Perforator versus Non-Perforator Flap-Based Vulvoperineal Reconstruction—A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Selection Process and Data Extraction
2.4. Clavien–Dindo Classification
2.5. Statistics
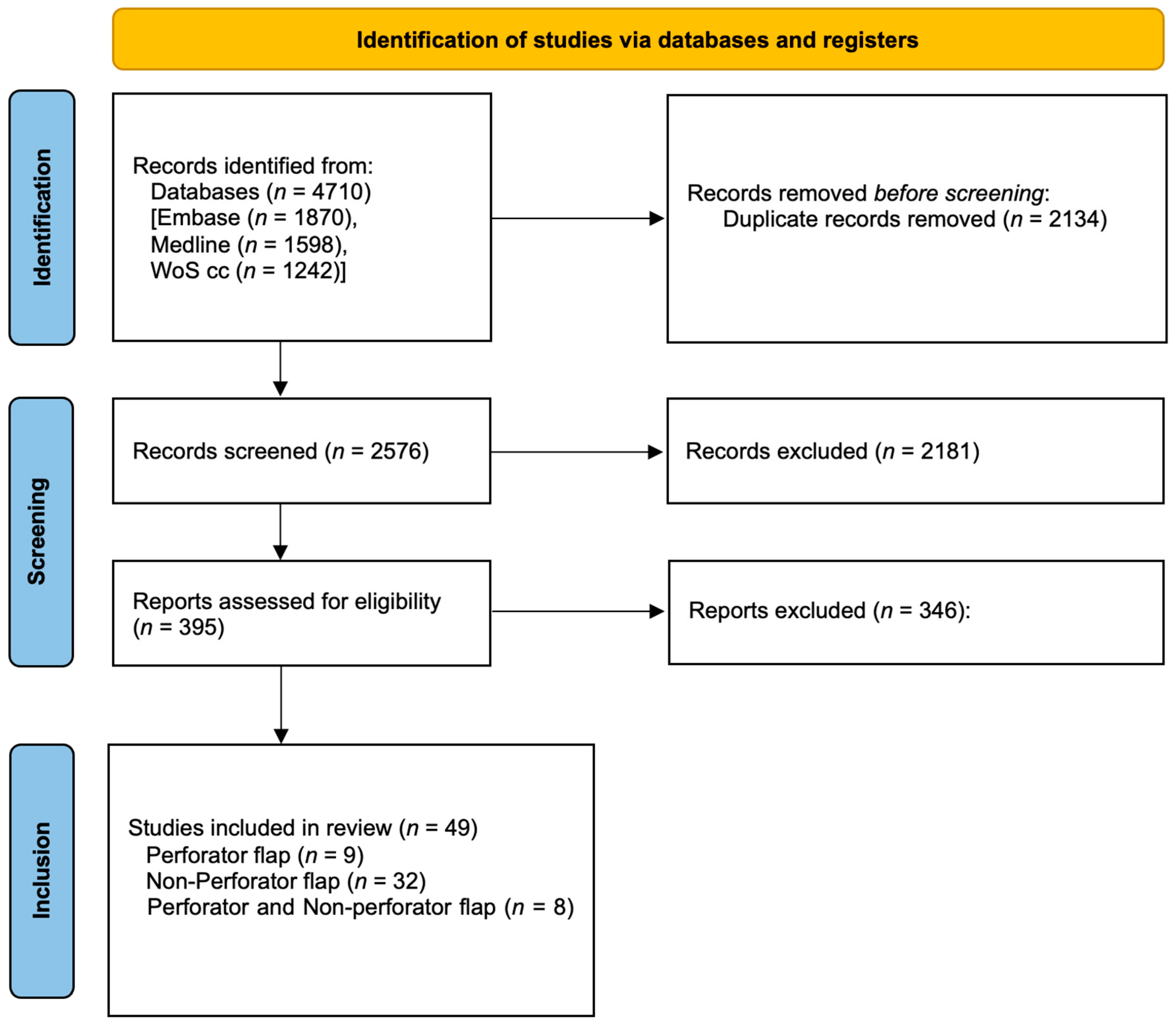
3. Results
3.1. Study Characteristics
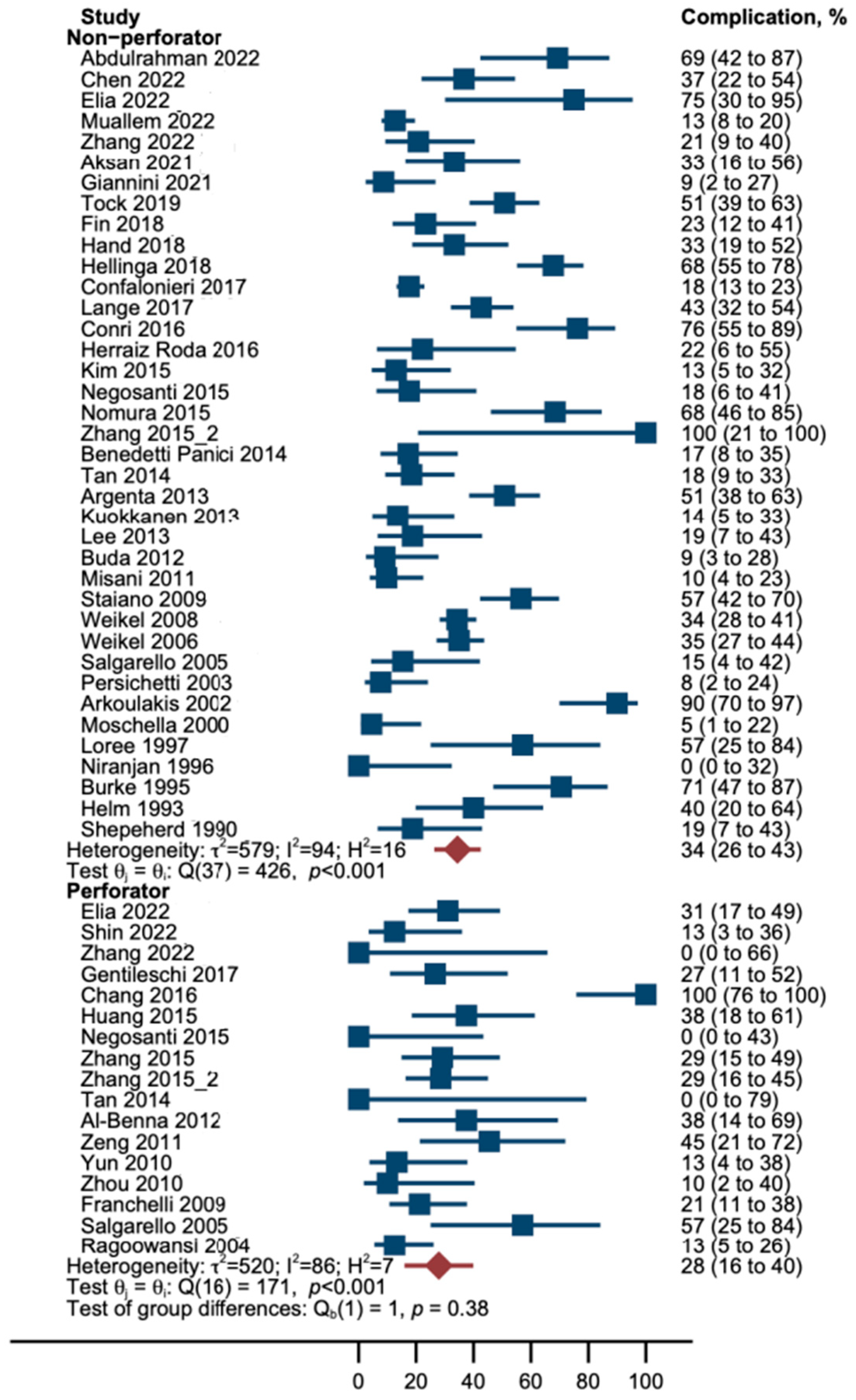
3.2. Overall Complications
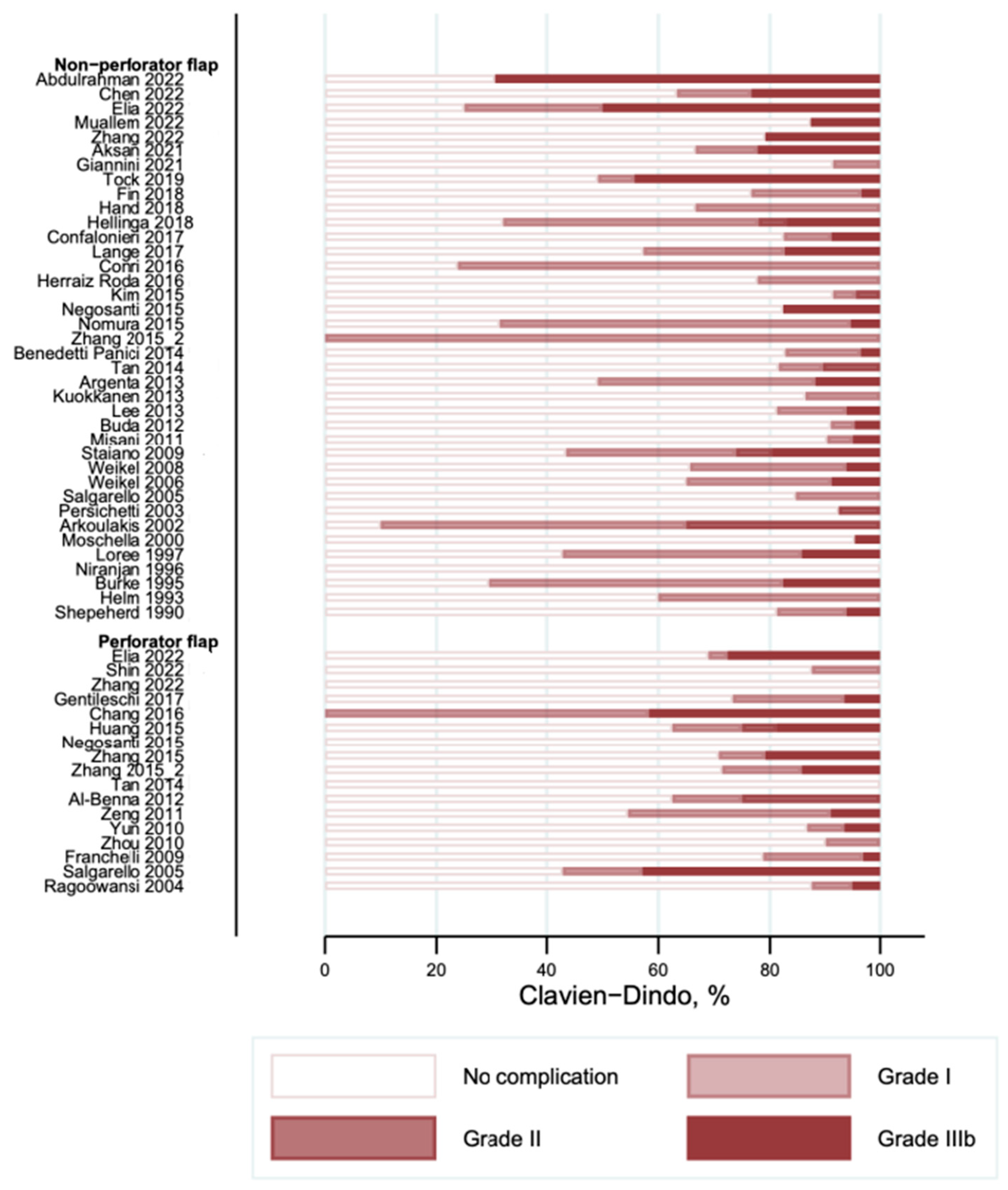
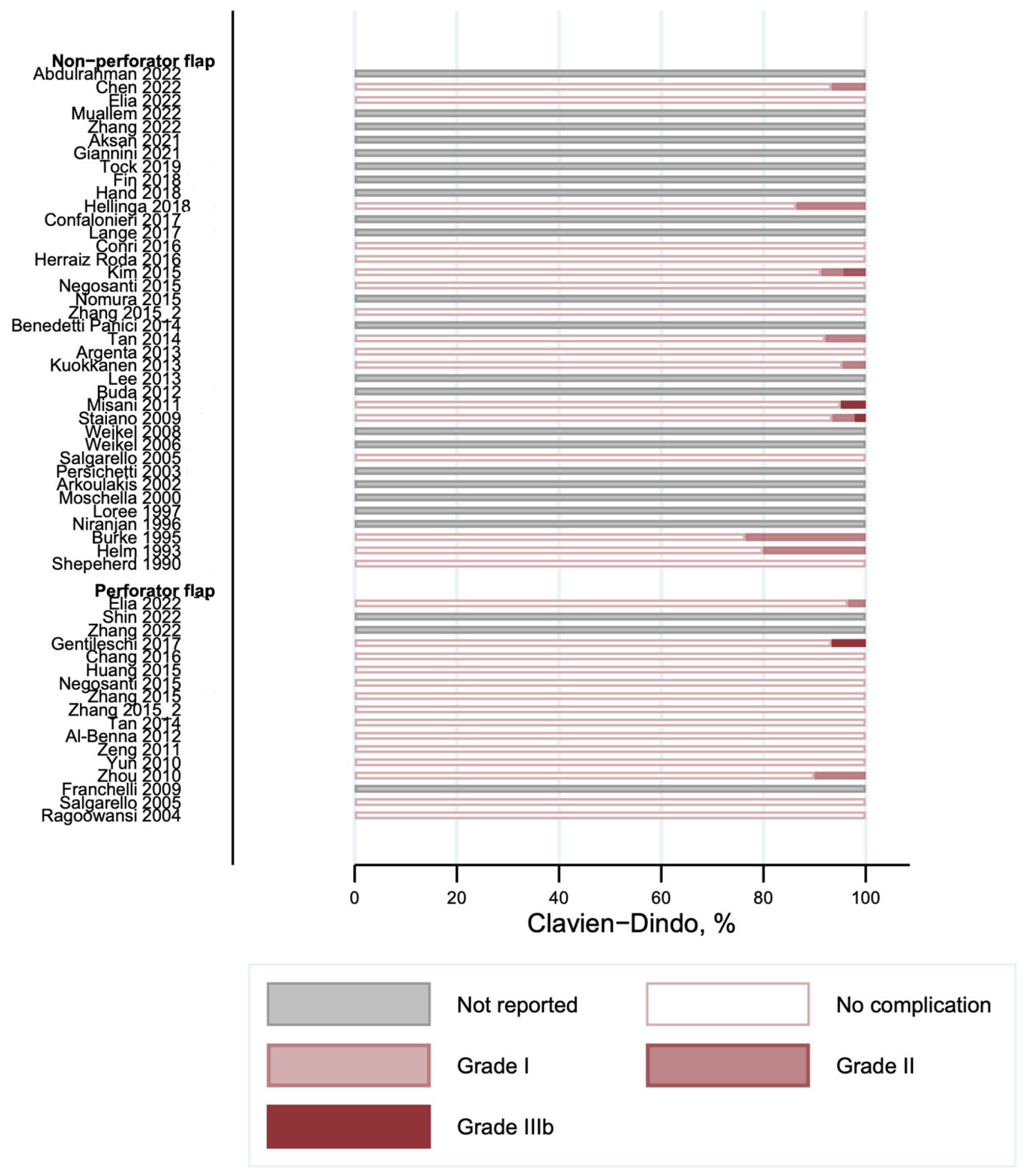
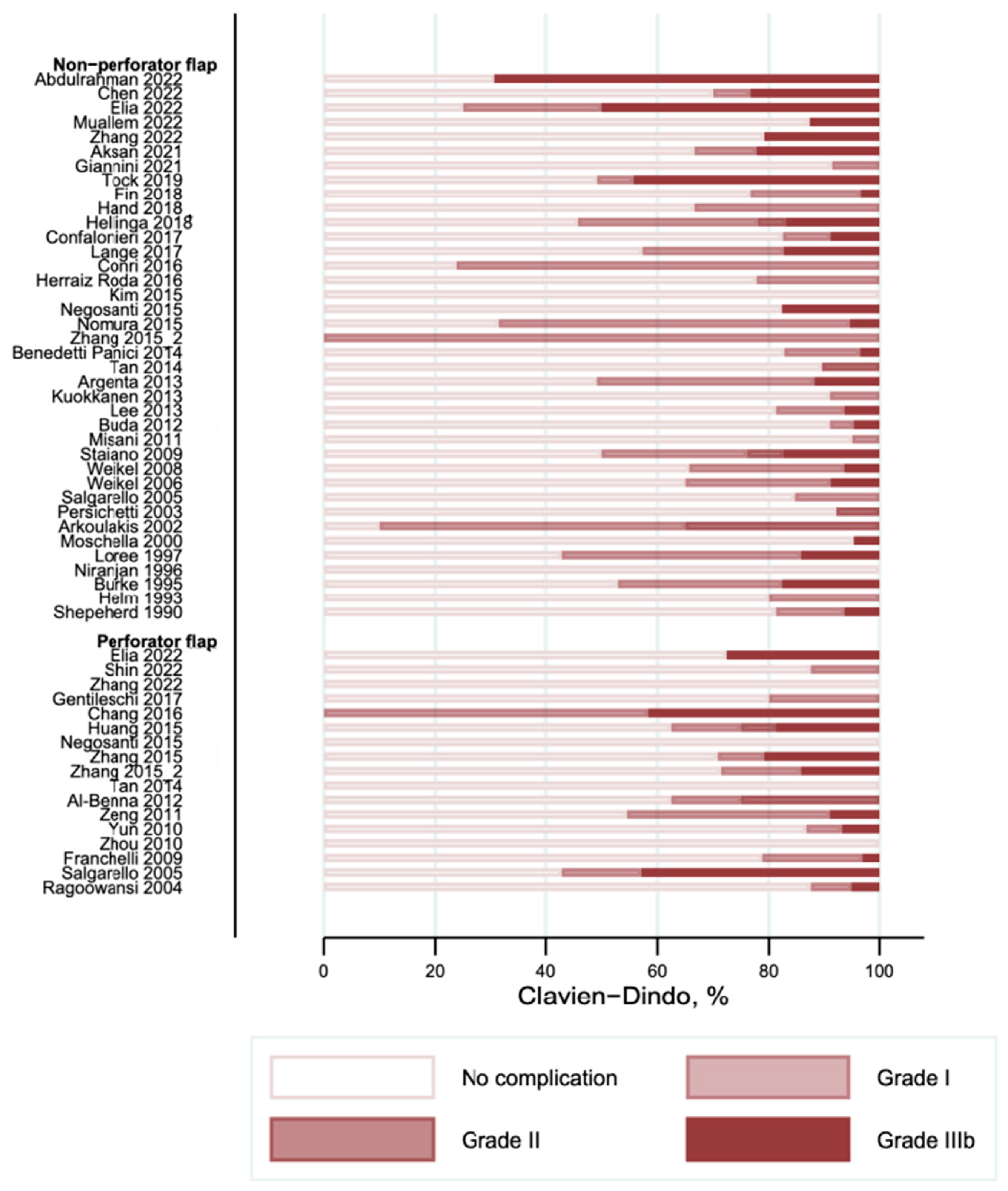
3.3. Complications According to the Clavien–Dindo Classification
3.4. Sensitivity Analysis
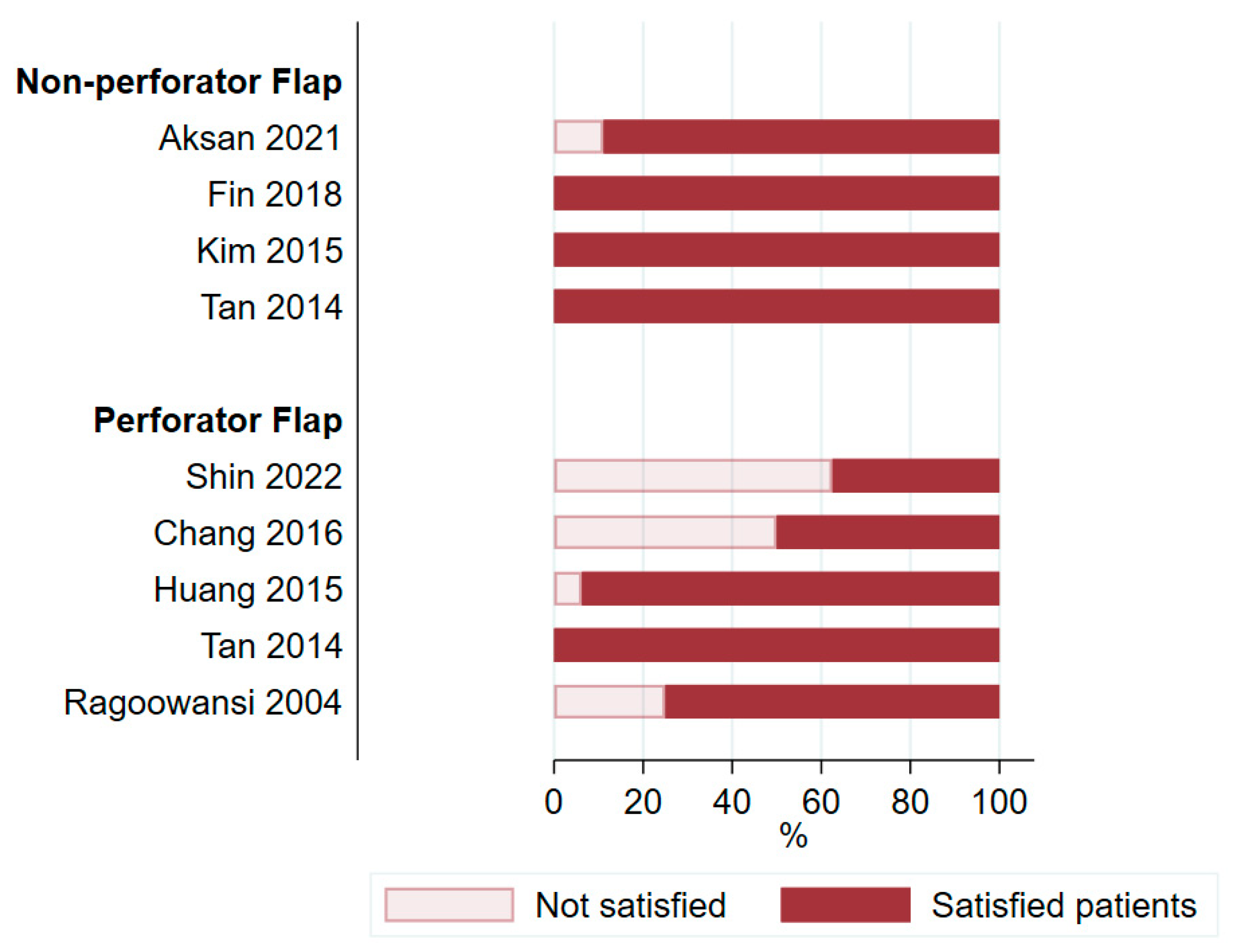
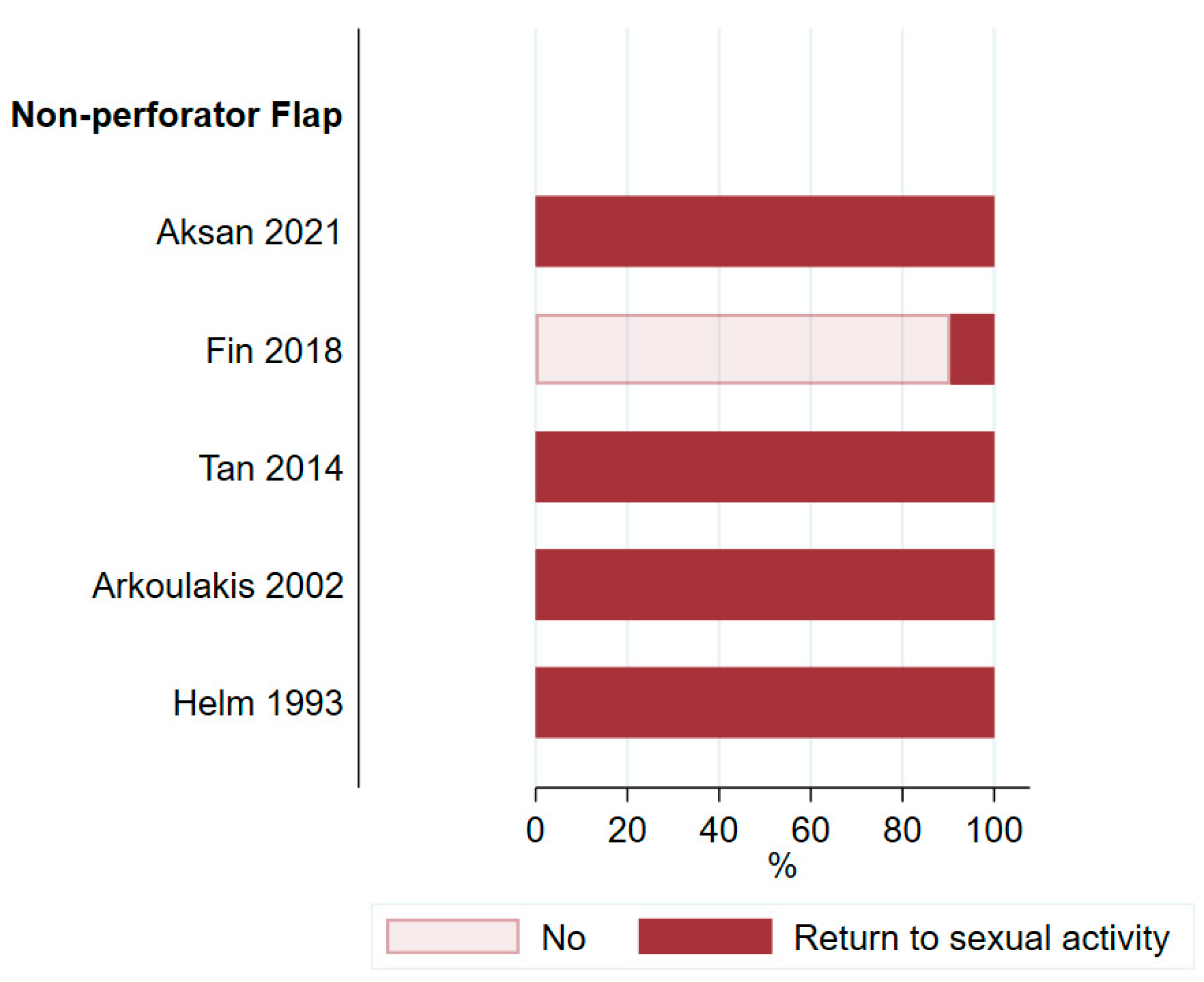
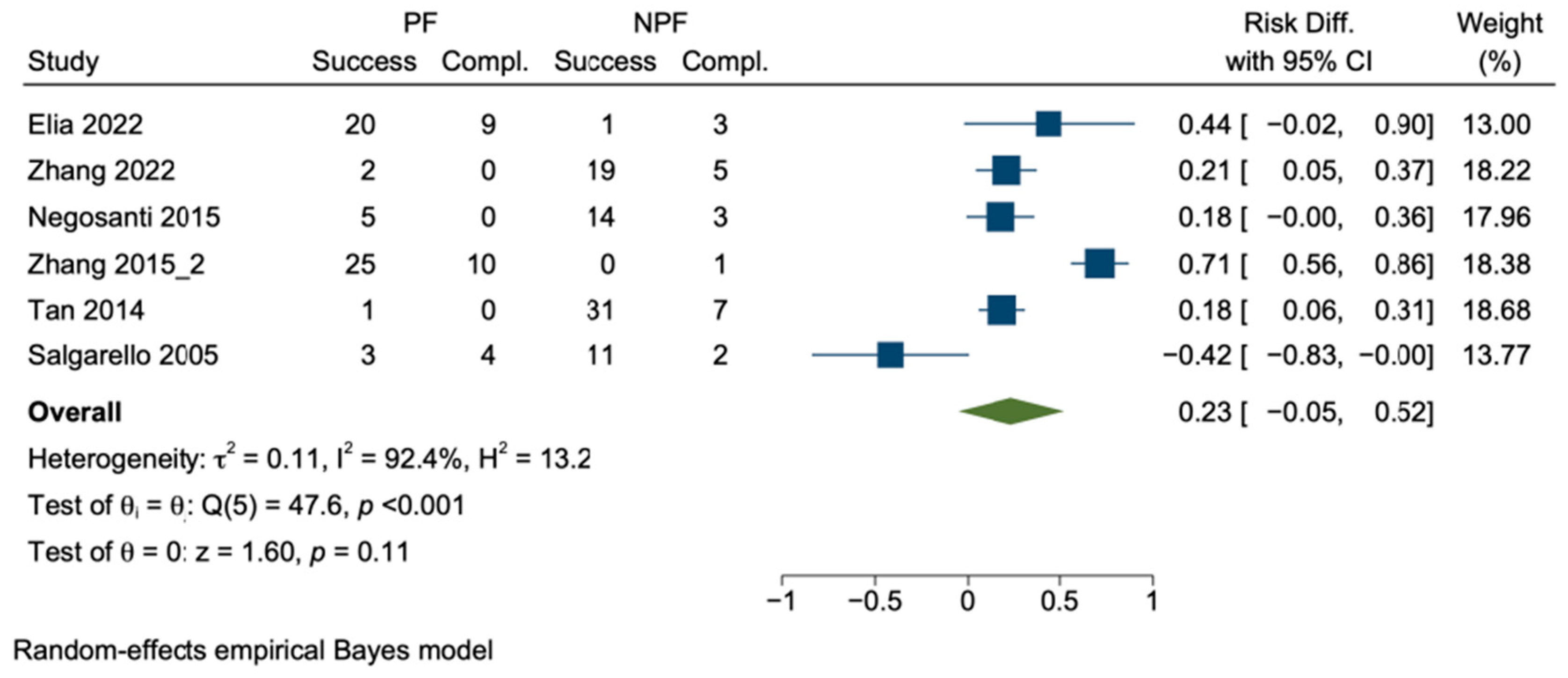
3.5. Satisfaction and Quality of Life
4. Discussion
4.1. Surgical Complications
4.2. Potential Importance of Perforator Flaps in Female Vulvoperineal Reconstruction
4.3. Assessment of Patient Satisfaction and Quality of Life
4.4. Limitations
4.5. Perforator Flaps as a Valid Choice in the Reconstructive Armamentarium
5. Conclusions and Future Direction
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| QoL | quality of life |
| PAP | profunda artery perforator |
| ALT | anterolateral thigh |
| SGAP | superior gluteal artery perforator |
| IGAP | inferior gluteal artery perforator |
| DIEP | deep inferior epigastric artery perforator |
| VRAM | vertical rectus abdominis myocutaneous |
References
- Kang, Y.-J.; Smith, M.; Barlow, E.; Coffey, K.; Hacker, N.; Canfell, K. Vulvar Cancer in High-Income Countries: Increasing Burden of Disease. Int. J. Cancer 2017, 141, 2174–2186. [Google Scholar] [CrossRef] [PubMed]
- Judson, P.L.; Habermann, E.B.; Baxter, N.N.; Durham, S.B.; Virnig, B.A. Trends in the Incidence of Invasive and In Situ Vulvar Carcinoma. Obstet. Gynecol. 2006, 107, 1018. [Google Scholar] [CrossRef] [PubMed]
- Olawaiye, A.B.; Cotler, J.; Cuello, M.A.; Bhatla, N.; Okamoto, A.; Wilailak, S.; Purandare, C.N.; Lindeque, G.; Berek, J.S.; Kehoe, S. FIGO Staging for Carcinoma of the Vulva: 2021 Revision. Int. J. Gynecol. Obstet. 2021, 155, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Bieber, A.K.; Stein, J.A.; Pomeranz, M.K. Diagnosis and Management of Vulvar Cancer: A Review. J. Am. Acad. Dermatol. 2019, 81, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Barton, D.P.J. The Prevention and Management of Treatment Related Morbidity in Vulval Cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2003, 17, 683–701. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. Management of Advanced Squamous Cell Carcinoma of the Vulva. Cancers 2021, 14, 167. [Google Scholar] [CrossRef]
- Morrison, J.; Baldwin, P.; Buckley, L.; Cogswell, L.; Edey, K.; Faruqi, A.; Ganesan, R.; Hall, M.; Hillaby, K.; Reed, N.; et al. British Gynaecological Cancer Society (BGCS) Vulval Cancer Guidelines: Recommendations for Practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 502–525. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, R.L.; Verleye, L.; Ratnavelu, N.; Galaal, K.; Fisher, A.; Naik, R. Locally Advanced Vulva Cancer: A Single Centre Review of Anovulvectomy and a Systematic Review of Surgical, Chemotherapy and Radiotherapy Alternatives. Is an International Collaborative RCT Destined for the “Too Difficult to Do” Box? Gynecol. Oncol. 2017, 144, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Hellinga, J.; Khoe, P.C.K.H.; Stenekes, M.W.; Eltahir, Y. Complications after Vulvar and Perineal Reconstruction with a Lotus Petal Flap. Ann. Plast. Surg. 2018, 80, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Confalonieri, P.L.; Gilardi, R.; Rovati, L.C.; Ceccherelli, A.; Lee, J.H.; Magni, S.; Del Bene, M.; Buda, A. Comparison of V-Y Advancement Flap Versus Lotus Petal Flap for Plastic Reconstruction After Surgery in Case of Vulvar Malignancies: A Retrospective Single Center Experience. Ann. Plast. Surg. 2017, 79, 186–191. [Google Scholar] [CrossRef]
- Hellinga, J.; Rots, M.; Werker, P.M.N.; Stenekes, M.W. Lotus Petal Flap and Vertical Rectus Abdominis Myocutaneous Flap in Vulvoperineal Reconstruction: A Systematic Review of Differences in Complications. J. Plast. Surg. Hand Surg. 2021, 55, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Geddes, C.R.; Morris, S.F.; Neligan, P.C. Perforator Flaps: Evolution, Classification, and Applications. Ann. Plast. Surg. 2003, 50, 90. [Google Scholar] [CrossRef] [PubMed]
- Largo, R.D.; Bhadkamkar, M.A.; Asaad, M.; Chu, C.K.; Garvey, P.B.; Butler, C.E.; Yu, P.; Hanasono, M.M.; Chang, E.I. The Profunda Artery Perforator Flap: A Versatile Option for Head and Neck Reconstruction. Plast. Reconstr. Surg. 2021, 147, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Mayo-Yáñez, M.; Rodríguez-Pérez, E.; Chiesa-Estomba, C.M.; Calvo-Henríquez, C.; Rodríguez-Lorenzo, A. Deep Inferior Epigastric Artery Perforator Free Flap in Head and Neck Reconstruction: A Systematic Review. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Z.; Azoury, S.C.; Matros, E.; Nelson, J.A.; Allen, R.J. Modern Approaches to Alternative Flap-Based Breast Reconstruction: Profunda Artery Perforator Flap. Clin. Plast. Surg. 2023, 50, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Zobec Logar, H.B. Long Term Results of Radiotherapy in Vulvar Cancer Patients in Slovenia between 1997–2004. Radiol. Oncol. 2017, 51, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, M.R.; Shen, A.H.; Lee, G.K.; Momeni, A.; Longaker, M.T.; Wan, D.C. Radiation-Induced Skin Fibrosis: Pathogenesis, Current Treatment Options, and Emerging Therapeutics. Ann. Plast. Surg. 2019, 83, S59–S64. [Google Scholar] [CrossRef] [PubMed]
- Bramer, W.M.; Rethlefsen, M.L.; Mast, F.; Kleijnen, J. Evaluation of a New Method for Librarian-mediated Literature Searches for Systematic Reviews. Res. Synth. Methods 2018, 9, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Lange, M.; Hage, J.J.; van Beurden, M. A Prospective Assessment of Surgical Risk Factors in 114 Gluteal Fold Flap Reconstructions After Oncological Vulvoperineal Resection. Ann. Plast. Surg. 2017, 79, 53–59. [Google Scholar] [CrossRef]
- Chang, T.N.-J.; Lee, C.-H.; Lai, C.-H.; Wu, C.-W.; Chang, C.-S.; Cheng, M.-H.; Huang, J.-J. Profunda Artery Perforator Flap for Isolated Vulvar Defect Reconstruction after Oncological Resection. J. Surg. Oncol. 2016, 113, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Abdulrahman, G.O.; Das, N.; Chandrasekaran, T.V.; Khot, U.; Drew, P.J.; Bose, P.; Vet, J.N.; Tofazzal, N.; Roberts, S.; Lutchman Singh, K. Pelvic Exenteration for the Treatment of Locally Advanced Vulvar Cancer in South West Wales. Cancers 2022, 14, 1767. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Dong, R.; Zeng, A.; Teng, Y.; Liu, Z.; Zhu, L.; Long, F.; Si, L.; Yu, N.; Wang, X. The Reconstructive Strategy for Pelvic Oncological Surgery with Various Types of MS-VRAM Flaps. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 2090–2097. [Google Scholar] [CrossRef] [PubMed]
- Muallem, M.Z.; Sehouli, J.; Miranda, A.; Plett, H.; Sayasneh, A.; Diab, Y.; Muallem, J.; Hatoum, I. Reconstructive Surgery versus Primary Closure Following Vulvar Cancer Excision: A Wide Single-Center Experience. Cancers 2022, 14, 1695. [Google Scholar] [CrossRef] [PubMed]
- Aksan, T.; Karateke, A.; Uzuneyüpoğlu, O.; Öztürk, M.B.; Küçükbaş, M. Reconstruction of Vulva and Perineal Defects After Gynecological Oncological Surgery and Effectiveness of Local Flaps. Düzce Tıp Fakültesi Derg. 2021, 23, 164–169. [Google Scholar] [CrossRef]
- Giannini, A.; Di Donato, V.; D’Oria, O.; Schiavi, M.C.; May, J.; Benedetti Panici, P.; Congiu, M.A. The V-Y Gluteal Fold Advancement Flap: Outcomes Following Radical Surgery for Vulvar Malignancies. Int. J. Gynecol. Obstet. 2021, 152, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Tock, S.; Wallet, J.; Belhadia, M.; Hudry, D.; Ghesquière, L.; Narducci, F.; Leblanc, E. Outcomes of the Use of Different Vulvar Flaps for Reconstruction during Surgery for Vulvar Cancer. Eur. J. Surg. Oncol. 2019, 45, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Fin, A.; Rampino Cordaro, E.; Guarneri, G.F.; Revesz, S.; Vanin, M.; Parodi, P.C. Experience with Gluteal V-Y Fasciocutaneous Advancement Flaps in Vulvar Reconstruction after Oncological Resection and a Modification to the Marking: Playing with Tension Lines. Int. Wound J. 2018, 16, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Hand, L.C.; Maas, T.M.; Baka, N.; Mercier, R.J.; Greaney, P.J.; Rosenblum, N.G.; Kim, C.H. Utilizing V-Y Fasciocutaneous Advancement Flaps for Vulvar Reconstruction. Gynecol. Oncol. Rep. 2018, 26, 24–28. [Google Scholar] [CrossRef]
- Conri, V.; Casoli, V.; Coret, M.; Houssin, C.; Trouette, R.; Brun, J.-L. Modified Gluteal Fold V-Y Advancement Flap for Reconstruction After Radical Vulvectomy. Int. J. Gynecol. Cancer 2016, 26, 1300–1306. [Google Scholar] [CrossRef]
- Herraiz Roda, J.L.; Llueca Abella, J.A.; Maazouzi, Y.; Bouché Babiloni, A.; Cañete Mota, A.; Guijarro Colomer, M.; Serra Rubert, A. Vulvar Reconstruction in Vulvar Cancer: “Lotus Petal” Suprafascial Flap. Gynecol. Surg. 2016, 13, 51–55. [Google Scholar] [CrossRef]
- Kim, S.W.; Lee, W.M.; Kim, J.T.; Kim, Y.H. Vulvar and Vaginal Reconstruction Using the “Angel Wing” Perforator-Based Island Flap. Gynecol. Oncol. 2015, 137, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Nomura, H.; Maeda, T.; Usami, T.; Abe, A.; Yamamoto, A.; Matoda, M.; Okamoto, S.; Kondo, E.; Omatsu, K.; Kato, K.; et al. Vulvar Reconstruction Following Surgery for Vulvar Cancer Using a Stepladder V-Y Advancement Medial Thigh Flap. Int. J. Gynecol. Cancer 2015, 25, 1484–1487. [Google Scholar] [CrossRef] [PubMed]
- Benedetti Panici, P.; Di Donato, V.; Bracchi, C.; Marchetti, C.; Tomao, F.; Palaia, I.; Perniola, G.; Muzii, L. Modified Gluteal Fold Advancement V-Y Flap for Vulvar Reconstruction after Surgery for Vulvar Malignancies. Gynecol. Oncol. 2014, 132, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Argenta, P.A.; Lindsay, R.; Aldridge, R.B.; Siddiqui, N.; Burton, K.; Telfer, J.R.C. Vulvar Reconstruction Using the “Lotus Petal” Fascio-Cutaneous Flap. Gynecol. Oncol. 2013, 131, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Kuokkanen, H.; Mikkola, A.; Nyberg, R.H.; Vuento, M.H.; Kaartinen, I.; Kuoppala, T. Reconstruction of the Vulva with Sensate Gluteal Fold Flaps. Scand. J. Surg. 2013, 102, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Shin, J.W.; Kim, S.W.; Oh, D.Y.; Park, J.S.; Hur, S.Y.; Rhie, J.W.; Ahn, S.T. Modified Gluteal Fold V-Y Advancement Flap for Vulvovaginal Reconstruction. Ann. Plast. Surg. 2013, 71, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Buda, A.; Confalonieri, P.L.; Rovati, L.C.V.; Fruscio, R.; Giuliani, D.; Signorelli, M.; Dell’Anna, T.; Pirovano, C.; Milani, R. Better Anatomical and Cosmetic Results Using Tunneled Lotus Petal Flap for Plastic Reconstruction after Demolitive Surgery for Vulvar Malignancy. Int. J. Gynecol. Cancer 2012, 22, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Misani, M.; Rovati, L.C.V.; Confalonieri, P.; Buda, A.; Giuliani, D.; Del Bene, M. Modified Lotus Petal Flap for Vulvo-Vaginal Reconstruction after Resection for Vulvar Cancer: A Single Institution Experience. Handchir. Mikrochir. Plast. Chir. 2011, 43, 250–254. [Google Scholar] [CrossRef]
- Staiano, J.J.; Wong, L.; Butler, J.; Searle, A.E.; Barton, D.P.J.; Harris, P.A. Flap Reconstruction Following Gynaecological Tumour Resection for Advanced and Recurrent Disease—A 12 Year Experience. J. Plast. Reconstr. Aesthetic Surg. 2009, 62, 346–351. [Google Scholar] [CrossRef]
- Weikel, W.; Schmidt, M.; Steiner, E.; Knapstein, P.-G.; Koelbl, H. Reconstructive Plastic Surgery in the Treatment of Vulvar Carcinomas. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 136, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Weikel, W.; Schmidt, M.; Steiner, E.; Knapstein, P.-G.; Koelbl, H. Surgical Therapy of Recurrent Vulvar Cancer. Am. J. Obstet. Gynecol. 2006, 195, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Persichetti, P.; Simone, P.; Berloco, M.; Casadei, R.M.; Marangi, G.F.; Cagli, B.; Di Lella, F. Vulvo-Perineal Reconstruction: Medial Thigh Septo-Fascio-Cutaneous Island Flap. Ann. Plast. Surg. 2003, 50, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Arkoulakis, N.S.; Angel, C.L.; DuBeshter, B.; Serletti, J.M. Reconstruction of an Extensive Vulvectomy Defect Using the Gluteus Maximus Fasciocutaneous V-Y Advancement Flap. Ann. Plast. Surg. 2002, 49, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Moschella, F.; Cordova, A. Innervated Island Flaps in Morphofunctional Vulvar Reconstruction. Plast. Reconstr. Surg. 2000, 105, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Loree, T.R.; Hempling, R.E.; Eltabbakh, G.H.; Recio, F.O.; Piver, M.S. The Inferior Gluteal Flap in the Difficult Vulvar and Perineal Reconstruction. Gynecol. Oncol. 1997, 66, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Yii, N.W.; Niranjan, N.S. Lotus Petal Flaps in Vulvo-Vaginal Reconstruction. Br. J. Plast. Surg. 1996, 49, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Burke, T.W.; Morris, M.; Roh, M.S.; Levenback, C.; Gershenson, D.M. Perineal Reconstruction Using Single Gracilis Myocutaneous Flaps. Gynecol. Oncol. 1995, 57, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Helm, C.W.; Hatch, K.D.; Partridge, E.E.; Shingleton, H.M. The Rhomboid Transposition Flap for Repair of the Perineal Defect after Radical Vulvar Surgery. Gynecol. Oncol. 1993, 50, 164–167. [Google Scholar] [CrossRef]
- Shepherd, J.H.; Van Dam, P.A.; Jobling, T.W.; Breach, N. The Use of Rectus Abdominis Myocutaneous Flaps Following Excision of Vulvar Cancer. Br. J. Obstet. Gynaecol. 1990, 97, 1020–1025. [Google Scholar] [CrossRef]
- Shin, J.; Kim, S.A.; Rhie, J.-W. Perineal Perforator Switch Flap for Three-Dimensional Vulvovaginal Reconstruction. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 3208–3216. [Google Scholar] [CrossRef]
- Gentileschi, S.; Servillo, M.; Garganese, G.; Simona, F.; Scambia, G.; Salgarello, M. Versatility of Pedicled Anterolateral Thigh Flap in Gynecologic Reconstruction after Vulvar Cancer Extirpative Surgery. Microsurgery 2017, 37, 516–524. [Google Scholar] [CrossRef]
- Huang, J.-J.; Chang, N.-J.; Chou, H.-H.; Wu, C.-W.; Abdelrahman, M.; Chen, H.-Y.; Cheng, M.-H. Pedicle Perforator Flaps for Vulvar Reconstruction—New Generation of Less Invasive Vulvar Reconstruction with Favorable Results. Gynecol. Oncol. 2015, 137, 66–72. [Google Scholar] [CrossRef]
- Al-Benna, S.; Tzakas, E. Postablative Reconstruction of Vulvar Defects with Local Fasciocutaneous Flaps and Superficial Fascial System Repair. Arch. Gynecol. Obstet. 2012, 286, 443–448. [Google Scholar] [CrossRef]
- Yun, I.S.; Lee, J.H.; Rah, D.K.; Lee, W.J. Perineal Reconstruction Using a Bilobed Pudendal Artery Perforator Flap. Gynecol. Oncol. 2010, 118, 313–316. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, X.; Yang, Y. The Application of Anterolateral Thigh Flap in Post-Operative Repairing of Vulva Tumor. Chin. -Ger. J. Clin. Oncol. 2010, 9, 539–542. [Google Scholar] [CrossRef]
- Franchelli, S.; Leone, M.S.; Bruzzone, M.; Muggianu, M.; Puppo, A.; Gustavino, C.; Di Capua, E.; Centurioni, M.G. The Gluteal Fold Fascio-Cutaneous Flap for Reconstruction after Radical Excision of Primary Vulvar Cancers. Gynecol. Oncol. 2009, 113, 245–248. [Google Scholar] [CrossRef]
- Ragoowansi, R.; Yii, N.; Niranjan, N. Immediate Vulvar and Vaginal Reconstruction Using the Gluteal-Fold Flap: Long-Term Results. Br. J. Plast. Surg. 2004, 57, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Elia, J.; Do, N.T.K.; Chang, T.N.-J.; Lai, C.-H.; Chou, H.-H.; Chang, F.C.-S.; Huang, J.-J. Redefining the Reconstructive Ladder in Vulvoperineal Reconstruction: The Role of Pedicled Perforator Flaps. J. Reconstr. Microsurg. 2022, 38, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.-K.; Kang, G.C.-W.; Tay, E.H.; Por, Y.C. Subunit Principle of Vulvar Reconstruction: Algorithm and Outcomes. Arch. Plast. Surg. 2014, 41, 379–386. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Ge, J.; Su, Z.; Xiao, X.; Chen, C.; Shi, F.; Wang, Y.; Zhang, J.; Liang, W. Experience with Flap Repair after Vulvar Carcinoma Resection: A Retrospective Observational Study of 26 Cases. Transl. Cancer Res. 2022, 11, 1740–1749. [Google Scholar] [CrossRef] [PubMed]
- Negosanti, L.; Sgarzani, R.; Fabbri, E.; Palo, S.; Oranges, C.M.; De Iaco, P.; Zannetti, G.; Contedini, F.; Cipriani, R. Vulvar Reconstruction by Perforator Flaps: Algorithm for Flap Choice Based on the Topography of the Defect. Int. J. Gynecol. Cancer 2015, 25, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zeng, A.; Yang, J.; Cao, D.; Huang, H.; Wang, X.; You, Y.; Chen, J.; Lang, J.; Shen, K. Outcome of Vulvar Reconstruction by Anterolateral Thigh Flap in Patients with Advanced and Recurrent Vulvar Malignancy. J. Surg. Oncol. 2015, 111, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zeng, A.; Yang, J.; Cao, D.; He, X.; Wang, X.; You, Y.; Chen, J.; Lang, J.; Shen, K. Outcome of Vulvar Reconstruction in Patients with Advanced and Recurrent Vulvar Malignancies. BMC Cancer 2015, 15, 851. [Google Scholar] [CrossRef]
- Salgarello, M.; Farallo, E.; Barone-Adesi, L.; Cervelli, D.; Scambia, G.; Salerno, G.; Margariti, P.A. Flap Algorithm in Vulvar Reconstruction after Radical, Extensive Vulvectomy. Ann. Plast. Surg. 2005, 54, 184–190. [Google Scholar] [CrossRef]
- Zeng, A.; Qiao, Q.; Zhao, R.; Song, K.; Long, X. Anterolateral Thigh Flap-Based Reconstruction for Oncologic Vulvar Defects. Plast. Reconstr. Surg. 2011, 127, 1939–1945. [Google Scholar] [CrossRef]
- Allen, R.J.; Treece, P. Deep Inferior Epigastric Perforator Flap for Breast Reconstruction. Ann. Plast. Surg. 1994, 32, 32. [Google Scholar] [CrossRef] [PubMed]
- Devulapalli, C.; Jia Wei, A.T.; DiBiagio, J.R.; Baez, M.L.; Baltodano, P.A.; Seal, S.M.; Sacks, J.M.; Cooney, C.M.; Rosson, G.D. Primary versus Flap Closure of Perineal Defects following Oncologic Resection: A Systematic Review and Meta-Analysis. Plast. Reconstr. Surg. 2016, 137, 1602. [Google Scholar] [CrossRef]
- Blondeel, P.N.; Van Landuyt, K.H.I.; Monstrey, S.J.M.; Hamdi, M.; Matton, G.E.; Allen, R.J.; Dupin, C.; Feller, A.-M.; Koshima, I.; Kostakoglu, N.; et al. The “Gent” Consensus on Perforator Flap Terminology: Preliminary Definitions. Plast. Reconstr. Surg. 2003, 112, 1378–1383, discussion 1384–1387; quiz 1383, 1516. [Google Scholar] [CrossRef]
- Eseme, E.A.; Scampa, M.; Viscardi, J.A.; Ebai, M.; Kalbermatten, D.F.; Oranges, C.M. Surgical Outcomes of VRAM vs. Gracilis Flaps in Vulvo-Perineal Reconstruction Following Oncologic Resection: A Proportional Meta-Analysis. Cancers 2022, 14, 4300. [Google Scholar] [CrossRef]
- Kim, E.; Fernando, C.; McCombie, A.; Bailey, W.; Frizelle, F.; Glyn, T.; Porter, C.; Wakeman, C.; Creagh, T. Abdominal and Perineal Hernia Rates Following Vertical Rectus Abdominis Myocutaneous (VRAM) Flap Reconstruction - a Supraregional Experience. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Xiong, L.; Li, J.; Sun, Y.; Sun, J.; Guo, N.; Wang, Z. A Systematic Review and Meta-Analysis on Microsurgical Safety and Efficacy of Profunda Artery Perforator Flap in Breast Reconstruction. J. Oncol. 2019, 2019, 9506720. [Google Scholar] [CrossRef] [PubMed]
- Knox, A.D.C.; Ho, A.L.; Leung, L.; Tashakkor, A.Y.; Lennox, P.A.; Van Laeken, N.; Macadam, S.A. Comparison of Outcomes following Autologous Breast Reconstruction Using the DIEP and Pedicled TRAM Flaps: A 12-Year Clinical Retrospective Study and Literature Review. Plast. Reconstr. Surg. 2016, 138, 16–28. [Google Scholar] [CrossRef]
- Tielemans, H.J.P.; van Kuppenveld, P.I.P.; Winters, H.; Hupkens, P.; Ulrich, D.J.O.; Hummelink, S. Breast Reconstruction with the Extended Profunda Artery Perforator Flap. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Jain, V.; Celik, N.; Chen, H.; Chuang, D.C.-C.; Lin, C. Have We Found an Ideal Soft-Tissue Flap? An Experience with 672 Anterolateral Thigh Flaps. Plast. Reconstr. Surg. 2002, 109, 2219–2226, discussion 2227–2230. [Google Scholar] [CrossRef] [PubMed]
- Chana, J.S.; Odili, J. Perforator Flaps in Head and Neck Reconstruction. Semin. Plast. Surg. 2010, 24, 237–254. [Google Scholar] [CrossRef]
- Witte, D.Y.S.; van Ramshorst, G.H.; Lapid, O.; Bouman, M.-B.; Tuynman, J.B. Flap Reconstruction of Perineal Defects after Pelvic Exenteration: A Systematic Description of Four Choices of Surgical Reconstruction Methods. Plast. Reconstr. Surg. 2021, 147, 1420–1435. [Google Scholar] [CrossRef]


| PICOS | Inclusion | Exclusion |
|---|---|---|
| Populations | Female adults with vulvoperineal reconstruction with perforator or non-perforator flap | Cadaveric, animal studies |
| Intervention | Perforator or non-perforator flap for vulvoperineal reconstruction following oncologic resection | Other reconstruction techniques like primary closure or net implementation |
| Comparator | The study analysis compared postoperative surgical outcome parameters | |
| Outcomes | Main outcome: complications like infection rate, dehiscence, partial or total flap necrosis graded according to Clavien–Dindo classification | Studies that do not report main outcome |
| Study design | Randomized controlled trials, comparative studies, and case series ≥7 patients | Reviews, meta-analyses, case reports, unpublished studies, and non-English language studies |
| Study | # Flaps | Non-Perforator | Perforator |
|---|---|---|---|
| Abdulrahman 2022 [22] | 18 | Gracilis myocutaneous flap (7), IGAM (1), local fasciocutaneous flap (2), VRAM (8) | |
| Chen 2022 [23] | 30 | VRAM (30) | |
| Elia 2022 [59] | 55 | Gracilis myocutaneous flap (3), local fasciocutaneous flap (2), VRAM (1) | ALT (3), local perforator fasciocutaneous flap (23), PAP (23) |
| Muallem 2022 [24] | 126 | Flap combination (22), local fasciocutaneous flap (84), myocutaneous flap (20) | |
| Shin 2022 [51] | 27 | Local perforator fasciocutaneous flap (27) | |
| Zhang 2022 [61] | 34 | Local fasciocutaneous flap (30), VRAM (2) | ALT (2) |
| Aksan 2021 [25] | 31 | Local fasciocutaneous flap (29), skin graft (1), VRAM (1) | |
| Giannini 2021 [26] | 40 | Local fasciocutaneous flap (40) | |
| Tock 2019 [27] | 61 | Gluteal thigh flap (16), local fasciocutaneous flap (45) | |
| Fin 2018 [28] | 59 | Local fasciocutaneous flap (59) | |
| Hand 2018 [29] | 42 | Local fasciocutaneous flap (42) | |
| Hellinga 2018 [9] | 89 | Local fasciocutaneous flap (89) | |
| Confalonieri 2017 [10] | 365 | Local fasciocutaneous flap (365) | |
| Gentileschi 2017 [52] | 16 | ALT (16) | |
| Lange 2017 [20] | 114 | Local fasciocutaneous flap (114) | |
| Chang 2016 [21] | 19 | PAP (19) | |
| Conri 2016 [30] | 36 | Local fasciocutaneous flap (36) | |
| Herraiz Roda 2016 [31] | 17 | Local fasciocutaneous flap (17) | |
| Huang 2015 [53] | 27 | Local perforator fasciocutaneous flap (16), PAP (11) | |
| Kim 2015 [32] | 41 | Local fasciocutaneous flap (41) | |
| Negosanti 2015 [62] | 33 | Local fasciocutaneous flap (28) | DIEP (5) |
| Nomura 2015 [33] | 19 | Local fasciocutaneous flap (16), non-specified flap (3) | |
| Zhang 2015 [63] | 27 | Gracilis myocutaneous flap (1), TRAM (1) | ALT (24), DIEP (1) |
| Zhang 2015_2 [64] | 40 | Gracilis myocutaneous flap (2), TRAM (1) | ALT (24), DIEP (6), pudendal thigh fasciocutaneous flap (7) |
| Benedetti Panici 2014 [34] | 29 | Local fasciocutaneous flap (29) | |
| Tan 2014 [60] | 72 | Gracilis myocutaneous flap (36), local fasciocutaneous flap (21), skin graft (11), VRAM (3) | ALT (1) |
| Argenta 2013 [35] | 80 | Local fasciocutaneous flap (80) | |
| Kuokkanen 2013 [36] | 22 | Local fasciocutaneous flap (22) | |
| Lee 2013 [37] | 27 | Local fasciocutaneous flap (27) | |
| Al-Benna 2012 [54] | 13 | Local perforator fasciocutaneous flap (13) | |
| Buda 2012 [38] | 38 | Local fasciocutaneous flap (38) | |
| Misani 2011 [39] | 69 | Local fasciocutaneous flap (69) | |
| Zeng 2011 [66] | 14 | Local fasciocutaneous flap (3) | ALT (11) |
| Yun 2010 [55] | 24 | PAP (24) | |
| Zhou 2010 [56] | 10 | ALT (10) | |
| Franchelli 2009 [57] | 53 | Local perforator fasciocutaneous flap (53) | |
| Staiano 2009 [40] | 53 | Gracilis myocutaneous flap (4), latissimus dorsi muscle flap (1), local fasciocutaneous flap (26), tensor fasciae latae flap (1), VRAM (21) | |
| Weikel 2008 [41] | 207 | Gluteal thigh flap (54), gracilis myocutaneous flap (5), local fasciocutaneous flap (123), tensor fasciae latae flap (9), VRAM (16) | |
| Weikel 2006 [42] | 123 | Gluteal thigh flap (40), gracilis myocutaneous flap (4), local fasciocutaneous flap (58), tensor fasciae latae flap (8), VRAM (13) | |
| Salgarello 2005 [65] | 31 | Local fasciocutaneous flap (18), VRAM (4) | Pudendal thigh fasciocutaneous flap (9) |
| Ragoowansi 2004 [58] | 56 | Local perforator fasciocutaneous flap (56) | |
| Persichetti 2003 [43] | 26 | Local fasciocutaneous flap (26) | |
| Arkoulakis 2002 [44] | 36 | Local fasciocutaneous flap (36) | |
| Moschella 2000 [45] | 22 | Local fasciocutaneous flap (22) | |
| Loree 1997 [46] | 13 | Gracilis (2), local fasciocutaneous flap (10), VRAM (1) | |
| Niranjan 1996 [47] | 13 | Local fasciocutaneous flap (13) | |
| Burke 1995 [48] | 18 | Gracilis myocutaneous flap (18) | |
| Helm 1993 [49] | 30 | Local fasciocutaneous flap (30) | |
| Shepeherd 1990 [50] | 16 | VRAM (16) |
| Variable | Association with Outcome | Model Fit | Heterogeneity among Studies | |||||
|---|---|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p | R2 | p * | I2 (%) | H2 | τ2 | p ** | |
| Treatment | −0.06 (−0.20 to 0.09) | 0.459 | 0 | 0.450 | 91 | 11 | 0.05 | <0.001 |
| Treatment | −0.06 (−0.21 to 0.08) | 0.413 | 1 | 0.299 | 90 | 10 | 0.05 | <0.001 |
| Year ≥ 2015 | 0.09 (−0.04 to 0.22) | 0.175 | ||||||
| Treatment | −0.04 (−0.20 to 0.11) | 0.599 | 0 | 0.617 | 90 | 10 | 0.05 | <0.001 |
| # Patients ≥ 50 | 0.06 (−0.13 to 0.25) | 0.540 | ||||||
| Treatment | −0.05 (−0.21 to 0.10) | 0.522 | 0 | 0.457 | 90 | 10 | 0.05 | <0.001 |
| Year ≥ 2015 | 0.09 (−0.05 to 0.22) | 0.203 | ||||||
| # Patients ≥ 50 | 0.04 (−0.14 to 0.23) | 0.654 | ||||||
| Association with Outcome | Model Fit | Heterogeneity among Studies | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Coefficient (95% CI) | p | R2 | *p | I2 (%) | H2 | τ2 | **p |
| Treatment | 0.01 (−0.14 to 0.17) | 0.880 | 0 | 0.871 | 91 | 11 | 0.05 | <0.001 |
| Treatment | 0.01 (−0.14 to 0.16) | 0.917 | 0 | 0.561 | 91 | 11 | 0.05 | <0.001 |
| Year ≥ 2015 | 0.06 (−0.07 to 0.20) | 0.368 | ||||||
| Treatment | 0.03 (−0.13 to 0.19) | 0.737 | 0 | 0.753 | 91 | 11 | 0.05 | <0.001 |
| # Patients ≥ 50 | 0.07 (−0.12 to 0.26) | 0.472 | ||||||
| Treatment | 0.02 (−0.14 to 0.18) | 0.792 | 0 | 0.745 | 91 | 10 | 0.06 | <0.001 |
| Year ≥ 2015 | 0.06 (−0.08 to 0.19) | 0.421 | ||||||
| # Patients ≥ 50 | 0.06 (−0.13 to 0.25) | 0.545 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wendelspiess, S.; Kouba, L.; Stoffel, J.; Speck, N.; Appenzeller-Herzog, C.; Gahl, B.; Montavon, C.; Heinzelmann-Schwarz, V.; Lariu, A.; Schaefer, D.J.; et al. Perforator versus Non-Perforator Flap-Based Vulvoperineal Reconstruction—A Systematic Review and Meta-Analysis. Cancers 2024, 16, 2213. https://doi.org/10.3390/cancers16122213
Wendelspiess S, Kouba L, Stoffel J, Speck N, Appenzeller-Herzog C, Gahl B, Montavon C, Heinzelmann-Schwarz V, Lariu A, Schaefer DJ, et al. Perforator versus Non-Perforator Flap-Based Vulvoperineal Reconstruction—A Systematic Review and Meta-Analysis. Cancers. 2024; 16(12):2213. https://doi.org/10.3390/cancers16122213
Chicago/Turabian StyleWendelspiess, Séverin, Loraine Kouba, Julia Stoffel, Nicole Speck, Christian Appenzeller-Herzog, Brigitta Gahl, Céline Montavon, Viola Heinzelmann-Schwarz, Ana Lariu, Dirk J. Schaefer, and et al. 2024. "Perforator versus Non-Perforator Flap-Based Vulvoperineal Reconstruction—A Systematic Review and Meta-Analysis" Cancers 16, no. 12: 2213. https://doi.org/10.3390/cancers16122213
APA StyleWendelspiess, S., Kouba, L., Stoffel, J., Speck, N., Appenzeller-Herzog, C., Gahl, B., Montavon, C., Heinzelmann-Schwarz, V., Lariu, A., Schaefer, D. J., Ismail, T., & Kappos, E. A. (2024). Perforator versus Non-Perforator Flap-Based Vulvoperineal Reconstruction—A Systematic Review and Meta-Analysis. Cancers, 16(12), 2213. https://doi.org/10.3390/cancers16122213