer Cells Are Refractory to Apoptosis and Cell Cycle Arrest in Response to Mortaparib(Plus), a Multimodal Small Molecule Inhibitor. Cancers 2021, 13, 3043. [Google Scholar] [CrossRef] [PubMed]
- Meidinna, H.N.; Shefrin, S.; Sari, A.N.; Zhang, H.; Dhanjal, J.K.; Kaul, S.C.; Sundar, D.; Wadhwa, R. Identification of a new member of Mortaparib class of inhibitors that target mortalin and PARP1. Front. Cell Dev. Biol. 2022, 10, 918970. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Prakash, J.; Zhang, Z.; Kaul, S.C.; Wadhwa, R. Three-Way Cell-Based Screening of Antistress Compounds: Identification, Validation, and Relevance to Old-Age-Related Pathologies. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 1569–1577. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, R.; Wu, X.; Zhang, M.; Zhang, S.; Jin, T. Prognostic value of Mortalin correlates with roles in epithelial-mesenchymal transition and angiogenesis in lung adenocarcinoma. Carcinogenesis 2022, 43, 40–51. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, Y.; Cui, M.; Wang, X.; Lin, Z. Mortalin contributes to colorectal cancer by promoting proliferation and epithelial-mesenchymal transition. IUBMB Life 2020, 72, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Cai, J.B.; Dong, R.Z.; Liu, L.X.; Zhang, C.; Zhang, P.F.; Zou, H.; Xie, N.; Zhang, L.; Zhang, X.Y.; et al. Mortalin promotes cell proliferation and epithelial mesenchymal transition of intrahepatic cholangiocarcinoma cells in vitro. J. Clin Pathol. 2017, 70, 677–683. [Google Scholar] [CrossRef]
- Wu, P.K.; Hong, S.K.; Starenki, D.; Oshima, K.; Shao, H.; Gestwicki, J.E.; Tsai, S.; Park, J.I. Mortalin/HSPA9 targeting selectively induces KRAS tumor cell death by perturbing mitochondrial membrane permeability. Oncogene 2020, 39, 4257–4270. [Google Scholar] [CrossRef]
- Chen, J.; Liu, W.B.; Jia, W.D.; Xu, G.L.; Ma, J.L.; Huang, M.; Deng, Y.R.; Li, J.S. Overexpression of Mortalin in hepatocellular carcinoma and its relationship with angiogenesis and epithelial to mesenchymal transition. Int. J. Oncol. 2014, 44, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Rajtak, A.; Czerwonka, A.; Pitter, M.; Kotarski, J.; Okla, K. Clinical Relevance of Mortalin in Ovarian Cancer Patients. Cells 2023, 12, 701. [Google Scholar] [CrossRef] [PubMed]
- Shankaranarayana, A.H.; Meduri, B.; Pujar, G.V.; Hariharapura, R.C.; Sethu, A.K.; Singh, M.; Bidye, D. Restoration of p53 functions by suppression of mortalin-p53 sequestration: An emerging target in cancer therapy. Future Med. Chem. 2023, 15, 2087–2112. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Vishwanathan, V.; Birje, A.; Sinha, D.; D’Silva, P. Evolving paradigms on the interplay of mitochondrial Hsp70 chaperone system in cell survival and senescence. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 517–536. [Google Scholar] [CrossRef] [PubMed]
- Benbrook, D.M.; Nammalwar, B.; Long, A.; Matsumoto, H.; Singh, A.; Bunce, R.A.; Berlin, K.D. SHetA2 interference with mortalin binding to p66shc and p53 identified using drug-conjugated magnetic microspheres. Investig. New Drugs 2014, 32, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Yfantis, A.; Mylonis, I.; Simos, G. Direct interaction between mortalin and HIF-1alpha at the mitochondria inhibits apoptosis by blocking recruitment of Bax. FEBS J. 2023, 290, 3764–3780. [Google Scholar] [CrossRef]
- Walker, C.; Bottger, S.; Low, B. Mortalin-based cytoplasmic sequestration of p53 in a nonmammalian cancer model. Am. J. Pathol. 2006, 168, 1526–1530. [Google Scholar] [CrossRef]
- Li, X.; Srinivasan, S.R.; Connarn, J.; Ahmad, A.; Young, Z.T.; Kabza, A.M.; Zuiderweg, E.R.; Sun, D.; Gestwicki, J.E. Analogues of the Allosteric Heat Shock Protein 70 (Hsp70) Inhibitor, MKT-077, as Anti-Cancer Agents. ACS Med. Chem. Lett. 2013, 4, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Pagliarone, A.C.; Castaneda, E.D.; Santana, J.P.P.; de Oliveira, C.A.B.; Robeldo, T.A.; Teixeira, F.R.; Borra, R.C. Mitochondrial heat shock protein mortalin as potential target for therapies based on oxidative stress. Photodiagnosis Photodyn. Ther. 2021, 34, 102256. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.K.; Starenki, D.; Johnson, O.T.; Gestwicki, J.E.; Park, J.I. Analogs of the Heat Shock Protein 70 Inhibitor MKT-077 Suppress Medullary Thyroid Carcinoma Cells. Int. J. Mol. Sci. 2022, 23, 1063. [Google Scholar] [CrossRef] [PubMed]
- Pham, M.Q.; Tran, T.H.V.; Pham, Q.L.; Gairin, J.E. In silico analysis of the binding properties of solasonine to mortalin and p53, and in vitro pharmacological studies of its apoptotic and cytotoxic effects on human HepG2 and Hep3b hepatocellular carcinoma cells. Fundam Clin. Pharmacol. 2019, 33, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Qin, S.; Yang, Z.; Li, Z.; Liang, Q.; Long, T.; Wang, W.; Zeng, D.; Zhao, Q.; Dai, Z.; et al. Targeting the DNA repair pathway for breast cancer therapy: Beyond the molecular subtypes. Biomed. Pharmacother. 2023, 169, 115877. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Sun, Y.; Li, C.; Xue, Y.; Ba, X. Targeting the DNA Damage Response for Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 15907. [Google Scholar] [CrossRef]
- Kanev, P.B.; Atemin, A.; Stoynov, S.; Aleksandrov, R. PARP1 roles in DNA repair and DNA replication: The basi(c)s of PARP inhibitor efficacy and resistance. Semin Oncol. 2024, 51, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Morganti, S.; Marra, A.; De Angelis, C.; Toss, A.; Licata, L.; Giugliano, F.; Taurelli Salimbeni, B.; Berton Giachetti, P.P.M.; Esposito, A.; Giordano, A.; et al. PARP Inhibitors for Breast Cancer Treatment: A Review. JAMA Oncol. 2024, 10, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.K.; Hong, S.K.; Park, J.I. Mortalin depletion induces MEK/ERK-dependent and ANT/CypD-mediated death in vemurafenib-resistant B-Raf(V600E) melanoma cells. Cancer Lett. 2021, 502, 25–33. [Google Scholar] [CrossRef]
- Sane, S.; Hafner, A.; Srinivasan, R.; Masood, D.; Slunecka, J.L.; Noldner, C.J.; Hanson, A.D.; Kruisselbrink, T.; Wang, X.; Wang, Y.; et al. UBXN2A enhances CHIP-mediated proteasomal degradation of oncoprotein mortalin-2 in cancer cells. Mol. Oncol. 2018, 12, 1753–1777. [Google Scholar] [CrossRef]
- Kalra, R.S.; Chaudhary, A.; Omar, A.; Cheung, C.T.; Garg, S.; Kaul, S.C.; Wadhwa, R. Stress-induced changes in CARF expression determine cell fate to death, survival, or malignant transformation. Cell Stress Chaperones 2020, 25, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Kalra, R.S.; Chaudhary, A.; Omar, A.; Li, X.; Khurana, M.; Kaul, S.C.; Wadhwa, R. Stress-induced changes in CARF expression serve as a quantitative predictive measure of cell proliferation fate. Exp. Cell Res. 2023, 429, 113669. [Google Scholar] [CrossRef] [PubMed]
- Shiota, M.; Ikeda, Y.; Kaul, Z.; Itadani, J.; Kaul, S.C.; Wadhwa, R. Internalizing antibody-based targeted gene delivery for human cancer cells. Hum. Gene Ther. 2007, 18, 1153–1160. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wadhwa, R.; Yang, S.; Meidinna, H.N.; Sari, A.N.; Bhargava, P.; Kaul, S.C. Mixtures of Three Mortaparibs with Enhanced Anticancer, Anti-Migration, and Antistress Activities: Molecular Characterization in p53-Null Cancer Cells. Cancers 2024, 16, 2239. https://doi.org/10.3390/cancers16122239
Wadhwa R, Yang S, Meidinna HN, Sari AN, Bhargava P, Kaul SC. Mixtures of Three Mortaparibs with Enhanced Anticancer, Anti-Migration, and Antistress Activities: Molecular Characterization in p53-Null Cancer Cells. Cancers. 2024; 16(12):2239. https://doi.org/10.3390/cancers16122239
Chicago/Turabian StyleWadhwa, Renu, Shi Yang, Hazna Noor Meidinna, Anissa Nofita Sari, Priyanshu Bhargava, and Sunil C. Kaul. 2024. "Mixtures of Three Mortaparibs with Enhanced Anticancer, Anti-Migration, and Antistress Activities: Molecular Characterization in p53-Null Cancer Cells" Cancers 16, no. 12: 2239. https://doi.org/10.3390/cancers16122239
APA StyleWadhwa, R., Yang, S., Meidinna, H. N., Sari, A. N., Bhargava, P., & Kaul, S. C. (2024). Mixtures of Three Mortaparibs with Enhanced Anticancer, Anti-Migration, and Antistress Activities: Molecular Characterization in p53-Null Cancer Cells. Cancers, 16(12), 2239. https://doi.org/10.3390/cancers16122239





