Extracellular Vesicle- and Mitochondria-Based Targeting of Non-Small Cell Lung Cancer Response to Radiation: Challenges and Perspectives
Abstract
Simple Summary
Abstract
1. Introduction
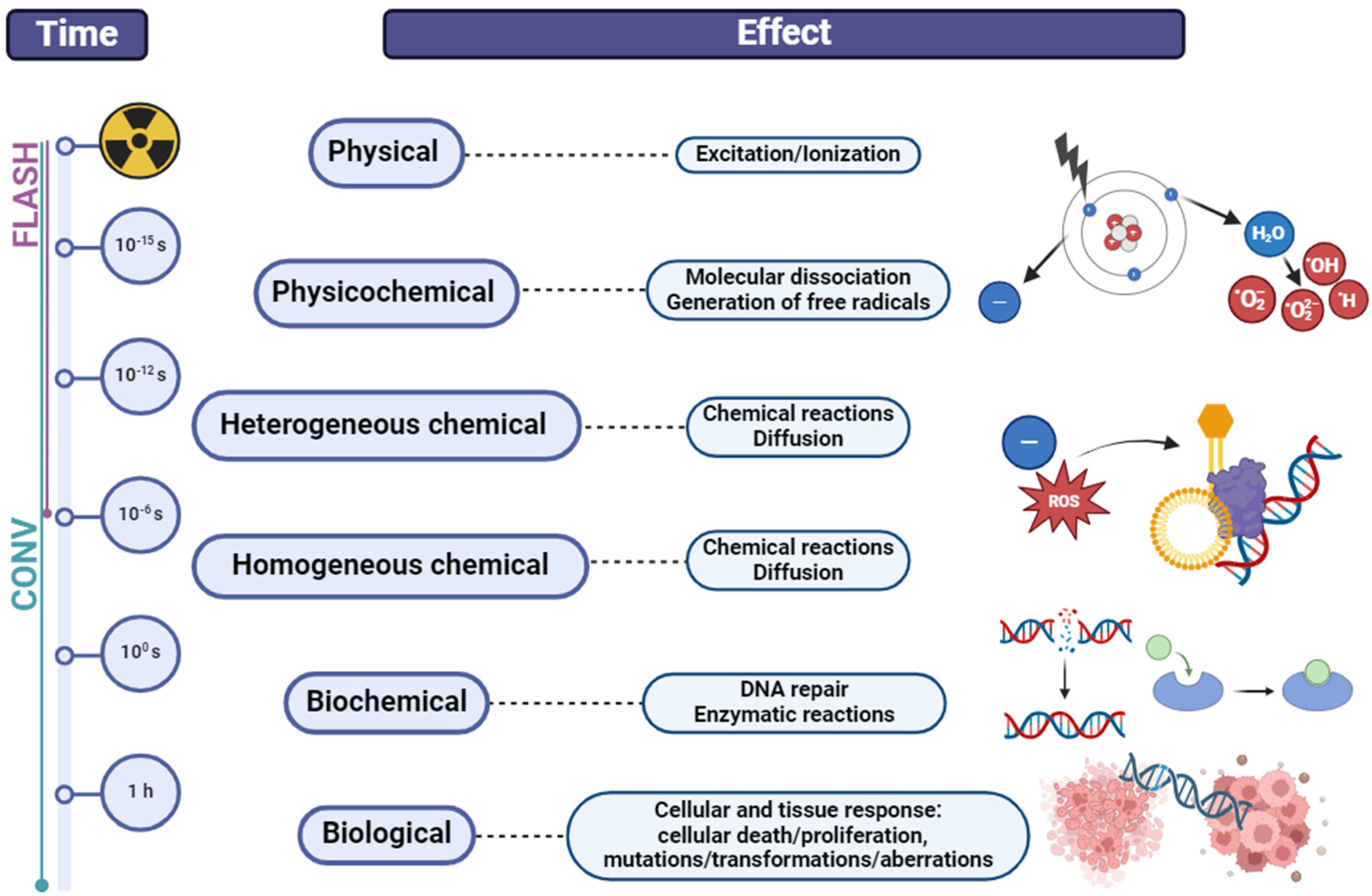
2. Modalities in Radiotherapy of Non-Small Cell Lung Cancer (NSCLC)
3. Radioresistance and Radiosensitivity—Current Controversies
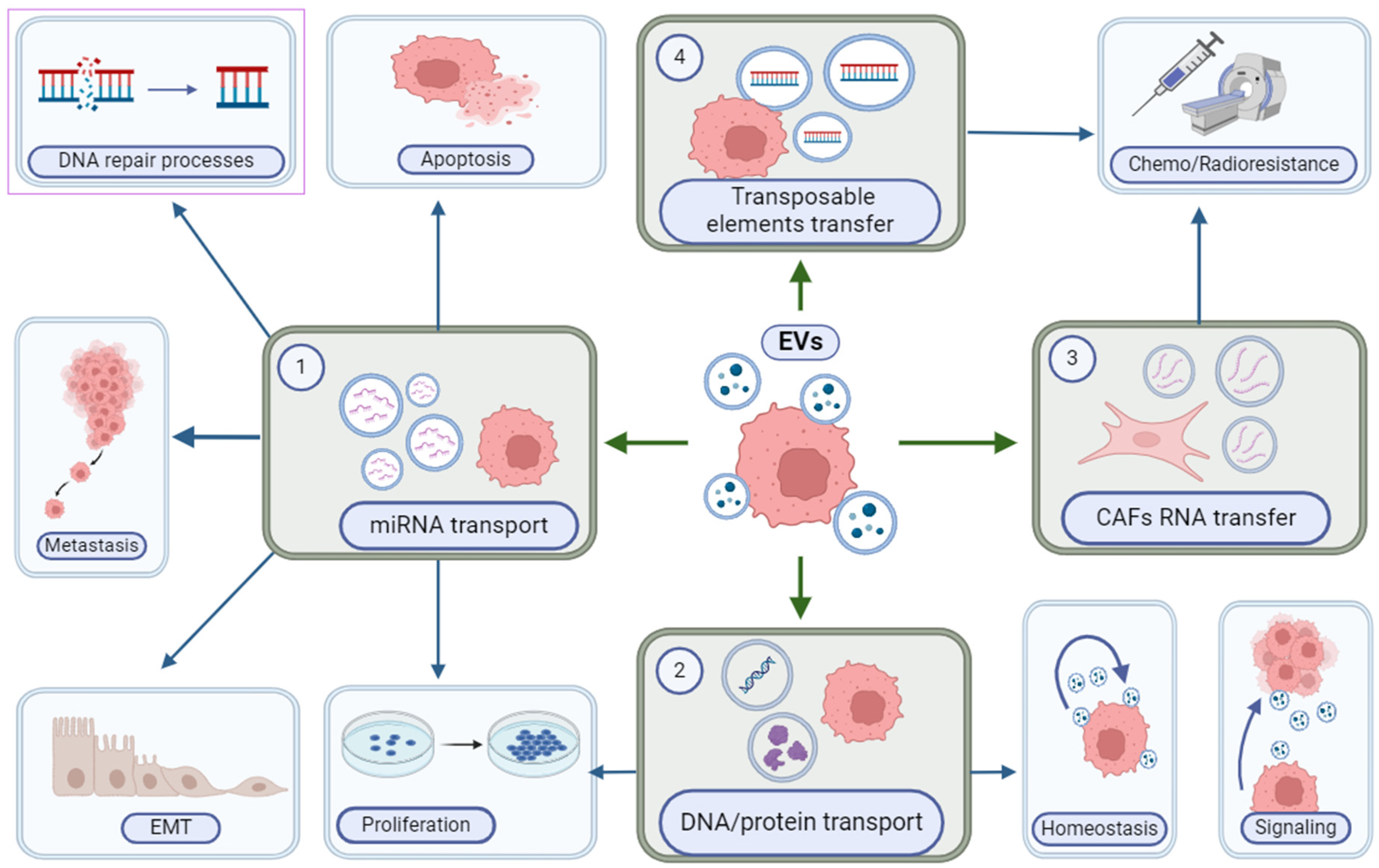
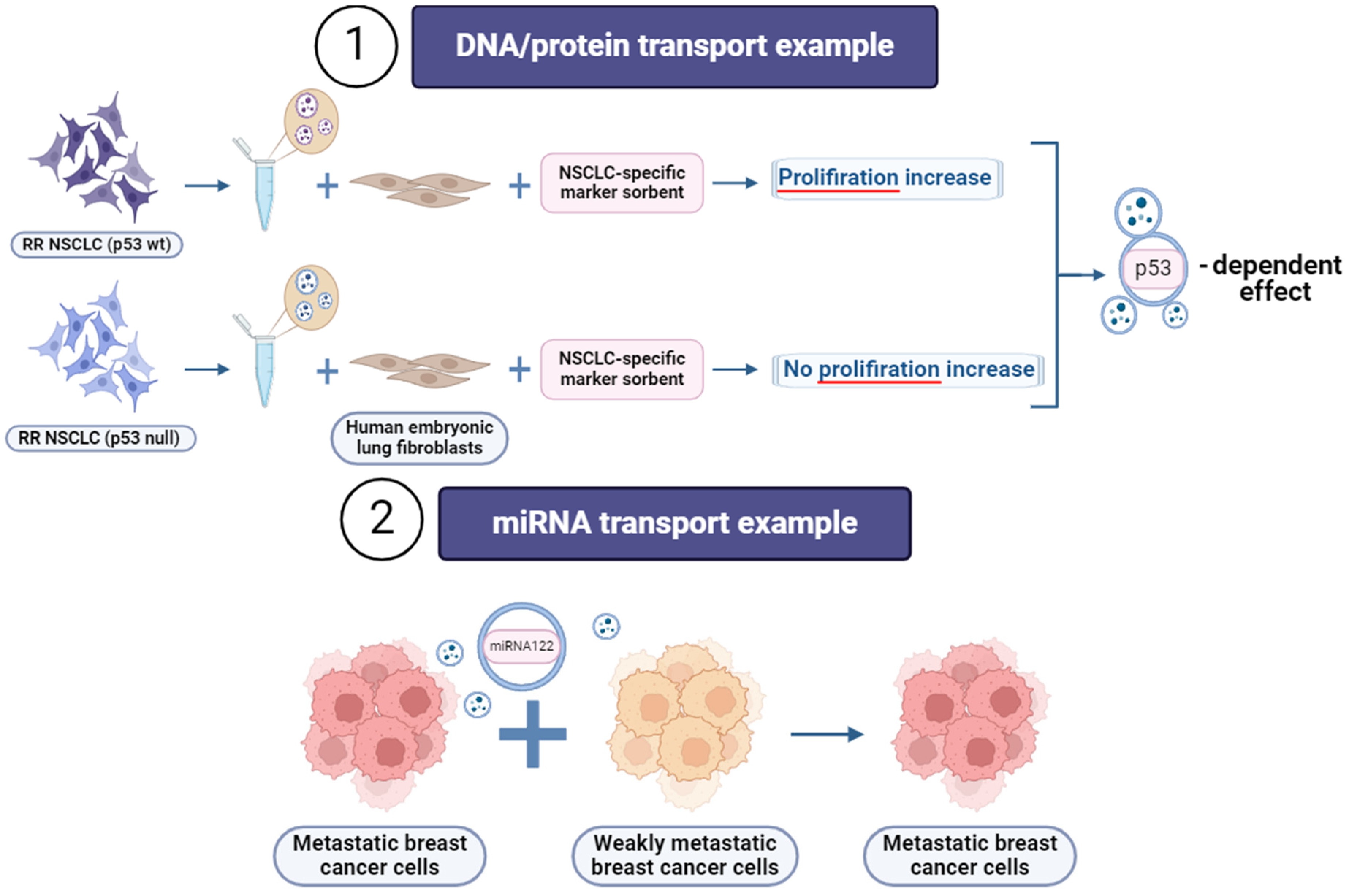
4. Extracellular Vesicles (EVs)
5. Mitochondria in Cancer Development and Progression—Extraction and Functional Characterization
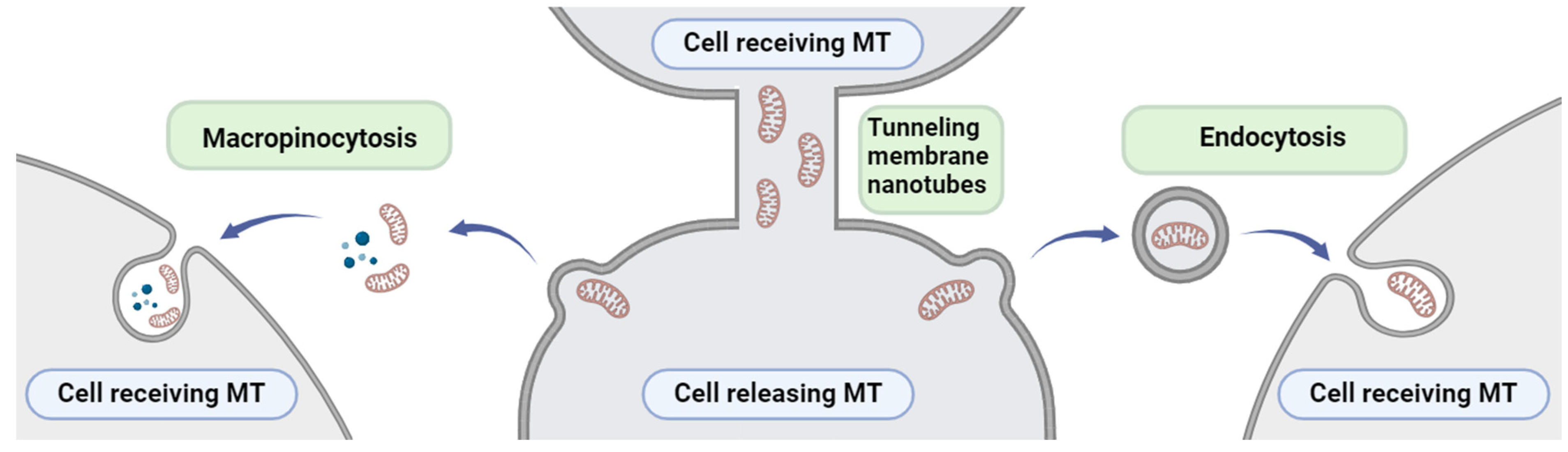
6. Mitochondria Transfer between Cells
7. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Vinod, S.K.; Hau, E. Radiotherapy Treatment for Lung Cancer: Current Status and Future Directions. Respirology 2020, 25, 61–71. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jeong, E.K.; Ju, M.K.; Jeon, H.M.; Kim, M.Y.; Kim, C.H.; Park, H.G.; Han, S.I.; Kang, H.S. Induction of Metastasis, Cancer Stem Cell Phenotype, and Oncogenic Metabolism in Cancer Cells by Ionizing Radiation. Mol. Cancer 2017, 16, 1–25. [Google Scholar] [CrossRef]
- de Miguel-Perez, D.; Ortega, F.G.; Tejada, R.G.; Martínez-Única, A.; Peterson, C.B.; Russo, A.; Gunasekaran, M.; Cardona, A.F.; Amezcua, V.; Lorente, J.A.; et al. Baseline Extracellular Vesicle MiRNA-30c and Autophagic CTCs Predict Chemoradiotherapy Resistance and Outcomes in Patients with Lung Cancer. Biomark. Res. 2023, 11, 98. [Google Scholar] [CrossRef]
- Zheng, Q.; Ding, H.; Wang, L.; Yan, Y.; Wan, Y.; Yi, Y.; Tao, L.; Zhu, C. Circulating Exosomal MiR-96 as a Novel Biomarker for Radioresistant Non-Small-Cell Lung Cancer. J. Oncol. 2021, 2021, 5893981. [Google Scholar] [CrossRef]
- Pustovalova, M.; Astrelina, T.A.; Grekhova, A.; Vorobyeva, N.; Tsvetkova, A.; Blokhina, T.; Nikitina, V.; Suchkova, Y.; Usupzhanova, D.; Brunchukov, V.; et al. Residual ΓH2AX Foci Induced by Low Dose X-ray Radiation in Bone Marrow Mesenchymal Stem Cells Do Not Cause Accelerated Senescence in the Progeny of Irradiated Cells. Aging 2017, 9, 2397–2410. [Google Scholar] [CrossRef][Green Version]
- Tesei, A.; Arienti, C.; Bossi, G.; Santi, S.; De Santis, I.; Bevilacqua, A.; Zanoni, M.; Pignatta, S.; Cortesi, M.; Zamagni, A.; et al. TP53 Drives Abscopal Effect by Secretion of Senescence-Associated Molecular Signals in Non-Small Cell Lung Cancer. J. Exp. Clin. Cancer Res. 2021, 40, 89. [Google Scholar] [CrossRef]
- Bian, C.; Zheng, Z.; Su, J.; Wang, H.; Chang, S.; Xin, Y.; Jiang, X. Targeting Mitochondrial Metabolism to Reverse Radioresistance: An Alternative to Glucose Metabolism. Antioxidants 2022, 11, 2202. [Google Scholar] [CrossRef]
- Lin, Y.T.; Chen, S.T.; Chang, J.C.; Teoh, R.J.; Liu, C.S.; Wang, G.J. Green Extraction of Healthy and Additive Free Mitochondria with a Conventional Centrifuge. Lab Chip 2019, 19, 3862–3869. [Google Scholar] [CrossRef]
- Gkerküçük, E.B.; Tramier, M.; Bertolin, G. Imaging Mitochondrial Functions: From Fluorescent Dyes to Genetically-Encoded Sensors. Genes 2020, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, L.X.; Silva-Almeida, C.; Rondeau, J.D.; Sonveaux, P. Mitochondrial Transfer in Cancer: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 3245. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The Ever-Increasing Importance of Cancer as a Leading Cause of Premature Death Worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Sleeman, J.P.; Nazarenko, I.; Thiele, W. Do All Roads Lead to Rome? Routes to Metastasis Development. Int. J. Cancer 2011, 128, 2511–2526. [Google Scholar] [CrossRef] [PubMed]
- Cairns, R.A.; Khokha, R.; Hill, R.P. Molecular Mechanisms of Tumor Invasion and Metastasis: An Integrated View. Curr. Mol. Med. 2003, 3, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Merkher, Y.; Kontareva, E.; Bogdan, E.; Achkasov, K.; Maximova, K.; Grolman, J.M.; Leonov, S. Encapsulation and Adhesion of Nanoparticles as a Potential Biomarker for TNBC Cells Metastatic Propensity. Sci. Rep. 2023, 13, 12289. [Google Scholar] [CrossRef] [PubMed]
- Casal-Mouriño, A.; Ruano-Ravina, A.; Lorenzo-González, M.; Rodríguez-Martínez, Á.; Giraldo-Osorio, A.; Varela-Lema, L.; Pereiro-Brea, T.; Miguel Barros-Dios, J.; Valdés-Cuadrado, L.; Pérez-Ríos, M. Epidemiology of Stage III Lung Cancer: Frequency, Diagnostic Characteristics, and Survival. Transl. Lung Cancer Res. 2021, 10, 506–518. [Google Scholar] [CrossRef] [PubMed]
- American Lung Association. State of Lung Cancer; American Lung Association: Chicago, IL, USA, 2023. [Google Scholar]
- Olivares-Urbano, M.A.; Griñán-Lisón, C.; Marchal, J.A.; Núñez, M.I. CSC Radioresistance: A Therapeutic Challenge to Improve Radiotherapy Effectiveness in Cancer. Cells 2020, 9, 1651. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.R.; Mangesius, J.; Skvortsova, I.I.; Ganswindt, U. The Role of Cancer Stem Cells in Radiation Resistance. Front. Oncol. 2020, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Gao, F.; Yang, Y.; Wu, D.; Zhang, Y.; Feng, G.; Dai, T.; Du, X. FLASH Radiotherapy: History and Future. Front. Oncol. 2021, 11, 644400. [Google Scholar] [CrossRef] [PubMed]
- Smyth, L.M.L.; Donoghue, J.F.; Ventura, J.A.; Livingstone, J.; Bailey, T.; Day, L.R.J.; Crosbie, J.C.; Rogers, P.A.W. Comparative Toxicity of Synchrotron and Conventional Radiation Therapy Based on Total and Partial Body Irradiation in a Murine Model. Sci. Rep. 2018, 8, 12044. [Google Scholar] [CrossRef]
- Hughes, J.R.; Parsons, J.L. FLASH Radiotherapy: Current Knowledge and Future Insights Using Proton-Beam Therapy. Int. J. Mol. Sci. 2020, 21, 6492. [Google Scholar] [CrossRef]
- Wei, H.J.; Upadhyayula, P.S.; Pouliopoulos, A.N.; Englander, Z.K.; Zhang, X.; Jan, C.I.; Guo, J.; Mela, A.; Zhang, Z.; Wang, T.J.C.; et al. Focused Ultrasound-Mediated Blood-Brain Barrier Opening Increases Delivery and Efficacy of Etoposide for Glioblastoma Treatment. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, J.; Montay-Gruel, P.; Gonçalves Jorge, P.; Bailat, C.; Petit, B.; Ollivier, J.; Jeanneret-Sozzi, W.; Ozsahin, M.; Bochud, F.; Moeckli, R.; et al. Clinical Translation of FLASH Radiotherapy: Why and How? Radiother. Oncol. 2019, 139, 11–17. [Google Scholar] [CrossRef]
- Bourhis, J.; Sozzi, W.J.; Jorge, P.G.; Gaide, O.; Bailat, C.; Duclos, F.; Patin, D.; Ozsahin, M.; Bochud, F.; Germond, J.F.; et al. Treatment of a First Patient with FLASH-Radiotherapy. Radiother. Oncol. 2019, 139, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh Dose-Rate FLASH Irradiation Increases the Differential Response between Normal and Tumor Tissue in Mice. Sci. Transl. Med. 2014, 6, 245ra93. [Google Scholar] [CrossRef] [PubMed]
- Petersson, K.; Adrian, G.; Butterworth, K.; McMahon, S.J. A Quantitative Analysis of the Role of Oxygen Tension in FLASH Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Montay-Gruel, P.; Acharya, M.M.; Petersson, K.; Alikhani, L.; Yakkala, C.; Allen, B.D.; Ollivier, J.; Petit, B.; Jorge, P.G.; Syage, A.R.; et al. Long-Term Neurocognitive Benefits of FLASH Radiotherapy Driven by Reduced Reactive Oxygen Species. Proc. Natl. Acad. Sci. USA 2019, 166, 10943–10951. [Google Scholar] [CrossRef]
- Fernet, M.; Ponette, V.; Deniaud-Alexandre, E.; Menissier-De Murcia, J.; De Murcia, G.; Giocanti, N.; Megnin-Chanet, F.; Favaudon, V. Poly(ADP-Ribose) Polymerase, a Major Determinant of Early Cell Response to Ionizing Radiation. Int. J. Radiat. Biol. 2000, 76, 1621–1629. [Google Scholar] [CrossRef]
- Ponette, V.; Le Pechoux, C.; Deniaud-Alexandre, E.; Fernet, M.; Giocanti, N.; Tourbez, H.; Favaudon, V. Hyperfast, Early Cell Response to Ionizing Radiation. Int. J. Radiat. Biol. 2000, 76, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Matuszak, N.; Suchorska, W.M.; Milecki, P.; Kruszyna-Mochalska, M.; Misiarz, A.; Pracz, J.; Malicki, J. FLASH Radiotherapy: An Emerging Approach in Radiation Therapy. Reports Pract. Oncol. Radiother. 2022, 27, 344–351. [Google Scholar] [CrossRef]
- Dietzel, F. Strahlensensibilisierung von Tumorzellen Durch Mikrowellen-Ende Des Sauerstoffproblems? Naturwissenschaften 1975, 62, 44–45. [Google Scholar] [CrossRef]
- Fu, C.; Zhou, H.; Tan, L.; Huang, Z.; Wu, Q.; Ren, X.; Ren, J.; Meng, X. Microwave-Activated Mn-Doped Zirconium Metal-Organic Framework Nanocubes for Highly Effective Combination of Microwave Dynamic and Thermal Therapies Against Cancer. ACS Nano 2018, 12, 2201–2210. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Schuchert, M.J.; Pennathur, A.; Gilbert, S.; Luketich, J.D. Ablative Treatments for Lung Tumors: Radiofrequency Ablation, Stereotactic Radiosurgery, and Microwave Ablation. Thorac. Surg. Clin. 2007, 17, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Livraghi, T.; Meloni, F.; Solbiati, L.; Zanus, G. Complications of Microwave Ablation for Liver Tumors: Results of a Multicenter Study. Cardiovasc. Intervent. Radiol. 2012, 35, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Kim, J.; Kim, J.; Kim, B.; Lee, J.; Kim, Y.; Li, M.; Kang, H.; Kim, J.S. Cancer Therapeutics Based on Diverse Energy Sources. Chem. Soc. Rev. 2022, 51, 8201–8215. [Google Scholar] [CrossRef] [PubMed]
- Komoshvili, K.; Becker, T.; Levitan, J.; Yahalom, A.; Barbora, A.; Liberman-Aronov, S. Morphological Changes in H1299 Human Lung Cancer Cells Following W-Band Millimeter-Wave Irradiation. Appl. Sci. 2020, 10, 3187. [Google Scholar] [CrossRef]
- Song, X.L.; Wang, C.H.; Hu, H.Y.; Yu, C.; Bai, C. Microwave Induces Apoptosis in A549 Human Lung Carcinoma Cell Line. Chin. Med. J. 2011, 124, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Rana, J.N.; Mumtaz, S.; Choi, E.H.; Han, I. ROS Production in Response to High-Power Microwave Pulses Induces P53 Activation and DNA Damage in Brain Cells: Radiosensitivity and Biological Dosimetry Evaluation. Front. Cell Dev. Biol. 2023, 11, 1067861. [Google Scholar] [CrossRef]
- Rana, J.N.; Mumtaz, S.; Han, I.; Choi, E.H. Formation of Reactive Species via High Power Microwave Induced DNA Damage and Promoted Intrinsic Pathway-Mediated Apoptosis in Lung Cancer Cells: An in Vitro Investigation. Fundam. Res. 2024; In Press, Corrected proofs. [Google Scholar] [CrossRef]
- Simon, C.J.; Dupuy, D.E.; Mayo-Smith, W.W. Microwave Ablation: Principles and Applications. In Proceedings of the Radiographics; RSNA: Phoenix, AZ, USA, 2005; Volume 25. [Google Scholar] [CrossRef]
- Shaverdian, N.; Lisberg, A.E.; Bornazyan, K.; Veruttipong, D.; Goldman, J.W.; Formenti, S.C.; Garon, E.B.; Lee, P. Previous Radiotherapy and the Clinical Activity and Toxicity of Pembrolizumab in the Treatment of Non-Small-Cell Lung Cancer: A Secondary Analysis of the KEYNOTE-001 Phase 1 Trial. Lancet Oncol. 2017, 18, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.M.; Ten Haken, R.K.; Schipper, M.; Frey, K.A.; Hayman, J.; Gross, M.; Ramnath, N.; Hassan, K.A.; Matuszak, M.; Ritter, T.; et al. Effect of Midtreatment PET/CT-Adapted Radiation Therapy with Concurrent Chemotherapy in Patients with Locally Advanced Non–Small-Cell Lung Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2017, 3, 1358–1365. [Google Scholar] [CrossRef]
- Sun, A.; Hu, C.; Wong, S.J.; Gore, E.; Videtic, G.; Dutta, S.; Suntharalingam, M.; Chen, Y.; Gaspar, L.E.; Choy, H. Prophylactic Cranial Irradiation vs Observation in Patients with Locally Advanced Non-Small Cell Lung Cancer: A Long-Term Update of the NRG Oncology/RTOG 0214 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 847–855. [Google Scholar] [CrossRef]
- Petty, W.J.; Urbanic, J.J.; Ahmed, T.; Hughes, R.; Levine, B.; Rusthoven, K.; Papagikos, M.; Ruiz, J.R.; Lally, B.E.; Chan, M.; et al. Long-Term Outcomes of a Phase 2 Trial of Chemotherapy With Consolidative Radiation Therapy for Oligometastatic Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 527–535. [Google Scholar] [CrossRef]
- Pustovalova, M.; Alhaddad, L.; Smetanina, N.; Chigasova, A.; Blokhina, T.; Chuprov-Netochin, R.; Osipov, A.N.; Leonov, S. The P53–53bp1-Related Survival of A549 and H1299 Human Lung Cancer Cells after Multifractionated Radiotherapy Demonstrated Different Response to Additional Acute x-Ray Exposure. Int. J. Mol. Sci. 2020, 21, 3342. [Google Scholar] [CrossRef] [PubMed]
- Ingram, W.J.; Crowther, L.M.; Little, E.B.; Freeman, R.; Harliwong, I.; Veleva, D.; Hassall, T.E.; Remke, M.; Taylor, M.D.; Hallahan, A.R. ABC Transporter Activity Linked to Radiation Resistance and Molecular Subtype in Pediatric Medulloblastoma. Exp. Hematol. Oncol. 2013, 2, 26. [Google Scholar] [CrossRef]
- Belkahla, S.; Khan, A.U.H.; Gitenay, D.; Alexia, C.; Gondeau, C.; Vo, D.N.; Orecchioni, S.; Talarico, G.; Bertolini, F.; Cartron, G.; et al. Changes in Metabolism Affect Expression of ABC Transporters through ERK5 and Depending on P53 Status. Oncotarget 2018, 9, 1114–1129. [Google Scholar] [CrossRef]
- Prabavathy, D.; Swarnalatha, Y.; Ramadoss, N. Lung Cancer Stem Cells-Origin, Characteristics and Therapy. Stem Cell Investig. 2018, 5, 6. [Google Scholar] [CrossRef]
- Gupta, A.; Hunt, C.R.; Chakraborty, S.; Pandita, R.K.; Yordy, J.; Ramnarain, D.B.; Horikoshi, N.; Pandita, T.K. Role of 53BP1 in the Regulation of DNA Double-Strand Break Repair Pathway Choice. Radiat. Res. 2014, 181, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, L.; Chen, C.; Ming, P.; Huang, Q.; Li, C.; Cao, D.; Xu, X.; Ge, W. The Extracellular Vesicles Secreted by Lung Cancer Cells in Radiation Therapy Promote Endothelial Cell Angiogenesis by Transferring MiR-23a. PeerJ 2017, 2017, e3627. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, X.; Yi, X. The Effects of A549 and H1299 Cell-Derived Exosomes on the Proliferation and Apoptosis of BEAS-2B Cells. Pharmazie 2021, 76, 379–387. [Google Scholar] [CrossRef]
- Sun, C.; Liu, X.; Wang, B.; Wang, Z.; Liu, Y.; Di, C.; Si, J.; Li, H.; Wu, Q.; Xu, D.; et al. Endocytosis-Mediated Mitochondrial Transplantation: Transferring Normal Human Astrocytic Mitochondria into Glioma Cells Rescues Aerobic Respiration and Enhances Radiosensitivity. Theranostics 2019, 9, 3595–3607. [Google Scholar] [CrossRef]
- Saha, T.; Dash, C.; Jayabalan, R.; Khiste, S.; Kulkarni, A.; Kurmi, K.; Mondal, J.; Majumder, P.K.; Bardia, A.; Jang, H.L.; et al. Intercellular Nanotubes Mediate Mitochondrial Trafficking between Cancer and Immune Cells. Nat. Nanotechnol. 2022, 17, 98–106. [Google Scholar] [CrossRef]
- Ramirez, R.D.; Sheridan, S.; Girard, L.; Sato, M.; Kim, Y.; Pollack, J.; Peyton, M.; Zou, Y.; Kurie, J.M.; DiMaio, J.M.; et al. Immortalization of Human Bronchial Epithelial Cells in the Absence of Viral Oncoproteins. Cancer Res. 2004, 64, 9027–9034. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Vaughan, M.B.; Girard, L.; Peyton, M.; Lee, W.; Shames, D.S.; Ramirez, R.D.; Sunaga, N.; Gazdar, A.F.; Shay, J.W.; et al. Multiple Oncogenic Changes (K-RASV12, P53 Knockdown, Mutant EGFRs, P16 Bypass, Telomerase) Are Not Sufficient to Confer a Full Malignant Phenotype on Human Bronchial Epithelial Cells. Cancer Res. 2006, 66, 2116–2128. [Google Scholar] [CrossRef] [PubMed]
- Leonov, S.; Inyang, O.; Achkasov, K.; Bogdan, E.; Kontareva, E.; Chen, Y.; Fu, Y.; Osipov, A.N.; Pustovalova, M.; Merkher, Y.; et al. Proteomic Markers for Mechanobiological Properties of Metastatic Cancer Cells. Int. J. Mol. Sci. 2023, 24, 4773. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Zhang, X.; Bai, J.; Li, Y.; Wang, X.; Zhou, Y. Tissue-Specific and Exosomal MiRNAs in Lung Cancer Radiotherapy: From Regulatory Mechanisms to Clinical Implications. Cancer Manag. Res. 2019, 11, 4413–4424. [Google Scholar] [CrossRef]
- Li, C.; Zhou, T.; Chen, J.; Li, R.; Chen, H.; Luo, S.; Chen, D.; Cai, C.; Li, W. The Role of Exosomal MiRNAs in Cancer. J. Transl. Med. 2022, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Melo, S.A.; Sugimoto, H.; O’Connell, J.T.; Kato, N.; Villanueva, A.; Vidal, A.; Qiu, L.; Vitkin, E.; Perelman, L.T.; Melo, C.A.; et al. Cancer Exosomes Perform Cell-Independent MicroRNA Biogenesis and Promote Tumorigenesis. Cancer Cell 2014, 26, 707–721. [Google Scholar] [CrossRef] [PubMed]
- Abd Elmageed, Z.Y.; Yang, Y.; Thomas, R.; Ranjan, M.; Mondal, D.; Moroz, K.; Fang, Z.; Rezk, B.M.; Moparty, K.; Sikka, S.C.; et al. Neoplastic Reprogramming of Patient-Derived Adipose Stem Cells by Prostate Cancer Cell-Associated Exosomes. Stem Cells 2014, 32, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Le, M.T.N.; Hamar, P.; Guo, C.; Basar, E.; Perdigão-Henriques, R.; Balaj, L.; Lieberman, J. MiR-200-Containing Extracellular Vesicles Promote Breast Cancer Cell Metastasis. J. Clin. Investig. 2014, 124, 5109–5128. [Google Scholar] [CrossRef]
- Fong, M.Y.; Zhou, W.; Liu, L.; Alontaga, A.Y.; Chandra, M.; Ashby, J.; Chow, A.; O’Connor, S.T.F.; Li, S.; Chin, A.R.; et al. Breast-Cancer-Secreted MiR-122 Reprograms Glucose Metabolism in Premetastatic Niche to Promote Metastasis. Nat. Cell Biol. 2015, 17, 183–194. [Google Scholar] [CrossRef]
- Luga, V.; Zhang, L.; Viloria-Petit, A.M.; Ogunjimi, A.A.; Inanlou, M.R.; Chiu, E.; Buchanan, M.; Hosein, A.N.; Basik, M.; Wrana, J.L. Exosomes Mediate Stromal Mobilization of Autocrine Wnt-PCP Signaling in Breast Cancer Cell Migration. Cell 2012, 151, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Boelens, M.C.; Wu, T.J.; Nabet, B.Y.; Xu, B.; Qiu, Y.; Yoon, T.; Azzam, D.J.; Twyman-Saint Victor, C.; Wiemann, B.Z.; Ishwaran, H.; et al. Exosome Transfer from Stromal to Breast Cancer Cells Regulates Therapy Resistance Pathways. Cell 2014, 159, 499–513. [Google Scholar] [CrossRef]
- Ciravolo, V.; Huber, V.; Ghedini, G.C.; Venturelli, E.; Bianchi, F.; Campiglio, M.; Morelli, D.; Villa, A.; Della Mina, P.; Menard, S.; et al. Potential Role of HER2-Overexpressing Exosomes in Countering Trastuzumab-Based Therapy. J. Cell. Physiol. 2012, 227, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Cesselli, D.; Parisse, P.; Aleksova, A.; Veneziano, C.; Cervellin, C.; Zanello, A.; Beltrami, A.P. Extracellular Vesicles: How Drug and Pathology Interfere with Their Biogenesis and Function. Front. Physiol. 2018, 9, 1394. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Sharifi, M. Exosomal MiRNAs as Novel Cancer Biomarkers: Challenges and Opportunities. J. Cell. Physiol. 2018, 233, 6370–6380. [Google Scholar] [CrossRef]
- Yeo, R.W.Y.; Lai, R.C.; Zhang, B.; Tan, S.S.; Yin, Y.; Teh, B.J.; Lim, S.K. Mesenchymal Stem Cell: An Efficient Mass Producer of Exosomes for Drug Delivery. Adv. Drug Deliv. Rev. 2013, 65, 336–341. [Google Scholar] [CrossRef]
- Katakowski, M.; Buller, B.; Zheng, X.; Lu, Y.; Rogers, T.; Osobamiro, O.; Shu, W.; Jiang, F.; Chopp, M. Exosomes from Marrow Stromal Cells Expressing MiR-146b Inhibit Glioma Growth. Cancer Lett. 2013, 335, 201–204. [Google Scholar] [CrossRef]
- Kordelas, L.; Rebmann, V.; Ludwig, A.K.; Radtke, S.; Ruesing, J.; Doeppner, T.R.; Epple, M.; Horn, P.A.; Beelen, D.W.; Giebel, B. MSC-Derived Exosomes: A Novel Tool to Treat Therapy-Refractory Graft-versus-Host Disease. Leukemia 2014, 28, 970–973. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wang, Z.; Liu, Y.; Liu, Y.; Li, H.; Di, C.; Wu, Z.; Gan, L.; Zhang, H. Carbon Ion Beams Induce Hepatoma Cell Death by NADPH Oxidase-Mediated Mitochondrial Damage. J. Cell. Physiol. 2014, 229, 100–107. [Google Scholar] [CrossRef]
- Elliott, R.; Barnett, B. Ultrastructural Observation of Mitochondria in Human Breast Carcinoma Cells. Microsc. Microanal. 2011, 17, 194–195. [Google Scholar] [CrossRef][Green Version]
- Cloos, C.R.; Daniels, D.H.; Kalen, A.; Matthews, K.; Du, J.; Goswami, P.C.; Cullen, J.J. Mitochondrial DNA Depletion Induces Radioresistance by Suppressing G 2 Checkpoint Activation in Human Pancreatic Cancer Cells. Radiat. Res. 2009, 171, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Pouliquen, D.; Boissard, A.; Coqueret, O.; Guette, C. Biomarkers of Tumor Invasiveness in Proteomics (Review). Int. J. Oncol. 2020, 57, 409–432. [Google Scholar] [CrossRef] [PubMed]
- Corcelli, A.; Saponetti, M.S.; Zaccagnino, P.; Lopalco, P.; Mastrodonato, M.; Liquori, G.E.; Lorusso, M. Mitochondria Isolated in Nearly Isotonic KCl Buffer: Focus on Cardiolipin and Organelle Morphology. Biochim. Biophys. Acta-Biomembr. 2010, 1798, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Nossal, N.G.; Heppel, L.A. The Release of Enzymes by Osmotic Shock from Escherichia Coli in Exponential Phase. J. Biol. Chem. 1966, 241, 3055–3062. [Google Scholar] [CrossRef]
- Belter, P.A.; Cussler, E.L.; Hu, W.-S. Bioseparations Downstream Processing for Biotechnology, Reprint Edition; Wiley: Hoboken, NJ, USA, 1994; ISBN 978-0-471-12113-8. [Google Scholar]
- Gross, V.S.; Greenberg, H.K.; Baranov, S.V.; Carlson, G.M.; Stavrovskaya, I.G.; Lazarev, A.V.; Kristal, B.S. Isolation of Functional Mitochondria from Rat Kidney and Skeletal Muscle without Manual Homogenization. Anal. Biochem. 2011, 418, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Hornig-Do, H.T.; Günther, G.; Bust, M.; Lehnartz, P.; Bosio, A.; Wiesner, R.J. Isolation of Functional Pure Mitochondria by Superparamagnetic Microbeads. Anal. Biochem. 2009, 389, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.H.; Xiao, Q.; Zhao, S.; Qu, F.; Chang, C.; Wei, A.C.; Ho, Y.P. Demarcating the Membrane Damage for the Extraction of Functional Mitochondria. Microsystems Nanoeng. 2018, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Azimzadeh, P.; Aghdaei, H.A.; Tarban, P.; Akhondi, M.M.; Shirazi, A.; Khorshid, H.R.K. Comparison of Three Methods for Mitochondria Isolation from the Human Liver Cell Line (HepG2). Gastroenterol. Hepatol. Bed Bench 2016, 9, 105–113. [Google Scholar] [CrossRef]
- Yin, Y.; Shen, H. Common Methods in Mitochondrial Research (Review). Int. J. Mol. Med. 2022, 50, 126. [Google Scholar] [CrossRef]
- Chao, J.; Zhu, D.; Zhang, Y.; Wang, L.; Fan, C. DNA Nanotechnology-Enabled Biosensors. Biosens. Bioelectron. 2016, 76, 68–79. [Google Scholar] [CrossRef]
- Slomovic, S.; Pardee, K.; Collins, J.J. Synthetic Biology Devices for in Vitro and in Vivo Diagnostics. Proc. Natl. Acad. Sci. USA 2015, 112, 14429–14435. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Liao, S.; Huang, T.; Wang, G.-J. A Biosensor Electrode with Self-Assembled Monolayer of Gold Nanoparticle on a Micro Hemisphere Array. J. Electrochem. Soc. 2019, 166, B349–B354. [Google Scholar] [CrossRef]
- Zhu, M.; Barbas, A.S.; Lin, L.; Scheuermann, U.; Bishawi, M.; Brennan, T.V. Mitochondria Released by Apoptotic Cell Death Initiate Innate Immune Responses. Immunohorizons 2018, 2, 384–397. [Google Scholar] [CrossRef]
- Du, Y.; Pan, D.; Jia, R.; Chen, Y.; Jia, C.; Wang, J.; Hu, B.; Martin, P.M. The Reduced Oligomerization of MAVS Mediated by ROS Enhances the Cellular Radioresistance. Oxid. Med. Cell. Longev. 2020, 2020, 2167129. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.K.; De Miroschedji, K.; Doeppner, T.R.; Börger, V.; Ruesing, J.; Rebmann, V.; Durst, S.; Jansen, S.; Bremer, M.; Behrmann, E.; et al. Precipitation with Polyethylene Glycol Followed by Washing and Pelleting by Ultracentrifugation Enriches Extracellular Vesicles from Tissue Culture Supernatants in Small and Large Scales. J. Extracell. Vesicles 2018, 7, 1528109. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.S.; Faruque, H.A.; Kim, J.H.; Kim, K.J.; Choi, J.E.; Kim, B.A.; Kim, B.; Kim, Y.J.; Woo, M.H.; Park, J.Y.; et al. Cd5l as an Extracellular Vesicle-Derived Biomarker for Liquid Biopsy of Lung Cancer. Diagnostics 2021, 11, 620. [Google Scholar] [CrossRef] [PubMed]
- Barenholz-Cohen, T.; Merkher, Y.; Haj, J.; Shechter, D.; Kirchmeier, D.; Shaked, Y.; Weihs, D. Lung Mechanics Modifications Facilitating Metastasis Are Mediated in Part by Breast Cancer-derived Extracellular Vesicles. Int. J. Cancer 2020, 147, 2924–2933. [Google Scholar] [CrossRef]
- Crescitelli, R.; Lässer, C.; Jang, S.C.; Cvjetkovic, A.; Malmhäll, C.; Karimi, N.; Höög, J.L.; Johansson, I.; Fuchs, J.; Thorsell, A.; et al. Subpopulations of Extracellular Vesicles from Human Metastatic Melanoma Tissue Identified by Quantitative Proteomics after Optimized Isolation. J. Extracell. Vesicles 2020, 9, 1722433. [Google Scholar] [CrossRef] [PubMed]
- Böing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.W.; Sturk, A.; Nieuwland, R. Single-Step Isolation of Extracellular Vesicles by Size-Exclusion Chromatography. J. Extracell. Vesicles 2014, 3, 23430. [Google Scholar] [CrossRef]
- Chandran, V.I.; Welinder, C.; Mansson, A.S.; Offer, S.; Freyhult, E.; Pernemalm, M.; Lund, S.M.; Pedersen, S.; Lehtio, J.; Marko-Varga, G.; et al. Ultrasensitive Immunoprofiling of Plasma Extracellular Vesicles Identifies Syndecan-1 as a Potential Tool for Minimally Invasive Diagnosis of Glioma. Clin. Cancer Res. 2019, 25, 3115–3127. [Google Scholar] [CrossRef]
- Frezza, C.; Cipolat, S.; Scorrano, L. Organelle Isolation: Functional Mitochondria from Mouse Liver, Muscle and Cultured Filroblasts. Nat. Protoc. 2007, 2, 287–295. [Google Scholar] [CrossRef]
- Gong, H.; Zhang, Q.; Komarla, A.; Wang, S.; Duan, Y.; Zhou, Z.; Chen, F.; Fang, R.H.; Xu, S.; Gao, W.; et al. Nanomaterial Biointerfacing via Mitochondrial Membrane Coating for Targeted Detoxification and Molecular Detection. Nano Lett. 2021, 21, 2603–2609. [Google Scholar] [CrossRef]
- McLaughlin, K.L.; McClung, J.M.; Fisher-Wellman, K.H. Bioenergetic Consequences of Compromised Mitochondrial DNA Repair in the Mouse Heart. Biochem. Biophys. Res. Commun. 2018, 504, 742–748. [Google Scholar] [CrossRef]
- Steinmark, I.E.; James, A.L.; Chung, P.H.; Morton, P.E.; Parsons, M.; Dreiss, C.A.; Lorenz, C.D.; Yahioglu, G.; Suhling, K. Targeted Fluorescence Lifetime Probes Reveal Responsive Organelle Viscosity and Membrane Fluidity. PLoS ONE 2019, 14, e0211165. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Beeson, G.; Beeson, C.; Rohrer, B. An Improved Method for Isolation of Mitochondria from Cell Lines That Enables Reconstitution of Calcium-Dependent Processes. Anal. Biochem. 2019, 577, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, S.; Li, L.; Zhong, X.; Chen, C.; Fan, Y.; Shen, W.; Zu, L.; Xue, F.; Wang, M.; et al. Comparative Mitochondrial Proteomic Analysis of Human Large Cell Lung Cancer Cell Lines with Different Metastasis Potential. Thorac. Cancer 2019, 10, 1111–1128. [Google Scholar] [CrossRef]
- Afanasyeva, M.A.; Ustiugova, A.S.; Golyshev, S.A.; Kopylov, A.T.; Bogolyubova, A.V.; Demin, D.E.; Belousov, P.V.; Schwartz, A.M. Isolation of Large Amounts of Highly Pure Mitochondria for “Omics” Studies. Biochemistry 2018, 83, 76–85. [Google Scholar] [CrossRef]
- Weerts, M.J.A.; Timmermans, E.C.; Vossen, R.H.A.M.; Van Strijp, D.; Van Den Hout-Van Vroonhoven, M.C.G.N.; Van Ijcken, W.F.J.; Van Der Zaag, P.J.; Anvar, S.Y.; Sleijfer, S.; Martens, J.W.M. Sensitive Detection of Mitochondrial DNA Variants for Analysis of Mitochondrial DNA-Enriched Extracts from Frozen Tumor Tissue. Sci. Rep. 2018, 8, 2261. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, A.Y.Y.; Budd, M.; Deng, D.; Gadawska, I.; Côté, H.C.F. A Monochrome Multiplex Real-Time Quantitative PCR Assay for the Measurement of Mitochondrial DNA Content. J. Mol. Diagn. 2018, 20, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Rosa, H.S.; Ajaz, S.; Gnudi, L.; Malik, A.N. A Case for Measuring Both Cellular and Cell-Free Mitochondrial DNA as a Disease Biomarker in Human Blood. FASEB J. 2020, 34, 12278–12288. [Google Scholar] [CrossRef]
- Kim, S.H.; Chi, J.G.; Reith, A.; Kadenbach, B. Quantitative Analysis of Mitochondrial DNA Deletion in Paraffin Embedded Muscle Tissues Form Patients with KSS and CPEO. Biochim. Biophys. Acta-Mol. Basis Dis. 1997, 1360, 193–195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kheirandish-Rostami, M.; Roudkenar, M.H.; Jahanian-Najafabadi, A.; Tomita, K.; Kuwahara, Y.; Sato, T.; Roushandeh, A.M. Mitochondrial Characteristics Contribute to Proliferation and Migration Potency of MDA-MB-231 Cancer Cells and Their Response to Cisplatin Treatment. Life Sci. 2020, 244, 117339. [Google Scholar] [CrossRef]
- Huang, T.; Lin, R.; Su, Y.; Sun, H.; Zheng, X.; Zhang, J.; Lu, X.; Zhao, B.; Jiang, X.; Huang, L.; et al. Efficient Intervention for Pulmonary Fibrosis via Mitochondrial Transfer Promoted by Mitochondrial Biogenesis. Nat. Commun. 2023, 14, 5781. [Google Scholar] [CrossRef]
- Liu, D.; Gao, Y.; Liu, J.; Huang, Y.; Yin, J.; Feng, Y.; Shi, L.; Meloni, B.P.; Zhang, C.; Zheng, M.; et al. Intercellular Mitochondrial Transfer as a Means of Tissue Revitalization. Signal Transduct. Target. Ther. 2021, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chi, X.; Wang, Y.; Heng, B.C.; Wei, Y.; Zhang, X.; Zhao, H.; Yin, Y.; Deng, X. Mitochondria Transfer Enhances Proliferation, Migration, and Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cell and Promotes Bone Defect Healing. Stem Cell Res. Ther. 2020, 11, 245. [Google Scholar] [CrossRef]
- Kesner, E.E.; Saada-Reich, A.; Lorberboum-Galski, H. Characteristics of Mitochondrial Transformation into Human Cells. Sci. Rep. 2016, 6, 26057. [Google Scholar] [CrossRef]
- Wang, X.; Gerdes, H.H. Transfer of Mitochondria via Tunneling Nanotubes Rescues Apoptotic PC12 Cells. Cell Death Differ. 2015, 22, 1181–1191. [Google Scholar] [CrossRef]
- Sansone, P.; Savini, C.; Kurelac, I.; Chang, Q.; Amato, L.B.; Strillacci, A.; Stepanova, A.; Iommarini, L.; Mastroleo, C.; Daly, L.; et al. Packaging and Transfer of Mitochondrial DNA via Exosomes Regulate Escape from Dormancy in Hormonal Therapy-Resistant Breast Cancer. Proc. Natl. Acad. Sci. USA 2017, 114, E9066–E9075. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.F.; Kovarova, J.; Bajzikova, M.; Bezawork-Geleta, A.; Svec, D.; Endaya, B.; Sachaphibulkij, K.; Coelho, A.R.; Sebkova, N.; Ruzickova, A.; et al. Horizontal Transfer of Whole Mitochondria Restores Tumorigenic Potential in Mitochondrial DNA-Deficient Cancer Cells. Elife 2017, 6, e22187. [Google Scholar] [CrossRef]
- Marlein, C.R.; Zaitseva, L.; Piddock, R.E.; Robinson, S.D.; Edwards, D.R.; Shafat, M.S.; Zhou, Z.; Lawes, M.; Bowles, K.M.; Rushworth, S.A. NADPH Oxidase-2 Derived Superoxide Drives Mitochondrial Transfer from Bone Marrow Stromal Cells to Leukemic Blasts. Blood 2017, 130, 1649–1660. [Google Scholar] [CrossRef]
- Chang, J.C.; Chang, H.S.; Wu, Y.C.; Cheng, W.L.; Lin, T.T.; Chang, H.J.; Kuo, S.J.; Chen, S.T.; Liu, C.S. Mitochondrial Transplantation Regulates Antitumour Activity, Chemoresistance and Mitochondrial Dynamics in Breast Cancer. J. Exp. Clin. Cancer Res. 2019, 38, 30. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, A.; Li, S.; Chatterjee, S.; Qi, R.; Segura-Ibarra, V.; Ferrari, M.; Gupte, A.; Blanco, E.; Hamilton, D.J. Polymer Functionalization of Isolated Mitochondria for Cellular Transplantation and Metabolic Phenotype Alteration. Adv. Sci. 2018, 5, 1700530. [Google Scholar] [CrossRef]
- Torralba, D.; Baixauli, F.; Sánchez-Madrid, F. Mitochondria Know No Boundaries: Mechanisms and Functions of Intercellular Mitochondrial Transfer. Front. Cell Dev. Biol. 2016, 4, 223255. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, A.D.; Benson, E.K.; Gone, S.; Liang, R.; Shim, J.; Lambertini, L.; Toloue, M.M.; Wigler, M.; Aaronson, S.A.; Sachidanandam, R. Stable Heteroplasmy at the Single-Cell Level Is Facilitated by Intercellular Exchange of MtDNA. Nucleic Acids Res. 2015, 43, 2177–2187. [Google Scholar] [CrossRef]
- Lu, J.; Zheng, X.; Li, F.; Yu, Y.; Chen, Z.; Liu, Z.; Wang, Z.; Xu, H.; Yang, W. Tunneling Nanotubes Promote Intercellular Mitochondria Transfer Followed by Increased Invasiveness in Bladder Cancer Cells. Oncotarget 2017, 8, 15539–15552. [Google Scholar] [CrossRef]
- Berridge, M.V.; Crasso, C.; Neuzil, J. Mitochondrial Genome Transfer to Tumor Cells Breaks The Rules and Establishes a New Precedent in Cancer Biology. Mol. Cell. Oncol. 2018, 5, 1023929. [Google Scholar] [CrossRef] [PubMed]
- Marlein, C.R.; Piddock, R.E.; Mistry, J.J.; Zaitseva, L.; Hellmich, C.; Horton, R.H.; Zhou, Z.; Auger, M.J.; Bowles, K.M.; Rushworth, S.A. CD38-Driven Mitochondrial Trafficking Promotes Bioenergetic Plasticity in Multiple Myeloma. Cancer Res. 2019, 79, 2285–2297. [Google Scholar] [CrossRef]
- Lee, T.H.; Chennakrishnaiah, S.; Audemard, E.; Montermini, L.; Meehan, B.; Rak, J. Oncogenic Ras-Driven Cancer Cell Vesiculation Leads to Emission of Double-Stranded DNA Capable of Interacting with Target Cells. Biochem. Biophys. Res. Commun. 2014, 451, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhang, L.Y.; He, J.Z.; Miao, Z.M.; Li, Y.Y.; Zhang, Y.M.; Liu, Z.W.; Zhang, S.Z.; Chen, Y.; Zhou, G.C.; et al. Review: Mechanisms and Perspective Treatment of Radioresistance in Non-Small Cell Lung Cancer. Front. Immunol. 2023, 14, 1133899. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. Mitochondrial Transfer as a Novel Therapeutic Approach in Disease Diagnosis and Treatment. Int. J. Mol. Sci. 2023, 24, 8848. [Google Scholar] [CrossRef]
- Sun, H.; Du, Y.; Yao, M.; Wang, Q.; Ji, K.; Du, L.; Xu, C.; He, N.; Wang, J.; Zhang, M.; et al. CIAP1/2 Are Involved in the Radiosensitizing Effect of Birinapant on NSCLC Cell Line in Vitro. J. Cell. Mol. Med. 2021, 25, 6125–6136. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Bushong, E.A.; Segawa, M.; Tiard, A.; Wong, A.; Brady, M.R.; Momcilovic, M.; Wolf, D.M.; Zhang, R.; Petcherski, A.; et al. Spatial Mapping of Mitochondrial Networks and Bioenergetics in Lung Cancer. Nature 2023, 615, 712–719. [Google Scholar] [CrossRef] [PubMed]


| Organelle | Extraction Method | Functional Characterization | Ref. |
|---|---|---|---|
| EVs | Precipitation | Western blotting | [89,90] |
| Differential ultracentrifugation | Nanoparticle tracking analysis | [91,92] | |
| Size exclusion chromatography | Electron microscopy imaging | [93,94] | |
| MtO | Differential ultracentrifugation | Biuret methods | [76,95] |
| Western blotting | [96,97] | ||
| Imaging | [98,99] | ||
| MtS | Differential ultracentrifugation | Mass spectrometry | [100,101] |
| Alkaline extraction | Multiplex PCR | [102,103] | |
| Precipitation | SYBR green-based PCR | [104,105] |
| Technique | Description | Result | Ref. |
|---|---|---|---|
| Co-incubation | Isolated mitochondria from healthy fibroblasts and mitochondrial-mutated cells were transferred into breast cancer (MCF7), embryonic kidney (HEK 293), and hepatocellular carcinoma (HepG2) cells | Mimic natural mitochondria transfer and persistent in the recipient cells for several days | [110] |
| Following apoptosis induction, CellTracker-labelled pheochromocytoma (PC12) cells were incubated with the conditioned medium from untreated and unlabeled PC12 cells | Rescue effect is nulled because there was no mitochondria transfer, indicating a contact-dependent mechanism | [111] | |
| Incubation of metastatic breast cancer cells (MCF7) with extracellular vesicles from murine cancer-associated fibroblasts (CAFs) | Transfer of therapy resistance to therapy-sensitive cells via mtDNA from EV in vivo and in vitro | [112] | |
| Microinjection | Mouse melanoma (B16ρ0) cells transfected with a plasmid coding for nuclear-targeted blue fluorescent protein (nBFP) were injected subcutaneously into C57BL/6Nsu9-DsRed2 mice (transgenic mice expressing red fluorescent protein in somatic cell mitochondria (the CAG/su9-DsRed2-transgene) | Double-positive cells with both red and blue fluorescence, prepared from a pre-tumor lesion, identifying mouse stromal cells as a source of mitochondria | [113] |
| Mitochondrial transfer from bone marrow-derived stromal cells (BMSCs) to primary human acute myeloid leukemia (AML) cells injected into immunodeficient NSG mice via acute myeloid leukemia AML-derived tunneling nanotube (TNT) | The transfer was enhanced by the treatment with hydrogen peroxide that drives the spike in ROS level in BMSC | [114] | |
| Injection of mCAF extracellular vesicles into tumor-bearing mice | Transfer of therapy resistance to therapy-sensitive cells via mtDNA from EV in vivo and in vitro | [112] | |
| Transplantation | Mitochondria were transported into MCF-7 breast cancer cells through passive uptake or peptide Pep-1-mediated delivery | Mitochondria and peptide showed significant induction of the nuclear translocation of apoptosis-inducing factor | [115] |
| In vitro mitochondrial transfer among bone marrow-derived mesenchymal stem cells (BM-MSCs) and two additional populations of MSCs sourced from healthy lung tissues (LT-MSCs) and bronchoalveolar lavage fluid of lung transplant recipients (BAL-MSCs) | LT-MSCs and BAL-MSCs exhibit the ability to donate spontaneously cytoplasmic content and mitochondria to healthy human bronchial epithelial cells with comparable efficiency through unidirectional transfer | [108] | |
| H9c2 rat heart myoblast cells and L929 mouse fibroblast cells were treated with uncoated or fluorescently coated mitochondria obtained from HeLa cells | Uptake and intracellular localization of HeLa-derived mitochondria in H9c2 cardiac myoblast cells were recorded | [116] |
| Method | Cells | Result | Evidence | Ref. |
|---|---|---|---|---|
| Transformation | B16ρ0SC B16ρ0CTC B16ρ0SCL | MtDNA is transferred from stromal cells to B16ρ0 cells within intact mitochondria | Acquisition of mtDNA by the trafficking of whole mitochondria from host donor cells to ρ0 cells resulting in long-lasting respiration recovery and efficient tumor formation | [113] |
| Conjugation | IMR90 WI-38 MDA-MB-157 U2OS A382 HCC1806 | The transfer of mtDNA most likely occurs through either the transfer of mitochondria-derived vesicles or intact mitochondrial organelles | Identification of variants exclusive to the non-GFP-labeled cell line within the co-cultured partner cell line indicates the transfer of mtDNA between the cells | [118] |
| Tunneling nanotubes (TNTs) | T24 RT4 | The distribution of mitochondria transferred from T24 cells was in good agreement with the original mitochondria in RT4 cells, which may indicate mitochondrial fusion | The indication that TNTs promote intercellular mitochondrial organelles transfer between heterogeneous cells and the transfer is unidirectional | [119] |
| Tunneling nanotubes (TNTs) | 4T1 4T1p0 | The displayed horizontal transfer of mtDNA from normal host cells to tumor cells lacking mtDNA was clearly established | The mtDNA transfer results in recovery of respiration, tumor initiation and metastasis | [120] |
| Tunneling nanotubes (TNTs) | Primary MM MM1S U266 | Increased level of ATP and oxidative phosphorylation in MM cells | CD38 is required for the formation of TNTs facilitating tumor mitochondrial transfer | [121] |
| Tunneling nanotubes (TNTs) | PC12 | Increased death rate of UV-treated cells co-cultured with ρ0 cells, compared with cells carrying functional mitochondria; indication of mitochondria transferred from untreated cells | Successful mitochondria transfer displayed its participation in the rescue effect by preventing apoptosis in its early stage in damaged cells, which form a novel type of TNTs | [111] |
| Extracellular vesicle (EV) | RAS-3 | Extracellular vesicles mediate intercellular transfer of oncogenic human H-ras DNA | The indication of an avid uptake of EVs | [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonov, S.; Dorfman, A.; Pershikova, E.; Inyang, O.; Alhaddad, L.; Wang, Y.; Pustovalova, M.; Merkher, Y. Extracellular Vesicle- and Mitochondria-Based Targeting of Non-Small Cell Lung Cancer Response to Radiation: Challenges and Perspectives. Cancers 2024, 16, 2235. https://doi.org/10.3390/cancers16122235
Leonov S, Dorfman A, Pershikova E, Inyang O, Alhaddad L, Wang Y, Pustovalova M, Merkher Y. Extracellular Vesicle- and Mitochondria-Based Targeting of Non-Small Cell Lung Cancer Response to Radiation: Challenges and Perspectives. Cancers. 2024; 16(12):2235. https://doi.org/10.3390/cancers16122235
Chicago/Turabian StyleLeonov, Sergey, Anna Dorfman, Elizaveta Pershikova, Olumide Inyang, Lina Alhaddad, Yuzhe Wang, Margarita Pustovalova, and Yulia Merkher. 2024. "Extracellular Vesicle- and Mitochondria-Based Targeting of Non-Small Cell Lung Cancer Response to Radiation: Challenges and Perspectives" Cancers 16, no. 12: 2235. https://doi.org/10.3390/cancers16122235
APA StyleLeonov, S., Dorfman, A., Pershikova, E., Inyang, O., Alhaddad, L., Wang, Y., Pustovalova, M., & Merkher, Y. (2024). Extracellular Vesicle- and Mitochondria-Based Targeting of Non-Small Cell Lung Cancer Response to Radiation: Challenges and Perspectives. Cancers, 16(12), 2235. https://doi.org/10.3390/cancers16122235







