Comparison of the Learning Curve between Uniportal and Robotic Thoracoscopic Approaches in Pulmonary Segmentectomy during the Implementation Period Using Cumulative Sum Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Surgical Procedures
2.2. Postoperative Treatment
2.3. Evaluation of the Learning Curve
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics and Perioperative Outcomes
3.2. Distributions of the Performed Segmentectomies in Both Groups
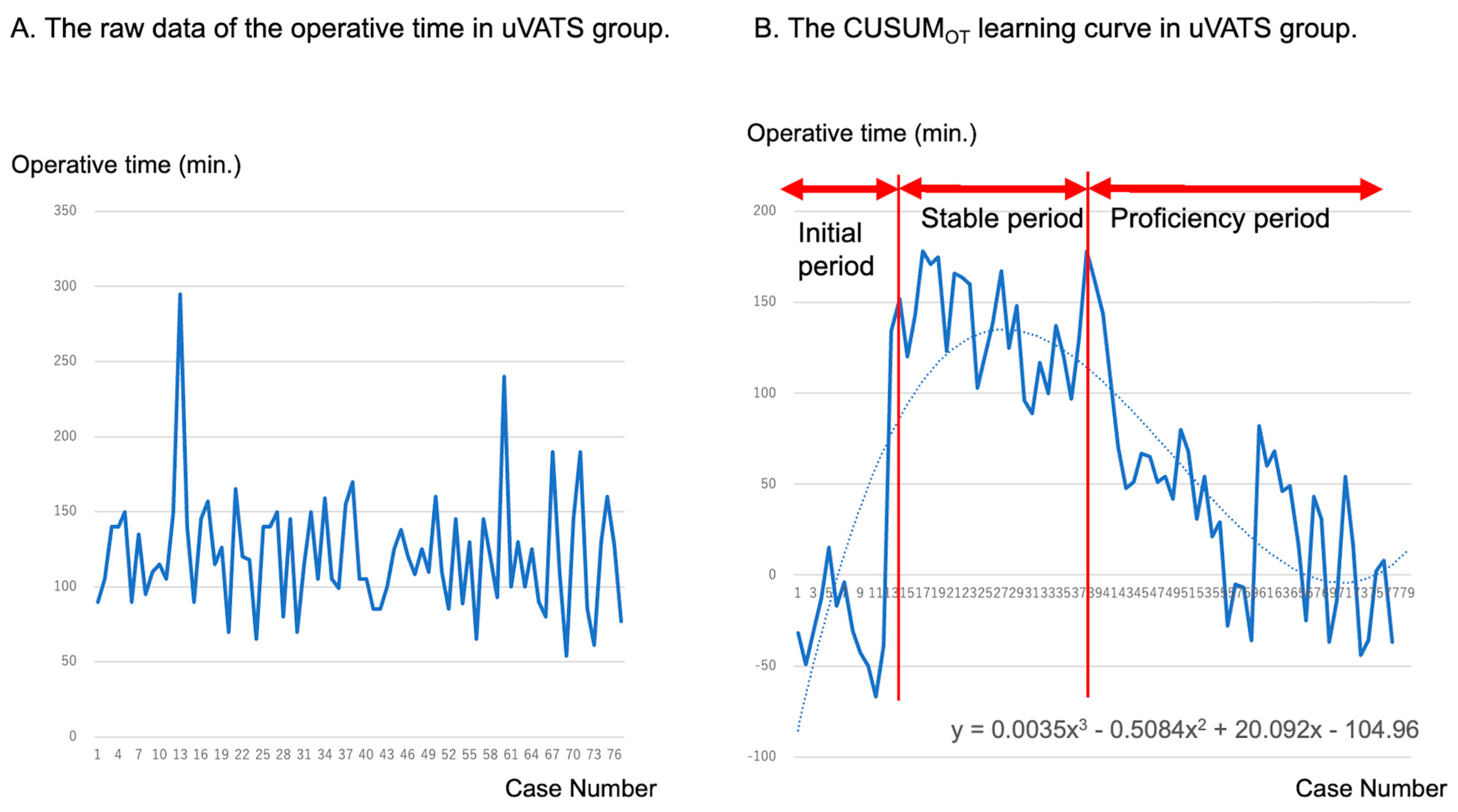
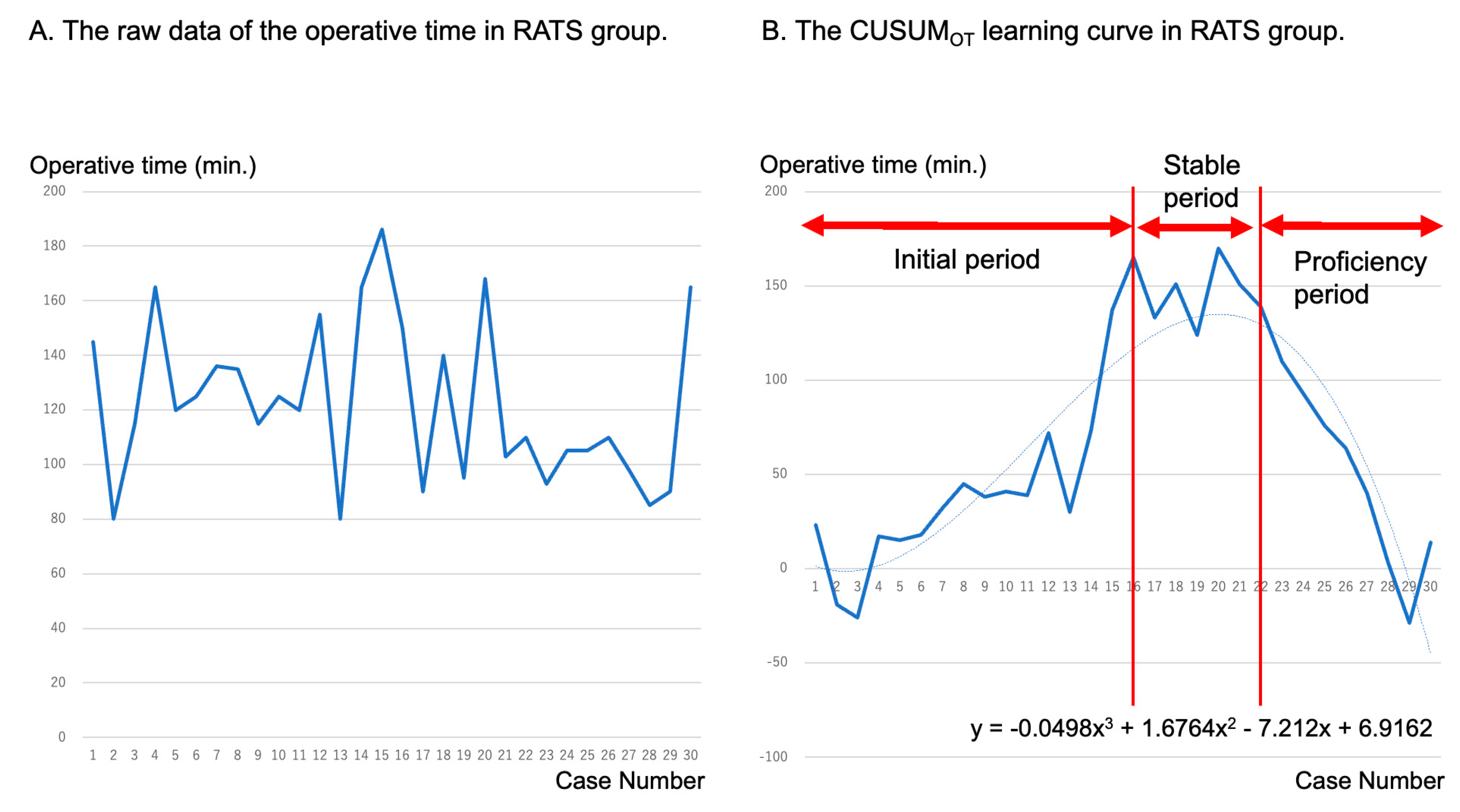
3.3. Learning Curves
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rocco, G.; Martin-Ucar, A.; Passera, E. Uniportal VATS wedge pulmonary resections. Ann. Thorac. Surg. 2004, 77, 726–728. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.; Paradela, M.; Garcia, J.; Dele Torre, M. Single-port video-assisted thoracoscopic lobectomy. Interact. Cardiovasc. Thorac. Surg. 2011, 12, 514–515. [Google Scholar] [CrossRef] [PubMed]
- Al-Ameri, M.; Sachs, E.; Sartipy, U.; Jackson, V. Uniportal versus multiportal video-assisted thoracic surgery for lung cancer. J. Thorac. Dis. 2019, 11, 5152–5161. [Google Scholar] [CrossRef] [PubMed]
- Bourdages-Pageau, E.; Vieira, A.; Lacasse, Y.; Figueroa, P.U. Outcomes of uniportal vs multiportal video-assisted thoracoscopic lobectomy. Semin. Thorac. Cardiovasc. Surg. 2020, 32, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Hirai, K.; Takeuchi, S.; Usuda, J. Single-incision thoracoscopic surgery and conventional video-assisted thoracoscopic surgery: A retrospective comparative study of perioperative clinical outcomes. Eur. J. Cardiothorac. Surg. 2016, 49, i37–i41. [Google Scholar] [CrossRef] [PubMed]
- Melfi, F.M.; Menconi, G.F.; Mariani, A.M.; Angeletti, C.A. Early experience with robotic technology for thoracocopic surgery. Eur. J. Cardiothorac. Surg. 2002, 64, 354–362. [Google Scholar]
- Kent, M.; Wang, T.; Whyte, R.; Curran, T.; Flores, R.; Gangadharan, S. Open, video-assisted thoracic surgery, and robotic lobectomy: Review of a national database. Ann. Thorac. Surg. 2014, 97, 236–242; discussion 242–244. [Google Scholar] [CrossRef]
- Oh, D.S.; Reddy, R.M.; Gorrepati, M.L.; Mehendale, S.; Reed, M.F. Robotic-assisted, video-assisted thoracoscopic and open lobectomy: Propensity matched analysis of recent premier data. Ann. Thorac. Surg. 2017, 104, 1733–1740. [Google Scholar] [CrossRef]
- Lee, B.E.; Korst, R.J.; Kletsman, E.; Rutledge, J.R. Transitioning from video-assisted thoracic surgical lobectomy to robotics for lung cancer: Are there outcomes advantages? J. Thorac. Cardiovasc. Surg. 2014, 147, 724–729. [Google Scholar] [CrossRef][Green Version]
- Saji, H.; Okada, M.; Tsuboi, M.; Nakajima, R.; Suzuki, K.; Aokage, K.; Aoki, T.; Okami, J.; Yoshino, I.; Ito, H.; et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJCOG4607L): A multicentre, open-label, phase 3, randomized, controlled, non-inferiority trial. Lancet 2022, 399, 1607–1617. [Google Scholar] [CrossRef]
- Altorki, N.; Wang, X.; Kozono, D.; Watt, C.; Landrenau, R.; Wigle, D.; Port, J.; Jones, D.R.; Conti, M.; Ashrafi, A.S.; et al. Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 388, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Oizumi, H.; Kanauchi, N.; Kato, H.; Endoh, M.; Takeda, S.; Suzuki, J.; Fukaya, K.; Sadahiro, M. Total thoracoscopic pulmonary segmentectomy. Eur. J. Cardiothorac. Surg. 2009, 36, 374–377; discussion 377. [Google Scholar] [CrossRef] [PubMed]
- Igai, H.; Matsuura, N.; Numajiri, K.; Ohsawa, F.; Kamiyoshihara, M. Early chest drain removal on the day of uniportal thoracoscopic segmentectomy. Gen. Thorac. Cardiovasc. Surg. 2023, 71, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Strasberg, S.M. Severity grading of surgical complications. Ann. Surg. 2009, 250, 197–198. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Shi, Y.; Qian, F.; Tang, B.; Hao, Y.; Zhao, Y.; Yu, P. Cumulative summation analysis of learning curve for robot-assisted gastrectomy in gastric cancer. J. Surg. Oncol. 2015, 111, 760–767. [Google Scholar] [CrossRef]
- Cela, V.; Freschi, L.; Simi, G.; Ruggiero, M.; Tana, R.; Pluchino, N. Robotic single-site hysterectomy: Feasibility, learning curve and surgical outcome. Surg. Endos. 2013, 27, 2638–2643. [Google Scholar] [CrossRef]
- Yang, S.; Guo, W.; Chen, X.; Wu, H.; Li, H. Early outcomes of robotic versus uniportal video-assisted thoracic surgery for lung cancer: A propensity score-matched study. Eur. J. Cardiothorac. Surg. 2018, 53, 348–352. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, L.; Lu, H.; Ma, A.; Wang, G. Short-Term Surgical Outcomes for Lobectomy Between Robot-Assisted Thoracic Surgery and Uniportal Video-Assisted Thoracoscopic Surgery. Front. Oncol. 2022, 12, 914059. [Google Scholar] [CrossRef]
- Chen, L.; Shen, Y.; Duan, S.; Jin, X.; Wang, Y.; Sang, Y.; Chen, Y. Learning curve for uniportal video-assisted thoracoscopic anatomical segmentectomy. Ann. Transl. Med. 2022, 10, 12. [Google Scholar] [CrossRef]
- Li, S.; Wu, J.; Wan, Z.; Chen, Y.; She, Y.; Xie, D.; Hu, X.; Zhao, D.; Chen, C. The learning curve for uniportal video-assisted thoracoscopic anatomical segmentectomy. J. Surg. Oncol. 2021, 124, 441–452. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, Z.; Feng, H.; Wen, H.; Su, K.; Xiao, F.; Liang, C.; Liu, D. Uniportal video-assisted anatomical segmentectomy: An analysis of the learning curve. World J. Surg. Oncol. 2023, 21, 232. [Google Scholar] [CrossRef] [PubMed]
- Drevet, G.; Ugalde Figueroa, P. Uniportal video-assisted thoracoscopic surgery: Safety, efficacy and learning curve during the first 250 cases in Quebec, Canada. Ann. Cardiothorac. Surg. 2016, 5, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Le Gac, C.; Gondé, H.; Gillibert, A.; Laurent, M.; Selim, J.; Bottet, B.; Varin, R.; Baste, J.M. Medico-economic impact of robot-assisted lung segmentectomy: What is the cost of the learning curve? Interact. Cardiovasc. Thorac. Surg. 2020, 30, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, S.; Han, Y.; Xiang, J.; Cerfolio, R.J.; Li, H. Robotic Anatomical Segmentectomy: An Analysis of the Learning Curve. Ann. Thorac. Surg. 2019, 107, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.Z.; Tan, Z.H.; Abbas, A.E.; Li, J.B.; Xie, C.L.; Long, H.; Zhang, L.J.; Fu, J.H.; Lin, P.; Yang, H.X. Defining the learning curve of robotic portal segmentectomy in small pulmonary lesions: A prospective observational study. J. Robot. Surg. 2023, 17, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, G.; Novellis, P.; Perroni, G. Overview of the outcomes of robotic segmentectomy and lobectomy. J. Thorac. Dis. 2021, 13, 6155–6162. [Google Scholar] [CrossRef] [PubMed]
- Power, A.D.; D’Souza, D.M.; Moffatt-Bruce, S.D.; Merritt, R.E.; Kneuertz, P.J. Defining the learning curve of robotic thoracic surgery: What does it take? Surg. Endosc. 2019, 33, 3880–3888. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Abdel-Rasoul, M.; Hekmat, R.; Merritt, R.E.; D’Souza, D.M.; Jackson, G.P.; Kneuertz, P.J. National learning curves among robotic thoracic surgeons in the United States: Quantifying the impact of procedural experience on efficiency and productivity gains. J. Thorac. Cardiovasc. Surg. 2023, in press. [Google Scholar] [CrossRef]
- Li, W.H.; Cheng, H.; Gan, X.F.; Li, X.J.; Wang, X.J.; Wu, X.W.; Zhong, H.C.; Wu, T.C.; Huo, W.W.; Ju, S.L.; et al. Learning curve of uniportal video-assisted thoracoscopic lobectomy: An analysis of the proficiency of 538 cases from a single centre. Interact. Cardiovasc. Thorac. Surg. 2022, 34, 799–807. [Google Scholar] [CrossRef]
- Gonzalez-Rivas, D.; Sihoe, A.D.L. Important Technical Details During Uniportal Video-Assisted Thoracoscopic Major Resections. Thorac. Surg. Clin. 2017, 27, 357–372. [Google Scholar] [CrossRef]
- Igai, H.; Furusawa, S.; Ohsawa, F.; Yazawa, T.; Matsuura, N.; Kamiyoshihara, M. Application of “suction-guided stapling” during uniportal thoracoscopic major lung resection. Gen. Thorac. Cardiovasc. Surg. 2022, 70, 204–205. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.E.; Ilonen, I.K.; Pälli, O.H.; Salo, J.A.; Räsänen, J.V. Learning curve in robotic-assisted lobectomy for non-small cell lung cancer is not steep after experience in video-assisted lobectomy; single-surgeon experience using cumulative sum analysis. Cancer Treat. Res. Commun. 2021, 27, 100362. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Hernández, M.T.; Fuentes, M.G.; Novoa, N.M.; Rodríguez, I.; Varela, G.; Jiménez, M.F. The robotic surgery learning curve of a surgeon experienced in video-assisted thoracoscopic surgery compared with his own video-assisted thoracoscopic surgery learning curve for anatomical lung resections. Eur. J. Cardiothorac. Surg. 2022, 61, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Saji, H.; Aokage, K.; Watanabe, S.I.; Okada, M.; Mizusawa, J.; Nakajima, R.; Tsuboi, M.; Nakamura, S.; Nakamura, K.; et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J. Thorac. Cardiovasc. Surg. 2019, 158, 895–907. [Google Scholar] [CrossRef]


| Variable | uVATS Group (n = 77) | RATS Group (n = 30) | p-Value |
|---|---|---|---|
| Age, years, median (IQR) | 70 (66–77) | 77 (62–79) | 0.59 |
| Sex | 1 | ||
| Female/male, n (%) | 38 (49.4)/39 (50.6) | 15 (50)/15 (50) | |
| Treated lobe | 0.18 | ||
| LUL, n (%) | 21 (27.3) | 15 (50) | |
| LLL, n (%) | 12 (15.6) | 4 (13.3) | |
| RUL, n (%) | 24 (31.2) | 6 (20) | |
| RML, n (%) | 0 (0) | 0 (0) | |
| RLL, n (%) | 20 (26) | 5 (16.7) | |
| ASA score, median (IQR) | 2 (2–2) | 2 (2–3) | 0.23 |
| Smoking index, pack-years, | 15 | 3 | 0.56 |
| median (IQR) | (0–46) | (0–39) | |
| Preoperative FEV1.0, mL, | 1890 | 1915 | 0.89 |
| median (IQR) | (1628–2390) | (1353–2808) | |
| Preoperative %FEV1.0, %, | 92 | 87 | 0.65 |
| median (IQR) | (77–103) | (69–107) | |
| Disease | 0.0084 | ||
| Primary lung cancer, n (%) | 52 (67.5) | 19 (63.3) | |
| Pulmonary metastasis, n (%) | 9 (11.7) | 9 (30) | |
| Other malignancy, n (%) | 0 (0) | 1 (3.3) | |
| Other benign, n (%) | 16 (20.8) | 1 (3.3) | |
| Type of segmentectomy | 0.96 | ||
| Intentional, n (%) | 36 (46.8) | 14 (46.7) | |
| Unintentional, n (%) | 18 (23.4) | 6 (20) | |
| Others, n (%) | 23 (29.9) | 10 (33.3) | |
| Operative time, minutes, median (IQR) | 118 (95–145) | 118 (99–144) | 0.72 |
| Blood loss, gram, median (IQR) | 0 (0–30) | 0 (0–0) | 0.22 |
| Postoperative drainage time, days, median (IQR) | 1 (1–1) | 0 (0–1) | <0.001 |
| Postoperative hospitalization time, days, median (IQR) | 2 (2–3) | 2 (1–3) | 0.0018 |
| Morbidity (Clavien–Dindo classification grade ≥ 3), n (%) | 2 (2.6) | 0 (0) | 1 |
| Readmission within 30 days after surgery, n (%) | 0 (0) | 0 (0) | — |
| Conversion to thoracotomy, n (%) | 2 (2.6) | 0 (0) | 1 |
| 30-day mortality, n (%) | 0 (0) | 0 (0) | — |
| 90-day mortality, n (%) | 0 (0) | 0 (0) | — |
| Variable | uVATS Group (n = 26) | RATS Group (n = 30) | p-Value |
|---|---|---|---|
| Postoperative drainage time, days, median (IQR) | 1 (0–1) | 0 (0–1) | 0.27 |
| Postoperative hospitalization time, days, median (IQR) | 2 (2–2) | 2 (1–3) | 0.45 |
| Variable | uVATS Group (n = 77) | RATS Group (n = 30) | p-Value |
|---|---|---|---|
| Simple/complex, n (%) | 35 (45.5)/42 (54.5) | 10 (33.3)/20 (66.7) | 0.28 |
| LUL | |||
| S1–3 | 12 | 3 | |
| S1 + 2 | 3 | 8 | |
| S1 + 2c | 0 | 1 | |
| S3 | 2 | 3 | |
| S4–5 | 4 | 0 | |
| LLL | |||
| S6 | 6 | 2 | |
| S8 | 3 | 0 | |
| S9 | 0 | 1 | |
| S9–10 | 2 | 0 | |
| S10 | 0 | 1 | |
| RUL | |||
| S1 | 7 | 2 | |
| S1 + 3 | 2 | 0 | |
| S2 | 9 | 3 | |
| S2 + S6a | 1 | 0 | |
| S3 | 5 | 1 | |
| RLL | |||
| S6 | 7 | 3 | |
| S7–8 | 1 | 0 | |
| S7–10 | 6 | 2 | |
| S8 | 1 | 0 | |
| S9–10 | 6 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Igai, H.; Numajiri, K.; Ohsawa, F.; Nii, K.; Kamiyoshihara, M. Comparison of the Learning Curve between Uniportal and Robotic Thoracoscopic Approaches in Pulmonary Segmentectomy during the Implementation Period Using Cumulative Sum Analysis. Cancers 2024, 16, 184. https://doi.org/10.3390/cancers16010184
Igai H, Numajiri K, Ohsawa F, Nii K, Kamiyoshihara M. Comparison of the Learning Curve between Uniportal and Robotic Thoracoscopic Approaches in Pulmonary Segmentectomy during the Implementation Period Using Cumulative Sum Analysis. Cancers. 2024; 16(1):184. https://doi.org/10.3390/cancers16010184
Chicago/Turabian StyleIgai, Hitoshi, Kazuki Numajiri, Fumi Ohsawa, Kazuhito Nii, and Mitsuhiro Kamiyoshihara. 2024. "Comparison of the Learning Curve between Uniportal and Robotic Thoracoscopic Approaches in Pulmonary Segmentectomy during the Implementation Period Using Cumulative Sum Analysis" Cancers 16, no. 1: 184. https://doi.org/10.3390/cancers16010184
APA StyleIgai, H., Numajiri, K., Ohsawa, F., Nii, K., & Kamiyoshihara, M. (2024). Comparison of the Learning Curve between Uniportal and Robotic Thoracoscopic Approaches in Pulmonary Segmentectomy during the Implementation Period Using Cumulative Sum Analysis. Cancers, 16(1), 184. https://doi.org/10.3390/cancers16010184






