Extracellular Vesicular miRNA in Pancreatic Cancer: From Lab to Therapy
Abstract
Simple Summary
Abstract
1. Introduction
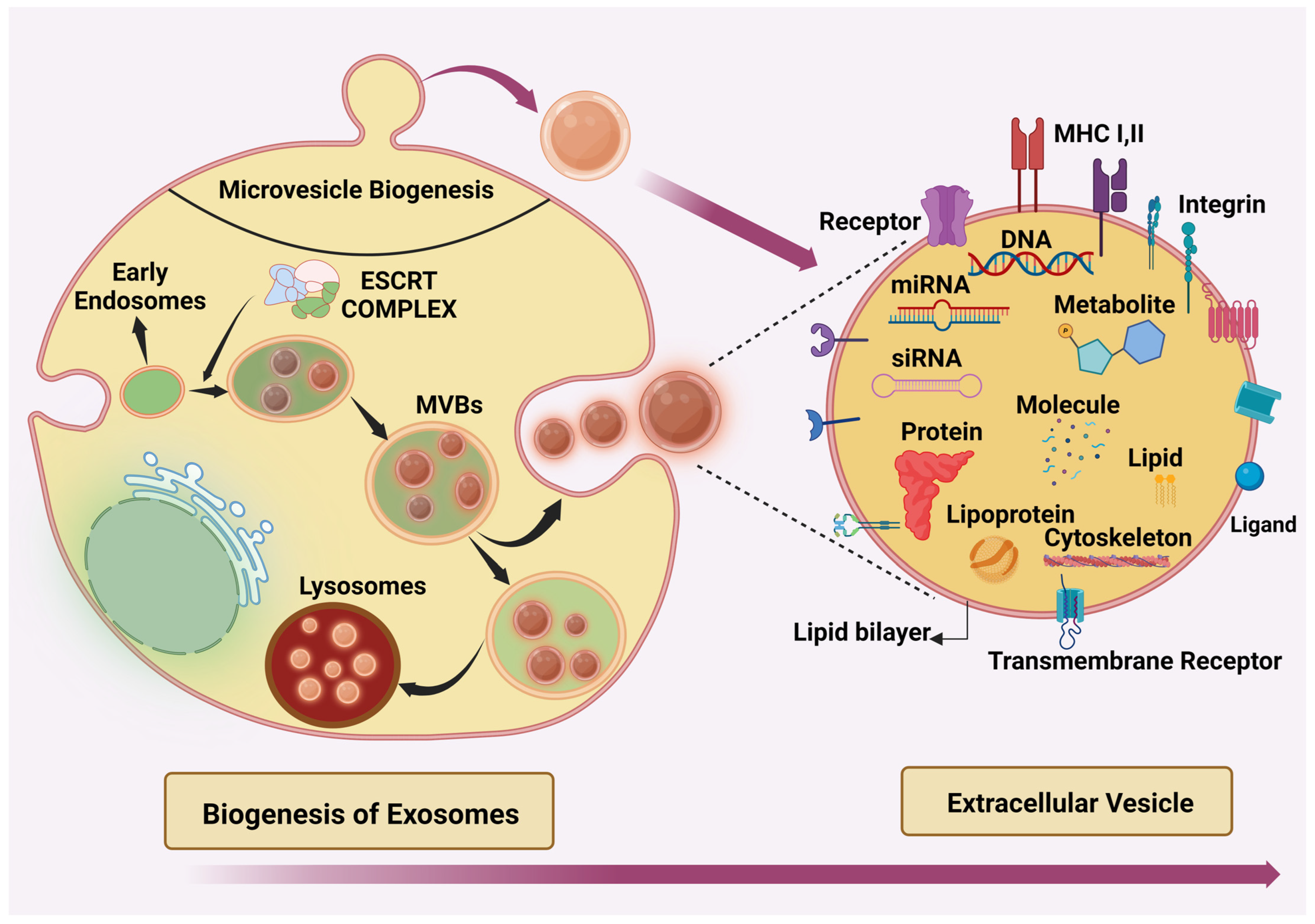
2. Extracellular Vesicle (EVs)
Biogenesis of Exosomes
3. Application of EV-miRNAs in Pancreatic Cancer
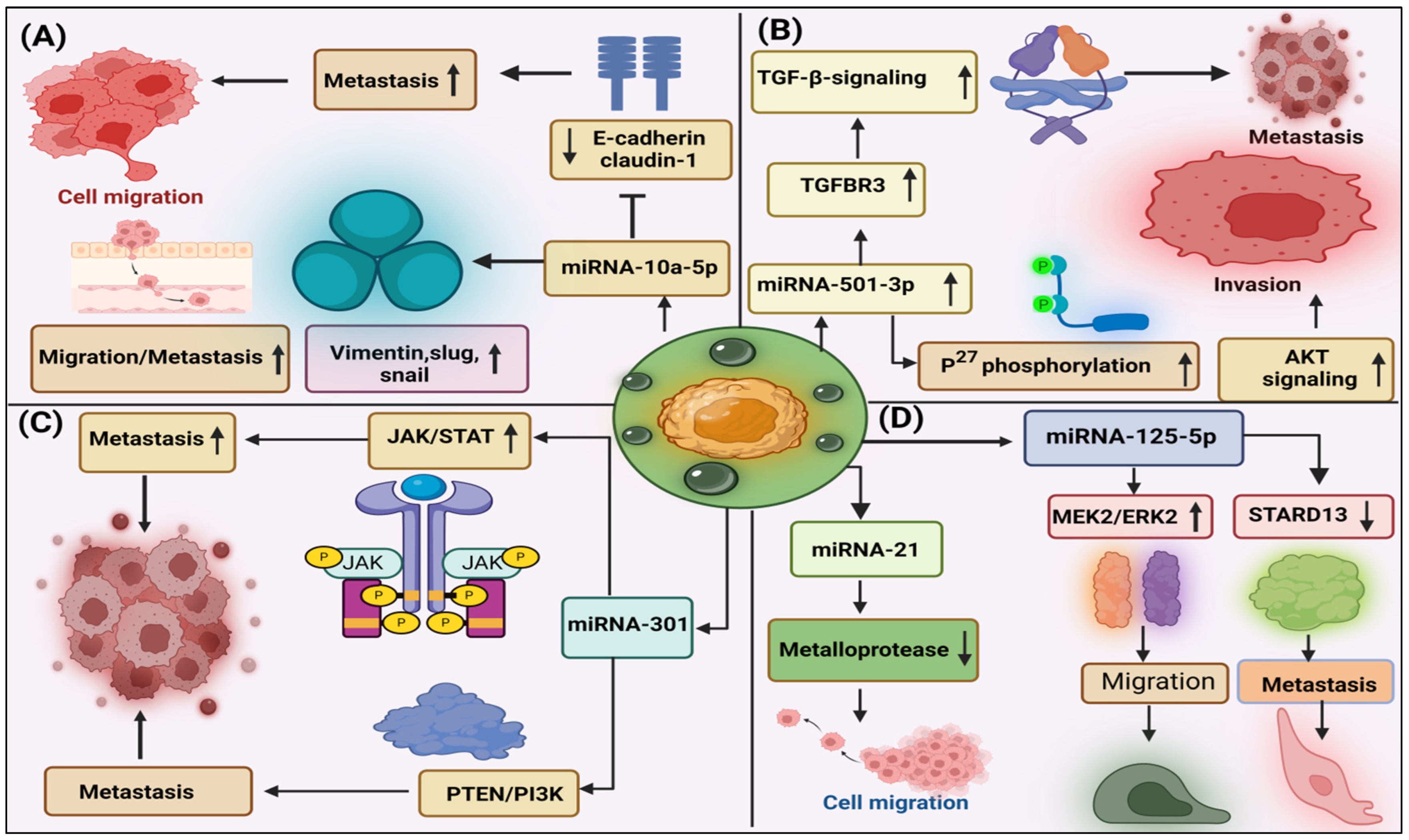
3.1. Role of Exosome-miRNAs in Progression of PDAC
3.2. Role of Exosome-miRNAs in Migration, Invasion, and Metastasis in PDAC
3.3. Role of Exosome-miRNAs in Proliferation and Angiogenesis of PDAC
3.4. Role of Exosome-miRNAs in Immune Escape in PDAC
3.5. Role of Exosome-miRNAs in PDAC as Diagnosis Biomarker
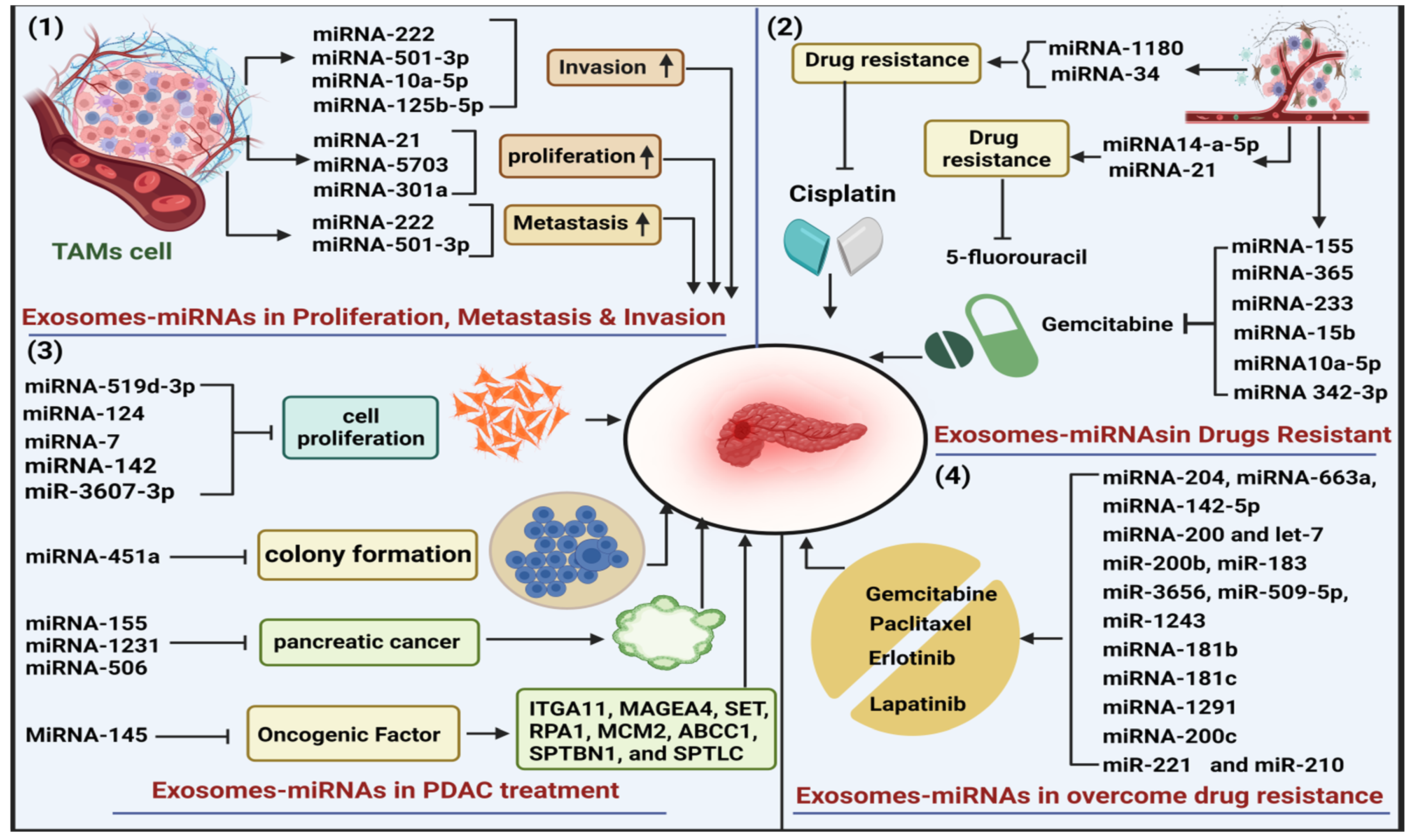
3.6. The Role of Exosome-miRNAs in Drug Resistance of PDAC
3.7. Role of Exosome-miRNAs in Overcome Drug Resistance
3.8. Role of Exosome-miRNAs in PDAC Treatment
3.9. Immune Checkpoint Blockade Therapy in PDAC
4. Exosome-Based Delivery Systems of miRNA
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tonini, V.; Zanni, M. Pancreatic Cancer in 2021: What You Need to Know to Win. World J. Gastroenterol. 2021, 27, 5851–5889. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Kumar, S.; Sandeep, K.; Patel, S.K.S. Therapeutic Approaches in Pancreatic Cancer: Recent Updates. Biomedicines 2023, 11, 1611. [Google Scholar] [CrossRef] [PubMed]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic Cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic Cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed]
- Papadakos, S.P.; Dedes, N.; Gkolemi, N.; Machairas, N.; Theocharis, S. The EPH/Ephrin System in Pancreatic Ductal Adenocarcinoma (PDAC): From Pathogenesis to Treatment. Int. J. Mol. Sci. 2023, 24, 3015. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wu, X.; Xia, M.; Wu, F.; Ding, J.; Jiao, Y.; Zhan, Q.; An, F. Upregulated Exosomic MiR-23b-3p Plays Regulatory Roles in the Progression of Pancreatic Cancer. Oncol. Rep. 2017, 38, 2182–2188. [Google Scholar] [CrossRef] [PubMed]
- Mendt, M.; Kamerkar, S.; Sugimoto, H.; McAndrews, K.M.; Wu, C.-C.; Gagea, M.; Yang, S.; Blanko, E.V.R.; Peng, Q.; Ma, X.; et al. Generation and Testing of Clinical-Grade Exosomes for Pancreatic Cancer. JCI Insight 2018, 3, e99263. [Google Scholar] [CrossRef] [PubMed]
- Mok, E.T.Y.; Chitty, J.L.; Cox, T.R. MiRNAs in Pancreatic Cancer Progression and Metastasis. Clin. Exp. Metastasis 2024. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhao, L.; Wu, Y.; Deng, S.; Cao, P.; Lei, X.; Yang, X. The Role of NcRNAs to Regulate Immune Checkpoints in Cancer. Front. Immunol. 2022, 13, 853480. [Google Scholar] [CrossRef]
- He, M.; Zhang, H.; Tang, Z.; Gao, S. Diagnostic and Therapeutic Potential of Exosomal MicroRNAs for Neurodegenerative Diseases. Neural Plast. 2021, 2021, 8884642. [Google Scholar] [CrossRef]
- Woith, E.; Fuhrmann, G.; Melzig, M.F. Extracellular Vesicles—Connecting Kingdoms. Int. J. Mol. Sci. 2019, 20, 5695. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular Vesicles as a Next-Generation Drug Delivery Platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Karn, V.; Ahmed, S.; Tsai, L.-W.; Dubey, R.; Ojha, S.; Singh, H.N.; Kumar, M.; Gupta, P.K.; Sadhu, S.; Jha, N.K.; et al. Extracellular Vesicle-Based Therapy for COVID-19: Promises, Challenges and Future Prospects. Biomedicines 2021, 9, 1373. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Yuzhakov, S.V.; Kumar, S.; Larson, N.B.; Enders, F.T.; Milliner, D.S.; Rule, A.D.; Lieske, J.C. Specific Populations of Urinary Extracellular Vesicles and Proteins Differentiate Type 1 Primary Hyperoxaluria Patients without and with Nephrocalcinosis or Kidney Stones. Orphanet J. Rare Dis. 2020, 15, 319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kumar, S.; Jayachandran, M.; Herrera Hernandez, L.P.; Wang, S.; Wilson, E.M.; Lieske, J.C. Excretion of Urine Extracellular Vesicles Bearing Markers of Activated Immune Cells and Calcium/Phosphorus Physiology Differ between Calcium Kidney Stone Formers and Non-Stone Formers. BMC Nephrol. 2021, 22, 204. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Greening, D.W.; Bolumar, D.; Balaguer, N.; Salamonsen, L.A.; Vilella, F. Extracellular Vesicles in Human Reproduction in Health and Disease. Endocr. Rev. 2018, 39, 292–332. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Jayachandran, M.; Haskic, Z.; Kumar, S.; Lieske, J.C. Differences of Uric Acid Transporters Carrying Extracellular Vesicles in the Urine from Uric Acid and Calcium Stone Formers and Non-Stone Formers. Int. J. Mol. Sci. 2022, 23, 10010. [Google Scholar] [CrossRef]
- Jin, Y.; Ma, L.; Zhang, W.; Yang, W.; Feng, Q.; Wang, H. Extracellular Signals Regulate the Biogenesis of Extracellular Vesicles. Biol. Res. 2022, 55, 35. [Google Scholar] [CrossRef]
- Schöneberg, J.; Lee, I.-H.; Iwasa, J.H.; Hurley, J.H. Reverse-Topology Membrane Scission by the ESCRT Proteins. Nat. Rev. Mol. Cell Biol. 2017, 18, 5–17. [Google Scholar] [CrossRef]
- Vietri, M.; Radulovic, M.; Stenmark, H. The Many Functions of ESCRTs. Nat. Rev. Mol. Cell Biol. 2020, 21, 25–42. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Lambert, M.; Benmoussa, A.; Provost, P. Small Non-Coding RNAs Derived from Eukaryotic Ribosomal RNA. Non-Coding RNA 2019, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Frédérick, P.-M.; Simard, M.J. Regulation and Different Functions of the Animal MicroRNA-Induced Silencing Complex. WIREs RNA 2022, 13, e1701. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xiang, H.; Ge, W.; Wang, H.; Wang, T.; Xiong, M. Expression and Functional Perspectives of MiR-184 in Pancreatic Ductal Adenocarcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 12313–12318. [Google Scholar]
- Cote, G.A.; Gore, J.A.; McElyea, S.D.; Heathers, L.E.; Xu, H.; Sherman, S.; Korc, M. A Pilot Study to Develop a Diagnostic Test for Pancreatic Ductal Adenocarcinoma Based on Differential Expression of Select MiRNA in Plasma and Bile. Off. J. Am. Coll. Gastroenterol. ACG 2014, 109, 1942. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, L.; Baddour, J.; Achreja, A.; Bernard, V.; Moss, T.; Marini, J.C.; Tudawe, T.; Seviour, E.G.; San Lucas, F.A.; et al. Tumor Microenvironment Derived Exosomes Pleiotropically Modulate Cancer Cell Metabolism. eLife 2016, 5, e10250. [Google Scholar] [CrossRef]
- Tang, P.; Tao, L.; Yuan, C.; Zhang, L.; Xiu, D. Serum Derived Exosomes from Pancreatic Cancer Patients Promoted Metastasis: An ITRAQ-Based Proteomic Analysis. OncoTargets Ther. 2019, 12, 9329–9339. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef]
- Stefanius, K.; Servage, K.; de Souza Santos, M.; Gray, H.F.; Toombs, J.E.; Chimalapati, S.; Kim, M.S.; Malladi, V.S.; Brekken, R.; Orth, K. Human Pancreatic Cancer Cell Exosomes, but Not Human Normal Cell Exosomes, Act as an Initiator in Cell Transformation. eLife 2019, 8, e40226. [Google Scholar] [CrossRef]
- Shang, S.; Wang, J.; Chen, S.; Tian, R.; Zeng, H.; Wang, L.; Xia, M.; Zhu, H.; Zuo, C. Exosomal MiRNA-1231 Derived from Bone Marrow Mesenchymal Stem Cells Inhibits the Activity of Pancreatic Cancer. Cancer Med. 2019, 8, 7728–7740. [Google Scholar] [CrossRef]
- Xiong, G.; Huang, H.; Feng, M.; Yang, G.; Zheng, S.; You, L.; Zheng, L.; Hu, Y.; Zhang, T.; Zhao, Y. MiR-10a-5p Targets TFAP2C to Promote Gemcitabine Resistance in Pancreatic Ductal Adenocarcinoma. J. Exp. Clin. Cancer Res. 2018, 37, 76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zha, Y.; Hu, W.; Huang, Z.; Gao, Z.; Zang, Y.; Chen, J.; Dong, L.; Zhang, J. The Autoregulatory Feedback Loop of MicroRNA-21/Programmed Cell Death Protein 4/Activation Protein-1 (MiR-21/PDCD4/AP-1) as a Driving Force for Hepatic Fibrosis Development. J. Biol. Chem. 2013, 288, 37082–37093. [Google Scholar] [CrossRef]
- Wu, M.; Tan, X.; Liu, P.; Yang, Y.; Huang, Y.; Liu, X.; Meng, X.; Yu, B.; Wu, Y.; Jin, H. Role of Exosomal MicroRNA-125b-5p in Conferring the Metastatic Phenotype among Pancreatic Cancer Cells with Different Potential of Metastasis. Life Sci. 2020, 255, 117857. [Google Scholar] [CrossRef]
- Nasimi Shad, A.; Fanoodi, A.; Maharati, A.; Akhlaghipour, I.; Moghbeli, M. Molecular Mechanisms of MicroRNA-301a during Tumor Progression and Metastasis. Pathol. Res. Pract. 2023, 247, 154538. [Google Scholar] [CrossRef]
- Li, Z.; Tao, Y.; Wang, X.; Jiang, P.; Li, J.; Peng, M.; Zhang, X.; Chen, K.; Liu, H.; Zhen, P.; et al. Tumor-Secreted Exosomal MiR-222 Promotes Tumor Progression via Regulating P27 Expression and Re-Localization in Pancreatic Cancer. Cell Physiol. Biochem. 2018, 51, 610–629. [Google Scholar] [CrossRef]
- Yin, Z.; Ma, T.; Huang, B.; Lin, L.; Zhou, Y.; Yan, J.; Zou, Y.; Chen, S. Macrophage-Derived Exosomal MicroRNA-501-3p Promotes Progression of Pancreatic Ductal Adenocarcinoma through the TGFBR3-Mediated TGF-β Signaling Pathway. J. Exp. Clin. Cancer Res. 2019, 38, 310. [Google Scholar] [CrossRef]
- Li, T.; Cheng, Y.; Wang, P.; Wang, W.; Hu, F.; Mo, X.; Lv, H.; Xu, T.; Han, W. CMTM4 Is Frequently Downregulated and Functions as a Tumour Suppressor in Clear Cell Renal Cell Carcinoma. J. Exp. Clin. Cancer Res. 2015, 34, 122. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Li, T.; Wang, P.; Mo, X.; Zhang, H.; Ding, S.; Ma, D.; Lv, W.; Zhang, J.; Han, W. CMTM4 Inhibits Cell Proliferation and Migration via AKT, ERK1/2, and STAT3 Pathway in Colorectal Cancer. Acta Biochim. Biophys. Sin. 2019, 51, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhou, Y.; Ma, T.; Chen, S.; Shi, N.; Zou, Y.; Hou, B.; Zhang, C. Down-Regulated LncRNA SBF2-AS1 in M2 Macrophage-Derived Exosomes Elevates MiR-122-5p to Restrict XIAP, Thereby Limiting Pancreatic Cancer Development. J. Cell. Mol. Med. 2020, 24, 5028–5038. [Google Scholar] [CrossRef]
- Luo, Q.; Hu, Z.; Zhao, H.; Fan, Y.; Tu, X.; Wang, Y.; Liu, X. The Role of TGF-β in the Tumor Microenvironment of Pancreatic Cancer. Genes Dis. 2023, 10, 1513–1524. [Google Scholar] [CrossRef]
- Wang, X.; Luo, G.; Zhang, K.; Cao, J.; Huang, C.; Jiang, T.; Liu, B.; Su, L.; Qiu, Z. Hypoxic Tumor-Derived Exosomal MiR-301a Mediates M2 Macrophage Polarization via PTEN/PI3Kγ to Promote Pancreatic Cancer Metastasis. Cancer Res. 2018, 78, 4586–4598. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, O.; Russo, A.; Scarpa, A.; Santini, D.; Reni, M.; Bittoni, A.; Azzariti, A.; Aprile, G.; Delcuratolo, S.; Signorile, M.; et al. MicroRNA in Pancreatic Adenocarcinoma: Predictive/Prognostic Biomarkers or Therapeutic Targets? Oncotarget 2015, 6, 23323–23341. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Liu, X. Macrophage-Derived Small Extracellular Vesicles in Multiple Diseases: Biogenesis, Function, and Therapeutic Applications. Front. Cell Dev. Biol. 2022, 10, 913110. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Guo, H.; Wang, Q.; Chen, K.; Marko, K.; Tian, X.; Yang, Y. Pancreatic Stellate Cells Derived Exosomal MiR-5703 Promotes Pancreatic Cancer by Downregulating CMTM4 and Activating PI3K/Akt Pathway. Cancer Lett. 2020, 490, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhou, W.-B.; Zhou, J.; Wei, Y.; Wang, H.-M.; Liu, X.-D.; Chen, X.-C.; Wang, W.; Ye, L.; Yao, L.C.; et al. Circulating Exosomal MicroRNAs as Novel Potential Detection Biomarkers in Pancreatic Cancer. Oncol. Lett. 2020, 20, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, J.; Cui, W.; Liu, Y.; Zhou, H.; Wang, Y.; Chen, X.; Chen, X.; Wang, Z. Serum Exosomal MiRNA-1226 as Potential Biomarker of Pancreatic Ductal Adenocarcinoma. OncoTargets Ther. 2021, 14, 1441–1451. [Google Scholar] [CrossRef]
- Wang, D.; Sang, Y.; Sun, T.; Kong, P.; Zhang, L.; Dai, Y.; Cao, Y.; Tao, Z.; Liu, W. Emerging Roles and Mechanisms of MicroRNA-222-3p in Human Cancer (Review). Int. J. Oncol. 2021, 58, 20. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhao, G.; Yang, S.; Zhu, S.; Zhang, S.; Li, P. The Significance of Exosomal RNAs in the Development, Diagnosis, and Treatment of Pancreatic Cancer. Cancer Cell Int. 2021, 21, 364. [Google Scholar] [CrossRef] [PubMed]
- Shang, D.; Xie, C.; Hu, J.; Tan, J.; Yuan, Y.; Liu, Z.; Yang, Z. Pancreatic Cancer Cell–Derived Exosomal MicroRNA-27a Promotes Angiogenesis of Human Microvascular Endothelial Cells in Pancreatic Cancer via BTG2. J. Cell. Mol. Med. 2020, 24, 588–604. [Google Scholar] [CrossRef]
- Khan, A.Q.; Kuttikrishnan, S.; Siveen, K.S.; Prabhu, K.S.; Shanmugakonar, M.; Al-Naemi, H.A.; Haris, M.; Dermime, S.; Uddin, S. RAS-Mediated Oncogenic Signaling Pathways in Human Malignancies. Semin. Cancer Biol. 2019, 54, 1–13. [Google Scholar] [CrossRef]
- Ma, Q.; Wu, H.; Xiao, Y.; Liang, Z.; Liu, T. Upregulation of Exosomal MicroRNA-21 in Pancreatic Stellate Cells Promotes Pancreatic Cancer Cell Migration and Enhances Ras/ERK Pathway Activity. Int. J. Oncol. 2020, 56, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Kurywchak, P.; Tavormina, J.; Kalluri, R. The Emerging Roles of Exosomes in the Modulation of Immune Responses in Cancer. Genome Med. 2018, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Gehrmann, U.; Näslund, T.I.; Hiltbrunner, S.; Larssen, P.; Gabrielsson, S. Harnessing the Exosome-Induced Immune Response for Cancer Immunotherapy. Semin. Cancer Biol. 2014, 28, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.J.; Lucien, F.; Sakemura, R.; Boysen, J.C.; Kim, Y.; Horvei, P.; Roman, C.M.; Hansen, M.J.; Tapper, E.E.; Siegler, E.L.; et al. Leukemic Extracellular Vesicles Induce Chimeric Antigen Receptor T Cell Dysfunction in Chronic Lymphocytic Leukemia. Mol. Ther. 2021, 29, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Tucci, M.; Passarelli, A.; Mannavola, F.; Felici, C.; Stucci, L.S.; Cives, M.; Silvestris, F. Immune System Evasion as Hallmark of Melanoma Progression: The Role of Dendritic Cells. Front. Oncol. 2019, 9, 1148. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Chen, J.; Zhou, L.; Chen, W.; Ding, G.; Cao, L. Pancreatic Cancer Derived Exosomes Regulate the Expression of TLR4 in Dendritic Cells via MiR-203. Cell Immunol. 2014, 292, 65–69. [Google Scholar] [CrossRef]
- Ding, G.; Zhou, L.; Qian, Y.; Fu, M.; Chen, J.; Chen, J.; Xiang, J.; Wu, Z.; Jiang, G.; Cao, L. Pancreatic Cancer-Derived Exosomes Transfer MiRNAs to Dendritic Cells and Inhibit RFXAP Expression via MiR-212-3p. Oncotarget 2015, 6, 29877–29888. [Google Scholar] [CrossRef] [PubMed]
- Soreide, K. Sweet Predictions Speak Volumes for Early Detection of Pancreatic Cancer. Gastroenterology 2018, 155, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sanagapalli, S.; Stoita, A. Challenges in Diagnosis of Pancreatic Cancer. World J. Gastroenterol. 2018, 24, 2047–2060. [Google Scholar] [CrossRef]
- Kahroba, H.; Hejazi, M.S.; Samadi, N. Exosomes: From Carcinogenesis and Metastasis to Diagnosis and Treatment of Gastric Cancer. Cell Mol. Life Sci. 2019, 76, 1747–1758. [Google Scholar] [CrossRef]
- Fitts, C.A.; Ji, N.; Li, Y.; Tan, C. Exploiting Exosomes in Cancer Liquid Biopsies and Drug Delivery. Adv. Healthc. Mater. 2019, 8, 1801268. [Google Scholar] [CrossRef]
- Karius, T.; Schnekenburger, M.; Dicato, M.; Diederich, M. MicroRNAs in Cancer Management and Their Modulation by Dietary Agents. Biochem. Pharmacol. 2012, 83, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Rawat, M.; Kadian, K.; Gupta, Y.; Kumar, A.; Chain, P.S.G.; Kovbasnjuk, O.; Kumar, S.; Parasher, G. MicroRNA in Pancreatic Cancer: From Biology to Therapeutic Potential. Genes 2019, 10, 752. [Google Scholar] [CrossRef] [PubMed]
- Takahasi, K.; Iinuma, H.; Wada, K.; Minezaki, S.; Kawamura, S.; Kainuma, M.; Ikeda, Y.; Shibuya, M.; Miura, F.; Sano, K. Usefulness of Exosome-Encapsulated MicroRNA-451a as a Minimally Invasive Biomarker for Prediction of Recurrence and Prognosis in Pancreatic Ductal Adenocarcinoma. J. Hepato-Biliary-Pancreat. Sci. 2018, 25, 155–161. [Google Scholar] [CrossRef]
- Guo, S.; Fesler, A.; Wang, H.; Ju, J. MicroRNA Based Prognostic Biomarkers in Pancreatic Cancer. Biomark. Res. 2018, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Schultz, N.A.; Andersen, K.K.; Roslind, A.; Willenbrock, H.; Wøjdemann, M.; Johansen, J.S. Prognostic MicroRNAs in Cancer Tissue from Patients Operated for Pancreatic Cancer—Five MicroRNAs in a Prognostic Index. World J. Surg. 2012, 36, 2699–2707. [Google Scholar] [CrossRef]
- Wald, P.; Liu, X.S.; Pettit, C.; Dillhoff, M.; Manilchuk, A.; Schmidt, C.; Wuthrick, E.; Chen, W.; Williams, T.M. Prognostic Value of MicroRNA Expression Levels in Pancreatic Adenocarcinoma: A Review of the Literature. Oncotarget 2017, 8, 73345–73361. [Google Scholar] [CrossRef][Green Version]
- Hannafon, B.N.; Carpenter, K.J.; Berry, W.L.; Janknecht, R.; Dooley, W.C.; Ding, W.-Q. Exosome-Mediated MicroRNA Signaling from Breast Cancer Cells Is Altered by the Anti-Angiogenesis Agent Docosahexaenoic Acid (DHA). Mol. Cancer 2015, 14, 133. [Google Scholar] [CrossRef]
- Kawamura, S.; Iinuma, H.; Wada, K.; Takahashi, K.; Minezaki, S.; Kainuma, M.; Shibuya, M.; Miura, F.; Sano, K. Exosome-Encapsulated MicroRNA-4525, MicroRNA-451a and MicroRNA-21 in Portal Vein Blood Is a High-Sensitive Liquid Biomarker for the Selection of High-Risk Pancreatic Ductal Adenocarcinoma Patients. J. Hepato-Biliary-Pancreat. Sci. 2019, 26, 63–72. [Google Scholar] [CrossRef]
- Yu, Z.; Zhao, S.; Wang, L.; Wang, J.; Zhou, J. MiRNA-339-5p Plays an Important Role in Invasion and Migration of Pancreatic Cancer Cells. Med. Sci. Monit. 2019, 25, 7509–7517. [Google Scholar] [CrossRef]
- Madhavan, B.; Yue, S.; Galli, U.; Rana, S.; Gross, W.; Müller, M.; Giese, N.A.; Kalthoff, H.; Becker, T.; Büchler, M.W.; et al. Combined Evaluation of a Panel of Protein and MiRNA Serum-Exosome Biomarkers for Pancreatic Cancer Diagnosis Increases Sensitivity and Specificity. Int. J. Cancer 2015, 136, 2616–2627. [Google Scholar] [CrossRef]
- Lai, X.; Wang, M.; McElyea, S.D.; Sherman, S.; House, M.; Korc, M. A MicroRNA Signature in Circulating Exosomes Is Superior to Exosomal Glypican-1 Levels for Diagnosing Pancreatic Cancer. Cancer Lett. 2017, 393, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Sadakari, Y.; Ohtsuka, T.; Okayama, T.; Nakashima, Y.; Gotoh, Y.; Saeki, K.; Mori, Y.; Nakata, K.; Miyasaka, Y.; et al. Pancreatic Juice Exosomal MicroRNAs as Biomarkers for Detection of Pancreatic Ductal Adenocarcinoma. Ann. Surg. Oncol. 2019, 26, 2104–2111. [Google Scholar] [CrossRef] [PubMed]
- Abue, M.; Yokoyama, M.; Shibuya, R.; Tamai, K.; Yamaguchi, K.; Sato, I.; Tanaka, N.; Hamada, S.; Shimosegawa, T.; Sugamura, K.; et al. Circulating MiR-483-3p and MiR-21 Is Highly Expressed in Plasma of Pancreatic Cancer. Int. J. Oncol. 2015, 46, 539–547. [Google Scholar] [CrossRef]
- Mortoglou, M.; Miralles, F.; Arisan, E.D.; Dart, A.; Jurcevic, S.; Lange, S.; Uysal-Onganer, P. MicroRNA-21 Regulates Stemness in Pancreatic Ductal Adenocarcinoma Cells. Int. J. Mol. Sci. 2022, 23, 1275. [Google Scholar] [CrossRef]
- Girolimetti, G.; Pelisenco, I.A.; Eusebi, L.H.; Ricci, C.; Cavina, B.; Kurelac, I.; Verri, T.; Calcagnile, M.; Alifano, P.; Salvi, A.; et al. Dysregulation of a Subset of Circulating and Vesicle-Associated MiRNA in Pancreatic Cancer. Non-Coding RNA 2024, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Bhandari, K.; Xu, C.; Morris, K.; Ding, W.-Q. MiR-18a and MiR-106a Signatures in Plasma Small EVs Are Promising Biomarkers for Early Detection of Pancreatic Ductal Adenocarcinoma. Int. J. Mol. Sci. 2023, 24, 7215. [Google Scholar] [CrossRef]
- Ohuchida, K.; Mizumoto, K.; Kayashima, T.; Fujita, H.; Moriyama, T.; Ohtsuka, T.; Ueda, J.; Nagai, E.; Hashizume, M.; Tanaka, M. MicroRNA Expression as a Predictive Marker for Gemcitabine Response after Surgical Resection of Pancreatic Cancer. Ann. Surg. Oncol. 2011, 18, 2381–2387. [Google Scholar] [CrossRef]
- Ren, Y.; Gao, J.; Liu, J.-Q.; Wang, X.-W.; Gu, J.-J.; Huang, H.-J.; Gong, Y.-F.; Li, Z.-S. Differential Signature of Fecal MicroRNAs in Patients with Pancreatic Cancer. Mol. Med. Rep. 2012, 6, 201–209. [Google Scholar] [CrossRef]
- Xu, Q.; Li, P.; Chen, X.; Zong, L.; Jiang, Z.; Nan, L.; Lei, J.; Duan, W.; Zhang, D.; Li, X.; et al. MiR-221/222 Induces Pancreatic Cancer Progression through the Regulation of Matrix Metalloproteinases. Oncotarget 2015, 6, 14153–14164. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, K.; Cen, G.; Jiang, T.; Cao, J.; Huang, K.; Huang, C.; Zhao, Q.; Qiu, Z. MicroRNA-301a-3p Promotes Pancreatic Cancer Progression via Negative Regulation of SMAD4. Oncotarget 2015, 6, 21046–21063. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.S.; Szafranska-Schwarzbach, A.E.; Wylie, D.; Doyle, L.A.; Bellizzi, A.M.; Kadiyala, V.; Suleiman, S.; Banks, P.A.; Andruss, B.F.; Conwell, D.L. Investigating MicroRNA Expression Profiles in Pancreatic Cystic Neoplasms. Clin. Transl. Gastroenterol. 2014, 5, e47. [Google Scholar] [CrossRef]
- Huang, W.-T.; Lin, T.-S.; Wu, J.-Y.; Hong, J.-M.; Chen, Y.-L.; Qiu, F.-N. Evaluation of MiR-429 as a Novel Serum Biomarker for Pancreatic Ductal Adenocarcinoma and Analysis Its Tumor Suppressor Function and Target Genes. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 4638–4653. [Google Scholar] [PubMed]
- Le Large, T.Y.S.; Meijer, L.L.; Mato Prado, M.; Kazemier, G.; Frampton, A.E.; Giovannetti, E. Circulating MicroRNAs as Diagnostic Biomarkers for Pancreatic Cancer. Expert Rev. Mol. Diagn. 2015, 15, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Amrutkar, M.; Gladhaug, I.P. Pancreatic Cancer Chemoresistance to Gemcitabine. Cancers 2017, 9, 157. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Yu, Y. The Role of MiRNAs in the Diagnosis, Chemoresistance, and Prognosis of Pancreatic Ductal Adenocarcinoma. Ther. Clin. Risk Manag. 2018, 14, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.K.; Khan, M.A.; Bhardwaj, A.; Srivastava, S.K.; Zubair, H.; Patton, M.C.; Singh, S.; Khushman, M.; Singh, A.P. Exosomes Confer Chemoresistance to Pancreatic Cancer Cells by Promoting ROS Detoxification and MiR-155-Mediated Suppression of Key Gemcitabine-Metabolising Enzyme, DCK. Br. J. Cancer 2017, 116, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhao, N.; Cui, J.; Wu, H.; Xiong, J.; Peng, T. Exosomes Derived from Cancer Stem Cells of Gemcitabine-Resistant Pancreatic Cancer Cells Enhance Drug Resistance by Delivering MiR-210. Cell Oncol. 2020, 43, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Binenbaum, Y.; Fridman, E.; Yaari, Z.; Milman, N.; Schroeder, A.; Ben David, G.; Shlomi, T.; Gil, Z. Transfer of MiRNA in Macrophage-Derived Exosomes Induces Drug Resistance in Pancreatic Adenocarcinoma. Cancer Res. 2018, 78, 5287–5299. [Google Scholar] [CrossRef]
- Ma, J.; Fang, B.; Zeng, F.; Ma, C.; Pang, H.; Cheng, L.; Shi, Y.; Wang, H.; Yin, B.; Xia, J.; et al. Down-Regulation of MiR-223 Reverses Epithelial-Mesenchymal Transition in Gemcitabine-Resistant Pancreatic Cancer Cells. Oncotarget 2015, 6, 1740–1749. [Google Scholar] [CrossRef]
- Zhang, W.-L.; Zhang, J.-H.; Wu, X.-Z.; Yan, T.; Lv, W. MiR-15b Promotes Epithelial-Mesenchymal Transition by Inhibiting SMURF2 in Pancreatic Cancer. Int. J. Oncol. 2015, 47, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, J.; Tanahashi, T.; Sato, Y.; Miyoshi, J.; Nakagawa, T.; Kimura, T.; Miyamoto, H.; Fujino, Y.; Nakamura, F.; Takehara, M.; et al. MicroRNA-296-5p Promotes Cell Invasion and Drug Resistance by Targeting Bcl2-Related Ovarian Killer, Leading to a Poor Prognosis in Pancreatic Cancer. Digestion 2019, 101, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Zhang, K.; Luo, G.; Cen, G.; Cao, J.; Huang, K.; Qiu, Z. Downregulation of MiR-301a-3p Sensitizes Pancreatic Cancer Cells to Gemcitabine Treatment via PTEN. Am. J. Transl. Res. 2017, 9, 1886–1895. [Google Scholar] [PubMed]
- Fang, Y.; Zhou, W.; Rong, Y.; Kuang, T.; Xu, X.; Wu, W.; Wang, D.; Lou, W. Exosomal MiRNA-106b from Cancer-Associated Fibroblast Promotes Gemcitabine Resistance in Pancreatic Cancer. Exp. Cell Res. 2019, 383, 111543. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Fan, Z.; Du, G.; Wang, H. Leptin-Elicited MiRNA-342-3p Potentiates Gemcitabine Resistance in Pancreatic Ductal Adenocarcinoma. Biochem. Biophys. Res. Commun. 2019, 509, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhao, S.; Shen, J.; Guo, L.; Sun, Y.; Zhu, Y.; Ma, Z.; Zhang, X.; Hu, Y.; Xiao, W.; et al. The MiR-135b–BMAL1–YY1 Loop Disturbs Pancreatic Clockwork to Promote Tumourigenesis and Chemoresistance. Cell Death Dis. 2018, 9, 149. [Google Scholar] [CrossRef]
- Iwagami, Y.; Eguchi, H.; Nagano, H.; Akita, H.; Hama, N.; Wada, H.; Kawamoto, K.; Kobayashi, S.; Tomokuni, A.; Tomimaru, Y.; et al. MiR-320c Regulates Gemcitabine-Resistance in Pancreatic Cancer via SMARCC1. Br. J. Cancer 2013, 109, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Cisplatin: The First Metal Based Anticancer Drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef] [PubMed]
- Makovec, T. Cisplatin and beyond: Molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol. Oncol. 2019, 53, 148–158. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, J.; Shi, M.; Peng, C. The Effects of MiRNA-1180 on Suppression of Pancreatic Cancer. Am. J. Transl. Res. 2017, 9, 2798–2806. [Google Scholar]
- Schreiber, R.; Mezencev, R.; Matyunina, L.V.; McDonald, J.F. Evidence for the Role of MicroRNA 374b in Acquired Cisplatin Resistance in Pancreatic Cancer Cells. Cancer Gene Ther. 2016, 23, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Hao, X.; Zhang, M.; Tang, W.; Yang, M.; Li, L.; Xiang, D.; DeSano, J.T.; Bommer, G.T.; Fan, D.; et al. MicroRNA MiR-34 Inhibits Human Pancreatic Cancer Tumor-Initiating Cells. PLoS ONE 2009, 4, e6816. [Google Scholar] [CrossRef] [PubMed]
- Koleini, N.; Nickel, B.E.; Edel, A.L.; Fandrich, R.R.; Ravandi, A.; Kardami, E. Oxidized Phospholipids in Doxorubicin-Induced Cardiotoxicity. Chem. Biol. Interact. 2019, 303, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Sritharan, S.; Sivalingam, N. A Comprehensive Review on Time-Tested Anticancer Drug Doxorubicin. Life Sci. 2021, 278, 119527. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, E.; Tang, Y.; Mao, J.; Shen, J.; Zheng, X.; Xie, S.; Zhang, S.; Wu, Y.; Liu, H.; et al. MiR-223 Overexpression Inhibits Doxorubicin-Induced Autophagy by Targeting FOXO3a and Reverses Chemoresistance in Hepatocellular Carcinoma Cells. Cell Death Dis. 2019, 10, 843. [Google Scholar] [CrossRef] [PubMed]
- Han, G.Y.; Cui, J.H.; Liang, S.; Li, H.L. Increased MiR-142 and Decreased DJ-1 Enhance the Sensitivity of Pancreatic Cancer Cell to Adriamycin. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7696–7703. [Google Scholar] [PubMed]
- Sethy, C.; Kundu, C.N. 5-Fluorouracil (5-FU) Resistance and the New Strategy to Enhance the Sensitivity against Cancer: Implication of DNA Repair Inhibition. Biomed. Pharmacother. 2021, 137, 111285. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, T.; Ramachandran, V.; Fournier, K.F.; Wang, H.; Marquis, L.; Abbruzzese, J.L.; Gallick, G.E.; Logsdon, C.D.; McConkey, D.J.; Choi, W. Epithelial to Mesenchymal Transition Contributes to Drug Resistance in Pancreatic Cancer. Cancer Res. 2009, 69, 5820–5828. [Google Scholar] [CrossRef]
- Xiong, G.; Feng, M.; Yang, G.; Zheng, S.; Song, X.; Cao, Z.; You, L.; Zheng, L.; Hu, Y.; Zhang, T.; et al. The Underlying Mechanisms of Non-Coding RNAs in the Chemoresistance of Pancreatic Cancer. Cancer Lett. 2017, 397, 94–102. [Google Scholar] [CrossRef]
- Meng, Q.; Liang, C.; Hua, J.; Zhang, B.; Liu, J.; Zhang, Y.; Wei, M.; Yu, X.; Xu, J.; Shi, S. A MiR-146a-5p/TRAF6/NF-KB P65 Axis Regulates Pancreatic Cancer Chemoresistance: Functional Validation and Clinical Significance. Theranostics 2020, 10, 3967–3979. [Google Scholar] [CrossRef]
- Huang, B.; Wang, J.; Chen, Q.; Qu, C.; Zhang, J.; Chen, E.; Zhang, Y.; Wang, Y.; Ni, L.; Liang, T. Gemcitabine Enhances OSI-027 Cytotoxicity by Upregulation of MiR-663a in Pancreatic Ductal Adenocarcinoma Cells. Am. J. Transl. Res. 2019, 11, 473–485. [Google Scholar]
- Zhang, P.; Sun, Y.; Ma, L. ZEB1: At the Crossroads of Epithelial-Mesenchymal Transition, Metastasis and Therapy Resistance. Cell Cycle 2015, 14, 481–487. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Lin, Y.; Wang, X.; Cui, X.; Zhang, Z.; Xian, G.; Qin, C. Novel Crosstalk between KLF4 and ZEB1 Regulates Gemcitabine Resistance in Pancreatic Ductal Adenocarcinoma. Int. J. Oncol. 2017, 51, 1239–1248, Erratum in Int. J. Oncol. 2021, 85, 278–279. [Google Scholar] [CrossRef]
- Yang, R.-M.; Zhan, M.; Xu, S.-W.; Long, M.-M.; Yang, L.-H.; Chen, W.; Huang, S.; Liu, Q.; Zhou, J.; Zhu, J.; et al. MiR-3656 Expression Enhances the Chemosensitivity of Pancreatic Cancer to Gemcitabine through Modulation of the RHOF/EMT Axis. Cell Death Dis. 2017, 8, e3129. [Google Scholar] [CrossRef]
- Li, Y.; VandenBoom, T.G., II; Kong, D.; Wang, Z.; Ali, S.; Philip, P.A.; Sarkar, F.H. Up-Regulation of MiR-200 and Let-7 by Natural Agents Leads to the Reversal of Epithelial-to-Mesenchymal Transition in Gemcitabine-Resistant Pancreatic Cancer Cells. Cancer Res. 2009, 69, 6704–6712. [Google Scholar] [CrossRef]
- Yang, X.; Wang, W.; Zhang, X.; Zou, Q.; Cai, L.; Yu, B. Downregulation of MiR-183 Inhibits the Growth of PANC-1 Pancreatic Cancer Cells in Vitro and in Vivo, and Increases Chemosensitivity to 5-fluorouracil and Gemcitabine. Exp. Ther. Med. 2019, 17, 1697–1705. [Google Scholar] [CrossRef]
- Cai, B.; An, Y.; Lv, N.; Chen, J.; Tu, M.; Sun, J.; Wu, P.; Wei, J.; Jiang, K.; Miao, Y. MiRNA-181b Increases the Sensitivity of Pancreatic Ductal Adenocarcinoma Cells to Gemcitabine in Vitro and in Nude Mice by Targeting BCL-2. Oncol. Rep. 2013, 29, 1769–1776. [Google Scholar] [CrossRef]
- Chen, M.; Wang, M.; Xu, S.; Guo, X.; Jiang, J. Upregulation of MiR-181c Contributes to Chemoresistance in Pancreatic Cancer by Inactivating the Hippo Signaling Pathway. Oncotarget 2015, 6, 44466–44479. [Google Scholar] [CrossRef]
- Tu, M.-J.; Ho, P.Y.; Zhang, Q.-Y.; Jian, C.; Qiu, J.-X.; Kim, E.J.; Bold, R.J.; Gonzalez, F.J.; Bi, H.; Yu, A.-M. Bioengineered MiRNA-1291 Prodrug Therapy in Pancreatic Cancer Cells and Patient-Derived Xenograft Mouse Models. Cancer Lett. 2019, 442, 82–90. [Google Scholar] [CrossRef]
- Tian, X.; Shivapurkar, N.; Wu, Z.; Hwang, J.J.; Pishvaian, M.J.; Weiner, L.M.; Ley, L.; Zhou, D.; Zhi, X.; Wellstein, A.; et al. Circulating MicroRNA Profile Predicts Disease Progression in Patients Receiving Second-Line Treatment of Lapatinib and Capecitabine for Metastatic Pancreatic Cancer. Oncol. Lett. 2016, 11, 1645–1650. [Google Scholar] [CrossRef]
- Liu, P.; Liang, H.; Xia, Q.; Li, P.; Kong, H.; Lei, P.; Wang, S.; Tu, Z. Resveratrol Induces Apoptosis of Pancreatic Cancers Cells by Inhibiting MiR-21 Regulation of BCL-2 Expression. Clin. Transl. Oncol. 2013, 15, 741–746. [Google Scholar] [CrossRef]
- Petrelli, F.; Parisi, A.; Tomasello, G.; Mini, E.; Arru, M.; Russo, A.; Garrone, O.; Khakoo, S.; Ardito, R.; Ghidini, M. Comparison of Different Second Line Treatments for Metastatic Pancreatic Cancer: A Systematic Review and Network Meta-Analysis. BMC Gastroenterol. 2023, 23, 212. [Google Scholar] [CrossRef]
- Caparello, C.; Meijer, L.L.; Garajova, I.; Falcone, A.; Le Large, T.Y.; Funel, N.; Kazemier, G.; Peters, G.J.; Vasile, E.; Giovannetti, E. FOLFIRINOX and Translational Studies: Towards Personalized Therapy in Pancreatic Cancer. World J. Gastroenterol. 2016, 22, 6987–7005. [Google Scholar] [CrossRef]
- Quiñonero, F.; Mesas, C.; Doello, K.; Cabeza, L.; Perazzoli, G.; Jimenez-Luna, C.; Rama, A.R.; Melguizo, C.; Prados, J. The Challenge of Drug Resistance in Pancreatic Ductal Adenocarcinoma: A Current Overview. Cancer Biol. Med. 2019, 16, 688–699. [Google Scholar] [CrossRef]
- Sun, H.; Shi, K.; Qi, K.; Kong, H.; Zhang, J.; Dai, S.; Ye, W.; Deng, T.; He, Q.; Zhou, M. Natural Killer Cell-Derived Exosomal MiR-3607-3p Inhibits Pancreatic Cancer Progression by Targeting IL-26. Front. Immunol. 2019, 10, 2819. [Google Scholar] [CrossRef]
- Ghaleb, A.M.; Yang, V.W. Krüppel-like Factor 4 (KLF4): What We Currently Know. Gene 2017, 611, 27–37. [Google Scholar] [CrossRef]
- Li, Y.; Sarkar, F.H. MicroRNA Targeted Therapeutic Approach for Pancreatic Cancer. Int. J. Biol. Sci. 2016, 12, 326–337. [Google Scholar] [CrossRef]
- Su, Z.; Zhao, J.; Rong, Z.; Geng, W.; Wang, Z. MiR-451, a Potential Prognostic Biomarker and Tumor Suppressor for Gastric Cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 9154–9160. [Google Scholar]
- Cheng, R.-F.; Wang, J.; Zhang, J.-Y.; Sun, L.; Zhao, Y.-R.; Qiu, Z.-Q.; Sun, B.-C.; Sun, Y. MicroRNA-506 Is up-Regulated in the Development of Pancreatic Ductal Adenocarcinoma and Is Associated with Attenuated Disease Progression. Chin. J. Cancer 2016, 35, 64. [Google Scholar] [CrossRef]
- Lu, Y.; Ji, N.; Wei, W.; Sun, W.; Gong, X.; Wang, X. MiR-142 Modulates Human Pancreatic Cancer Proliferation and Invasion by Targeting Hypoxia-Inducible Factor 1 (HIF-1α) in the Tumor Microenvironments. Biol. Open 2017, 6, 252–259. [Google Scholar] [CrossRef]
- Fathi, M.; Ghafouri-Fard, S.; Abak, A.; Taheri, M. Emerging Roles of MiRNAs in the Development of Pancreatic Cancer. Biomed. Pharmacother. 2021, 141, 111914. [Google Scholar] [CrossRef]
- Wang, P.; Chen, L.; Zhang, J.; Chen, H.; Fan, J.; Wang, K.; Luo, J.; Chen, Z.; Meng, Z.; Liu, L. Methylation-Mediated Silencing of the MiR-124 Genes Facilitates Pancreatic Cancer Progression and Metastasis by Targeting Rac1. Oncogene 2014, 33, 514–524. [Google Scholar] [CrossRef]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes Facilitate Therapeutic Targeting of Oncogenic KRAS in Pancreatic Cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef]
- Xia, J.; Cao, T.; Ma, C.; Shi, Y.; Sun, Y.; Wang, Z.P.; Ma, J. MiR-7 Suppresses Tumor Progression by Directly Targeting MAP3K9 in Pancreatic Cancer. Mol. Ther. Nucleic Acids 2018, 13, 121–132. [Google Scholar] [CrossRef]
- Sureban, S.M.; May, R.; Qu, D.; Weygant, N.; Chandrakesan, P.; Ali, N.; Lightfoot, S.A.; Pantazis, P.; Rao, C.V.; Postier, R.G.; et al. DCLK1 Regulates Pluripotency and Angiogenic Factors via MicroRNA-Dependent Mechanisms in Pancreatic Cancer. PLoS ONE 2013, 8, e73940. [Google Scholar] [CrossRef]
- Liang, J.; Liu, Y.; Zhang, L.; Tan, J.; Li, E.; Li, F. Overexpression of MicroRNA-519d-3p Suppressed the Growth of Pancreatic Cancer Cells by Inhibiting Ribosomal Protein S15A-Mediated Wnt/β-Catenin Signaling. Chem. Biol. Interact. 2019, 304, 1–9. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- de Jong, O.G.; Kooijmans, S.A.A.; Murphy, D.E.; Jiang, L.; Evers, M.J.W.; Sluijter, J.P.G.; Vader, P.; Schiffelers, R.M. Drug Delivery with Extracellular Vesicles: From Imagination to Innovation. Acc. Chem. Res. 2019, 52, 1761–1770. [Google Scholar] [CrossRef]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as Therapeutic Drug Carriers and Delivery Vehicles across Biological Membranes: Current Perspectives and Future Challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef]
- Butreddy, A.; Kommineni, N.; Dudhipala, N. Exosomes as Naturally Occurring Vehicles for Delivery of Biopharmaceuticals: Insights from Drug Delivery to Clinical Perspectives. Nanomaterials 2021, 11, 1481. [Google Scholar] [CrossRef]
- Munir, J.; Yoon, J.K.; Ryu, S. Therapeutic MiRNA-Enriched Extracellular Vesicles: Current Approaches and Future Prospects. Cells 2020, 9, 2271. [Google Scholar] [CrossRef] [PubMed]
- de Castilla, P.E.M.; Tong, L.; Huang, C.; Sofias, A.M.; Pastorin, G.; Chen, X.; Storm, G.; Schiffelers, R.M.; Wang, J.-W. Extracellular Vesicles as a Drug Delivery System: A Systematic Review of Preclinical Studies. Adv. Drug Deliv. Rev. 2021, 175, 113801. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, W.; Chen, X.; Wang, Q.; Li, C.; Chen, Q.; Zhang, Y.; Lu, Y.; Ding, X.; Jiang, C. Bone Marrow Mesenchymal Stem Cells-Derived Exosomes for Penetrating and Targeted Chemotherapy of Pancreatic Cancer. Acta Pharm. Sin. B 2020, 10, 1563–1575. [Google Scholar] [CrossRef]
| miRNA | Expression Status | Source | Role of miRNA | Reference |
|---|---|---|---|---|
| miRNA-222 | Up-regulation | PDAC cells | Promote PDAC cell invasion. | [42] |
| miRNA-501-3p | Overexpression | PDAC cells | promote PC cell invasion, migration, and metastasis. | [43] |
| miRNA-5703 | Overexpression | Pancreatic stellate cells (PSCs) | Increase cell proliferation and migration. | [44] |
| miRNA-10a-5p | Overexpression | AsPC-1 and T3M4 cells | Enhance migration and invasion abilities. | [31] |
| miRNA-21 | Overexpression | PDAC cells | Increase proliferation. | [32] |
| miRNA-125b-5p | Overexpression | Pancreatic cancer cells (PC-1.0) | Increase invasion and metastasis. | [45] |
| miRNA-301a | Overexpression | PDAC cells | Increase cell proliferation and metastasis. | [34] |
| miRNA-222 | Overexpression | Pancreatic cancer cells | Promote invasion and metastasis. | [46] |
| miRNA-501-3p | Overexpression | TAMs cell | Enhance tumorigenesis and metastasis. | [36] |
| S.No. | Cell Line/Number of Patients | Up-Regulated miRNA | Down-Regulated miRNA | Role of miRNA | Sources | Reference |
|---|---|---|---|---|---|---|
| 1. | Patients samples 29 | miR-10b, miR-21, miR-30c, and miR-181a | miR-let7a | Differentiate PDAC from Healthy. | [72] | |
| 2. | Only cell lines such as MiaPaca-2, Panc-1, and BxPC3 | miR-21, miR-155, miR-221 | miR-126 | Differentiate between (PDAC) and normal pancreatic ductal epithelial cells. | Cell line | [75] |
| 3. | Cell lines such as MIA PaCa-2, ANC-1, YAPC, and BxPC-3/Patients samples 15 | miR-27a-3p, miR-221-3p, miR-23b-3p | miR-155-5p, let-7a-5p, miR-193a-3p | Predict poor prognosis of PDAC. | Serum/Plasma | [76] |
| 4. | miR-30a-3p, miR-105 | - | Predict better prognosis in PDAC patients. | PDAC specimens | [65] | |
| 5. | Patients samples 20 | miR-106a, miR-18a | - | Significantly discriminated in PDAC patients and healthy. | PDAC plasma | [77] |
| 6. | Patients samples 90 | miR-142-5p and miR-204 | Associated with better overall survival in pancreatic cancer. | Pancreatic tissue | [78] | |
| 7. | Patients samples 225 | miR-212 and miR-675 | miR-148a, miR-187, and Let-7g | Predict overall survival. | Pancreatic tissue | [66] |
| 8. | miR-181b and miR-210 | Discriminated PCa from normal individuals. | stool sample | [79] | ||
| 9. | Cell line such as SW-1990, Panc-1, Miapaca-2 and Bxpc-3/Patients samples 21 | miR-221/222 | - | Differentiate between pancreatic ductal adenocarcinoma (PDAC) tissues and adjacent normal pancreatic tissues, as well as between invasive and nonaggressive pancreatic cancer cell lines. | Pancreatic tissue and cell line | [80] |
| 10. | Only cell lines such as Aspc-1, BxPC-3 | miR-301a-3p | Worse survival. | PDAC specimens and cell line | [81] | |
| 11. | Only Patients samples 69 | 21-5p, miR-485-3p, miR-708-5p, and miR-375 | Distinguished PDAC from IPMN with a sensitivity and specificity of 95% and 85%, respectively. | FFPE pancreatic specimens | [82] | |
| 12. | Only Patients samples 90 | miR-429 | Significantly discriminated in PDAC patients and healthy. | PDAC plasma | [83] | |
| 13. | Only Patients samples 56 | miR-451a | Prediction of recurrence and prognosis in PDAC patients. | PDAC plasma | [64] | |
| 14. | Only Patients samples 457 | miR-486-5p and miR-938 | Differentiate PDAC from healthy and chronic pancreatitis. | Plasma samples | [84] | |
| 15. | Only Patients samples 131 | miR-1246, miR-4644, miR-3976, miR-4306 | Differentiate PDAC from healthy. | serum-exosomes and exosome-depleted serum | [71] |
| MiRNA | Patients/Cell Line | Expression | Function | Reference |
|---|---|---|---|---|
| miRNA-663a | Panc-1, BxPC-3, T3-M4, MIApaca-2 | Overexpression | Overcome drug resistance to gemcitabine | [111] |
| miRNA-200b,miRNA200c and let-7 | MiaPaCa-2, Panc-1, and Aspc-1 | Up-regulation | Increase sensitivity to gemcitabine | [115] |
| miR-183 | H6C7 | Down-regulation | Increase sensitivity to 5-fluorouracil and gemcitabine | [116] |
| miR-3656, miR-509-5p, and miR-1243 | No.of patients 157/FFPE(Pancreatic tissue) | Up-regulation | Increase sensitivity to gemcitabine | [114] |
| miRNA-181b | PDAC SW1990 and CFPAC-1 | Up-regulation | Overcome drug resistance to gemcitabine | [117] |
| miRNA-181c | 124 FFPE(Pancreatic tissue)/PANC-1 and BXPC3 | Overexpression | Increase resistance to paclitaxel | [118] |
| miRNA-1291 | PANC-1 xenograft and three different PC patient-derived xenograft (PDX) tumor mouse models | Increases sensitivity to gemcitabine-nab-paclitaxel combination therapy | [119] | |
| miR-221 and miR-210 | 21/PANC-1, MIA PaCa-2, BXCP-3 | Overexpression | Increase resistance to Lapatinib | [120] |
| miRNA | Patients/Cell Line | Expression | Target | Function | Reference |
|---|---|---|---|---|---|
| miR-3607-3p | 40/Mia PaCa-2 and PANC-1 | Overexpression | Interleukin-26 (IL-26) in PC | Inhibit proliferation, migration, and invasion | [125] |
| miRNA-1231 | BxPC-3 and MIA PaCa-2 | Overexpression | Suppress aggressiveness of pancreatic cancer cells | [30] | |
| miRNA-145 | AsPC-1 | OCT4, SOX2, NANOG, and KLF4 | Decrease expression ITGA11, MAGEA4, SET, RPA1, MCM2, ABCC1, SPTBN1, and SPTLC. Inhibit pancreas carcinogenesis | [135] | |
| miRNA-155 | RInk-1 | Knock down | EGFR, MT1-MMP, and K-ras | Reduce pancreatic cancer | [127] |
| miRNA-506 | 113 | Up-regulation | Reduce disease progression | [129] | |
| miRNA-142 | 42/PANC-1, SW1990, Hup, CFPAC-1 and a normal cell line HPC-Y5 | Overexpression | HIF-1α | Inhibit and consequently reduce invasion and proliferation | [130] |
| miRNA-519d-3p | AsPC-1, BxPC3, PANC-1,SW1990, and HPNE | Up-regulation | RPS15A | Inhibit proliferation | [136] |
| miRNA-124 | Overexpression | Rac1 | Inhibit cell proliferation | [132] | |
| miRNA-7 | BxPC-3, PANC-1, and Patu-8988 cells, and HEK293T | Overexpression | MAP3K9 | Inhibit cell proliferation | [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwari, P.K.; Shanmugam, P.; Karn, V.; Gupta, S.; Mishra, R.; Rustagi, S.; Chouhan, M.; Verma, D.; Jha, N.K.; Kumar, S. Extracellular Vesicular miRNA in Pancreatic Cancer: From Lab to Therapy. Cancers 2024, 16, 2179. https://doi.org/10.3390/cancers16122179
Tiwari PK, Shanmugam P, Karn V, Gupta S, Mishra R, Rustagi S, Chouhan M, Verma D, Jha NK, Kumar S. Extracellular Vesicular miRNA in Pancreatic Cancer: From Lab to Therapy. Cancers. 2024; 16(12):2179. https://doi.org/10.3390/cancers16122179
Chicago/Turabian StyleTiwari, Prashant Kumar, Poojhaa Shanmugam, Vamika Karn, Saurabh Gupta, Richa Mishra, Sarvesh Rustagi, Mandeep Chouhan, Devvret Verma, Niraj Kumar Jha, and Sanjay Kumar. 2024. "Extracellular Vesicular miRNA in Pancreatic Cancer: From Lab to Therapy" Cancers 16, no. 12: 2179. https://doi.org/10.3390/cancers16122179
APA StyleTiwari, P. K., Shanmugam, P., Karn, V., Gupta, S., Mishra, R., Rustagi, S., Chouhan, M., Verma, D., Jha, N. K., & Kumar, S. (2024). Extracellular Vesicular miRNA in Pancreatic Cancer: From Lab to Therapy. Cancers, 16(12), 2179. https://doi.org/10.3390/cancers16122179








