Clinical Outcomes of Immune Checkpoint Inhibitors in Unique Cohorts Underrepresented in Clinical Trials
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients and Outcomes
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.1.1. Entire Cohort
3.1.2. Non-Small Cell Lung Cancer Cohort
3.2. Safety Analysis
3.2.1. Entire Cohort
3.2.2. Anti-PD-(L)1 Monotherapy Non-Small Cell Lung Cancer Cohort
3.3. Efficacy Analysis
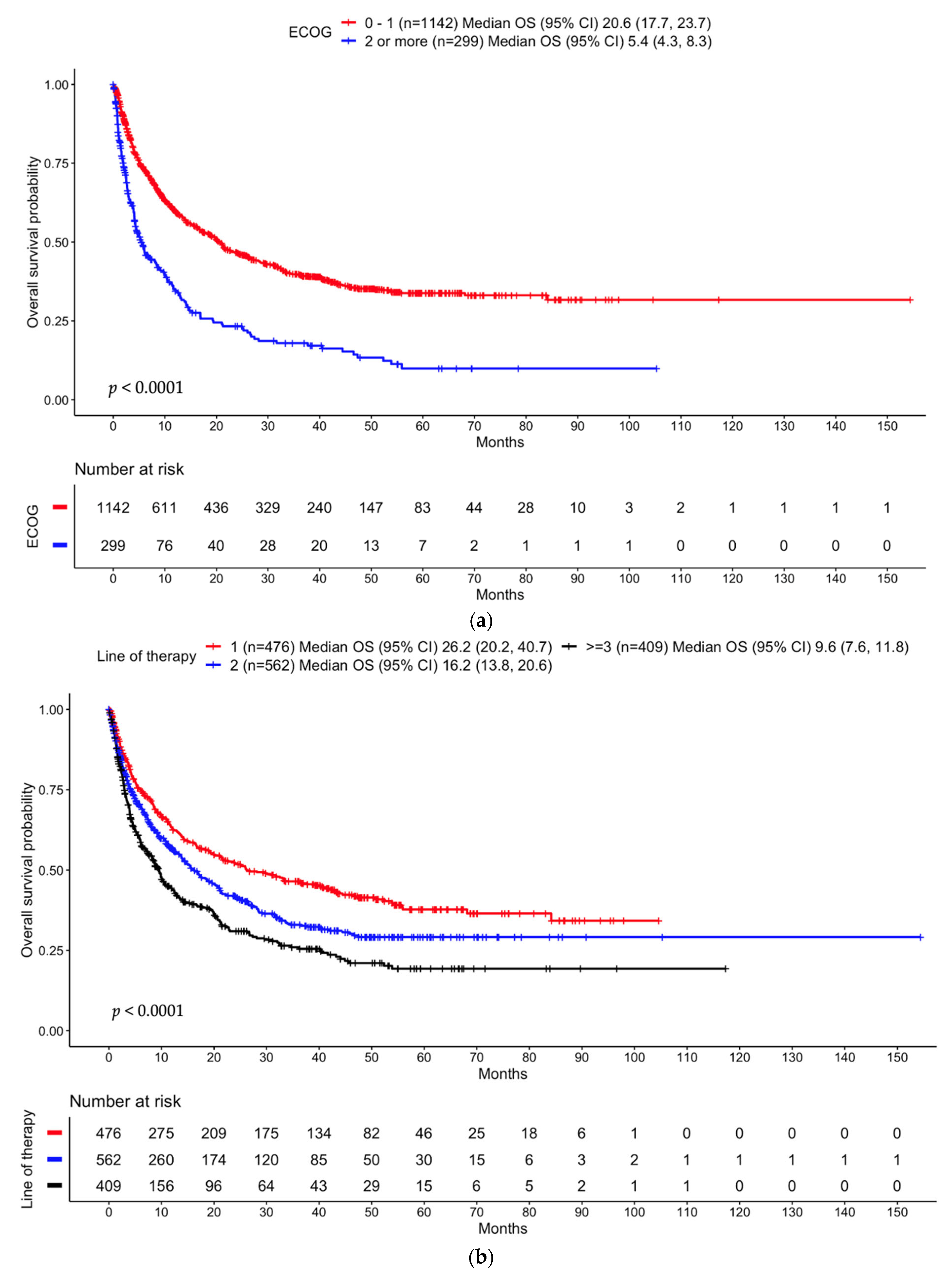
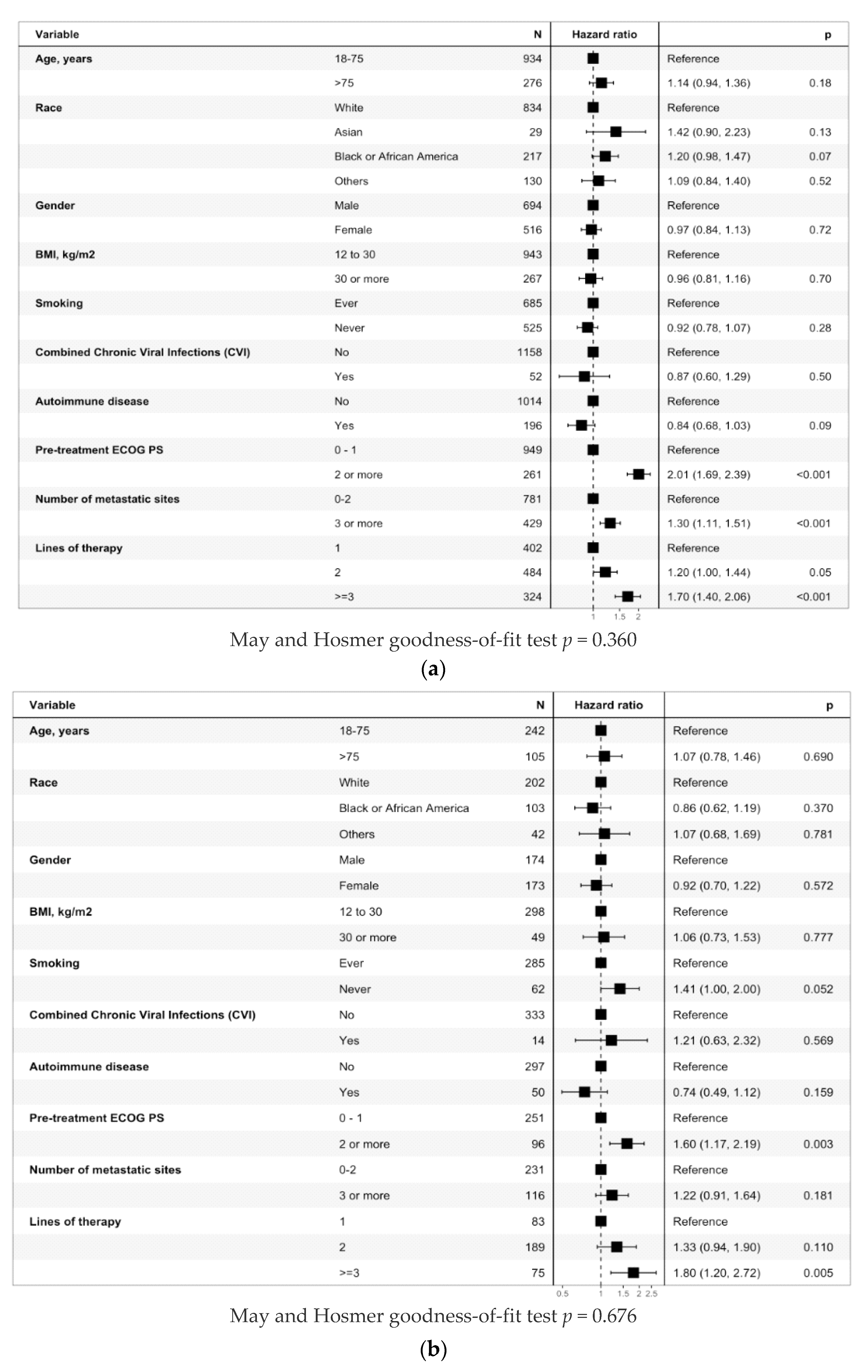
3.3.1. Entire Cohort
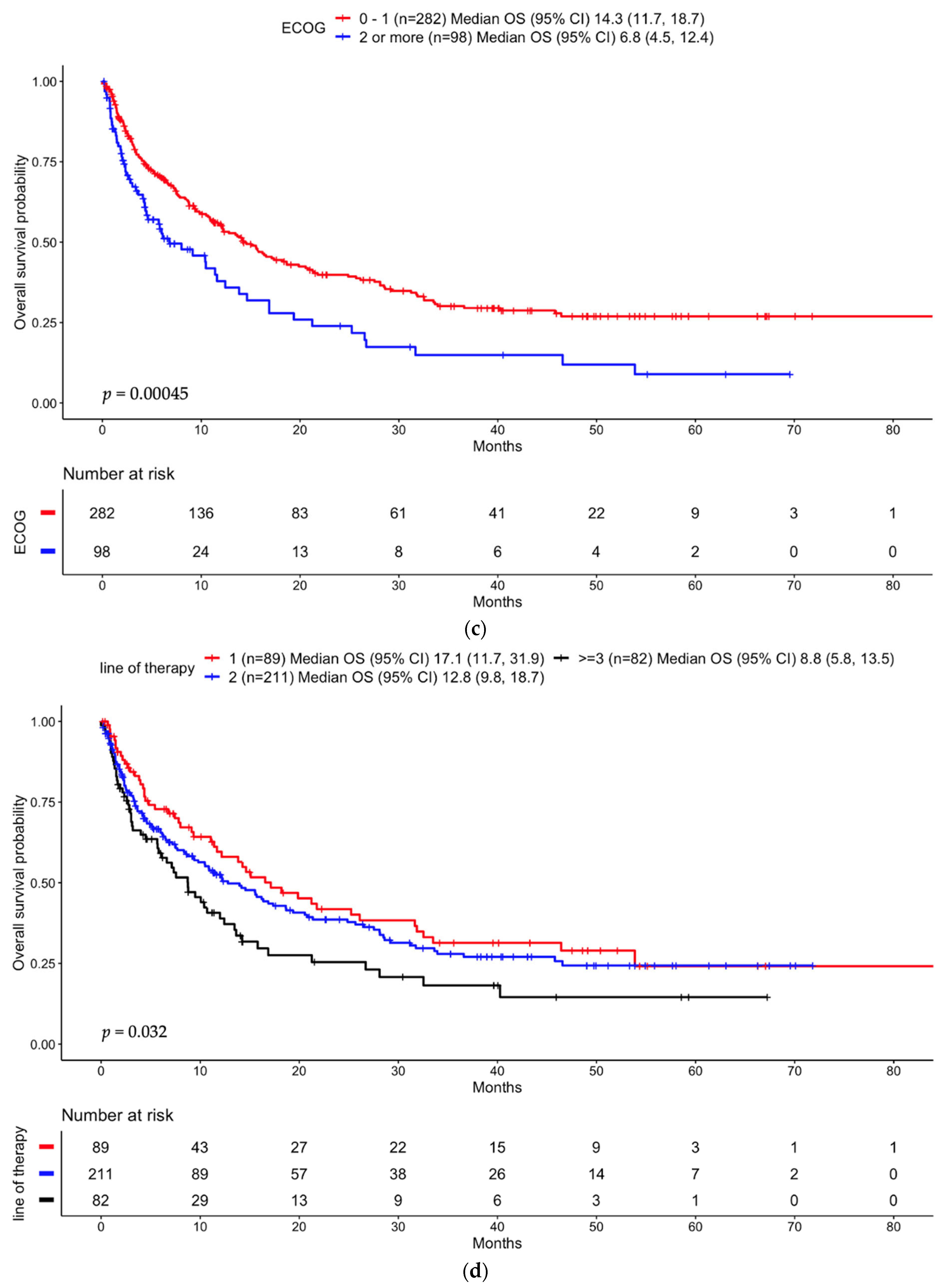
3.3.2. Anti-PD-(L)1 Monotherapy Non-Small Cell Lung Cancer Cohort
3.4. Time to Treatment Failure (TTF)
3.4.1. Entire Cohort
3.4.2. Anti-PD-(L)1 Monotherapy Non-Small Cell Lung Cancer Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356, Erratum in N. Engl. J. Med. 2018, 379, 2185. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.A.; Reed, K.; et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013, 369, 122–133, Erratum in N. Engl. J. Med. 2018, 379, 2185. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Kennedy, E.B.; Alarcon-Rozas, A.E.; Alcindor, T.; Bartley, A.N.; Malowany, A.B.; Bhadkamkar, N.A.; Deighton, D.C.; Janjigian, Y.; Karippot, A.; et al. Immunotherapy and Targeted Therapy for Advanced Gastroesophageal Cancer: ASCO Guideline. J. Clin. Oncol. 2023, 41, 1470–1491, Erratum in J. Clin. Oncol. 2023, JCO2300441. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.J.; Sura, S.D.; Shinde, R.; Shi, J.; Singhal, P.K.; Robert, N.J.; Vogelzang, N.J.; Perini, R.F.; Motzer, R.J. Real-world Treatment Patterns and Clinical Outcomes for Metastatic Renal Cell Carcinoma in the Current Treatment Era. Eur. Urol. Open Sci. 2023, 49, 110–118. [Google Scholar] [CrossRef]
- Gul, A.; Stewart, T.F.; Mantia, C.M.; Shah, N.J.; Gatof, E.S.; Long, Y.; Allman, K.D.; Ornstein, M.C.; Hammers, H.J.; McDermott, D.F.; et al. Salvage Ipilimumab and Nivolumab in Patients with Metastatic Renal Cell Carcinoma After Prior Immune Checkpoint Inhibitors. J. Clin. Oncol. 2020, 38, 3088–3094. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef]
- Spigel, D.R.; McCleod, M.; Jotte, R.M.; Einhorn, L.; Horn, L.; Waterhouse, D.M.; Creelan, B.; Babu, S.; Leighl, N.B.; Chandler, J.C.; et al. Safety, Efficacy, and Patient-Reported Health-Related Quality of Life and Symptom Burden with Nivolumab in Patients with Advanced Non-Small Cell Lung Cancer, Including Patients Aged 70 Years or Older or with Poor Performance Status (CheckMate 153). J. Thorac. Oncol. 2019, 14, 1628–1639. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Ardizzoni, A.; Ciuleanu, T.; Cobo, M.; Laktionov, K.; Szilasi, M.; Califano, R.; Carcereny, E.; Griffiths, R.; Paz-Ares, L.; et al. CheckMate 171: A phase 2 trial of nivolumab in patients with previously treated advanced squamous non-small cell lung cancer, including ECOG PS 2 and elderly populations. Eur. J. Cancer 2020, 127, 160–172. [Google Scholar] [CrossRef]
- Nosaki, K.; Saka, H.; Hosomi, Y.; Baas, P.; de Castro, G., Jr.; Reck, M.; Wu, Y.L.; Brahmer, J.R.; Felip, E.; Sawada, T.; et al. Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD-L1-positive advanced non-small-cell lung cancer: Pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies. Lung Cancer 2019, 135, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, N.; Ochi, N.; Nakagawa, N.; Nagasaki, Y.; Taoka, M.; Ichiyama, N.; Mimura, A.; Nakanishi, H.; Kohara, H.; Yamane, H. Do Elderly Lung Cancer Patients Aged ≥75 Years Benefit from Immune Checkpoint Inhibitors? Cancers 2020, 12, 1995. [Google Scholar] [CrossRef]
- Grossi, F.; Crinò, L.; Logroscino, A.; Canova, S.; Delmonte, A.; Melotti, B.; Proto, C.; Gelibter, A.; Cappuzzo, F.; Turci, D.; et al. Use of nivolumab in elderly patients with advanced squamous non-small-cell lung cancer: Results from the Italian cohort of an expanded access programme. Eur. J. Cancer 2018, 100, 126–134. [Google Scholar] [CrossRef]
- Nebhan, C.A.; Cortellini, A.; Ma, W.; Ganta, T.; Song, H.; Ye, F.; Irlmeier, R.; Debnath, N.; Saeed, A.; Radford, M.; et al. Clinical Outcomes and Toxic Effects of Single-Agent Immune Checkpoint Inhibitors among Patients Aged 80 Years or Older with Cancer: A Multicenter International Cohort Study. JAMA Oncol. 2021, 7, 1856–1861. [Google Scholar] [CrossRef]
- Tomasik, B.; Bieńkowski, M.; Braun, M.; Popat, S.; Dziadziuszko, R. Effectiveness and safety of immunotherapy in NSCLC patients with ECOG PS score ≥2—Systematic review and meta-analysis. Lung Cancer 2021, 158, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Nazha, B.; Goyal, S.; Chen, Z.; Engelhart, A.; Carlisle, J.W.; Beardslee, T.J.; Gill, H.; Odikadze, L.; Liu, Y.; Mishra, M.K.; et al. Efficacy and safety of immune checkpoint blockade in self-identified Black patients with advanced non-small cell lung cancer. Cancer 2020, 126, 5040–5049. [Google Scholar] [CrossRef]
- Florez, M.A.; Kemnade, J.O.; Chen, N.; Du, W.; Sabichi, A.L.; Wang, D.Y.; Huang, Q.; Miller-Chism, C.N.; Jotwani, A.; Chen, A.C.; et al. Persistent ethnicity-associated disparity in anti-tumor effectiveness of immune checkpoint inhibitors despite equal access. Cancer Res. Commun. 2022, 2022, 806–813. [Google Scholar] [CrossRef]
- Wang, Z.; Aguilar, E.G.; Luna, J.I.; Dunai, C.; Khuat, L.T.; Le, C.T.; Mirsoian, A.; Minnar, C.M.; Stoffel, K.M.; Sturgill, I.R.; et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 2019, 25, 141–151. [Google Scholar] [CrossRef]
- Cortellini, A.; Bersanelli, M.; Buti, S.; Cannita, K.; Santini, D.; Perrone, F.; Giusti, R.; Tiseo, M.; Michiara, M.; Di Marino, P.; et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: When overweight becomes favorable. J. Immunother. Cancer 2019, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- McQuade, J.L.; Daniel, C.R.; Hess, K.R.; Mak, C.; Wang, D.Y.; Rai, R.R.; Park, J.J.; Haydu, L.E.; Spencer, C.; Wongchenko, M.; et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: A retrospective, multicohort analysis. Lancet Oncol. 2018, 19, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Shah, N.J.; Cook, M.; Blackburn, M.; Serzan, M.T.; Advani, S.; Potosky, A.L.; Madhavan, S.; Belouali, A.; Atkins, M.B.; et al. Association between Body Mass Index and Immune-Related Adverse Events (irAEs) among Advanced-Stage Cancer Patients Receiving Immune Checkpoint Inhibitors: A Pan-Cancer Analysis. Cancers 2021, 13, 6109, Erratum in Cancers 2022, 14, 4525. [Google Scholar] [CrossRef] [PubMed]
- Kichenadasse, G.; Miners, J.O.; Mangoni, A.A.; Rowland, A.; Hopkins, A.M.; Sorich, M.J. Association Between Body Mass Index and Overall Survival with Immune Checkpoint Inhibitor Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2020, 6, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, D.; Bajaj, S.; Yu, J.; Hsu, M.; Balar, A.; Pavlick, A.; Weber, J.; Osman, I.; Zhong, J. The complex relationship between body mass index and response to immune checkpoint inhibition in metastatic melanoma patients. J. Immunother. Cancer 2019, 7, 222. [Google Scholar] [CrossRef] [PubMed]
- Roccuzzo, G.; Moirano, G.; Fava, P.; Maule, M.; Ribero, S.; Quaglino, P. Obesity and immune-checkpoint inhibitors in advanced melanoma: A meta-analysis of survival outcomes from clinical studies. Semin. Cancer Biol. 2023, 91, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Han, C.Y.; Fitzgerald, C.; Lee, M.; Valero, C.; Gönen, M.; Shoushtari, A.; Morris, L.G.T. Association Between Toxic Effects and Survival in Patients with Cancer and Autoimmune Disease Treated With Checkpoint Inhibitor Immunotherapy. JAMA Oncol. 2022, 8, 1352–1354. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Pruitt, S.L.; Xuan, L.; Gerber, D.E. Prevalence of Autoimmune Disease Among Patients with Lung Cancer: Implications for Immunotherapy Treatment Options. JAMA Oncol. 2016, 2, 1507–1508. [Google Scholar] [CrossRef] [PubMed]
- Tison, A.; Quéré, G.; Misery, L.; Funck-Brentano, E.; Danlos, F.; Routier, E.; Robert, C.; Loriot, Y.; Lambotte, O.; Bonniaud, B.; et al. Safety and Efficacy of Immune Checkpoint Inhibitors in Patients with Cancer and Preexisting Autoimmune Disease: A Nationwide, Multicenter Cohort Study. Arthritis Rheumatol. 2019, 71, 2100–2111. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.C.; Bhatia, S.; Thompson, J.A.; Grivas, P. Preexisting Autoimmune Disease: Implications for Immune Checkpoint Inhibitor Therapy in Solid Tumors. J. Natl. Compr. Cancer Netw. 2019, 17, 750–757. [Google Scholar] [CrossRef]
- Zarif, T.E.; Nassar, A.; Adib, E.; Fitzgerald, B.; Huang, J.; Mouhieddine, T.; Nonato, T.; McKay, R.; Li, M.; Mittra, A.; et al. 437 Safety and efficacy of immune checkpoint inhibitors (ICI) in patients living with HIV (PLWH) and metastatic non-small cell lung cancer (NSCLC): A matched cohort study from the international CATCH-IT consortium. J. Immuno Therapy Cancer 2022, 10 (Suppl. S2), A457–A458. [Google Scholar]
- Shah, N.J.; Al-Shbool, G.; Blackburn, M.; Cook, M.; Belouali, A.; Liu, S.V.; Madhavan, S.; He, A.R.; Atkins, M.B.; Gibney, G.T.; et al. Safety and efficacy of immune checkpoint inhibitors (ICIs) in cancer patients with HIV, hepatitis B, or hepatitis C viral infection. J. Immunother. Cancer 2019, 7, 353. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.R.; Kim, C. Safety and Efficacy of Immune Checkpoint Inhibitor Therapy in Patients with HIV Infection and Advanced-Stage Cancer: A Systematic Review. JAMA Oncol. 2019, 5, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- CTCAE V4.03. Available online: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf (accessed on 1 March 2024).
- May, S.; Hosmer, D.W. A simplified method of calculating an overall goodness-of-fit test for the Cox proportional hazards model. Lifetime Data Anal. 1998, 4, 109–120. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. 2022. Available online: https://www.r-project.org/ (accessed on 1 March 2024).
- Kim, C.M.; Lee, J.B.; Shin, S.J.; Ahn, J.B.; Lee, M.; Kim, H.S. The efficacy of immune checkpoint inhibitors in elderly patients: A meta-analysis and meta-regression. ESMO Open 2022, 7, 100577. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Mazzaschi, G.; Barbieri, F.; Passiglia, F.; Mazzoni, F.; Berardi, R.; Proto, C.; Cecere, F.L.; Pilotto, S.; Scotti, V.; et al. First-line pembrolizumab in advanced non-small cell lung cancer patients with poor performance status. Eur. J. Cancer 2020, 130, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Tapia Rico, G.; Chan, M.M.; Loo, K.F. The safety and efficacy of immune checkpoint inhibitors in patients with advanced cancers and pre-existing chronic viral infections (Hepatitis B/C, HIV): A review of the available evidence. Cancer Treat. Rev. 2020, 86, 102011. [Google Scholar] [CrossRef]
- Atkins, M.B.; Lee, S.J.; Chmielowski, B.; Tarhini, A.A.; Cohen, G.I.; Truong, T.-G.; Moon, H.H.; Davar, D.; O’Rourke, M.; Stephenson, J.J.; et al. Combination Dabrafenib and Trametinib Versus Combination Nivolumab and Ipilimumab for Patients with Advanced BRAF-Mutant Melanoma: The DREAMseq Trial-ECOG-ACRIN EA6134. J. Clin. Oncol. 2023, 41, 186–197. [Google Scholar] [CrossRef]
| Characteristics | Entire Cohort N = 1453 n (%) | PD-(L)1 Monotherapy NSCLC Cohort N = 384 n (%) |
|---|---|---|
| Age, median (IQR) years | 65.8 (56.6, 74.3) | 70.1 (61.8, 76.3) |
| 18–75 | 1118 (77.2) | 264 (68.9) |
| >75 | 330 (22.8) | 119 (31.1) |
| Race | ||
| Asian | 39 (2.7) | 13 (3.4) |
| Black | 237 (16.3) | 111 (28.9) |
| White a | 1012 (69.6) | 227 (59.1) |
| Others | 165 (11.4) | 33 (8.6) |
| Gender | ||
| Male | 838 (57.8) | 195 (50.8) |
| Female | 612 (42.2) | 189 (49.2) |
| BMI, kg/m2 | ||
| 12 ≤ BMI < 30 | 1110 (77.9) | 322 (85) |
| BMI ≥ 30 | 315 (22.1) | 57 (15) |
| Smoking Status | ||
| Ever Smoker b | 817 (56.5) | 319 (83.1) |
| Never Smoker | 628 (43.5) | 65 (16.9) |
| Chronic Viral Infections (CVI) | ||
| Combined CVI c | 68 (4.7) | 16 (4.2) |
| Hepatitis B (HBV) | 25 (1.7) | 3 (0.8) |
| Hepatitis C (HCV) | 32 (2.2) | 6 (1.6) |
| HIV | 18 (1.2) | 8 (2.1) |
| History of AID d | 228 (15.7) | 54 (14.1) |
| Pre-treatment ECOG PS | ||
| 0 | 383 (26.5) | 78 (20.4) |
| 1 | 761 (52.7) | 205 (53.7) |
| ≥2 | 300 (20.8) | 99 (25.9) |
| ICIs | ||
| Atezolizumab | 47 (3.2) | 26 (6.8) |
| Avelumab | 3 (0.2) | 1 (0.3) |
| Durvalumab | 17 (1.2) | 9 (2.3) |
| Ipilimumab | 163 (11.2) | - |
| Nivolumab | 539 (37.1) | 245 (63.8) |
| Nivolumab + ipilimumab | 192 (13.2) | - |
| Pembrolizumab | 323 (22.2) | 103 (26.8) |
| Pembrolizumab + ipilimumab | 14 (1) | - |
| IO plus chemo | 38 (2.6) | - |
| Others e | 117 (8.1) | - |
| Cancer types | ||
| Lung cancer | 499 (34.4) f | 384 (100) |
| Adenocarcinoma | 312 (62.5) | 256 (66.7) |
| Squamous | 116 (23.2) | 105 (27.3) |
| Others | 69 (13.8) | - |
| Melanoma | 403 (27.8) g | - |
| Cutaneous | 293 (72.7) | - |
| Others | 75 (18.6) | - |
| GI cancers | 104 (7.2) | - |
| Kidney cancers | 100 (6.9) | - |
| Others | 346 (23.8) | - |
| Characteristic | Entire Cohort | |||
|---|---|---|---|---|
| Any Grade irAEs OR (95% CI) | p-Value | Grade ≥ 3 irAEs OR (95% CI) | p-Value | |
| Age, years | 0.446 | 0.425 | ||
| 18–75 | ref | ref | ||
| >75 | 1.11 (0.85, 1.43) | 0.85 (0.57, 1.25) | ||
| Race | ||||
| Asian | 0.43 (0.19, 0.90) | 0.033 | 0.56 (0.13, 1.60) | 0.344 |
| Black | 0.54 (0.39, 0.73) | <0.001 | 0.49 (0.28, 0.81) | 0.008 |
| White | ref | - | ref | - |
| Other | 0.81 (0.57, 1.14) | 0.233 | 0.79 (0.46, 1.3) | 0.383 |
| Gender | 0.356 | 0.439 | ||
| Male | ref | ref | ||
| Female | 1.11 (0.89, 1.38) | 0.88 (0.63, 1.21) | ||
| BMI, kg/m2 | <0.001 | 0.001 | ||
| 12 ≤ BMI < 30 | ref | ref | ||
| BMI ≥ 30 | 1.44 (1.11, 1.86) | 1.61 (1.13, 2.28) | ||
| ECOG PS | <0.001 | 0.001 | ||
| 0–1 | ref | ref | ||
| ≥2 | 0.46 (0.34, 0.62) | 0.45 (0.27, 0.71) | ||
| Characteristic | Anti-PD-(L)1 NSCLC Cohort | |||
|---|---|---|---|---|
| Any Grade irAEs OR (95% CI) | p-Value | Grade ≥ 3 irAEs OR (95% CI) | p-Value | |
| Age, years | 0.097 | 0.093 | ||
| 18–75 | ref | ref | ||
| >75 | 1.52 (0.93, 2.47) | 1.98 (0.89, 4.42) | ||
| Race | ||||
| Black | 0.53 (0.30, 0.90) | 0.023 | 0.44 (0.14, 1.16) | 0.123 |
| White | ref | - | ref | - |
| Other | 0.56 (0.25, 1.15) | 0.128 | 0.70 (0.16, 2.21) | 0.583 |
| Gender | 0.136 | 0.292 | ||
| Male | ref | ref | ||
| Female | 1.43 (0.89, 2.28) | 1.53 (0.70, 3.50) | ||
| BMI, kg/m2 | - | - | 0.252 | |
| 12 ≤ BMI < 30 | ref | |||
| BMI ≥ 30 | 1.72 (0.64, 4.17) | |||
| Combined CVI a | 0.006 | 0.041 | ||
| Yes | ref | ref | ||
| No | 0.22 (0.07, 0.64) | 0.23 (0.06, 1.12) | ||
| History of AID b | 0.037 | 0.293 | ||
| Yes | 1.93 (1.03, 3.59) | 1.65 (0.61, 4.00) | ||
| No | ref | ref | ||
| ECOG PS | 0.036 | - | - | |
| 0–1 | ref | |||
| ≥2 | 0.55 (0.31, 0.95) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, N.J.; Della Pia, A.; Wu, T.; Williams, A.; Weber, M.; Sinclaire, B.; Gourna Paleoudis, E.; Alaoui, A.; Lev-Ari, S.; Adams, S.; et al. Clinical Outcomes of Immune Checkpoint Inhibitors in Unique Cohorts Underrepresented in Clinical Trials. Cancers 2024, 16, 2223. https://doi.org/10.3390/cancers16122223
Shah NJ, Della Pia A, Wu T, Williams A, Weber M, Sinclaire B, Gourna Paleoudis E, Alaoui A, Lev-Ari S, Adams S, et al. Clinical Outcomes of Immune Checkpoint Inhibitors in Unique Cohorts Underrepresented in Clinical Trials. Cancers. 2024; 16(12):2223. https://doi.org/10.3390/cancers16122223
Chicago/Turabian StyleShah, Neil J., Alexandra Della Pia, Tianmin Wu, Aquino Williams, Melinda Weber, Brittany Sinclaire, Elli Gourna Paleoudis, Adil Alaoui, Shaked Lev-Ari, Shari Adams, and et al. 2024. "Clinical Outcomes of Immune Checkpoint Inhibitors in Unique Cohorts Underrepresented in Clinical Trials" Cancers 16, no. 12: 2223. https://doi.org/10.3390/cancers16122223
APA StyleShah, N. J., Della Pia, A., Wu, T., Williams, A., Weber, M., Sinclaire, B., Gourna Paleoudis, E., Alaoui, A., Lev-Ari, S., Adams, S., Kaufman, J., Parikh, S. B., Tonti, E., Muller, E., Serzan, M., Cheruku, D., Lee, A., Sridhar, A., Hee, B. P., ... Atkins, M. B. (2024). Clinical Outcomes of Immune Checkpoint Inhibitors in Unique Cohorts Underrepresented in Clinical Trials. Cancers, 16(12), 2223. https://doi.org/10.3390/cancers16122223






