Different Patterns of Care and Survival Outcomes in Transplant-Centre Managed Patients with Early-Stage HCC: Real-World Data from an Australian Multi-Centre Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Statistical Analysis
3. Results
3.1. Patients
3.2. Transplantation
3.3. Resection
3.4. Locoregional Treatment
3.5. All-Cause Mortality
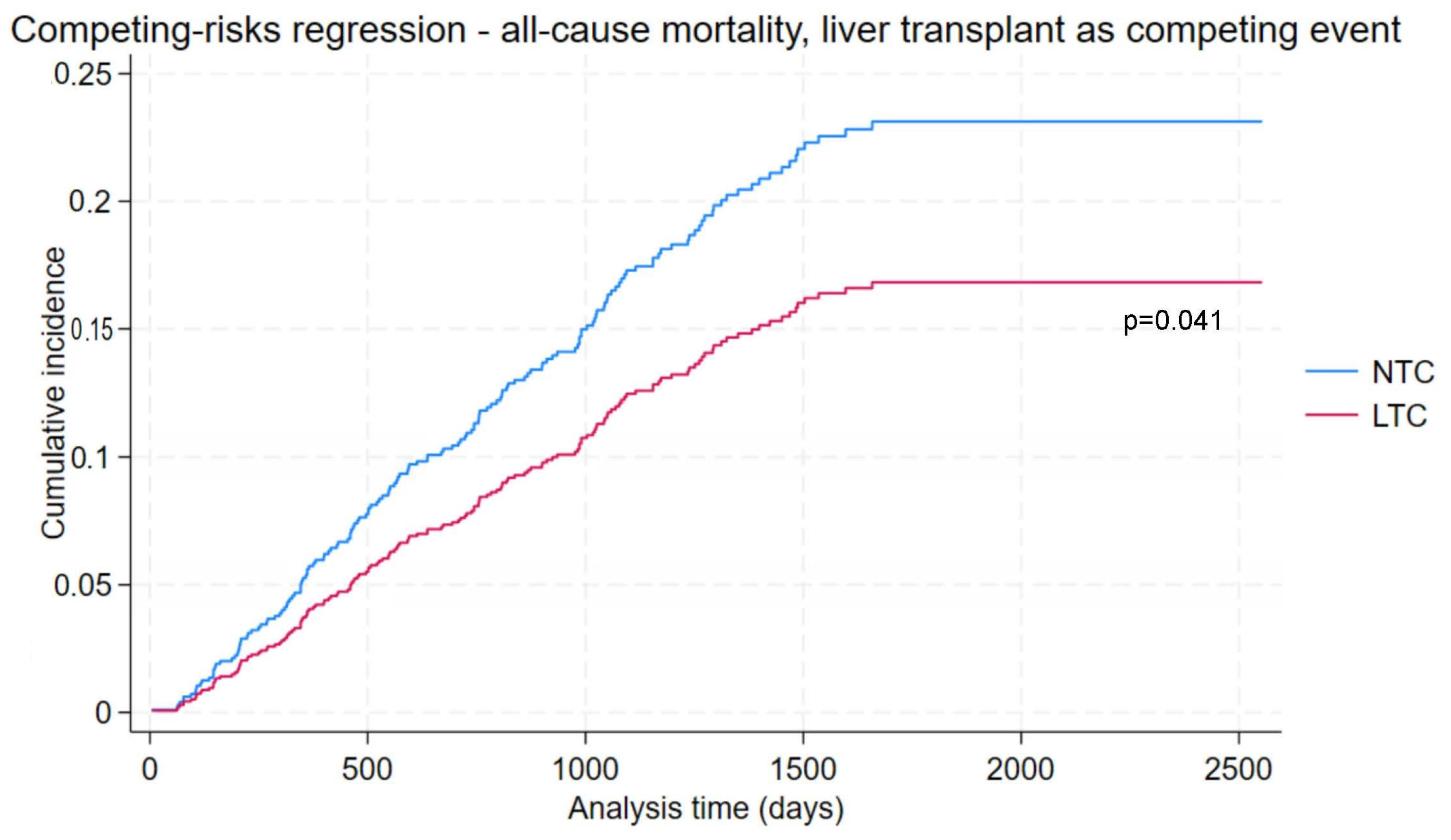
3.6. All-Cause Mortality with Liver Transplant as Competing Risk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Ref | Data Item | Field Type and Values |
|---|---|---|
| Participant Details | ||
| 1.1.1 | Record ID | Text |
| PARTICIPANT DETAILS | ||
| 1.2.1 | Recruiting Hospital | Dropdown 2, Alfred Health|6, Austin Health|102, Eastern Health|9, Monash Health|221208, Prince of Wales Hospital|1, Royal Melbourne Hospital|220208, Royal Prince Alfred Hospital|3, St Vincent’s Hospital Melbourne|106, Western Health|109, John Hunter Hospital (Hunter New England) |
| 1.2.2 | Date of Birth | Date |
| 1.2.3 | Sex at Birth | Dropdown 1, Male|2, Female|3, Intersex or indeterminate|−99, Not stated/inadequately described |
| 1.2.4 | Postcode | Text |
| 1.2.5 | Country of Birth | Radio 1, Australia|2, Country other than Australia |
| 1.2.6 | Country of birth | Text |
| 1.2.7 | Estimated first arrival year to Australia | Text |
| 1.2.8 | Ethnicity | Dropdown 1, Australian Indigenous|2, African|3, Caucasian (Australia, Europe, UK, Nth America etc.)|4, North−East Asian (China, Japan, Sth/Nth Korea, Mongolia, Taiwan)|5, Hispanic (Central, South American, North American)|6, Middle Eastern/North African|7, Polynesian/Pacific Islander|8, Southern Asian (Indian, Pakistan, Bangladesh, Nepal, Afghanistan)|9, South−East Asian (Vietnamese, Thai, Burmese, Khmer etc.)|98, Other|−99, Unknown |
| 1.2.9 | Other ethnicity | Text |
| 1.2.10 | Aboriginal and Torres Strait Islander status | Dropdown 4, Neither Aboriginal nor Torres Strait Islander origin|1, Aboriginal but not Torres Strait Islander|2, Torres Strait Islander but not Aboriginal|3, Both Aboriginal and Torres Strait Islander origin|−99, Not stated/inadequately described |
| FORM STATUS | ||
| 1.3.1 | Complete? | Dropdown 0, Incomplete|1, Unverified|2, Complete |
| Health Status and End of Life Details | ||
| HEALTH STATUS AND END OF LIFE | ||
| 2.1.1 | Was patient alive at 31 December 2020? | Radio 1, Yes|2, No|−99, Unknown |
| 2.1.2 | Date of death | Date |
| 2.1.3 | Cause of death | Radio 1, Directly related to HCC|2, Related to underlying liver disease|3, Related to combination HCC and underlying liver disease|4, Non−Liver related|5, Not ascertained but probably/definitely related to HCC|6, Not ascertained but unlikely or not related to HCC|7, Unable to be ascertained |
| 2.1.4 | If non-liver related, specify cause of death | Text |
| FORM STATUS | ||
| 2.2.1 | Complete? | Dropdown 0, Incomplete|1, Unverified|2, Complete |
| Risk Factors | ||
| RISK FACTORS | ||
| 3.1.1 | Risk Factors | Checkbox 1, rf___1 Cirrhosis|2, rf___2 Alcohol|3, rf___3 NAFLD/MAFLD|4, rf___4 Smoking history|5, rf___5 Diabetes|6, rf___6 HCV positive|7, rf___7 HBV positive|8, rf___8 Autoimmune hepatitis|9, rf___9 PSC|10, rf___10 PBC|11, rf___11 Alpha 1 anti−trypsin deficiency|12, rf___12 Wilsons disease|13, rf___13 Family History|14, rf___14 Other: {rf_other}|15, rf___15 None of the above|−99, rf____99 Unknown − factors contributing to HCC unknown |
| 3.1.2 | Alcohol | Dropdown 1, Current heavy user|4, Current non−heavy user|2, Past heavy alcohol use|3, Never consumed alcohol|−99, Unknown − consumption not reported |
| 3.1.3 | Family History Type | Dropdown 1, First degree relative|2, Second degree relative |
| 3.1.4 | Other | Text |
| 3.1.5 | Smoking status | Radio 1, Current smoker|2, Ex−smoker|3, Never smoked|4, Non−smoker (no further specification)|−99, Unknown/Not documented |
| 3.1.6 | Past HCC | Radio 1, Yes|2, No |
| 3.1.7 | Date of past HCC | Date |
| 3.1.8 | Was the past HCC in the same location as the current one—i.e., is this a recurrence? | Dropdown 1, Yes|2, No|−99, Unknown |
| FORM STATUS | ||
| 3.2.1 | Complete? | Dropdown 0, Incomplete|1, Unverified|2, Complete |
| Diagnosis Details | ||
| DIAGNOSIS DETAILS | ||
| 4.1.1 | Date of HCC diagnosis | Date |
| 4.1.2 | Mode of HCC Diagnosis | Radio 1, Histopathology (includes biopsy, surgical resection)|2, Imaging |
| 4.1.3 | Histology type | Dropdown 1, Biopsy|2, Surgical Specimen |
| 4.1.4 | Imaging type | Radio 1, Multiphase CT|2, MRI Liver|3, CEUS|4, Other: clinical: {dx_other} |
| 4.1.5 | Other mode of diagnosis | Text |
| 4.1.6 | Method or presentation of HCC Diagnosis | Radio 1, Screening/surveillance−please specify reason for doing so: {dx_screen_reason}|2, Incidental−please specify how so: {dx_incident_how}|3, Symptoms|4, Other: {dx_other_method} |
| 4.1.7 | Reason for screening/surveillance | Text |
| 4.1.8 | Incidental (how) | Text |
| 4.1.9 | Other (method or presentation) | Text |
| 4.1.10 | Tumour Size (largest lesion measured in cm) | Text |
| 4.1.11 | Site of the largest lesion(s) | Checkbox 1, dx_largelesion_location___1 Seg 1 (caudate lobe)|2, dx_largelesion_location___2 Seg 2|3, dx_largelesion_location___3 Seg 3|4, dx_largelesion_location___4 Seg 4a|5, dx_largelesion_location___5 Seg 4b|6, dx_largelesion_location___6 Seg 5|7, dx_largelesion_location___7 Seg 6|8, dx_largelesion_location___8 Seg 7|9, dx_largelesion_location___9 Seg 8|10, dx_largelesion_location___10 Diffuse type not easily determined|11, dx_largelesion_location___11 Right Lobe (segment not specified)|12, dx_largelesion_location___12 Left Lobe (segment not specified)|13, dx_largelesion_location___13 None of the above|−99, dx_largelesion_location____99 Not recorded |
| 4.1.12 | Number of HCC lesions | Text |
| 4.1.13 | Total lobes with lesion(s) | Dropdown 1, one lobe only|2, both lobes|−99, unknown site of lesion(s) |
| 4.1.14 | Child–Pugh Class | Dropdown 1, A (5−6)|2, B (7−9)|3, C (10−15)|−99 Unknown–unknown result |
| 4.1.15 | You have selected: [dx_childpugh_class] This equates to: [calc_childpugh_c2s] | Descriptive |
| 4.1.16 | Calculation—Class to Score | Text |
| 4.1.17 | Child–Pugh Score | Text |
| 4.1.18 | You have selected: [dx_childpugh_score] This equates to: [calc_childpugh_s2c] | Descriptive |
| 4.1.19 | Calculation—Score to Class | Text |
| 4.1.20 | BCLC staging score | Dropdown 0, 0−Very early (single < 2 cm)|1, A−Early (single, 3 nodules ≤ 3 cm)|2, B−Intermediate (multinodular)|3, C−Advanced (portal invasion)|4, D−End−stage|−99, Unknown−unknown result |
| 4.1.21 | Other comorbidities | Dropdown 1, Yes−see next field for details|2, No−no other known comorbidities |
| 4.1.22 | Charlson Comorbidity Index | Checkbox 1, dx_comorbidet___1 Prior myocardial infarction|2, dx_comorbidet___2 Congestive heart failure|3, dx_comorbidet___3 Peripheral vascular disease|4, dx_comorbidet___4 Cerebrovascular disease or Transient ischemic attack (TIA)|5, dx_comorbidet___5 Dementia|6, dx_comorbidet___6 Chronic obstructive pulmonary disease|7, dx_comorbidet___7 Rheumatologic disease or Connective tissue disease|8, dx_comorbidet___8 Peptic ulcer disease|9, dx_comorbidet___9 Mild liver disease|10, dx_comorbidet___10 Moderate or severe liver disease (Severe = cirrhosis and portal hypertension with variceal bleeding history, moderate = cirrhosis and portal hypertension but no variceal bleeding history, mild = chronic hepatitis (or cirrhosis without portal hypertension))|11, dx_comorbidet___11 Diabetes with chronic complications|12, dx_comorbidet___12 Cerebrovascular (hemiplegia) event|13, dx_comorbidet___13 Moderate−to−severe chronic renal/kidney disease (Severe = on dialysis, status post kidney transplant, uraemia, moderate = creatinine > 3 mg/dL (0.27 mmol/L))|14, dx_comorbidet___14 Cancer without metastases/localised solid tumour|15, dx_comorbidet___15 Metastatic solid tumour|16, dx_comorbidet___16 Leukaemia|17, dx_comorbidet___17 Lymphoma|18, dx_comorbidet___18 Acquired immuno−deficiency syndrome (AIDS)|19, dx_comorbidet___19 Other: {dx_other_comorbidity}|20, dx_comorbidet___20 Atrial fibrillation (AF)/Supraventricular tachycardia (SVT)|21, dx_comorbidet___21 Uncomplicated diabetes|22, dx_comorbidet___22 None of the above|−99, dx_comorbidet____99 Unknown |
| 4.1.23 | Other comorbidity | Text |
| 4.1.24 | Diabetes at time of diagnosis | Dropdown 0, No−did not have diabetes|1, T1DM−had type 1 diabetes mellitus|2, T2DM−had type 2 diabetes mellitus (NIDDM)|3, T2IDM−had type 2 insulin−dependent diabetes mellitus (IDDM)|4, Yes−unspecified−known to have diabetes but specific type missing|−99, Unknown−diabetes status unknown |
| 4.1.25 | Portal hypertension | Dropdown 1, Yes−had portal hypertension at diagnosis|2, No−did not have portal hypertension at time of diagnosis|−99, Unknown |
| 4.1.26 | AFP measured | Text |
| 4.1.27 | AFP result—unit of measurement | Dropdown 1, μg/mL|2, ng/ml or μg/L|3, Other|−99, Unknown−units unknown |
| 4.1.28 | Platelets × 109/L | Text |
| 4.1.29 | Albumin | Text |
| 4.1.30 | Other AFP Unit Measurement | Text |
| 4.1.31 | ECOG at time of diagnosis | Dropdown 0, 0 = Fully active, able to carry on all pre−disease performance without restriction|1, 1 = Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e.g., light house work, office work|2, 2 = Ambulatory and capable of all selfcare but unable to carry out any work activities; up and about more than 50% of waking hours|3, 3 = Capable of only limited selfcare; confined to bed or chair more than 50% of waking hours|4, 4 = Completely disabled; cannot carry on any selfcare; totally confined to bed or chair|5, 5− Dead|−99, ECOG not documented |
| 4.1.32 | Presence of ascites | Radio 1, Yes|2, No|−99, Not recorded |
| 4.1.33 | Presence of hepatic encephalopathy | Radio 1, Yes|2, No|−99, Not recorded |
| FORM STATUS | ||
| 4.2.1 | Complete? | Dropdown 0, Incomplete|1, Unverified|2, Complete |
| Viral Status at Diagnosis | ||
| HEPATITS B STATUS | ||
| 5.1.1 | Hepatitis B virus (HBV) | Dropdown 1, Yes (either past or present)− had HBV at diagnosis|0, No−(neither past nor present)|−99, Unknown−results unknown |
| 5.1.2 | Hepatitis B viral treatment after HCC diagnosis | Dropdown 1, Yes–on HBV treatment|2, No–not on HBV treatment|−99, Unknown–treatment status unknown |
| HEPATITIS C STATUS | ||
| 5.2.1 | Hepatitis C virus (HCV) | Dropdown 1, Current infection (i.e HCV RNA PCR positive) at diagnosis|2, Past infection (i.e HCV RNA PCR negative AND HCV Ab positive) at diagnosis|0, No current or past HCV−HCV at diagnosis|−99, Unknown−results unknown |
| 5.2.2 | Hepatitis C virus treatment history | Dropdown 1, Naïve−never treated|2, Non−responder−treated but still RNA PCR positive|3, Ongoing−on treatment at time of diagnosis|4, Relapse−treated, end−of−treatment RNA PCR negative but subsequently RNA PCR positive|5, SVR (sustained virological response)−treated, end−of−treatment RNA PCR negative and maintains RNA PCR negative|−99, Unknown−HCV treatment history |
| 5.2.3 | Date of past HCV cure | Date |
| COINFECTION | ||
| 5.3.1 | Viral coinfection | Dropdown 1, Yes|0, No |
| 5.3.2 | Viral coinfection type | Checkbox 1, dx_coinf_yes___1 HDV (only if hepatitis B sAg positive)|2, dx_coinf_yes___2 HIV |
| FORM STATUS | ||
| 5.4.1 | Complete? | Dropdown 0, Incomplete|1, Unverified|2, Complete |
| Treatment | ||
| TREATMENT | ||
| 6.1.1 | Modality of initial treatment | Dropdown 1, Resection|2, Transplantation|3, Locoregional|4, Systemic |
| 6.1.2 | First HCC treatment type | Checkbox 1, rx_1type___1 Conventional Transarterial chemoembolization (cTACE)|2, rx_1type___2 Drug Eluting Bead (DEB)−TACE|3, rx_1type___3 Radiofrequency ablation (RFA)|4, rx_1type___4 Irreversible electroporation|5, rx_1type___5 Percutaneous Ethanol Injection (PEI)|6, rx_1type___6 Hepatic Resection|7, rx_1type___7 Microwave ablation|8, rx_1type___8 Medication|9, rx_1type___9 Stereotactic Body Ablation Radiotherapy|10, rx_1type___10 Liver Transplant|11, rx_1type___11 Selective Internal Radiation Therapy (SIRT)|12, rx_1type___12 No Treatment|13, rx_1type___13 Other {rx_1other}cc|14, rx_1type___14 Distant hepatic recurrence|15, rx_1type___15 None of the above |
| 6.1.3 | Medications | Checkbox 1, rx_medications___1 Sorafenib|2, rx_medications___2 Lenvima (Lenvatinib)|3, rx_medications___3 Atezolizumab|4, rx_medications___4 Others: please specify: {rx_medications_other}|5, rx_medications___5 Clinical trial medication: please specify: {rx_medications_clintrial} |
| 6.1.4 | Other medications | Text |
| 6.1.5 | Clinical trial medications | Text |
| 6.1.6 | Date of treatment 1 | Date |
| 6.1.7 | Other | Text |
| 6.1.8 | Reason no treatment | Checkbox 1, rx_1notreat___1 Patient unable to tolerate treatment|2, rx_1notreat___2 Patient moved before treatment|3, rx_1notreat___3 Patient lost to follow-up|4, rx_1notreat___4 Patient died before treatment |
| 6.1.9 | Curative intent | Dropdown 1, Yes|2, No|−99, Unknown |
| 6.1.10 | Treatment response at time interval 1 | Dropdown 1, PD−progressive disease (An increase of at least 20% in the sum of the diameters of viable (enhancing) target lesions, taking as reference the smallest sum of the diameters of viable (enhancing) target lesions recorded since treatment started)|2, SD−stable disease (Any cases that do not qualify for either partial response or progressive disease)|3, PR−partial response (At least a 30% decrease in the sum of diameters of viable (enhancement in the arterial phase) target lesions, taking as reference the baseline sum of the diameters of target lesions)|4, CR−complete response (Disappearance of any intratumoral arterial enhancement in all target lesions)|−99, Not recorded/not measurable |
| 6.1.11 | Date of response assessment to treatment 1 | Date |
| 6.1.12 | Date complete response confirmed | Date |
| 6.1.13 | If complete response, was there a recurrence? | Dropdown 1, Yes|2, No|−99, Unknown |
| 6.1.14 | Date of recurrence | Date |
| 6.1.15 | Type of recurrence: liver | Dropdown 1, Yes|2, No|−99, Unknown |
| 6.1.16 | Liver recurrence | Dropdown 1, Local|2, Distant|3, Vascular invasion|−99, Unknown |
| 6.1.17 | Extrahepatic | Dropdown 1, Yes|2, No|−99, Unknown |
| 6.1.18 | Where is the extrahepatic spread? | Text |
| 6.1.19 | Complications after initial treatment | Checkbox 1, rx_complications___1 Liver related morbidity|2, rx_complications___2 Post procedural infections|3, rx_complications___3 Post procedural bleeding|4, rx_complications___4 Bile duct injury|5, rx_complications___5 Respiratory events|6, rx_complications___6 Local events|7, rx_complications___7 Other {comp1_other} |
| 6.1.20 | Other complications | Text |
| 6.1.21 | Secondary therapies | Checkbox 1, rx_2type___1 Conventional Transarterial chemoembolization (cTACE)|2, rx_2type___2 Drug Eluting Bead (DEB)−TACE|3, rx_2type___3 Radiofrequency ablation (RFA)|4, rx_2type___4 Irreversible electroporation|5, rx_2type___5 Percutaneous Ethanol Injection (PEI)|6, rx_2type___6 Hepatic Resection|7, rx_2type___7 Microwave ablation|8, rx_2type___8 Medication|9, rx_2type___9 Stereotactic Body Ablation Radiotherapy|10, rx_2type___10 Liver Transplant|11, rx_2type___11 Selective Internal Radiation Therapy (SIRT)|12, rx_2type___12 No Treatment|13, rx_2type___13 Other {rx_2other}|14, rx_2type___14 Distant hepatic recurrence|15, rx_2type___15 None of the above |
| 6.1.22 | Medications | Checkbox 1, rx_medications_2___1 Sorafenib|2, rx_medications_2___2 Lenvima (Lenvatinib)|3, rx_medications_2___3 Atezolizumab|4, rx_medications_2___4 Others: please specify: {rx_medications_other_2}|5, rx_medications_2___5 Clinical trial medication: please specify: {rx_medications_clintrial_2} |
| 6.1.23 | Other medications | Text |
| 6.1.24 | Clinical trial medications | Text |
| 6.1.25 | Date of treatment 2 | Date |
| 6.1.26 | Other | Text |
| 6.1.27 | Reason no treatment | Checkbox 1, rx_2notreat___1 Patient unable to tolerate treatment|2, rx_2notreat___2 Patient moved before treatment|3, rx_2notreat___3 Patient lost to follow-up|4, rx_2notreat___4 Patient died before treatment |
| 6.1.28 | Treatment response at time interval 2 | Dropdown 1, PD−progressive disease (An increase of at least 20% in the sum of the diameters of viable (enhancing) target lesions, taking as reference the smallest sum of the diameters of viable (enhancing) target lesions recorded since treatment started)|2, SD−stable disease (Any cases that do not qualify for either partial response or progressive disease)|3, PR−partial response (At least a 30% decrease in the sum of diameters of viable (enhancement in the arterial phase) target lesions, taking as reference the baseline sum of the diameters of target lesions)|4, CR−complete response (Disappearance of any intratumoral arterial enhancement in all target lesions)|−99, Not recorded/not measurable |
| 6.1.29 | Date of response assessment to treatment 2 | Date |
| 6.1.30 | Date complete response confirmed | Date |
| 6.1.31 | If complete response, was there a recurrence? | Dropdown 1, Yes|2, No|−99, Unknown |
| 6.1.32 | Date of recurrence | Date |
| 6.1.33 | Type of recurrence: liver | Dropdown 1, Yes|2, No|−99, Unknown |
| 6.1.34 | Liver recurrence | Dropdown 1, Local|2, Distant|3, Vascular invasion|−99, Unknown |
| 6.1.35 | Extrahepatic | Dropdown 1, Yes|2, No|−99, Unknown |
| 6.1.36 | Where is the extrahepatic spread? | Text |
| 6.1.37 | Complications after second treatment | Checkbox 1, rx_complications_2___1 Liver related morbidity|2, rx_complications_2___2 Post procedural infections|3, rx_complications_2___3 Post procedural bleeding|4, rx_complications_2___4 Bile duct injury|5, rx_complications_2___5 Respiratory events|6, rx_complications_2___6 Local events|7, rx_complications_2___7 Other {comp2_other} |
| 6.1.38 | Other complications | Text |
| 6.1.39 | Third therapies | Checkbox 1, rx_3type___1 Conventional Transarterial chemoembolization (cTACE)|2, rx_3type___2 Drug Eluting Bead (DEB)−TACE|3, rx_3type___3 Radiofrequency ablation (RFA)|4, rx_3type___4 Irreversible electroporation|5, rx_3type___5 Percutaneous Ethanol Injection (PEI)|6, rx_3type___6 Hepatic Resection|7, rx_3type___7 Microwave ablation|8, rx_3type___8 Medication|9, rx_3type___9 Stereotactic Body Ablation Radiotherapy|10, rx_3type___10 Liver Transplant|11, rx_3type___11 Selective Internal Radiation Therapy (SIRT)|12, rx_3type___12 No Treatment|13, rx_3type___13 Other {rx_3other}|14, rx_3type___14 Distant hepatic recurrence|15, rx_3type___15 None of the above |
| 6.1.40 | Medications | Checkbox 1, rx_medications_3___1 Sorafenib|2, rx_medications_3___2 Lenvima (Lenvatinib)|3, rx_medications_3___3 Atezolizumab|4, rx_medications_3___4 Others: please specify: {rx_medications_other_3}|5, rx_medications_3___5 Clinical trial medication: please specify: {rx_medications_clintrial_3} |
| 6.1.41 | Other medications | Text |
| 6.1.42 | Clinical trial medications | Text |
| 6.1.43 | Date of treatment 3 | Date |
| 6.1.44 | Other | Text |
| 6.1.45 | Reason no treatment | Checkbox 1, rx_3notreat___1 Patient unable to tolerate treatment|2, rx_3notreat___2 Patient moved before treatment|3, rx_3notreat___3 Patient lost to follow-up|4, rx_3notreat___4 Patient died before treatment |
| 6.1.46 | Treatment response at time interval 3 | Dropdown 1, PD−progressive disease (an increase of at least 20% in the sum of the diameters of viable (enhancing) target lesions, taking as reference the smallest sum of the diameters of viable (enhancing) target lesions recorded since treatment started)|2, SD—stable disease (any cases that do not qualify for either partial response or progressive disease)|3, PR—partial response (at least a 30% decrease in the sum of diameters of viable (enhancement in the arterial phase) target lesions, taking as reference the baseline sum of the diameters of target lesions)|4, CR—complete response (disappearance of any intratumoral arterial enhancement in all target lesions)|−99, not recorded/not measurable |
| 6.1.47 | Date of response assessment to treatment 3 | Date |
| 6.1.48 | Date complete response confirmed | Date |
| 6.1.49 | If complete response, was there a recurrence? | Dropdown 1, Yes|2, No|−99, Unknown |
| 6.1.50 | Date of recurrence | Date |
| 6.1.51 | Type of recurrence: liver | Dropdown 1, Yes|2, No|−99, Unknown |
| 6.1.52 | Liver recurrence | Dropdown 1, Local|2, Distant|3, Vascular invasion|−99, Unknown |
| 6.1.53 | Extrahepatic | Dropdown 1, Yes|2, No|−99, Unknown |
| 6.1.54 | Where is the extrahepatic spread? | Text |
| 6.1.55 | Complications after third treatment | Checkbox 1, rx_complications_3___1 Liver related morbidity|2, rx_complications_3___2 Post procedural infections|3, rx_complications_3___3 Post procedural bleeding|4, rx_complications_3___4 Bile duct injury|5, rx_complications_3___5 Respiratory events|6, rx_complications_3___6 Local events|7, rx_complications_3___7 Other {comp3_other} |
| 6.1.56 | Other complications | Text |
| 6.1.57 | Did the patient receive additional treatments beyond those above? | yesno 1, Yes|0, No |
| 6.1.58 | Fourth therapy(ies) | Checkbox 1, rx_4type___1 Conventional Transarterial chemoembolization (cTACE)|2, rx_4type___2 Drug Eluting Bead (DEB)−TACE|3, rx_4type___3 Radiofrequency ablation (RFA)|4, rx_4type___4 Irreversible electroporation|5, rx_4type___5 Percutaneous Ethanol Injection (PEI)|6, rx_4type___6 Hepatic Resection|7, rx_4type___7 Microwave ablation|8, rx_4type___8 Medication|9, rx_4type___9 Stereotactic Body Ablation Radiotherapy|10, rx_4type___10 Liver Transplant|11, rx_4type___11 Selective Internal Radiation Therapy (SIRT)|12, rx_4type___12 No Treatment|13, rx_4type___13 Other {rx_4other}|14, rx_4type___14 Distant hepatic recurrence|15, rx_4type___15 None of the above |
| 6.1.59 | Other | Text |
| 6.1.60 | Medications | Checkbox 1, rx_medications_4___1 Sorafenib|2, rx_medications_4___2 Lenvima (Lenvatinib)|3, rx_medications_4___3 Atezolizumab|4, rx_medications_4___4 Others: please specify: {rx_medications_other_4}|5, rx_medications_4___5 Clinical trial medication: please specify: {rx_medications_clintrial_4} |
| 6.1.61 | Other medications | Text |
| 6.1.62 | Clinical trial medications | Text |
| 6.1.63 | Date of treatment 4 | Date |
| 6.1.64 | Reason no treatment | Checkbox 1, rx_4notreat___1 Patient unable to tolerate treatment|2, rx_4notreat___2 Patient moved before treatment|3, rx_4notreat___3 Patient lost to follow-up|4, rx_4notreat___4 Patient died before treatment |
| 6.1.65 | Treatment response at time interval 4 | Dropdown 1, PD−progressive disease (An increase of at least 20% in the sum of the diameters of viable (enhancing) target lesions, taking as reference the smallest sum of the diameters of viable (enhancing) target lesions recorded since treatment started)|2, SD−stable disease (Any cases that do not qualify for either partial response or progressive disease)|3, PR−partial response (At least a 30% decrease in the sum of diameters of viable (enhancement in the arterial phase) target lesions, taking as reference the baseline sum of the diameters of target lesions)|4, CR−complete response (Disappearance of any intratumoral arterial enhancement in all target lesions)|−99, Not recorded/not measurable |
| 6.1.66 | Date of response assessment to treatment 4 | Date |
| 6.1.67 | Date complete response confirmed | Date |
| 6.1.68 | If complete response, was there a recurrence? | Dropdown 1, Yes|2, No|−99, Unknown |
| 6.1.69 | Date of recurrence | Date |
| 6.1.70 | Type of recurrence: liver | Dropdown 1, Yes|2, No|−99, Unknown |
| 6.1.71 | Liver recurrence | Dropdown 1, Local|2, Distant|3, Vascular invasion|−99, Unknown |
| 6.1.72 | Extrahepatic | Dropdown 1, Yes|2, No|−99, Unknown |
| 6.1.73 | Where is the extrahepatic spread? | Text |
| 6.1.74 | Complications after fourth treatment | Checkbox 1, rx_complications_4___1 Liver related morbidity|2, rx_complications_4___2 Post procedural infections|3, rx_complications_4___3 Post procedural bleeding|4, rx_complications_4___4 Bile duct injury|5, rx_complications_4___5 Respiratory events|6, rx_complications_4___6 Local events|7, rx_complications_4___7 Other {comp4_other} |
| 6.1.75 | Other complications | Text |
| 6.1.76 | Fifth therapy(ies) | Checkbox 1, rx_5type___1 Conventional Transarterial chemoembolization (cTACE)|2, rx_5type___2 Drug Eluting Bead (DEB)−TACE|3, rx_5type___3 Radiofrequency ablation (RFA)|4, rx_5type___4 Irreversible electroporation|5, rx_5type___5 Percutaneous Ethanol Injection (PEI)|6, rx_5type___6 Hepatic Resection|7, rx_5type___7 Microwave ablation|8, rx_5type___8 Medication|9, rx_5type___9 Stereotactic Body Ablation Radiotherapy|10, rx_5type___10 Liver Transplant|11, rx_5type___11 Selective Internal Radiation Therapy (SIRT)|12, rx_5type___12 No Treatment|13, rx_5type___13 Other {rx_5other}|14, rx_5type___14 Distant hepatic recurrence|15, rx_5type___15 None of the above |
| 6.1.77 | Other | Text |
| 6.1.78 | Medications | Checkbox 1, rx_medications_5___1 Sorafenib|2, rx_medications_5___2 Lenvima (Lenvatinib)|3, rx_medications_5___3 Atezolizumab|4, rx_medications_5___4 Others: please specify: {rx_medications_other_5}|5, rx_medications_5___5 Clinical trial medication: please specify: {rx_medications_clintrial_5} |
| 6.1.79 | Other medications | Text |
| 6.1.80 | Clinical trial medications | Text |
| 6.1.81 | Date of treatment 5 | Date |
| 6.1.82 | Reason no treatment | Checkbox 1, rx_5notreat___1 Patient unable to tolerate treatment|2, rx_5notreat___2 Patient moved before treatment|3, rx_5notreat___3 Patient lost to follow-up|4, rx_5notreat___4 Patient died before treatment |
| 6.1.83 | Treatment response at time interval 5 | Dropdown 1, PD−progressive disease (An increase of at least 20% in the sum of the diameters of viable (enhancing) target lesions, taking as reference the smallest sum of the diameters of viable (enhancing) target lesions recorded since treatment started)|2, SD−stable disease (Any cases that do not qualify for either partial response or progressive disease)|3, PR−partial response (At least a 30% decrease in the sum of diameters of viable (enhancement in the arterial phase) target lesions, taking as reference the baseline sum of the diameters of target lesions)|4, CR−complete response (Disappearance of any intratumoral arterial enhancement in all target lesions)|−99, Not recorded/not measurable |
| 6.1.84 | Date of response assessment to treatment 5 | Date |
| 6.1.85 | Date complete response confirmed | Date |
| 6.1.86 | If complete response, was there a recurrence? | Dropdown 1, Yes|2, No|−99, Unknown |
| 6.1.87 | Date of recurrence | Date |
| 6.1.88 | Type of recurrence: liver | Dropdown 1, Yes|2, No|−99, Unknown |
| 6.1.89 | Liver recurrence | Dropdown 1, Local|2, Distant|3, Vascular invasion|−99, Unknown |
| 6.1.90 | Extrahepatic | Dropdown 1, Yes|2, No|−99, Unknown |
| 6.1.91 | Where is the extrahepatic spread? | Text |
| 6.1.92 | Complications after fifth treatment | Checkbox 1, rx_complications_5___1 Liver related morbidity|2, rx_complications_5___2 Post procedural infections|3, rx_complications_5___3 Post procedural bleeding|4, rx_complications_5___4 Bile duct injury|5, rx_complications_5___5 Respiratory events|6, rx_complications_5___6 Local events|7, rx_complications_5___7 Other {comp5_other} |
| 6.1.93 | Other complications | Text |
| 6.1.94 | Sixth therapy(ies) | Checkbox 1, rx_6type___1 Conventional Transarterial chemoembolization (cTACE)|2, rx_6type___2 Drug Eluting Bead (DEB)−TACE|3, rx_6type___3 Radiofrequency ablation (RFA)|4, rx_6type___4 Irreversible electroporation|5, rx_6type___5 Percutaneous Ethanol Injection (PEI)|6, rx_6type___6 Hepatic Resection|7, rx_6type___7 Microwave ablation|8, rx_6type___8 Medication|9, rx_6type___9 Stereotactic Body Ablation Radiotherapy|10, rx_6type___10 Liver Transplant|11, rx_6type___11 Selective Internal Radiation Therapy (SIRT)|12, rx_6type___12 No Treatment|13, rx_6type___13 Other {rx_6other}|14, rx_6type___14 Distant hepatic recurrence|15, rx_6type___15 None of the above |
| 6.1.95 | Other | Text |
| 6.1.96 | Medications | Checkbox 1, rx_medications_6___1 Sorafenib|2, rx_medications_6___2 Lenvima (Lenvatinib)|3, rx_medications_6___3 Atezolizumab|4, rx_medications_6___4 Others: please specify: {rx_medications_other_6}|5, rx_medications_6___5 Clinical trial medication: please specify: {rx_medications_clintrial_6} |
| 6.1.97 | Other medications | Text |
| 6.1.98 | Clinical trial medications | Text |
| 6.1.99 | Date of treatment 6 | Date |
| 6.1.100 | Reason no treatment | Checkbox 1, rx_6notreat___1 Patient unable to tolerate treatment|2, rx_6notreat___2 Patient moved before treatment|3, rx_6notreat___3 Patient lost to follow-up|4, rx_6notreat___4 Patient died before treatment |
| 6.1.101 | Treatment response at time interval 6 | Dropdown 1, PD−progressive disease (An increase of at least 20% in the sum of the diameters of viable (enhancing) target lesions, taking as reference the smallest sum of the diameters of viable (enhancing) target lesions recorded since treatment started)|2, SD−stable disease (Any cases that do not qualify for either partial response or progressive disease)|3, PR−partial response (At least a 30% decrease in the sum of diameters of viable (enhancement in the arterial phase) target lesions, taking as reference the baseline sum of the diameters of target lesions)|4, CR−complete response (Disappearance of any intratumoral arterial enhancement in all target lesions)|−99, Not recorded/not measurable |
| 6.1.102 | Date of response assessment to treatment 6 | Date |
| 6.1.103 | Date complete response confirmed | Date |
| 6.1.104 | If complete response, was there a recurrence? | Dropdown 1, Yes|2, No|−99, Unknown |
| 6.1.105 | Date of recurrence | Date |
| 6.1.106 | Type of recurrence: liver | Dropdown 1, Yes|2, No|−99, Unknown |
| 6.1.107 | Liver recurrence | Dropdown 1, Local|2, Distant|3, Vascular invasion|−99, Unknown |
| 6.1.108 | Extrahepatic | Dropdown 1, Yes|2, No|−99, Unknown |
| 6.1.109 | Where is the extrahepatic spread? | Text |
| 6.1.110 | Complications after sixth treatment | Checkbox 1, rx_complications_6___1 Liver related morbidity|2, rx_complications_6___2 Post procedural infections|3, rx_complications_6___3 Post procedural bleeding|4, rx_complications_6___4 Bile duct injury|5, rx_complications_6___5 Respiratory events|6, rx_complications_6___6 Local events|7, rx_complications_6___7 Other {comp5_other} |
| 6.1.111 | Other complications | Text |
| FORM STATUS | ||
| 6.2.1 | Complete? | Dropdown 0, Incomplete|1, Unverified|2, Complete |
| Subsequent Treatments | ||
| 7.1.1 | This is a repeating form. If the patient had multiple subsequent treatments beyond the previous six, please complete this and if needed, add a new repeating form or instance by either clicking the dropdown arrow next to “Current instance:” above and select “+Add new” OR at the bottom, press the blue drop down arrow and select “Save & Add New Instance”. | Descriptive |
| SUBSEQUENT TREATMENT | ||
| 7.2.1 | Subsequent Treatment | Checkbox 1, rx_sub___1 Conventional Transarterial chemoembolization (cTACE)|2, rx_sub___2 Drug Eluting Bead (DEB)−TACE|3, rx_sub___3 Radiofrequency ablation (RFA)|4, rx_sub___4 Irreversible electroporation|5, rx_sub___5 Percutaneous Ethanol Injection (PEI)|6, rx_sub___6 Hepatic Resection|7, rx_sub___7 Microwave ablation|8, rx_sub___8 Medication|9, rx_sub___9 Stereotactic Body Ablation Radiotherapy|10, rx_sub___10 Liver Transplant|11, rx_sub___11 Selective Internal Radiation Therapy (SIRT)|12, rx_sub___12 No Treatment|13, rx_sub___13 Other {rx_sub_other}|14, rx_sub___14 Distant hepatic recurrence|15, rx_sub___15 None of the above |
| 7.2.2 | Other | Text |
| 7.2.3 | Medications | Checkbox 1, rx_submed___1 Sorafenib|2, rx_ submed ___2 Lenvima (Lenvatinib)|3, rx_ submed ___3 Atezolizumab|4, rx_ submed ___4 Others: please specify: {rx_ submed _other}|5, rx_ submed ___5 Clinical trial medication: please specify: {rx_ submed _clintrial} |
| 7.2.4 | Other medications | Text |
| 7.2.5 | Clinical trial medications | Text |
| 7.2.6 | Date of subsequent treatment | Date |
| 7.2.7 | Reason no treatment | Checkbox 1, rx_sub_notreat___1 Patient unable to tolerate treatment|2, rx_sub_notreat___2 Patient moved before treatmen|3, rx_sub_notreat___3 Patient lost to follow-up|4, rx_sub_notreat___4 Patient died before treatment |
| 7.2.8 | Treatment response at time interval of subsequent treatment | Dropdown 1, PD−progressive disease (An increase of at least 20% in the sum of the diameters of viable (enhancing) target lesions, taking as reference the smallest sum of the diameters of viable (enhancing) target lesions recorded since treatment started)|2, SD−stable disease (Any cases that do not qualify for either partial response or progressive disease)|3, PR−partial response (At least a 30% decrease in the sum of diameters of viable (enhancement in the arterial phase) target lesions, taking as reference the baseline sum of the diameters of target lesions)|4, CR−complete response (Disappearance of any intratumoral arterial enhancement in all target lesions)|−99, Not recorded/not measurable |
| 7.2.9 | Date of response assessment to subsequent treatment | Date |
| 7.2.10 | Date complete response confirmed after subsequent treatment | Date |
| 7.2.11 | If complete response, was there a recurrence? | Dropdown 1, Yes|2, No|−99, Unknown |
| 7.2.12 | Date of recurrence | Date |
| 7.2.13 | Type of recurrence: liver | Dropdown 1, Yes|2, No|−99, Unknown |
| 7.2.14 | Liver recurrence | Dropdown 1, Local|2, Distant|3, Vascular invasion|−99, Unknown |
| 7.2.15 | Extrahepatic | Dropdown 1, Yes|2, No|−99, Unknown |
| 7.2.16 | Where is the extrahepatic spread? | Text |
| 7.2.17 | Complications after subsequent treatment | Checkbox 1, rx_sub_complications___1 Liver related morbidity|2, rx_sub_complications___2 Post procedural infections|3, rx_sub_complications___3 Post procedural bleeding|4, rx_sub_complications___4 Bile duct injury|5, rx_sub_complications___5 Respiratory events|6, rx_sub_complications___6 Local events|7, rx_sub_complications___7 Other {compsub_other}|8, rx_sub_complications___8 Systemic treatment (chemotherapy) |
| 7.2.18 | Other complications | Text |
| FORM STATUS | ||
| 7.3.1 | Complete? | Dropdown 0, Incomplete|1, Unverified|2, Complete |
References
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018; 4, 1553–1568.
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Ryerson, A.B.; Eheman, C.R.; Altekruse, S.F.; Ward, J.W.; Jemal, A.; Sherman, R.L.; Henley, S.J.; Holtzman, D.; Lake, A.; Noone, A.; et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer 2016, 122, 1312–1337. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef] [PubMed]
- Mathers, C.D.; Loncar, D. Projections of Global Mortality and Burden of Disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, L.; Chagas, A.L.; Alencar, R.S.; Tani, C.; Diniz, M.A.; D’Albuquerque, L.A.; Carrilho, F.J. Adherence to BCLC recommendations for the treatment of hepatocellular carcinoma: Impact on survival according to stage. Clinics 2017, 72, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Borzio, M.; Fornari, F.; De Sio, I.; Andriulli, A.; Terracciano, F.; Parisi, G.; Francica, G.; Salvagnini, M.; Marignani, M.; Salmi, A.; et al. Adherence to American Association for the Study of Liver Diseases guidelines for the management of hepatocellular carcinoma: Results of an Italian field practice multicenter study. Futur. Oncol. 2013, 9, 283–294. [Google Scholar] [CrossRef]
- Guarino, M.; Tortora, R.; de Stefano, G.; Coppola, C.; Morisco, F.; Megna, A.S.; Izzo, F.; Nardone, G.; Piai, G.; Adinolfi, L.E.; et al. Adherence to Barcelona Clinic Liver Cancer guidelines in field practice: Results of Progetto Epatocarcinoma Campania. J. Gastroenterol. Hepatol. 2018, 33, 1123–1130. [Google Scholar] [CrossRef]
- Leoni, S.; Piscaglia, F.; Serio, I.; Terzi, E.; Pettinari, I.; Croci, L.; Marinelli, S.; Benevento, F.; Golfieri, R.; Bolondi, L. Adherence to AASLD guidelines for the treatment of hepatocellular carcinoma in clinical practice: Experience of the Bologna Liver Oncology Group. Dig. Liver Dis. 2014, 46, 549–555. [Google Scholar] [CrossRef]
- Tabrizian, P.; Jibara, G.; Shrager, B.; Schwartz, M.; Roayaie, S. Recurrence of Hepatocellular Cancer after Resection: Patterns, Treatments, and Prognosis. Ann. Surg. 2015, 261, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.W.H.; Zhong, J.; Berhane, S.; Toyoda, H.; Cucchetti, A.; Shi, K.; Tada, T.; Chong, C.C.N.; Xiang, B.-D.; Li, L.-Q.; et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J. Hepatol. 2018, 69, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.K.C.; Chok, K.S.H.; Chan, A.C.Y.; Cheung, T.T.; Wong, T.C.L.; Fung, J.Y.Y.; Yuen, J.; Poon, R.T.P.; Fan, S.T.; Lo, C.M. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br. J. Surg. 2017, 104, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Beecroft, S.; O’connell, M.; Nassar, A.; Noon, K.; Pollock, K.G.; Palmer, D.; Cross, T.J.S. Major variation in hepatocellular carcinoma treatment and outcomes in England: A retrospective cohort study. Front. Gastroenterol. 2023, 14, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.R.; Karalis, J.D.; Chansard, M.; Augustine, M.M.; Mortensen, E.; Wang, S.C.; Polanco, P.M. Variation of Hepatocellular Carcinoma Treatment Patterns and Survival Across Geographic Regions in a Veteran Population. Ann. Surg. Oncol. 2022, 29, 8413–8420. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, A.D.; Lubel, J.; Lam, E.; Clark, P.J.; Duncan, O.; George, J.; Jeffrey, G.P.; Lipton, L.; Liu, H.; McCaughan, G.; et al. Monitoring quality of care in hepatocellular carcinoma: A modified Delphi consensus. Hepatol. Commun. 2022, 6, 3260–3271. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; Ghabril, M.S.; Kuo, A.; Merriman, R.B.; Morgan, T.; Parikh, N.D.; Ovchinsky, N.; Kanwal, F.; Volk, M.L.; Ho, C.; et al. Quality measures in HCC care by the Practice Metrics Committee of the American Association for the Study of Liver Diseases. Hepatology 2022, 75, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Bruix, J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Taddei, T.H. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023, 78, 1922–1965. [Google Scholar] [CrossRef]
- Lubel, J.S.; Roberts, S.K.; Strasser, S.I.; Shackel, N. Australian recommendations for the management of hepatocellular carcinoma. Med. J. Aust. 2020, 215, 334.e1. [Google Scholar] [CrossRef]
- Omata, M.; Cheng, A.-L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.-H.; Chawla, Y.K.; Shiina, S.; et al. Asia–Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.-W.; Zhang, Y.-J.; Chen, M.-S.; Xu, L.; Liang, H.-H.; Lin, X.-J.; Guo, L.-P.; Zhang, Y.-Q.; Lau, W.Y. Radiofrequency Ablation with or Without Transcatheter Arterial Chemoembolization in the Treatment of Hepatocellular Carcinoma: A Prospective Randomized Trial. J. Clin. Oncol. 2013, 31, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Cao, Y.; Ma, H.; Kan, X.; Zhou, C.; Liu, J.; Shi, Q.; Feng, G.; Xiong, B.; Zheng, C. Improved clinical outcome using transarterial chemoembolization combined with radiofrequency ablation for patients in Barcelona clinic liver cancer stage A or B hepatocellular carcinoma regardless of tumor size: Results of a single-center retrospective case control study. BMC Cancer 2019, 19, 983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Chen, M.S.; Chen, Y.; Lau, W.Y.; Peng, Z. Long-term Outcomes of Transcatheter Arterial Chemoembolization Combined With Radiofrequency Ablation as an Initial Treatment for Early-Stage Hepatocellular Carcinoma. JAMA Netw. Open 2021, 4, e2126992. [Google Scholar] [CrossRef] [PubMed]

| LTC n = 433 | NTC n = 454 | p-Value | |
|---|---|---|---|
| Age * | 63.6 ± 10.0 | 65.5 ± 11.7 | 0.011 |
| Sex Male Female | 351 (81.1%) 82 (18.9%) | 362 (79.7%) 92 (20.3%) | 0.247 |
| Aetiology Alcohol HBV HCV MASLD Other metALD HBV/HCV HCV + SLD HBV + SLD | 62 (14.3%) 53 (12.2%) 74 (17.1%) 49 (11.3%) 28 (6.5%) 26 (6.0%) 13 (3.0%) 119 (27.5%) 9 (2.1%) | 72 (15.9%) 57 (12.6%) 66 (14.5%) 63 (13.9%) 26 (5.7%) 29 (6.4%) 19 (4.2%) 95 (20.9%) 27 (5.9%) | 0.047 |
| Smoking Yes No | 102 (76.4%) 331 (23.6%) | 151 (66.7%) 303 (33.3%) | 0.001 |
| Charlson Comorbidity Index ** | 5 (3 to 6) | 4 (3 to 6) | 0.155 |
| Cirrhosis Yes No | 369 (85.2%) 64 (14.8%) | 372 (81.9%) 82 (18.1%) | 0.188 |
| Platelets ** | 117 (80 to 164) | 137 (92 to 204) | <0.001 |
| Child–Pugh Score 5 6 7 8 9 | 243 (56.1%) 100 (23.1%) 44 (10.2%) 25 (5.8%) 21 (4.8%) | 248 (54.6%) 132 (29.1%) 48 (10.6%) 17 (3.7%) 9 (2.0%) | 0.033 |
| Tumour Burden Category Single ≤ 2 cm Single > 2 cm, ≤3 cm Single > 3 cm, ≤5 cm Single > 5 cm Multinodular, all ≤ 3 cm | 121 (27.9%) 95 (21.9%) 69 (15.9%) 46 (10.6%) 102 (23.6%) | 141 (31.1%) 129 (28.4%) 72 (15.9%) 35 (7.7%) 77 (17.0%) | 0.024 |
| Initial Treatment Allocation Resection Ablation TACE Other | 79 (18.2%) 74 (17.1%) 256 (59.1%) 24 (5.5%) | 120 (26.4%) 185 (40.7%) 111 (24.4%) 38 (8.4%) | <0.001 |
| Follow-up ablation after TACE After first TACE After second TACE After third or subsequent TACE No ablation during follow-up | 80 (31.3%) 12 (4.7%) 14 (5.5%) 150 (58.6%) | 18 (16.2%) 6 (5.4%) 4 (3.6%) 83 (74.8%) | 0.016 |
| LTC n = 35 | NTC n = 7 | p-Value | |
|---|---|---|---|
| Age * | 59.8 ± 5.1 | 53.7 ± 7.0 | 0.062 |
| Sex Male Female | 32 (91.4%) 3 (8.6%) | 5 (71.4%) 2 (28.6%) | 0.136 |
| Aetiology Alcohol HBV HCV MASLD Other metALD HBV/HCV HCV + SLD | 4 (11.4%) 4 (11.4%) 3 (8.6%) 4 (11.4%) 1 (2.9%) 4 (11.4%) 1 (2.9%) 14 (40.0%) | 0 3 (42.9%) 1 (14.3%) 0 1 (14.3%) 0 1 (14.3%) 1 (14.3%) | 0.170 |
| Charlson Comorbidity Index ** | 5 (4 to 6) | 3 (2 to 4) | 0.028 |
| Initial Child–Pugh Score 5 6 7 8 9 | 16 (45.7%) 5 (14.3%) 3 (8.6%) 4 (11.4%) 7 (20.0%) | 3 (42.9%) 2 (28.6%) 2 (28.6%) 0 0 | 0.299 |
| Initial Tumour Burden Category Single ≤ 2 cm Single > 2 cm, ≤3 cm Single > 3 cm, ≤5 cm Single > 5 cm Multinodular, all ≤ 3 cm | 8 (22.9%) 9 (25.7%) 4 (11.4%) 2 (5.7%) 12 (34.3%) | 1 (14.3%) 2 (28.6%) 0 0 4 (57.1%) | 0.696 |
| Initial Treatment Resection Ablation TACE Other | 3 (8.6%) 5 (14.3%) 27 (77.1%) 0 | 1 (14.3%) 4 (57.1%) 1 (14.3%) 1 (14.3%) | 0.003 |
| Indication for Transplant Salvage Recurrence | 3 (8.6%) 32 (91.4%) | 1 (14.3%) 6 (85.7%) | 0.562 |
| Time from initial diagnosis to transplant (days) ** | 504 (349 to 1019) | 729 (546 to 743) | 0.446 |
| LTC n = 79 | NTC n = 120 | p-Value | |
|---|---|---|---|
| Age * | 64.0 ± 8.5 | 62.7 ± 10.3 | 0.346 |
| Sex Male Female | 62 (78.5%) 17 (21.5%) | 95 (79.2%) 25 (20.8%) | 0.908 |
| Aetiology Alcohol HBV HCV MASLD Other metALD HBV/HCV HCV + SLD HBV + SLD | 10 (12.7%) 21 (26.6%) 18 (22.8%) 8 (10.1%) 5 (6.3%) 3 (3.8%) 2 (2.5%) 12 (15.2%) 0 | 8 (6.7%) 30 (25.0%) 18 (15.0%) 15 (12.5%) 8 (6.7%) 3 (2.5%) 3 (2.5%) 25 20.8(%) 10 (8.3%) | 0.178 |
| Smoking Yes No | 18 (22.8%) 61 (77.2%) | 41 (34.2%) 79 (65.8%) | 0.085 |
| Charlson Comorbidity Index ** | 3 (2 to 4) | 3 (2 to 4) | 0.372 |
| Cirrhosis Yes No | 43 (54.4%) 36 (45.6%) | 72 (60.0%) 48 (40.0%) | 0.436 |
| Platelets ** | 161 (131 to 216) | 194 (142 to 242.5) | 0.068 |
| Child–Pugh Score 5 6 7 8 | 66 (83.5%) 10 (12.7%) 2 (2.5%) 1 (1.3%) | 98 (81.7%) 19 (15.8%) 2 (1.7%) 1 (0.8%) | 0.893 |
| Tumour Burden Category Single ≤ 2 cm Single > 2 cm, ≤3 cm Single > 3 cm, ≤5 cm Single > 5 cm Multinodular, all ≤ 3 cm | 19 (24.1%) 22 (27.8%) 17 (21.5%) 16 (20.3%) 5 (6.3%) | 32 (26.7%) 35 (29.2%) 34 (29.3%) 12 (28.3%) 7 (6.8%) | 0.331 |
| Adjusted OR | 95% CI | p-Value | |
|---|---|---|---|
| Centre Type | |||
| NTC | Reference | − | − |
| LTC | 0.75 | 0.50 to 1.11 | 0.153 |
| Age | 1.00 | 0.98 to 1.02 | 0.922 |
| Sex | |||
| Male | Reference | − | − |
| Female | 1.22 | 0.75 to 1.99 | 0.432 |
| Diabetes | |||
| No | Reference | − | − |
| Yes | 0.69 | 0.41 to 1.17 | 0.170 |
| Smoking | |||
| No | Reference | − | − |
| Yes | 1.30 | 0.82 to 2.06 | 0.262 |
| HBV | |||
| No | Reference | − | − |
| Yes | 1.14 | 0.72 to 1.81 | 0.571 |
| Alcohol | |||
| No | Reference | − | − |
| Yes | 0.87 | 0.55 to 1.36 | 0.535 |
| Charlson Comorbidity Index | 0.73 | 0.64 to 0.83 | <0.001 |
| Cirrhosis | |||
| No | Reference | − | − |
| Yes | 0.35 | 0.21 to 0.57 | <0.001 |
| Platelets | 1.00 | 1.00 to 1.01 | 0.003 |
| Child–Pugh Score | 0.50 | 0.37 to 0.69 | <0.001 |
| Tumour Burden Category | |||
| Single ≤ 2 cm | Reference | − | − |
| Single > 2 cm, ≤3 cm | 1.35 | 0.82 to 2.22 | 0.233 |
| Single > 3 cm, ≤5 cm | 2.03 | 1.16 to 3.55 | 0.013 |
| Single > 5 cm | 0.94 | 0.47 to 1.88 | 0.855 |
| Multinodular, all ≤ 3 cm | 0.35 | 0.17 to 0.73 | 0.004 |
| LTC n = 354 | NTC n = 334 | p-Value | |
|---|---|---|---|
| Single ≤ 2 cm Ablation TACE Other | 41 (40.2%) 61 (59.8%) 0 | 84 (77.1%) 16 (14.7%) 9 (8.3%) | <0.001 |
| Single > 2 cm, ≤3 cm Ablation TACE Other | 23 (31.5%) 46 (63.0%) 4 (5.5%) | 53 (56.4%) 24 (25.5%) 17 (18.1%) | <0.001 |
| Single > 3 cm, ≤5 cm Ablation TACE Other | 0 49 (94.2%) 3 (5.8%) | 13 (34.2%) 23 (60.5%) 2 (52.6%) | <0.001 |
| Single > 5 cm Ablation TACE Other | 0 16 (53.3%) 14 (46.7%) | 3 (13.0%) 13 (56.5%) 7 (30.4%) | 0.091 |
| Multinodular, all ≤ 3 cm Ablation TACE Other | 10 (9.7%) 84 (86.6%) 3 (3.1%) | 32 (45.7%) 35 (50.0%) 3 (4.3%) | <0.001 |
| Adjusted OR | 95% CI | p-Value | |
|---|---|---|---|
| Centre Type | |||
| NTC | Reference | − | − |
| LTC | 0.19 | 0.13 to 0.28 | <0.001 |
| Age | 1.00 | 0.98 to 1.02 | 0.799 |
| Sex | |||
| Male | Reference | − | − |
| Female | 1.07 | 0.67 to 1.70 | 0.789 |
| Diabetes | |||
| No | Reference | − | − |
| Yes | 1.05 | 0.68 to 1.61 | 0.835 |
| Smoking | |||
| No | Reference | − | − |
| Yes | 1.29 | 0.84 to 1.96 | 0.242 |
| HBV | |||
| No | Reference | − | − |
| Yes | 0.97 | 0.58 to 1.63 | 0.904 |
| Alcohol | |||
| No | Reference | − | − |
| Yes | 0.96 | 0.64 to 1.45 | 0.854 |
| Charlson Comorbidity Index | 1.02 | 0.91 to 1.14 | 0.740 |
| Cirrhosis | |||
| No | Reference | − | − |
| Yes | 1.94 | 0.92 to 4.08 | 0.082 |
| Platelets | 1.00 | 1.00 to 1.00 | 0.800 |
| Child–Pugh Score | 0.82 | 0.69 to 0.99 | 0.037 |
| Tumour Burden Category | |||
| Single ≤ 2 cm | Reference | − | − |
| Single > 2 cm, ≤3 cm | 0.50 | 0.32 to 0.79 | 0.003 |
| Single > 3 cm, ≤5 cm | 0.10 | 0.05 to 0.21 | <0.001 |
| Single > 5 cm | 0.04 | 0.01 to 0.13 | <0.001 |
| Multinodular, all ≤ 3 cm | 0.22 | 0.14 to 0.36 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelmalak, J.; Strasser, S.I.; Ngu, N.L.; Dennis, C.; Sinclair, M.; Majumdar, A.; Collins, K.; Bateman, K.; Dev, A.; Abasszade, J.H.; et al. Different Patterns of Care and Survival Outcomes in Transplant-Centre Managed Patients with Early-Stage HCC: Real-World Data from an Australian Multi-Centre Cohort Study. Cancers 2024, 16, 1966. https://doi.org/10.3390/cancers16111966
Abdelmalak J, Strasser SI, Ngu NL, Dennis C, Sinclair M, Majumdar A, Collins K, Bateman K, Dev A, Abasszade JH, et al. Different Patterns of Care and Survival Outcomes in Transplant-Centre Managed Patients with Early-Stage HCC: Real-World Data from an Australian Multi-Centre Cohort Study. Cancers. 2024; 16(11):1966. https://doi.org/10.3390/cancers16111966
Chicago/Turabian StyleAbdelmalak, Jonathan, Simone I. Strasser, Natalie L. Ngu, Claude Dennis, Marie Sinclair, Avik Majumdar, Kate Collins, Katherine Bateman, Anouk Dev, Joshua H. Abasszade, and et al. 2024. "Different Patterns of Care and Survival Outcomes in Transplant-Centre Managed Patients with Early-Stage HCC: Real-World Data from an Australian Multi-Centre Cohort Study" Cancers 16, no. 11: 1966. https://doi.org/10.3390/cancers16111966
APA StyleAbdelmalak, J., Strasser, S. I., Ngu, N. L., Dennis, C., Sinclair, M., Majumdar, A., Collins, K., Bateman, K., Dev, A., Abasszade, J. H., Valaydon, Z., Saitta, D., Gazelakis, K., Byers, S., Holmes, J., Thompson, A. J., Pandiaraja, D., Bollipo, S., Sharma, S., ... Roberts, S. K. (2024). Different Patterns of Care and Survival Outcomes in Transplant-Centre Managed Patients with Early-Stage HCC: Real-World Data from an Australian Multi-Centre Cohort Study. Cancers, 16(11), 1966. https://doi.org/10.3390/cancers16111966







