Implementing Multifactorial Risk Assessment with Polygenic Risk Scores for Personalized Breast Cancer Screening in the Population Setting: Challenges and Opportunities
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
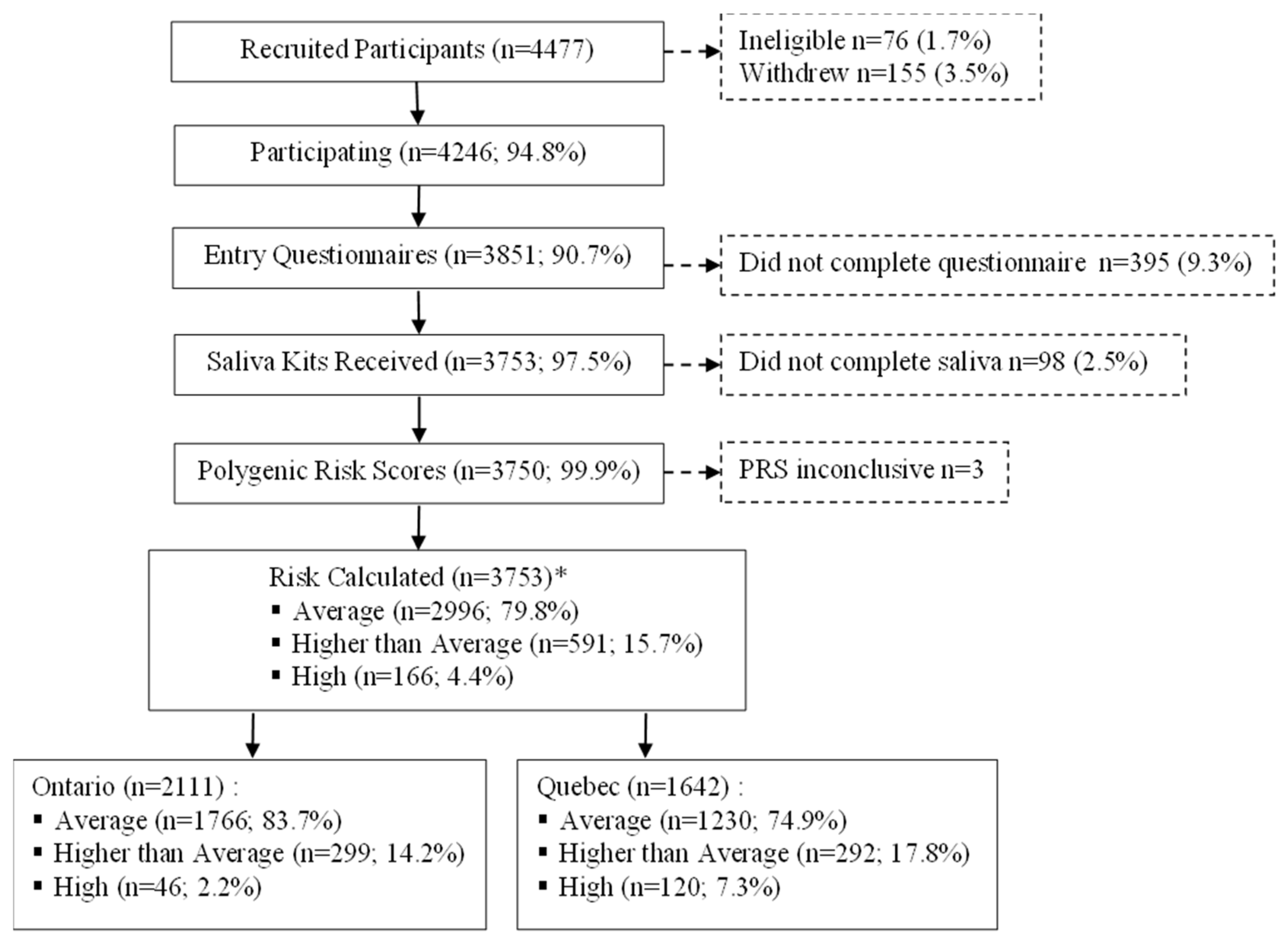
2.2. Study Population and Recruitment
2.3. Data Collection
2.3.1. Questionnaires
2.3.2. Saliva Samples and Polygenic Risk Scores
2.3.3. Breast Density
2.4. Breast Cancer Risk Estimation
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Supplementary Methods
Appendix A.1.1. Alcohol Intake
| Type of Drink | Strength (ABV %) | Volume (mL) |
| Bottle of beer, cider or cooler | 5 | 341 |
| Pint of cider or beer | 4 | 586 |
| Glass of wine | 11 | 142 |
| Shot of liquor | 40 | 43 |
- (a)
- Calculate the units: strength (ABV) × volume (mL)/1000 = units
- 11 × 142/1000 = 1.562 units
- (b)
- Calculate the grams of alcohol: assume that 1 unit = 8 g of alcohol
- 1.562 × 8 = 12.5 g
- (c)
- Calculate grams per day based on the reported number of drinks and frequency (daily, weekly, monthly)
- a.
- Daily = 12.5 g
- b.
- Weekly (divide by 7) = 1.8 g/day
- c.
- Monthly (divide by 30) = 0.4 g/day
Appendix A.1.2. Calculation of Standardized Difference
| Characteristics | PERSPECTIVE I&I Participants N = 3753 | CanPath Ontario– Quebec Participants * N = 72,314 | Standardized Difference (%) | CanPath Canada Participants * N = 114,599 | Standardized Difference (%) |
|---|---|---|---|---|---|
| N (%) † | N (%) † | N (%) † | |||
| Age at study entry (years) | |||||
| 40–49 | 544 (14.5) | 26,949 (37.3) | −53.9 | 38,773 (33.8) | −46.3 |
| 50–59 | 1600 (42.6) | 28,657 (39.6) | 6.1 | 45,673 (39.9) | 5.5 |
| 60–69 | 1609 (42.9) | 16,708 (23.1) | 43.1 | 30,153 (26.3) | 35.4 |
| Born in Canada | |||||
| Yes | 3191 (85.5) | 55,040 (81.8) | 10.0 | 88,326 (82.6) | 7.9 |
| No | 540 (14.5) | 12,266 (18.2) | −10.0 | 18,555 (17.4) | −7.9 |
| Missing | 22 | 5008 | 7718 | ||
| Visible minority | |||||
| Not a visible minority ‡ | 3416 (92.8) | 56,393 (88.9) | 13.6 | 89,798 (90.8) | 7.3 |
| Visible minority | 264 (7.2) | 7057 (11.1) | −13.6 | 9058 (9.2) | −7.3 |
| Do not know/missing | 73 | 8864 | 15,743 | ||
| Race/ethnicity | |||||
| Black | 35 (1.0) | 817 (1.3) | −2.8 | 912 (0.9) | 1.0 |
| East Asian | 92 (2.5) | 1730 (2.7) | −1.7 | 2451 (2.5) | 0.0 |
| Indigenous | 43 (1.2) | 1645 (2.6) | −10.3 | 2801 (2.8) | −11.5 |
| Latin American/Hispanic | 41 (1.1) | 414 (0.7) | 4.2 | 544 (0.6) | 5.5 |
| Arab | 5 (0.1) | 326 (0.5) | −7.3 | 360 (0.4) | −6.0 |
| West Asian | 11 (0.3) | 93 (0.1) | 4.5 | 157 (0.2) | 2.0 |
| South Asian | 24 (0.7) | 801 (1.3) | −6.0 | 979 (1.0) | −3.3 |
| Southeast Asian | 30 (0.8) | 440 (0.7) | 1.2 | 610 (0.6) | 2.4 |
| White | 3361 (91.3) | 54,577 (86.0) | 16.8 | 86,718 (87.7) | 11.8 |
| Other/Mixed § | 38 (1.0) | 2607 (4.1) | −19.8 | 3324 (3.4) | −16.4 |
| Do not know/missing | 73 | 8864 | 15,743 | ||
| Marital status | |||||
| Married/common law | 2791 (75.1) | 49,901 (69.2) | 13.2 | 79,149 (69.8) | 11.9 |
| Single/widowed/divorced/separated | 925 (24.9) | 22,237 (30.8) | −13.2 | 34,194 (30.2) | −11.9 |
| Missing | 37 | 176 | 1256 | ||
| Highest level of education | |||||
| University Bachelor’s degree or above | 1904 (51.2) | 26,409 (36.9) | 29.1 | 41,664 (36.7) | 29.5 |
| College/Registered Apprenticeship/trades certificate | 1336 (35.9) | 28,715 (40.1) | −8.7 | 45,681 (40.3) | −9.1 |
| High school diploma or below | 479 (12.9) | 16,424 (23.0) | −26.6 | 26,034 (23.0) | −26.6 |
| Prefer not to answer/missing | 34 | 766 | 1220 | ||
| Employment status ** | |||||
| Employed | 2277 (61.0) | 44,248 (63.2) | −4.5 | 70,001 (63.1) | −4.3 |
| Not employed/retired | 1458 (39.0) | 25,808 (36.8) | 4.5 | 40,979 (36.9) | 4.3 |
| Prefer not to answer/missing | 18 | 2258 | 3619 |
| Risk Factors | PERSPECTIVE I&I Participants N = 3753 | CanPath Ontario–Quebec Participants * N = 72,314 | Standardized Difference (%) | CanPath Canada Participants * N = 114,599 | Standardized Difference (%) |
|---|---|---|---|---|---|
| N (%) † | N (%) † | N (%) † | |||
| Height (cm) | |||||
| ≤152.90 | 208 (5.6) | 2907 (5.9) | −1.3 | 4073 (5.8) | −0.9 |
| 152.91–159.64 | 711 (19.0) | 9205 (18.6) | 1.0 | 13,757 (19.6) | −1.5 |
| 159.65–165.95 | 1614 (43.1) | 20,174 (40.9) | 4.5 | 28,339 (40.4) | 5.5 |
| 165.96–172.69 | 842 (22.5) | 11,112 (22.5) | 0.0 | 16,047 (22.9) | −1.0 |
| ≥172.70 | 372 (9.9) | 5986 (12.1) | −7.0 | 7851 (11.2) | −4.2 |
| Missing ‡ | 6 | 22,930 | 44,532 | ||
| BMI (kg/m2) § | |||||
| <18.5 | 60 (1.6) | 649 (1.5) | 0.8 | 899 (1.4) | 1.7 |
| 18.5–<25 | 1673 (44.7) | 18,530 (42.6) | 4.2 | 27,170 (42.7) | 4.0 |
| 25–<30 | 1128 (30.2) | 13,403 (30.8) | −1.3 | 19,758 (31.1) | −2.0 |
| ≥30 | 878 (23.5) | 10,948 (25.2) | −4.0 | 15,756 (24.8) | −3.0 |
| Missing | 14 | 28,784 | 51,016 | ||
| Age at menarche (years) | |||||
| <11 | 198 (5.5) | 4282 (6.4) | −3.8 | 6241 (5.8) | −1.3 |
| 11 | 479 (13.2) | 9223 (13.8) | −1.8 | 14,365 (13.5) | −0.9 |
| 12 | 1004 (27.6) | 17,718 (26.6) | 2.3 | 28,667 (26.8) | 1.8 |
| 13 | 1011 (27.8) | 18,105 (27.2) | 1.3 | 29,803 (27.9) | −0.2 |
| 14 | 557 (15.3) | 9872 (14.8) | 1.4 | 15,771 (14.8) | 1.4 |
| 15 | 219 (6.0) | 4150 (6.2) | −0.8 | 6872 (6.4) | −1.7 |
| >15 | 164 (4.5) | 3261 (4.9) | −1.9 | 5050 (4.7) | −1.0 |
| Missing ** | 121 | 5703 | 7830 | ||
| Age at menopause (years) †† | |||||
| <40 | 185 (7.1) | 4407 (12.3) | −17.6 | 7597 (12.6) | −18.5 |
| 40–44 | 204 (7.9) | 4267 (11.9) | −13.4 | 6977 (11.6) | −12.5 |
| 45–49 | 508 (19.6) | 8778 (24.5) | −11.8 | 14,354 (23.8) | −10.2 |
| 50–54 | 1239 (47.8) | 14,248 (39.8) | 16.2 | 24,153 (40.1) | 15.6 |
| ≥55 | 456 (17.6) | 4091 (11.4) | 17.7 | 7104 (11.8) | 16.4 |
| Missing ‡‡ | 260 | 2737 | 4511 | ||
| Oral contraceptive use | |||||
| Never | 377 (10.2) | 9758 (14.0) | −11.7 | 13,869 (12.5) | −7.3 |
| Ever §§ | 3322(89.8) | 59,860 (86.0) | 11.7 | 97,392 (87.5) | 7.3 |
| Missing | 54 | 2696 | 3338 | ||
| Menopausal hormone therapy use †† | |||||
| Never | 1889 (66.9) | 24,336 (63.6) | 6.9 | 38,980 (60.7) | 12.9 |
| Ever | 934 (33.1) | 13,913 (36.4) | −6.9 | 25,274 (39.3) | −12.9 |
| Missing | 29 | 279 | 442 | ||
| Parity (number of live births) | |||||
| Nulliparous *** | 816 (21.7) | 13,475 (19.8) | 4.7 | 21,187 (19.5) | 5.4 |
| 1 birth | 566 (15.1) | 11,102 (16.3) | −3.3 | 16,634 (15.3) | −0.6 |
| 2 births | 1625 (43.3) | 27,840 (40.9) | 4.9 | 44,997 (41.3) | 4.1 |
| >2 births | 746 (19.9) | 15,581 (22.9) | −7.3 | 26,044 (23.9) | −9.7 |
| Missing | 0 | 4316 | 5737 | ||
| Alcohol intake per day (grams) | |||||
| 0–<5 | 1672 (46.9) | 38,620 (58.4) | −23.2 | 61,935 (59.6) | −25.7 |
| 5–<15 | 1167 (32.8) | 15,381 (23.3) | 21.3 | 24,067 (23.2) | 21.5 |
| 15–<25 | 340 (9.5) | 6495 (9.8) | −1.0 | 9519 (9.2) | 1.0 |
| 25–<35 | 229 (6.4) | 2704 (4.1) | 10.3 | 4052 (3.9) | 11.3 |
| 35–<45 | 91 (2.6) | 1279 (1.9) | 4.7 | 1924 (1.9) | 4.7 |
| ≥45 | 63 (1.8) | 1651 (2.5) | −4.8 | 2425 (2.3) | −3.5 |
| Missing ††† | 191 | 6184 | 10,677 | ||
| Breast and ovarian cancer among first-degree relatives ‡‡‡ | |||||
| Breast cancer only | 883 (23.5) | 6921(12.2) | 29.8 | 12,042 (13.0) | 27.4 |
| Ovarian cancer only | 109 (2.9) | 1077 (1.9) | 6.5 | 1901 (2.0) | 5.8 |
| Both breast and ovarian cancer | 28 (0.7) | 261 (0.5) | 2.6 | 420 (0.5) | 2.6 |
| None | 2733 (72.8) | 48,440 (85.4) | −31.4 | 78,600 (84.5) | −28.9 |
| Missing | 0 | 15,615 | 21,636 |
| Characteristics | PERSPECTIVE Total | Census * Canada | Standardized Difference (%) |
|---|---|---|---|
| N (%) † | N (%) † | ||
| Age at study entry (years) | |||
| 40–49 | 544 (14.5) | 2,398,515 (32.3) | −43.0 |
| 50–59 | 1600 (42.6) | 2,554,035 (34.4) | 16.9 |
| 60–69 | 1609 (42.9) | 2,463,910 (33.2) | 20.1 |
| Born in Canada | |||
| Yes | 3191 (85.5) | 13,580,610 (73.8) | 29.4 |
| No | 540 (14.5) | 4,810,705 (26.2) | −29.4 |
| Missing | 22 | ||
| Visible minority | |||
| Not a visible minority ‡ | 3416 (92.8) | 13,464,900 (73.2) | 54.1 |
| Chinese | 78 (2.1) | 907,035 (4.9) | −15.3 |
| Black | 40 (1.1) | 793,765 (4.3) | −19.8 |
| Filipino | 22 (0.6) | 529,600 (2.9) | −17.6 |
| Latin American | 41 (1.1) | 299,200 (1.6) | −4.3 |
| South Asian | 32 (0.9) | 1,247,275 (6.8) | −31.0 |
| Multiple visible minorities § | 11 (0.3) | 171,010 (0.9) | −7.8 |
| Other visible minorities ** | 40 (1.1) | 978,525 (5.3) | −24.0 |
| Do not know/prefer not to answer/missing | 73 | ||
| Marital status †† | |||
| Married/common law | 2791 (75.1) | 8,812,595 (55.6) | 41.9 |
| Single/widowed/divorced/separated | 925 (24.9) | 7,026,860 (44.4) | −41.9 |
| Missing | 37 | ||
| Highest level of education ‡‡ | |||
| University Bachelor’s degree or above | 1904 (51.2) | 3,594,215 (36.1) | 30.8 |
| College/Registered Apprenticeship/trades certificate | 1336 (35.9) | 3,364,885 (33.8) | 4.4 |
| High school diploma or below | 479 (12.9) | 2,988,825 (30.0) | −42.6 |
| Prefer not to answer/missing | 34 | ||
| Employment status §§ | |||
| Employed | 2277 (61.0) | 8,265,025 (53.4) | 15.4 |
| Not employed/retired | 1458 (39.0) | 7,209,650 (46.6) | −15.4 |
| Prefer not to answer/missing | 18 |
| Characteristics | PERSPECTIVE Ontario | Census * Ontario | Standardized Difference (%) | PERSPECTIVE Quebec | Census * Quebec | Standardized Difference (%) |
|---|---|---|---|---|---|---|
| N (%) † | N (%) † | N (%) † | N (%) † | |||
| Age at study entry (years) | ||||||
| 40–49 | 12 (0.6) | 926,300 (32.3) | −94.6 | 532 (32.4) | 543,065 (31.8) | 1.3 |
| 50–59 | 943 (44.7) | 1,018,310 (35.5) | 18.9 | 657 (40.0) | 568,790 (33.3) | 13.9 |
| 60–69 | 1156 (54.8) | 925,515 (32.2) | 46.8 | 453 (27.6) | 595,850 (34.9) | −15.8 |
| Born in Canada | ||||||
| Yes | 1617 (77.3) | 4,745,495 (66.4) | 24.4 | 1574 (96.0) | 3,467,705 (82.9) | 43.7 |
| No | 475 (22.7) | 2,398,690 (33.6) | −24.4 | 65 (4.0) | 713,915 (17.1) | −43.7 |
| Missing | 19 | 3 | ||||
| Visible minority | ||||||
| Not a visible minority ‡ | 1848 (88.4) | 4,676,955 (65.5) | 56.5 | 1568 (98.6) | 3,502,955 (83.8) | 54.1 |
| Chinese | 78 (3.7) | 431,935 (6.0) | −10.7 | ≤5 (0.0) | 63,865 (1.5) | −17.5 |
| Black | 34 (1.6) | 402,940 (5.6) | −21.6 | 6 (0.4) | 216,070 (5.2) | −5.7 |
| Filipino | 22 (1.1) | 206,070 (2.9) | −12.9 | ≤5 (0.0) | 25,580 (0.6) | −11.0 |
| Latin American | 27 (1.3) | 129,830 (1.8) | −4.1 | 14 (0.9) | 87,880 (2.1) | 30.5 |
| South Asian | 32 (1.5) | 736,020 (10.3) | −38.0 | ≤5 (0.0) | 60,850 (1.5) | −17.5 |
| Multiple visible minorities § | 11 (0.5) | 93,750 (1.3) | −8.5 | ≤5 (0.0) | 17,875 (0.4) | −9.0 |
| Other visible minorities ** | 38 (1.8) | 466,685 (6.5) | −23.7 | 2 (0.1) | 206,545 (4.9) | −23.2 |
| Do not know/prefer not to answer/missing | 21 | 52 | ||||
| Marital status †† | ||||||
| Married/common law | 1550 (74.6) | 3,385,240 (55.0) | 41.9 | 1241 (75.8) | 1,981,145 (54.7) | 45.4 |
| Single/widowed/divorced/separated | 529 (25.4) | 2,771,690 (45.0) | −41.9 | 396 (24.2) | 1,640,660 (45.3) | −45.4 |
| Missing | 32 | 5 | ||||
| Highest level of education ‡‡ | ||||||
| University Bachelor’s degree or above | 1088 (52.3) | 1,900,925 (31.4) | 43.4 | 816 (49.8) | 733,395 (33.0) | 34.6 |
| College/Registered Apprenticeship/trades certificate | 689 (33.1) | 1,657,230 (27.4) | 12.4 | 647 (39.5) | 906,335 (40.7) | −2.5 |
| High school diploma or below | 303 (14.6) | 2,491,315 (41.2) | −62.1 | 176 (10.7) | 586,045 (26.3) | −41.0 |
| Prefer not to answer/missing | 31 | 3 | ||||
| Employment status §§ | ||||||
| Employed | 1152 (54.9) | 3,075,940 (50.8) | 8.2 | 1125 (68.8) | 1,974,095 (56.3) | 26.0 |
| Not employed/retired | 947 (45.1) | 2,973,520 (49.2) | −8.2 | 511 (31.2) | 1,530,115 (43.7) | −26.0 |
| Prefer not to answer/missing | 12 | 6 |
References
- Canadian Cancer Statistics, 2021. Available online: https://cancer.ca/en/research/cancer-statistics/past-editions (accessed on 17 August 2023).
- Canadian Task Force on Preventive Health Care. Recommendations on screening for breast cancer in women aged 40-74 years who are not at increased risk for breast cancer. CMAJ 2018, 190, E1441–E1451. [Google Scholar] [CrossRef]
- Nelson, H.D.; Fu, R.; Cantor, A.; Pappas, M.; Daeges, M.; Humphrey, L. Effectiveness of Breast Cancer Screening: Systematic Review and Meta-analysis to Update the 2009 U.S. Preventive Services Task Force Recommendation. Ann. Intern. Med. 2016, 164, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Siu, A.L.; U.S. Preventive Services Task Force. Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2016, 164, 279–296, Erratum in Ann. Intern. Med. 2016, 164, 448. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Kharazmi, E.; Fallah, M. Race and Ethnicity-Adjusted Age Recommendation for Initiating Breast Cancer Screening. JAMA Netw. Open 2023, 6, e238893. [Google Scholar] [CrossRef] [PubMed]
- U.S. Preventive Services Task Force. Draft Recommendation: Breast Cancer: Screening. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-screening (accessed on 16 October 2023).
- US Preventive Services Task Force; Nicholson, W.K.; Silverstein, M.; Wong, J.B.; Barry, M.J.; Chelmow, D.; Coker, T.R.; Davis, E.M.; Jaén, C.R.; Krousel-Wood, M.; et al. Screening for Breast Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2024, Online ahead of print. [Google Scholar] [CrossRef]
- Pashayan, N.; Antoniou, A.C.; Ivanus, U.; Esserman, L.J.; Easton, D.F.; French, D.; Sroczynski, G.; Hall, P.; Cuzick, J.; Evans, D.G.; et al. Personalized early detection and prevention of breast cancer: ENVISION consensus statement. Nat. Rev. Clin. Oncol. 2020, 17, 687–705, Erratum in Nat. Rev. Clin. Oncol. 2020, 17, 716. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, L.; Evans, D.G.; Payne, K.; Harrison, F.; Howell, A.; Howell, S.J.; French, D.P.; Breast Screening Risk-Stratification Agenda Setting Group. Implementing Risk-Stratified Breast Screening in England: An Agenda Setting Meeting. Cancers 2022, 14, 4636. [Google Scholar] [CrossRef] [PubMed]
- Pashayan, N.; Antoniou, A.C.; Lee, A.; Wolfson, M.; Chiquette, J.; Eloy, L.; Eisen, A.; Stockley, T.L.; Nabi, H.; Brooks, J.D.; et al. Should Age-Dependent Absolute Risk Thresholds Be Used for Risk Stratification in Risk-Stratified Breast Cancer Screening? J. Pers. Med. 2021, 11, 916. [Google Scholar] [CrossRef] [PubMed]
- Pashayan, N.; Morris, S.; Gilbert, F.J.; Pharoah, P.D.P. Cost-effectiveness and Benefit-to-Harm Ratio of Risk-Stratified Screening for Breast Cancer: A Life-Table Model. JAMA Oncol. 2018, 4, 1504–1510, Erratum in JAMA Oncol. 2022, 8, 484. [Google Scholar] [CrossRef]
- Yang, X.; Eriksson, M.; Czene, K.; Lee, A.; Leslie, G.; Lush, M.; Wang, J.; Dennis, J.; Dorling, L.; Carvalho, S.; et al. Prospective validation of the BOADICEA multifactorial breast cancer risk prediction model in a large prospective cohort study. J. Med. Genet. 2022, 59, 1196–1205. [Google Scholar] [CrossRef]
- Lee, A.; Mavaddat, N.; Wilcox, A.N.; Cunningham, A.P.; Carver, T.; Hartley, S.; de Villiers, C.B.; Izquierdo, A.; Simard, J.; Schmidt, M.K.; et al. BOADICEA: A comprehensive breast cancer risk prediction model incorporating genetic and nongenetic risk factors. Genet Med. 2019, 21, 1708–1718. [Google Scholar] [CrossRef] [PubMed]
- Mbuya-Bienge, C.; Pashayan, N.; Kazemali, C.D.; Lapointe, J.; Simard, J.; Nabi, H. A Systematic Review and Critical Assessment of Breast Cancer Risk Prediction Tools Incorporating a Polygenic Risk Score for the General Population. Cancers 2023, 15, 5380. [Google Scholar] [CrossRef] [PubMed]
- Shieh, Y.; Eklund, M.; Madlensky, L.; Sawyer, S.D.; Thompson, C.K.; Fiscalini, A.S.; Ziv, E.; van’t Veer, L.J.; Esserman, L.J.; Tice, J.A.; et al. Breast Cancer Screening in the Precision Medicine Era: Risk-Based Screening in a Population-Based Trial. J. Natl. Cancer Inst. 2017, 109, djw290. [Google Scholar] [CrossRef] [PubMed]
- Roux, A.; Cholerton, R.; Sicsic, J.; Moumjid, N.; French, D.P.; Rossi, P.G.; Balleyguier, C.; Guindy, M.; Gilbert, F.J.; Burrion, J.-B.; et al. Study protocol comparing the ethical, psychological and socio-economic impact of personalised breast cancer screening to that of standard screening in the “My Personal Breast Screening” (MyPeBS) randomised clinical trial. BMC Cancer 2022, 22, 507. [Google Scholar] [CrossRef] [PubMed]
- Gabrielson, M.; Eriksson, M.; Hammarström, M.; Borgquist, S.; Leifland, K.; Czene, K.; Hall, P. Cohort Profile: The Karolinska Mammography Project for Risk Prediction of Breast Cancer (KARMA). Int. J. Epidemiol. 2017, 46, 1740–1741g. [Google Scholar] [CrossRef] [PubMed]
- Rainey, L.; van der Waal, D.; Broeders, M.J.M. Dutch women’s intended participation in a risk-based breast cancer screening and prevention programme: A survey study identifying preferences, facilitators and barriers. BMC Cancer 2020, 20, 965. [Google Scholar] [CrossRef] [PubMed]
- Giordano, L.; Gallo, F.; Petracci, E.; Chiorino, G.; Segnan, N.; Andromeda Working Group. The ANDROMEDA prospective cohort study: Predictive value of combined criteria to tailor breast cancer screening and new opportunities from circulating markers: Study protocol. BMC Cancer 2017, 17, 785. [Google Scholar] [CrossRef]
- Evans, D.G.; Donnelly, L.S.; Harkness, E.F.; Astley, S.M.; Stavrinos, P.; Dawe, S.; Watterson, D.; Fox, L.; Sergeant, J.C.; Ingham, S.; et al. Breast cancer risk feedback to women in the UK NHS breast screening population. Br. J. Cancer 2016, 114, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.D.; Nabi, H.; Andrulis, I.L.; Antoniou, A.C.; Chiquette, J.; Després, P.; Devilee, P.; Dorval, M.; Droit, A.; Easton, D.F.; et al. Personalized Risk Assessment for Prevention and Early Detection of Breast Cancer: Integration and Implementation (PERSPECTIVE I&I). J. Pers. Med. 2021, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Mavaddat, N.; Michailidou, K.; Dennis, J.; Lush, M.; Fachal, L.; Lee, A.; Tyrer, J.P.; Chen, T.-H.; Wang, Q.; Bolla, M.K.; et al. Polygenic Risk Scores for Prediction of Breast Cancer and Breast Cancer Subtypes. Am. J. Hum. Genet. 2019, 104, 21–34. [Google Scholar] [CrossRef]
- Mavaddat, N.; Ficorella, L.; Carver, T.; Lee, A.; Cunningham, A.P.; Lush, M.; Dennis, J.; Tischkowitz, M.; Downes, K.; Hu, D.; et al. Incorporating Alternative Polygenic Risk Scores into the BOADICEA Breast Cancer Risk Prediction Model. Cancer Epidemiol. Biomarkers Prev. 2023, 32, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Carver, T.; Hartley, S.; Lee, A.; Cunningham, A.P.; Archer, S.; de Villiers, C.B.; Roberts, J.; Ruston, R.; Walter, F.M.; Tischkowitz, M.; et al. CanRisk Tool-A Web Interface for the Prediction of Breast and Ovarian Cancer Risk and the Likelihood of Carrying Genetic Pathogenic Variants. Cancer Epidemiol. Biomarkers Prev. 2021, 30, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Visible Minority and Population Group Reference Guide, Census of Population, 2021. Available online: https://www12.statcan.gc.ca/census-recensement/2021/ref/98-500/006/98-500-x2021006-eng.cfm (accessed on 16 October 2023).
- Sickles, E.A.; D’Orsi, C.J.; Bassett, L.W.; Appleton, C.M.; Berg, W.A.; Burnside, E.S. ACR BI-RADS mammography. In ACR BIRADS atlas, Breast Imaging Reporting and Data System, 5th ed.; D’Orsi, C.J., Sickles, E.A., Mendelson, E.B., Morris, E.A., Eds.; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- SAS Institute Inc. Statistical Analysis Software (ed. 9.4); SAS Institute: Cary, NC, USA, 2013. [Google Scholar]
- Nutbeam, D.; Lloyd, J.E. Understanding and Responding to Health Literacy as a Social Determinant of Health. Annu. Rev. Public. Health 2021, 42, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Brewer, N.T.; Richman, A.R.; DeFrank, J.T.; Reyna, V.F.; Carey, L.A. Improving communication of breast cancer recurrence risk. Breast Cancer Res. Treat. 2012, 133, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.C.; Williams, M.V.; Marin, E.; Parker, R.M.; Glass, J. Health literacy and cancer communication. CA Cancer J. Clin. 2002, 52, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Lea, D.H.; Kaphingst, K.A.; Bowen, D.; Lipkus, I.; Hadley, D.W. Communicating genetic and genomic information: Health literacy and numeracy considerations. Public. Health Genomics 2011, 14, 279–289. [Google Scholar] [CrossRef]
- Baccolini, V.; Isonne, C.; Salerno, C.; Giffi, M.; Migliara, G.; Mazzalai, E.; Turatto, F.; Sinopoli, A.; Rosso, A.; De Vito, C.; et al. The association between adherence to cancer screening programs and health literacy: A systematic review and meta-analysis. Prev. Med. 2022, 155, 106927. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.; Hayes, A.E. Cancer Health Literacy in Black Women with Breast Cancer: A Comprehensive Literature Review. Clin. J. Oncol. Nurs. 2023, 27, 507–513. [Google Scholar] [PubMed]
- A Vision for a Health Literate Canada: Report of the Expert Panel on Health Literacy. Available online: https://www.cpha.ca/sites/default/files/uploads/resources/healthlit/report_e.pdf (accessed on 16 October 2023).
- Cortez, S.; Milbrandt, M.; Kaphingst, K.; James, A.; Colditz, G. The readability of online breast cancer risk assessment tools. Breast Cancer Res Treat. 2015, 154, 191–199. [Google Scholar] [CrossRef]
- Lamb, L.R.; Baird, G.L.; Roy, I.T.; Choi, P.H.S.; Lehman, C.D.; Miles, R.C. Are English-language online patient education materials related to breast cancer risk assessment understandable, readable, and actionable? Breast 2022, 61, 29–34. [Google Scholar] [CrossRef]
- Vahabi, M.; Lofters, A.; Kumar, M.; Glazier, R.H. Breast cancer screening disparities among urban immigrants: A population-based study in Ontario, Canada. BMC Public Health 2015, 15, 679. [Google Scholar] [CrossRef] [PubMed]
- Lofters, A.K.; Kopp, A.; Vahabi, M.; Glazier, R.H. Understanding those overdue for cancer screening by five years or more: A retrospective cohort study in Ontario, Canada. Prev. Med. 2019, 129, 105816. [Google Scholar] [CrossRef] [PubMed]
- Tungasuvvingat Inuit and Cancer Care Ontario. Cancer Risk Factors and Screening Among Inuit in Ontario and Other Canadian Regions; Cancer Care Ontario: Toronto, ON, Canada, 2017. [Google Scholar]
- Métis Nation of Ontario and Cancer Care Ontario. Cancer in the Métis people of Ontario: Risk Factors and Screening Behaviours; Cancer Care Ontario: Ottawa, ON, Canada, 2015. [Google Scholar]
- Withrow, D.R.; Amartey, A.; Marrett, L.D. Cancer risk factors and screening in the off-reserve First Nations, Métis and non-Aboriginal populations of Ontario. Chronic Dis. Inj. Can. 2014, 34, 103–112. [Google Scholar] [CrossRef] [PubMed]
- OurCare–National Survey Data. Available online: https://www.ourcare.ca/nationalsurvey (accessed on 16 October 2023).
| Characteristics | Total N = 3753 | Quebec N = 1642 | Ontario N = 2111 | Odds Ratio Ontario vs. Quebec (95% CI) | p-Value |
|---|---|---|---|---|---|
| N (%) * | N (%) * | N (%) * | |||
| Age at study entry (years) | |||||
| 40–49 | 544 (14.5) | 532 (32.4) | 12 (0.6) | 0.02 (0.01 to 0.03) | <0.0001 |
| 50–59 | 1600 (42.6) | 657 (40.0) | 943 (44.7) | 1.00 (Referent) | -- |
| 60–69 | 1609 (42.9) | 453 (27.6) | 1156 (54.8) | 1.78 (1.53 to 2.06) | <0.0001 |
| Born in Canada | |||||
| Yes | 3191 (85.5) | 1574 (96.0) | 1617 (77.3) | 1.00 (Referent) | -- |
| No | 540 (14.5) | 65 (4.0) | 475 (22.7) | 7.11 (5.44 to 9.30) | <0.0001 |
| Missing | 22 | 3 | 19 | ||
| Visible minority | |||||
| Not a visible minority | 3416 (92.8) | 1568 (98.6) | 1848 (88.4) | 1.00 (Referent) | -- |
| Visible minority | 264 (7.2) | 22 (1.4) | 242 (11.6) | 9.33 (6.00 to 14.52) | <0.0001 |
| Do not know/prefer not to answer/missing | 73 | 52 | 21 | ||
| Marital status | |||||
| Married/common law | 2791 (75.1) | 1241 (75.8) | 1550 (74.6) | 1.00 (Referent) | -- |
| Single/widowed/divorced/separated | 925 (24.9) | 396 (24.2) | 529 (25.4) | 1.07 (0.92 to 1.24) | 0.38 |
| Prefer not to answer/missing | 37 | 5 | 32 | ||
| Highest level of education | |||||
| University Bachelor’s degree or above | 1904 (51.2) | 816 (49.8) | 1088 (52.3) | 1.00 (Referent) | -- |
| College/Registered Apprenticeship/trades certificate | 1336 (35.9) | 647 (39.5) | 689 (33.1) | 0.80 (0.69 to 0.92) | 0.002 |
| High school diploma or below | 479 (12.9) | 176 (10.7) | 303 (14.6) | 1.29 (1.05 to 1.59) | 0.02 |
| Prefer not to answer/missing | 34 | 3 | 31 | ||
| Employment status | |||||
| Employed | 2277 (61.0) | 1125 (68.8) | 1152 (54.9) | 1.00 (Referent) | |
| Not employed | 256 (6.9) | 86 (5.3) | 170 (8.1) | 1.93 (1.47 to 2.53) | <0.0001 |
| Retired | 1202 (32.2) | 425 (26.0) | 777 (37.0) | 1.79 (1.55 to 2.06) | <0.0001 |
| Prefer not to answer/missing | 18 | 6 | 12 | ||
| Overall health | |||||
| Excellent/very good/good | 3547 (94.8) | 1578 (96.2) | 1969 (93.7) | 1.00 (Referent) | |
| Fair/poor | 194 (5.2) | 62 (3.8) | 132 (6.3) | 1.71 (1.25 to 2.32) | <0.001 |
| Do not know/missing | 12 | 2 | 10 |
| Risk Factors | Total N = 3753 | Quebec N = 1642 | Ontario N = 2111 | Age-Adjusted Odds Ratio (95% CI) † | p-Value |
|---|---|---|---|---|---|
| N (%) * | N (%) * | N (%) * | |||
| Height (cm) | |||||
| ≤152.90 | 209 (5.6) | 79 (4.8) | 130 (6.2) | 0.78 (0.56 to 1.08) | 0.13 |
| 152.91–159.64 | 711 (19.0) | 327 (19.9) | 384 (18.2) | 1.10 (0.90 to 1.34) | 0.35 |
| 159.65–165.95 | 1614 (43.1) | 747 (45.5) | 867(41.1) | 1.00 (Referent) | -- |
| 165.96–172.69 | 842 (22.5) | 359 (21.9) | 483 (22.9) | 0.72 (0.59 to 0.88) | 0.001 |
| ≥172.70 | 373 (9.9) | 130 (7.9) | 243 (11.5) | 0.46 (0.35 to 0.61) | <0.0001 |
| Missing | 4 | 0 | 4 | ||
| BMI (kg/m2) | |||||
| <18.5 | 60 (1.6) | 24 (1.5) | 36 (1.7) | 0.77 (0.41 to 1.46) | 0.43 |
| 18.5–<25.0 | 1673 (44.7) | 721 (43.9) | 952 (45.1) | 0.96 (0.80 to 1.16) | 0.70 |
| 25.0–<30.0 | 1130 (30.2) | 513 (31.2) | 617 (29.2) | 1.15 (0.94 to 1.40) | 0.18 |
| ≥30.0 | 876 (23.5) | 384 (23.4) | 492 (23.3) | 1.00 (Referent) | -- |
| Missing | 14 | 0 | 14 | ||
| Age at menarche (years) | |||||
| <11 | 198 (5.5) | 99 (6.3) | 99 (4.8) | 1.29 (0.91 to 1.83) | 0.15 |
| 11 | 479 (13.2) | 224 (14.2) | 255 (12.4) | 1.17 (0.91 to 1.50) | 0.22 |
| 12 | 1004 (27.6) | 439 (27.9) | 565 (27.5) | 1.00 (Referent) | -- |
| 13 | 1011 (27.8) | 384 (24.4) | 627 (30.5) | 0.86 (0.70 to 1.05) | 0.13 |
| 14 | 557 (15.3) | 263 (16.7) | 294 (14.3) | 1.25 (0.99 to 1.58) | 0.07 |
| 15 | 219 (6.0) | 101 (6.4) | 118 (5.7) | 1.27 (0.92 to 1.75) | 0.16 |
| >15 | 164 (4.5) | 66 (4.2) | 98 (4.8) | 0.91 (0.63 to 1.33) | 0.63 |
| Missing | 121 | 66 | 55 | ||
| Menopausal status | |||||
| Premenopausal | 895 (23.9) | 643 (39.3) | 252 (11.9) | 1.09 (0.88 to 1.36) | 0.44 |
| Menopausal | 2852 (76.1) | 994 (60.7) | 1858 (88.1) | 1.00 (Referent) | -- |
| Missing | 6 | 5 | 1 | ||
| Age at menopause (years) | |||||
| <40 | 185 (7.1) | 91 (10.4) | 94 (5.4) | 2.16 (1.50 to 3.11) | <0.0001 |
| 40–44 | 204 (7.9) | 90 (10.4) | 114 (6.6) | 1.61 (1.13 to 2.32) | 0.009 |
| 45–49 | 508 (19.5) | 194 (22.2) | 314 (18.2) | 1.41 (1.07 to 1.86) | 0.02 |
| 50–54 | 1239 (47.7) | 371 (42.8) | 868 (50.2) | 1.07 (0.84 to 1.36) | 0.61 |
| ≥55 | 464 (17.8) | 126 (14.3) | 338 (19.6) | 1.00 (Referent) | |
| Missing | 252 | 122 | 130 | ||
| Oral contraceptive use | |||||
| Never | 377 (10.2) | 108 (6.6) | 269 (13.1) | 0.57 (0.44 to 0.73) | <0.0001 |
| Former | 3145 (85.0) | 1383 (84.3) | 1762 (85.6) | 1.00 (Referent) | -- |
| Current | 177 (4.8) | 149 (9.1) | 28 (1.4) | 2.33 (1.44 to 3.75) | <0.001 |
| Missing | 54 | 2 | 52 | ||
| Menopausal hormone therapy use | |||||
| Never | 1889 (66.9) | 565 (57.0) | 1324 (72.3) | 1.00 (Referent) | -- |
| Former (any type) | 358 (12.7) | 125 (12.6) | 233 (12.7) | 1.51 (1.18 to 1.92) | 0.001 |
| Current (E-type) | 272 (9.6) | 116 (11.7) | 156 (8.5) | 1.83 (1.40 to 2.38) | <0.0001 |
| Current (other/unknown type(including combined type)) | 304 (10.8) | 185 (18.7) | 119 (6.5) | 3.80 (2.95 to 4.91) | <0.0001 |
| Unspecified/missing | 29 | 3 | 26 | ||
| Parity (number of live births) | |||||
| Nulliparous | 816 (21.7) | 306 (18.6) | 510 (24.2) | 1.00 (Referent) | -- |
| 1 birth | 565 (15.1) | 257 (15.7) | 308 (14.6) | 1.24 (0.97 to 1.58) | 0.08 |
| 2 births | 1626 (43.3) | 749 (45.6) | 877 (41.5) | 1.22 (1.01 to 1.48) | 0.04 |
| >2 births | 746 (19.9) | 330 (20.1) | 416 (19.7) | 1.19 (0.95 to 1.49) | 0.13 |
| Missing | 0 | 0 | 0 | ||
| Age at first live birth (years) | |||||
| <20 | 100 (3.4) | 27 (2.0) | 73 (4.6) | 0.64 (0.37 to 1.11) | 0.11 |
| 20–24 | 549 (18.7) | 294 (22.0) | 255 (15.9) | 2.37 (1.88 to 3.00) | <0.0001 |
| 25–29 | 1178 (40.1) | 587 (43.9) | 591 (36.9) | 1.82 (1.50 to 2.20) | <0.0001 |
| ≥30 | 1110 (37.8) | 428 (32.0) | 682 (42.6) | 1.00 (Referent) | -- |
| Missing | 0 | 0 | 0 | ||
| Alcohol intake per day (grams) | |||||
| 0 | 453 (12.7) | 141 (8.9) | 312 (15.8) | 1.00 (Referent) | -- |
| >0–<5 | 1219 (34.2) | 498 (31.3) | 721 (36.6) | 1.44 (1.11 to 1.87) | 0.006 |
| 5–<15 | 1167 (32.8) | 611 (38.4) | 556 (28.2) | 2.33 (1.80 to3.03) | <0.0001 |
| 15–<25 | 340 (9.5) | 187 (11.7) | 153 (7.8) | 2.79 (2.01 to 3.86) | <0.0001 |
| 25–<35 | 229 (6.4) | 85 (5.3) | 144 (7.3) | 1.47 (1.01 to 2.14) | 0.04 |
| 35–<45 | 91 (2.6) | 36 (2.3) | 55 (2.8) | 1.73 (1.04 to 2.89) | 0.04 |
| ≥45 | 63 (1.8) | 35 (2.2) | 28 (1.4) | 3.23 (1.81 to 5.77) | <0.0001 |
| Missing | 191 | 49 | 142 | ||
| Family history of breast, ovarian, pancreatic and prostate cancer | |||||
| First- and second-degree | 615 (16.4) | 362 (22.0) | 253 (12.0) | 2.16 (1.74 to 2.69) | <0.0001 |
| First-degree only | 701 (18.7) | 289 (17.6) | 412 (19.5) | 1.33 (1.09 to 1.64) | 0.006 |
| Second-degree only | 1005 (26.8) | 502 (30.6) | 503 (23.8) | 1.56 (1.30 to 1.88) | <0.0001 |
| None | 1432 (38.2) | 489 (29.8) | 943 (44.7) | 1.00 (Referent) | -- |
| Family history of any breast cancer | |||||
| First- and second-degree | 314 (8.4) | 213 (13.0) | 101 (4.8) | 3.06 (2.31 to 4.05) | <0.0001 |
| First-degree only | 542 (14.4) | 259 (15.8) | 283 (13.4) | 1.41 (1.14 to 1.75) | 0.002 |
| Second-degree only | 830 (22.1) | 439 (26.7) | 391 (18.5) | 1.64 (1.36 to 1.97) | <0.0001 |
| None | 2067 (55.1) | 731 (44.5) | 1336 (63.3) | 1.00 (Referent) | -- |
| BIRADS® density | |||||
| a, b, or c | 3232 (87.4) | 1333 (83.9) | 1899 (90.0) | 1.00 (Referent) | -- |
| d | 468 (12.6) | 256 (16.1) | 212 (10.0) | 1.30 (1.04 to 1.63) | 0.02 |
| Unknown | 53 | 53 | 0 | ||
| Polygenic risk score | |||||
| −3.831 to −1.223 | 374 (10.0) | 167 (10.2) | 207 (9.8) | 1.31 (0.94 to 1.83) | 0.11 |
| −1.222 to 0.141 | 1503 (40.0) | 671 (40.9) | 832 (39.4) | 1.34 (1.02 to 1.74) | 0.03 |
| 0.142 to 1.477 | 1498 (39.9) | 654 (39.8) | 844 (40.0) | 1.29 (0.99 to 1.69) | 0.06 |
| 1.478 to 3.805 | 375 (10.0) | 149 (9.1) | 226 (10.7) | 1.00 (Referent) | -- |
| Missing | 3 | 1 | 2 |
| Characteristics | Total N = 2111 | Online N = 1537 (72.8%) | Paper/Phone N = 574 (27.2%) | Adjusted Odds Ratio (95% CI) † | p-Value |
|---|---|---|---|---|---|
| N (%) * | N (%) * | N (%) * | |||
| Age at study entry (years) | |||||
| 40–49 | 12 (0.6) | 7 (0.5) | 5 (0.9) | 2.79 (0.75 to10.34) | 0.13 |
| 50–59 | 943 (44.7) | 726 (47.2) | 217 (37.8) | 1.00 (Referent) | -- |
| 60–69 | 1156 (54.8) | 804 (52.3) | 352 (61.3) | 1.32 (1.04 to 1.67) | 0.02 |
| Born in Canada | |||||
| Yes | 1617 (77.3) | 1198 (78.6) | 419 (73.9) | 1.00 (Referent) | -- |
| No | 475 (22.7) | 327 (21.4) | 148 (26.1) | 1.28 (1.01 to 1.62) | 0.04 |
| Missing | 19 | 12 | 7 | -- | |
| Visible minority | |||||
| Not a visible minority | 1848 (88.4) | 1368 (89.7) | 480 (85.0) | 1.00 (Referent) | -- |
| Visible minority | 242 (11.6) | 157 (10.3) | 85 (15.0) | 1.51 (1.10 to 2.07) | 0.01 |
| Do not know/prefer not to answer/missing | 21 | 12 | 9 | ||
| Marital status | |||||
| Married/common law | 1550 (74.6) | 1167 (76.5) | 383 (69.1) | 1.00 (Referent) | -- |
| Single/widowed/divorced/separated | 529 (25.4) | 358 (23.5) | 171 (30.9) | 1.47 (1.17 to 1.84) | 0.001 |
| Prefer not to answer/missing | 32 | 12 | 20 | ||
| Highest level of education | |||||
| University Bachelor’s degree or above | 1088 (52.3) | 881 (57.7) | 207 (37.4) | 1.00 (Referent) | -- |
| College/Registered Apprenticeship/trades certificate | 689 (33.1) | 456 (29.9) | 233 (42.1) | 2.18 (1.74 to 2.74) | <0.0001 |
| High school diploma or below | 303 (14.6) | 189 (12.4) | 114 (20.6) | 2.32 (1.73 to 3.12) | <0.0001 |
| Prefer not to answer/missing | 31 | 11 | 20 | ||
| Employment status | |||||
| Employed | 1152 (54.9) | 868 (56.7) | 284 (50.0) | 1.00 (Referent) | -- |
| Not employed | 170 (8.1) | 108 (7.1) | 62 (10.9) | 1.26 (0.86 to 1.84) | 0.24 |
| Retired | 777 (37.0) | 555 (36.3) | 222 (39.1) | 1.02 (0.80 to 1.30) | 0.90 |
| Prefer not to answer/missing | 12 | 6 | 6 | ||
| Overall health | |||||
| Excellent/very good/good | 1969 (93.7) | 1466 (95.6) | 503 (88.6) | 1.00 (Referent) | -- |
| Fair/poor | 132 (6.3) | 67 (4.4) | 65 (11.4) | 2.15 (1.46 to 3.17) | <0.001 |
| Do not know/missing | 10 | 4 | 6 |
| Characteristics | Total N = 3618 * | No Verification N = 2375 (65.6%) | Verification Required N = 1243 (34.4%) | Adjusted Odds Ratio (95% CI) ‡ | p-Value |
|---|---|---|---|---|---|
| N (%) † | N (%) † | N (%) † | |||
| Study Site | |||||
| Quebec | 1600 (44.2) | 935 (39.4) | 665 (53.5) | 1.90 (1.62 to 2.24) | <0.0001 |
| Ontario | 2018 (55.8) | 1440 (60.6) | 578 (46.5) | 1.00 (Referent) | -- |
| Age at study entry (years) | |||||
| 40–49 | 531 (14.7) | 306 (12.9) | 225 (18.1) | 1.07 (0.86 to 1.34) | 0.54 |
| 50–59 | 1526 (42.2) | 1018 (42.9) | 508 (40.9) | 1.00 (Referent) | -- |
| 60–69 | 1561 (43.1) | 1051 (44.3) | 510 (41.0) | 1.14 (0.95 to 1.36) | 0.16 |
| Born in Canada | |||||
| Yes | 3094 (86.0) | 2023 (85.6) | 1071 (86.7) | 1.00 (Referent) | -- |
| No | 503 (14.0) | 339 (14.4) | 164 (13.3) | 1.14 (0.92 to 1.41) | 0.24 |
| Missing | 21 | 13 | 8 | ||
| Visible minority | |||||
| Not a visible minority | 3305 (93.2) | 2179 (93.6) | 1126 (92.5) | 1.00 (Referent) | -- |
| Visible minority | 241 (6.8) | 150 (6.4) | 91 (7.5) | 1.46 (1.10 to 1.95) | 0.009 |
| Do not know/prefer not to answer/missing | 72 | 46 | 26 | ||
| Marital status | |||||
| Married/common law | 2691 (75.0) | 1774 (75.4) | 917 (74.4) | 1.00 (Referent) | -- |
| Single/widowed/divorced/separated | 896 (25.0) | 580 (24.6) | 316 (25.6) | 1.03 (0.88 to 1.22) | 0.70 |
| Prefer not to answer/missing | 31 | 21 | 10 | ||
| Highest level of education | |||||
| University Bachelor’s degree or above | 1831 (51.1) | 1254 (53.1) | 577 (47.1) | 1.00 (Referent) | -- |
| College/Registered Apprenticeship/trades certificate | 1292 (36.0) | 816 (34.6) | 476 (38.9) | 1.26 (1.08 to 1.47) | 0.0030.003 |
| High school diploma or below | 462 (12.9) | 290 (12.3) | 172 (14.0) | 1.37 (1.09 to 1.71) | 0.006 |
| Prefer not to answer/missing | 33 | 15 | 18 | ||
| Employment status | |||||
| Employed | 2186 (60.7) | 1418 (60.0) | 768 (62.2) | 1.00 (Referent) | -- |
| Not employed | 246 (6.8) | 143 (6.0) | 103 (8.3) | 1.29 (0.97 to 1.71) | 0.08 |
| Retired | 1168 (32.4) | 804 (34.0) | 364 (29.5) | 0.84 (0.70 to 1.01) | 0.07 |
| Prefer not to answer/missing | 18 | 10 | 8 | ||
| Overall health | |||||
| Excellent/very good/good | 3424 (94.9) | 2263 (95.4) | 1161 (93.9) | 1.00 (Referent) | -- |
| Fair/poor | 183 (5.1) | 108 (4.6) | 75 (6.1) | 1.30 (0.95 to 1.78) | 0.11 |
| Do not know/missing | 11 | 4 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walker, M.J.; Blackmore, K.M.; Chang, A.; Lambert-Côté, L.; Turgeon, A.; Antoniou, A.C.; Bell, K.A.; Broeders, M.J.M.; Brooks, J.D.; Carver, T.; et al. Implementing Multifactorial Risk Assessment with Polygenic Risk Scores for Personalized Breast Cancer Screening in the Population Setting: Challenges and Opportunities. Cancers 2024, 16, 2116. https://doi.org/10.3390/cancers16112116
Walker MJ, Blackmore KM, Chang A, Lambert-Côté L, Turgeon A, Antoniou AC, Bell KA, Broeders MJM, Brooks JD, Carver T, et al. Implementing Multifactorial Risk Assessment with Polygenic Risk Scores for Personalized Breast Cancer Screening in the Population Setting: Challenges and Opportunities. Cancers. 2024; 16(11):2116. https://doi.org/10.3390/cancers16112116
Chicago/Turabian StyleWalker, Meghan J., Kristina M. Blackmore, Amy Chang, Laurence Lambert-Côté, Annie Turgeon, Antonis C. Antoniou, Kathleen A. Bell, Mireille J. M. Broeders, Jennifer D. Brooks, Tim Carver, and et al. 2024. "Implementing Multifactorial Risk Assessment with Polygenic Risk Scores for Personalized Breast Cancer Screening in the Population Setting: Challenges and Opportunities" Cancers 16, no. 11: 2116. https://doi.org/10.3390/cancers16112116
APA StyleWalker, M. J., Blackmore, K. M., Chang, A., Lambert-Côté, L., Turgeon, A., Antoniou, A. C., Bell, K. A., Broeders, M. J. M., Brooks, J. D., Carver, T., Chiquette, J., Després, P., Easton, D. F., Eisen, A., Eloy, L., Evans, D. G., Fienberg, S., Joly, Y., Kim, R. H., ... Chiarelli, A. M. (2024). Implementing Multifactorial Risk Assessment with Polygenic Risk Scores for Personalized Breast Cancer Screening in the Population Setting: Challenges and Opportunities. Cancers, 16(11), 2116. https://doi.org/10.3390/cancers16112116









