Phospholipid Acyltransferases: Characterization and Involvement of the Enzymes in Metabolic and Cancer Diseases
Abstract
Simple Summary
Abstract
1. Introduction
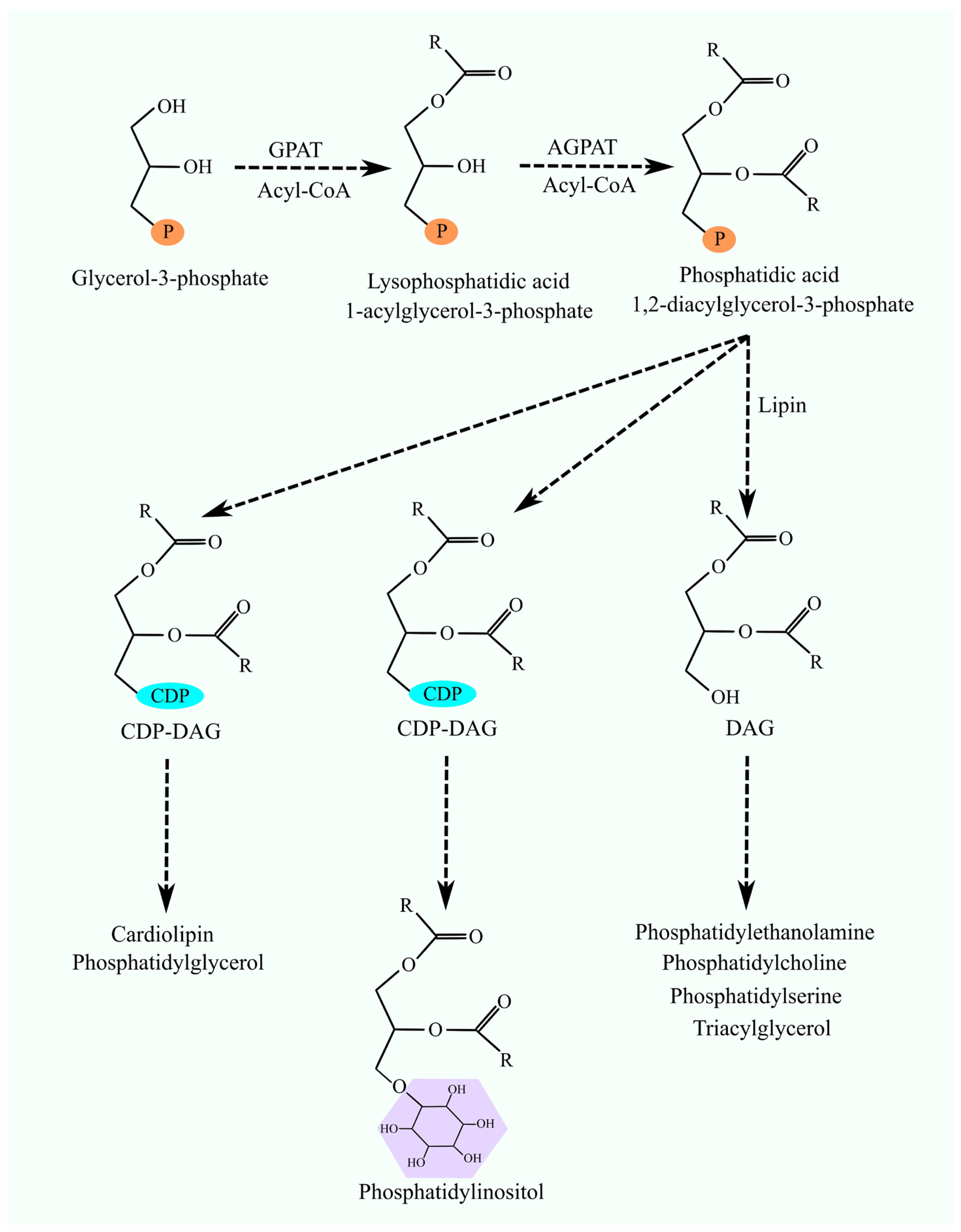
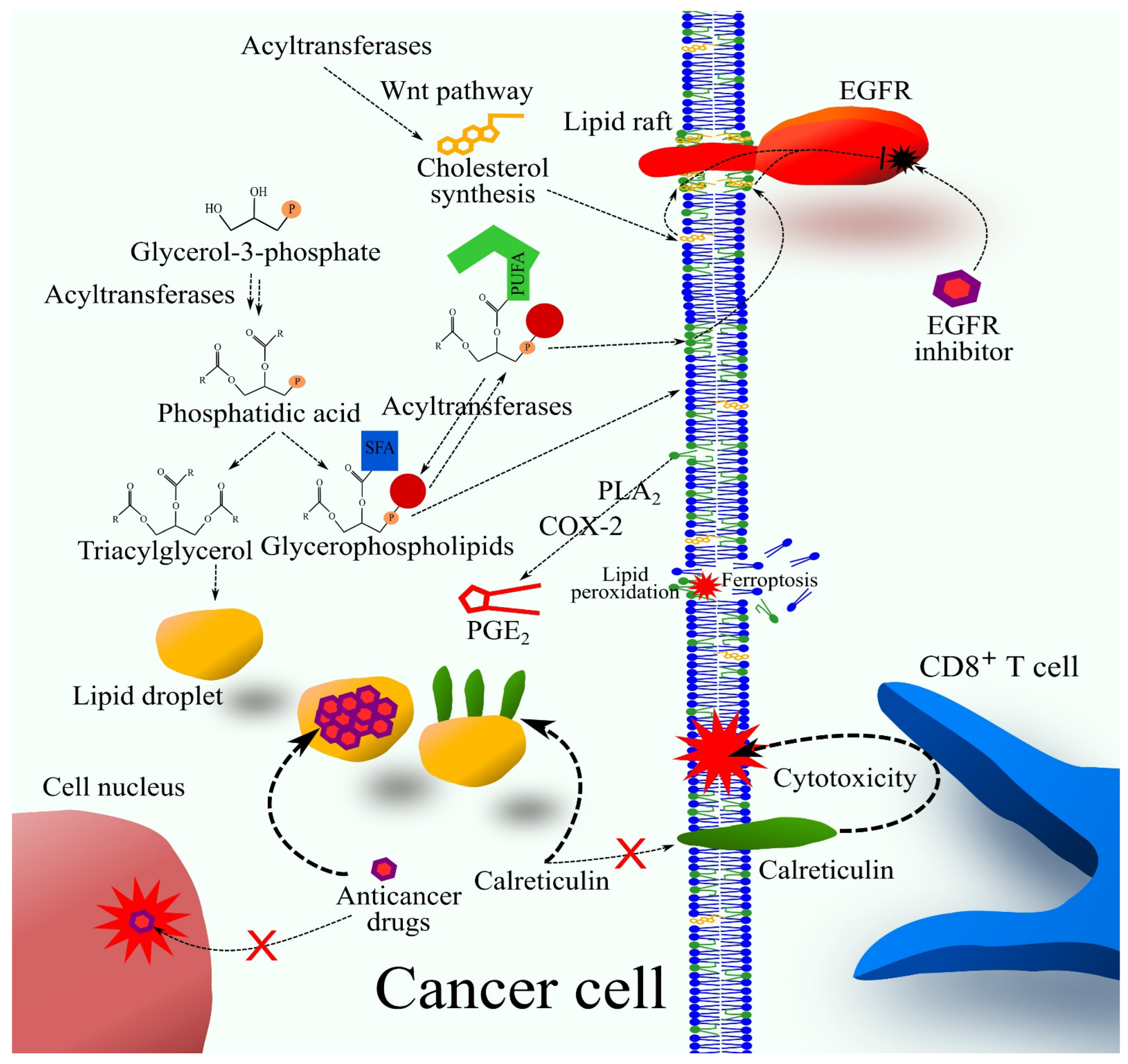
2. Glycerol-3-Phosphate Acyltransferases
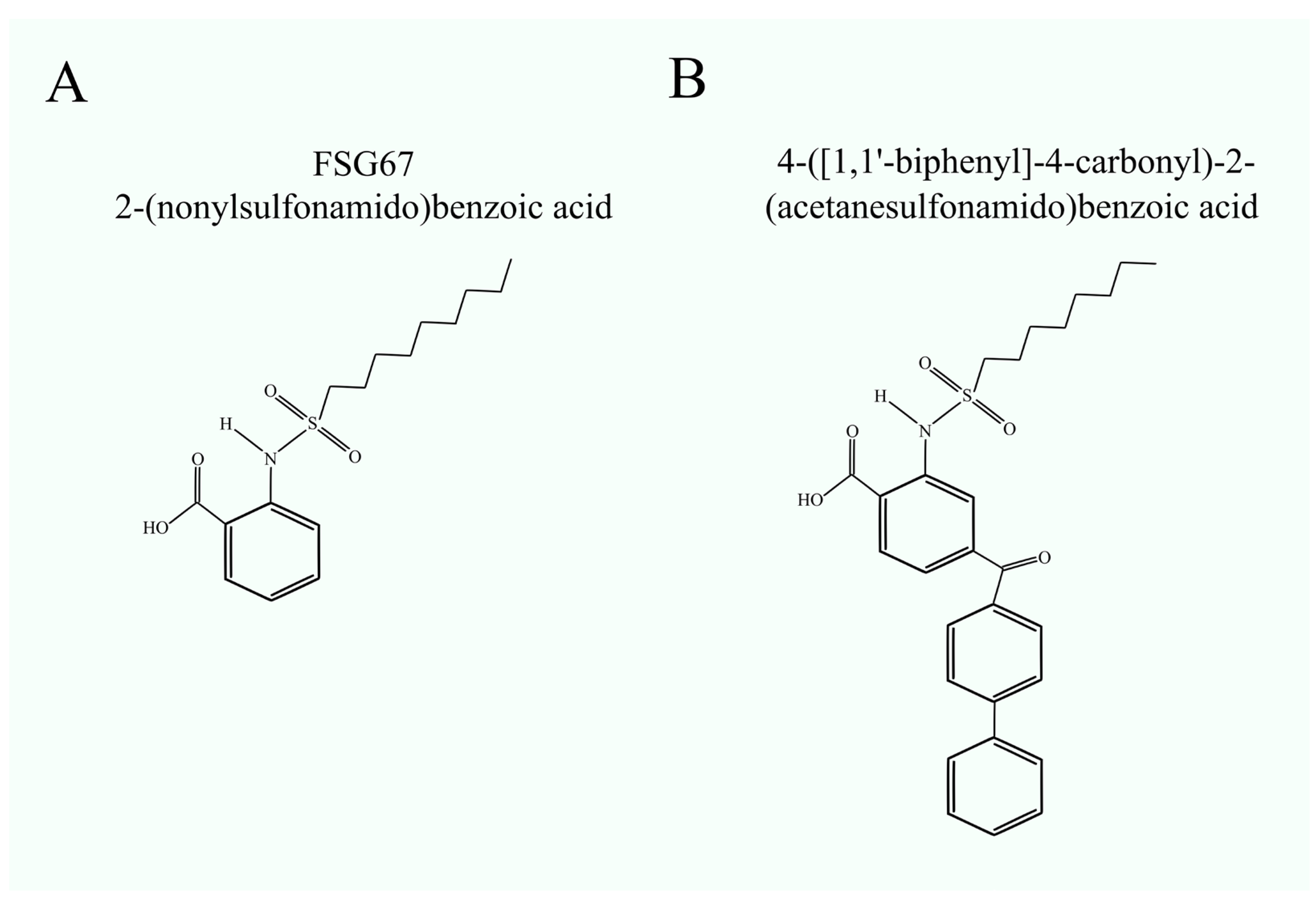
3. Glycerol-3-Phosphate Acyltransferase Inhibitors
4. 1-Acylglycerol-3-phosphate O-Acyltransferases and Lysophospholipids Acyltransferases
4.1. AGPAT1
4.2. AGPAT2
4.3. AGPAT3
4.4. AGPAT4
4.5. AGPAT5
4.6. LPCAT1
4.7. LPCAT2
4.8. LPCAT3
4.9. LPCAT4
4.10. LCLAT1
4.11. LPGAT1
4.12. MBOAT1
4.13. MBOAT2
4.14. MBOAT7
5. Bioinformatics Analysis of the Significance of Enzymes in Cancer
- -
- the expression level of the selected gene in the tumor relative to adjacent tumor tissue and healthy tissue,
- -
- analysis of the association between the expression level of the selected gene in the tumor and the prognosis of patients with the selected cancer type,
- -
- the correlation between the expression of two genes in the tumor of selected cancer types.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kennedy, E.P.; Weiss, S.B. The function of cytidine coenzymes in the biosynthesis of phospholipides. J. Biol. Chem. 1956, 222, 193–214. [Google Scholar] [CrossRef] [PubMed]
- Wendel, A.A.; Lewin, T.M.; Coleman, R.A. Glycerol-3-phosphate acyltransferases: Rate limiting enzymes of triacylglycerol biosynthesis. Biochim. Biophys. Acta 2009, 1791, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.K. Lysophospholipid acyltransferases: 1-acylglycerol-3-phosphate O-acyltransferases. From discovery to disease. Curr. Opin. Lipidol. 2012, 23, 290–302. [Google Scholar] [CrossRef]
- Blunsom, N.J.; Cockcroft, S. CDP-Diacylglycerol Synthases (CDS): Gateway to Phosphatidylinositol and Cardiolipin Synthesis. Front. Cell Dev. Biol. 2020, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Shindou, H.; Shimizu, T. Acyl-CoA:lysophospholipid acyltransferases. J. Biol. Chem. 2009, 284, 1–5. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, V.B. New appreciation for an old pathway: The Lands Cycle moves into new arenas in health and disease. Biochem. Soc. Trans. 2022, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, I.; Cui, Y.; Amiri, A.; Ding, Y.; Campbell, R.E.; Maysinger, D. Pharmacological inhibition of lipid droplet formation enhances the effectiveness of curcumin in glioblastoma. Eur. J. Pharm. Biopharm. 2016, 100, 66–76. [Google Scholar] [CrossRef]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Qatanani, M.; Lazar, M.A. Mechanisms of obesity-associated insulin resistance: Many choices on the menu. Genes Dev. 2007, 21, 1443–1455. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, T.; Zhang, S.; Zhou, L. Associations of Different Adipose Tissue Depots with Insulin Resistance: A Systematic Review and Meta-analysis of Observational Studies. Sci. Rep. 2015, 5, 18495. [Google Scholar] [CrossRef]
- Jiang, J.; Cai, X.; Pan, Y.; Du, X.; Zhu, H.; Yang, X.; Zheng, D.; Gaisano, H.; Wei, T.; He, Y. Relationship of obesity to adipose tissue insulin resistance. BMJ Open Diabetes Res. Care 2020, 8, e000741. [Google Scholar] [CrossRef]
- Park, J.; Morley, T.S.; Kim, M.; Clegg, D.J.; Scherer, P.E. Obesity and cancer-mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol. 2014, 10, 455–465. [Google Scholar] [CrossRef]
- Sohn, W.; Lee, H.W.; Lee, S.; Lim, J.H.; Lee, M.W.; Park, C.H.; Yoon, S.K. Obesity and the risk of primary liver cancer: A systematic review and meta-analysis. Clin. Mol. Hepatol. 2021, 27, 157–174. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, J.; Zhu, Y.; Luo, L.; He, T.; Hu, H.; Liu, H.; Zhang, Y.; Luo, D.; Xu, S.; et al. Abdominal obesity and colorectal cancer risk: Systematic review and meta-analysis of prospective studies. Biosci. Rep. 2017, 37, BSR20170945. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Petrelli, F.; Cortellini, A.; Indini, A.; Tomasello, G.; Ghidini, M.; Nigro, O.; Salati, M.; Dottorini, L.; Iaculli, A.; Varricchio, A.; et al. Association of Obesity with Survival Outcomes in Patients with Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e213520. [Google Scholar] [CrossRef] [PubMed]
- The Gene Expression Profiling Interactive Analysis. Available online: http://gepia.cancer-pku.cn/detail.php (accessed on 9 November 2023).
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef]
- Hammond, L.E.; Gallagher, P.A.; Wang, S.; Hiller, S.; Kluckman, K.D.; Posey-Marcos, E.L.; Maeda, N.; Coleman, R.A. Mitochondrial glycerol-3-phosphate acyltransferase-deficient mice have reduced weight and liver triacylglycerol content and altered glycerolipid fatty acid composition. Mol. Cell. Biol. 2002, 22, 8204–8214. [Google Scholar] [CrossRef] [PubMed]
- Lewin, T.M.; Schwerbrock, N.M.; Lee, D.P.; Coleman, R.A. Identification of a new glycerol-3-phosphate acyltransferase isoenzyme, mtGPAT2, in mitochondria. J. Biol. Chem. 2004, 279, 13488–13495. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Kuo, M.S.; Li, S.; Bui, H.H.; Peake, D.A.; Sanders, P.E.; Thibodeaux, S.J.; Chu, S.; Qian, Y.W.; Zhao, Y.; et al. AGPAT6 is a novel microsomal glycerol-3-phosphate acyltransferase. J. Biol. Chem. 2008, 283, 10048–10057. [Google Scholar] [CrossRef]
- Nagle, C.A.; Vergnes, L.; Dejong, H.; Wang, S.; Lewin, T.M.; Reue, K.; Coleman, R.A. Identification of a novel sn-glycerol-3-phosphate acyltransferase isoform, GPAT4, as the enzyme deficient in Agpat6−/− mice. J. Lipid Res. 2008, 49, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Collison, L.W.; Kannan, L.; Onorato, T.M.; Knudsen, J.; Haldar, D.; Jolly, C.A. Aging reduces glycerol-3-phosphate acyltransferase activity in activated rat splenic T-lymphocytes. Biochim. Biophys. Acta 2005, 1687, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Bronnikov, G.E.; Aboulaich, N.; Vener, A.V.; Strålfors, P. Acute effects of insulin on the activity of mitochondrial GPAT1 in primary adipocytes. Biochem. Biophys. Res. Commun. 2008, 367, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Onorato, T.M.; Chakraborty, S.; Haldar, D. Phosphorylation of rat liver mitochondrial glycerol-3-phosphate acyltransferase by casein kinase 2. J. Biol. Chem. 2005, 280, 19527–19534. [Google Scholar] [CrossRef] [PubMed]
- Collison, L.W.; Jolly, C.A. Phosphorylation regulates mitochondrial glycerol-3-phosphate-1 acyltransferase activity in T-lymphocytes. Biochim. Biophys. Acta 2006, 1761, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Li, J.L.; Wu, L.; Li, D.; Hurov, J.; Tobin, J.F.; Gimeno, R.E.; Cao, J. GPAT3 and GPAT4 are regulated by insulin-stimulated phosphorylation and play distinct roles in adipogenesis. J. Lipid Res. 2010, 51, 1971–1981. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, S.; Barnes, R.I.; Garg, A.; Agarwal, A.K. Functional characterization of the human 1-acylglycerol-3-phosphate-O-acyltransferase isoform 10/glycerol-3-phosphate acyltransferase isoform 3. J. Mol. Endocrinol. 2009, 42, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Wilfling, F.; Wang, H.; Haas, J.T.; Krahmer, N.; Gould, T.J.; Uchida, A.; Cheng, J.X.; Graham, M.; Christiano, R.; Fröhlich, F.; et al. Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Dev. Cell 2013, 24, 384–399. [Google Scholar] [CrossRef] [PubMed]
- Seal, R.L.; Braschi, B.; Gray, K.; Jones, T.E.M.; Tweedie, S.; Haim-Vilmovsky, L.; Bruford, E.A. Genenames.org: The HGNC resources in 2023. Nucleic Acids Res. 2023, 51, D1003–D1009. [Google Scholar] [CrossRef] [PubMed]
- Beigneux, A.P.; Vergnes, L.; Qiao, X.; Quatela, S.; Davis, R.; Watkins, S.M.; Coleman, R.A.; Walzem, R.L.; Philips, M.; Reue, K.; et al. Agpat6—A novel lipid biosynthetic gene required for triacylglycerol production in mammary epithelium. J. Lipid Res. 2006, 47, 734–744. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, K.; Lin, S.; Lin, X. Glycerol-3-phosphate acyltransferases and metabolic syndrome: Recent advances and future perspectives. Expert Rev. Mol. Med. 2022, 24, e30. [Google Scholar] [CrossRef]
- Kojta, I.; Zabielski, P.; Roszczyc-Owsiejczuk, K.; Imierska, M.; Sokołowska, E.; Błachnio-Zabielska, A. GPAT Gene Silencing in Muscle Reduces Diacylglycerols Content and Improves Insulin Action in Diet-Induced Insulin Resistance. Int. J. Mol. Sci. 2020, 21, 7369. [Google Scholar] [CrossRef]
- Nagle, C.A.; An, J.; Shiota, M.; Torres, T.P.; Cline, G.W.; Liu, Z.X.; Wang, S.; Catlin, R.L.; Shulman, G.I.; Newgard, C.B.; et al. Hepatic overexpression of glycerol-sn-3-phosphate acyltransferase 1 in rats causes insulin resistance. J. Biol. Chem. 2007, 282, 14807–14815. [Google Scholar] [CrossRef] [PubMed]
- Ghouse, J.; Sveinbjörnsson, G.; Vujkovic, M.; Seidelin, A.S.; Gellert-Kristensen, H.; Ahlberg, G.; Tragante, V.; Rand, S.A.; Brancale, J.; Vilarinho, S.; et al. Integrative common and rare variant analyses provide insights into the genetic architecture of liver cirrhosis. Nat. Genet. 2024, 56, 827–837. [Google Scholar] [CrossRef]
- Lewin, T.M.; de Jong, H.; Schwerbrock, N.J.; Hammond, L.E.; Watkins, S.M.; Combs, T.P.; Coleman, R.A. Mice deficient in mitochondrial glycerol-3-phosphate acyltransferase-1 have diminished myocardial triacylglycerol accumulation during lipogenic diet and altered phospholipid fatty acid composition. Biochim. Biophys. Acta 2008, 1781, 352–358. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; Yang, X.; Liu, X.; Guo, Z.; Lin, X.; Li, L.; Huang, Z. Glycerol-3-phosphate acyltransferase 3-mediated lipid droplets accumulation confers chemoresistance of colorectal cancer. MedComm 2020, 5, e486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cooper, D.E.; Grevengoed, T.J.; Li, L.O.; Klett, E.L.; Eaton, J.M.; Harris, T.E.; Coleman, R.A. Glycerol-3-phosphate acyltransferase-4-deficient mice are protected from diet-induced insulin resistance by the enhanced association of mTOR and rictor. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E305–E315. [Google Scholar] [CrossRef]
- Wydysh, E.A.; Medghalchi, S.M.; Vadlamudi, A.; Townsend, C.A. Design and synthesis of small molecule glycerol 3-phosphate acyltransferase inhibitors. J. Med. Chem. 2009, 52, 3317–3327. [Google Scholar] [CrossRef]
- Outlaw, V.K.; Wydysh, E.A.; Vadlamudi, A.; Medghalchi, S.M.; Townsend, C.A. Design, Synthesis, and Evaluation of 4- and 5-Substituted o-(Octanesulfonamido)benzoic Acids as Inhibitors of Glycerol-3-Phosphate Acyltransferase. MedChemComm 2014, 5, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Kuhajda, F.P.; Aja, S.; Tu, Y.; Han, W.F.; Medghalchi, S.M.; El Meskini, R.; Landree, L.E.; Peterson, J.M.; Daniels, K.; Wong, K.; et al. Pharmacological glycerol-3-phosphate acyltransferase inhibition decreases food intake and adiposity and increases insulin sensitivity in diet-induced obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R116–R130. [Google Scholar] [CrossRef]
- McFadden, J.W.; Aja, S.; Li, Q.; Bandaru, V.V.; Kim, E.K.; Haughey, N.J.; Kuhajda, F.P.; Ronnett, G.V. Increasing fatty acid oxidation remodels the hypothalamic neurometabolome to mitigate stress and inflammation. PLoS ONE 2014, 9, e115642. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Irifune, H.; Kochi, Y.; Miyamoto, T.; Sakoda, T.; Kato, K.; Kunisaki, Y.; Akashi, K.; Kikushige, Y. GPAM mediated lysophosphatidic acid synthesis regulates mitochondrial dynamics in acute myeloid leukemia. Cancer Sci. 2023, 114, 3247–3258. [Google Scholar] [CrossRef] [PubMed]
- Irifune, H. Identification of GPAT1-dependent mitochondrial metabolism as a novel therapeutic target for AML. Rinsho Ketsueki 2022, 63, 353–362. [Google Scholar] [PubMed]
- Marchan, R.; Büttner, B.; Lambert, J.; Edlund, K.; Glaeser, I.; Blaszkewicz, M.; Leonhardt, G.; Marienhoff, L.; Kaszta, D.; Anft, M.; et al. Glycerol-3-phosphate Acyltransferase 1 Promotes Tumor Cell Migration and Poor Survival in Ovarian Carcinoma. Cancer Res. 2017, 77, 4589–4601. [Google Scholar] [CrossRef] [PubMed]
- Brockmöller, S.F.; Bucher, E.; Müller, B.M.; Budczies, J.; Hilvo, M.; Griffin, J.L.; Orešič, M.; Kallioniemi, O.; Iljin, K.; Loibl, S.; et al. Integration of metabolomics and expression of glycerol-3-phosphate acyltransferase (GPAM) in breast cancer-link to patient survival, hormone receptor status, and metabolic profiling. J. Proteome Res. 2012, 11, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Collison, L.W.; Murphy, E.J.; Jolly, C.A. Glycerol-3-phosphate acyltransferase-1 regulates murine T-lymphocyte proliferation and cytokine production. Am. J. Physiol. Cell Physiol. 2008, 295, C1543–C1549. [Google Scholar] [CrossRef]
- Prasad, S.S.; Garg, A.; Agarwal, A.K. Enzymatic activities of the human AGPAT isoform 3 and isoform 5: Localization of AGPAT5 to mitochondria. J. Lipid Res. 2011, 52, 451–462. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Sukumaran, S.; Bartz, R.; Barnes, R.I.; Garg, A. Functional characterization of human 1-acylglycerol-3-phosphate-O-acyltransferase isoform 9: Cloning, tissue distribution, gene structure, and enzymatic activity. J. Endocrinol. 2007, 193, 445–457. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Garg, A. Enzymatic activity of the human 1-acylglycerol-3-phosphate-O-acyltransferase isoform 11: Upregulated in breast and cervical cancers. J. Lipid Res. 2010, 51, 2143–2152. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Barnes, R.I.; Garg, A. Functional characterization of human 1-acylglycerol-3-phosphate acyltransferase isoform 8: Cloning, tissue distribution, gene structure, and enzymatic activity. Arch. Biochem. Biophys. 2006, 449, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.K.; Sukumaran, S.; Cortés, V.A.; Tunison, K.; Mizrachi, D.; Sankella, S.; Gerard, R.D.; Horton, J.D.; Garg, A. Human 1-acylglycerol-3-phosphate O-acyltransferase isoforms 1 and 2: Biochemical characterization and inability to rescue hepatic steatosis in Agpat2−/− gene lipodystrophic mice. J. Biol. Chem. 2011, 286, 37676–37691. [Google Scholar] [CrossRef]
- Subauste, A.R.; Elliott, B.; Das, A.K.; Burant, C.F. A role for 1-acylglycerol-3-phosphate-O-acyltransferase-1 in myoblast differentiation. Differentiation 2010, 80, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, N.; Ahmed, M.; Raffan, E.; Stewart, C.L.; O’Rahilly, S.; Semple, R.K.; Raef, H.; Rochford, J.J. Identification and Characterisation of a Novel Pathogenic Mutation in the Human Lipodystrophy Gene AGPAT2:C48R: A Novel Mutation in AGPAT2. JIMD Rep. 2013, 9, 73–80. [Google Scholar] [PubMed]
- Haghighi, A.; Razzaghy-Azar, M.; Talea, A.; Sadeghian, M.; Ellard, S.; Haghighi, A. Identification of a novel nonsense mutation and a missense substitution in the AGPAT2 gene causing congenital generalized lipodystrophy type 1. Eur. J. Med. Genet. 2012, 55, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Ceccarini, G.; Magno, S.; Pelosini, C.; Ferrari, F.; Sessa, M.R.; Scabia, G.; Maffei, M.; Jéru, I.; Lascols, O.; Vigouroux, C.; et al. Congenital Generalized Lipoatrophy (Berardinelli-Seip Syndrome) Type 1: Description of Novel AGPAT2 Homozygous Variants Showing the Highly Heterogeneous Presentation of the Disease. Front. Endocrinol. 2020, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Niesporek, S.; Denkert, C.; Weichert, W.; Köbel, M.; Noske, A.; Sehouli, J.; Singer, J.W.; Dietel, M.; Hauptmann, S. Expression of lysophosphatidic acid acyltransferase beta (LPAAT-beta) in ovarian carcinoma: Correlation with tumour grading and prognosis. Br. J. Cancer 2005, 92, 1729–1736. [Google Scholar] [CrossRef]
- Diefenbach, C.S.; Soslow, R.A.; Iasonos, A.; Linkov, I.; Hedvat, C.; Bonham, L.; Singer, J.; Barakat, R.R.; Aghajanian, C.; Dupont, J. Lysophosphatidic acid acyltransferase-beta (LPAAT-beta) is highly expressed in advanced ovarian cancer and is associated with aggressive histology and poor survival. Cancer 2006, 107, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllou, E.A.; Georgatsou, E.; Mylonis, I.; Simos, G.; Paraskeva, E. Expression of AGPAT2, an enzyme involved in the glycerophospholipid/triacylglycerol biosynthesis pathway, is directly regulated by HIF-1 and promotes survival and etoposide resistance of cancer cells under hypoxia. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1142–1152. [Google Scholar] [CrossRef]
- Song, L.; Duan, P.; Gan, Y.; Li, P.; Zhao, C.; Xu, J.; Zhang, Z.; Zhou, Q. Silencing LPAATβ inhibits tumor growth of cisplatin-resistant human osteosarcoma in vivo and in vitro. Int. J. Oncol. 2017, 50, 535–544. [Google Scholar] [CrossRef]
- Gong, B.; Hong, F.; Kohm, C.; Bonham, L.; Klein, P. Synthesis and SAR of 2-arylbenzoxazoles, benzothiazoles and benzimidazoles as inhibitors of lysophosphatidic acid acyltransferase-beta. Bioorg. Med. Chem. Lett. 2004, 14, 1455–1459. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Hong, F.; Kohm, C.; Jenkins, S.; Tulinsky, J.; Bhatt, R.; De Vries, P.; Singer, J.W.; Klein, P. Synthesis, SAR, and antitumor properties of diamino-C,N-diarylpyrimidine positional isomers: Inhibitors of lysophosphatidic acid acyltransferase-beta. Bioorg. Med. Chem. Lett. 2004, 14, 2303–2308. [Google Scholar] [CrossRef] [PubMed]
- La Rosée, P.; Jia, T.; Demehri, S.; Härtel, N.; de Vries, P.; Bonham, L.; Hollenback, D.; Singer, J.W.; Melo, J.V.; Druker, B.J.; et al. Antileukemic activity of lysophosphatidic acid acyltransferase-beta inhibitor CT32228 in chronic myelogenous leukemia sensitive and resistant to imatinib. Clin. Cancer Res. 2006, 12, 6540–6546. [Google Scholar] [CrossRef] [PubMed]
- Valentine, W.J.; Tokuoka, S.M.; Hishikawa, D.; Kita, Y.; Shindou, H.; Shimizu, T. LPAAT3 incorporates docosahexaenoic acid into skeletal muscle cell membranes and is upregulated by PPARδ activation. J. Lipid Res. 2018, 59, 184–194. [Google Scholar] [CrossRef]
- Iizuka-Hishikawa, Y.; Hishikawa, D.; Sasaki, J.; Takubo, K.; Goto, M.; Nagata, K.; Nakanishi, H.; Shindou, H.; Okamura, T.; Ito, C.; et al. Lysophosphatidic acid acyltransferase 3 tunes the membrane status of germ cells by incorporating docosahexaenoic acid during spermatogenesis. J. Biol. Chem. 2017, 292, 12065–12076. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Fick, K.; Patel, V.; Hilton, L.R.; Kim, H.W.; Bagi, Z.; Weintraub, N.L.; Chen, W. AGPAT3 Deficiency Impairs Adipocyte Differentiation and Leads to a Lean Phenotype in Mice. Am. J. Physiol. Endocrinol. Metab. 2024, 8. [Google Scholar]
- Malik, M.A.; Saqib, M.A.N.; Mientjes, E.; Acharya, A.; Alam, M.R.; Wallaard, I.; Schrauwen, I.; Bamshad, M.J.; Santos-Cortez, R.L.P.; Elgersma, Y.; et al. A loss of function variant in AGPAT3 underlies intellectual disability and retinitis pigmentosa (IDRP) syndrome. Eur. J. Hum. Genet. 2023, 31, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Viggiano, M.; Ceroni, F.; Visconti, P.; Posar, A.; Scaduto, M.C.; Sandoni, L.; Baravelli, I.; Cameli, C.; Rochat, M.J.; Maresca, A.; et al. Genomic analysis of 116 autism families strengthens known risk genes and highlights promising candidates. NPJ Genom. Med. 2024, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Shi, R.; Xiang, W.; Kang, X.; Tang, B.; Li, C.; Gao, L.; Zhang, X.; Zhang, L.; Dai, R.; et al. The Agpat4/LPA axis in colorectal cancer cells regulates antitumor responses via p38/p65 signaling in macrophages. Signal Transduct. Target. Ther. 2020, 5, 24. [Google Scholar] [CrossRef]
- Xiong, Z.; Lin, Y.; Yu, Y.; Zhou, X.; Fan, J.; Rog, C.J.; Cai, K.; Wang, Z.; Chang, Z.; Wang, G.; et al. Exploration of Lipid Metabolism in Gastric Cancer: A Novel Prognostic Genes Expression Profile. Front. Oncol. 2021, 11, 712746. [Google Scholar] [CrossRef]
- Eto, M.; Shindou, H.; Shimizu, T. A novel lysophosphatidic acid acyltransferase enzyme (LPAAT4) with a possible role for incorporating docosahexaenoic acid into brain glycerophospholipids. Biochem. Biophys. Res. Commun. 2014, 443, 718–724. [Google Scholar] [CrossRef]
- Bradley, R.M.; Marvyn, P.M.; Aristizabal Henao, J.J.; Mardian, E.B.; George, S.; Aucoin, M.G.; Stark, K.D.; Duncan, R.E. Acylglycerophosphate acyltransferase 4 (AGPAT4) is a mitochondrial lysophosphatidic acid acyltransferase that regulates brain phosphatidylcholine, phosphatidylethanolamine, and phosphatidylinositol levels. Biochim. Biophys. Acta 2015, 1851, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Sun, J.; Xiu, W.; Liu, X.; Chai, Y.; Zhou, Y. Low Expression of AGPAT5 Is Associated with Clinical Stage and Poor Prognosis in Colorectal Cancer and Contributes to Tumour Progression. Clin. Med. Insights Oncol. 2022, 16, 11795549221137399. [Google Scholar] [CrossRef] [PubMed]
- Harayama, T.; Shindou, H.; Shimizu, T. Biosynthesis of phosphatidylcholine by human lysophosphatidylcholine acyltransferase 1. J. Lipid Res. 2009, 50, 1824–1831. [Google Scholar] [CrossRef]
- Li, Z.; Hu, Y.; Zheng, H.; Li, M.; Liu, Y.; Feng, R.; Li, X.; Zhang, S.; Tang, M.; Yang, M.; et al. LPCAT1-mediated membrane phospholipid remodelling promotes ferroptosis evasion and tumour growth. Nat. Cell Biol. 2024, 26, 811–824. [Google Scholar] [CrossRef]
- Akagi, S.; Kono, N.; Ariyama, H.; Shindou, H.; Shimizu, T.; Arai, H. Lysophosphatidylcholine acyltransferase 1 protects against cytotoxicity induced by polyunsaturated fatty acids. FASEB J. 2016, 30, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Yu, G.; Mao, Y.; Song, S.; Li, L.; Zhou, L.; Wang, Z.; Liu, Y.; Li, M.; Xu, B. LPCAT1 enhances castration resistant prostate cancer progression via increased mRNA synthesis and PAF production. PLoS ONE 2020, 15, e0240801. [Google Scholar] [CrossRef]
- Zou, C.; Ellis, B.M.; Smith, R.M.; Chen, B.B.; Zhao, Y.; Mallampalli, R.K. Acyl-CoA:lysophosphatidylcholine acyltransferase I (Lpcat1) catalyzes histone protein O-palmitoylation to regulate mRNA synthesis. J. Biol. Chem. 2011, 286, 28019–28025. [Google Scholar] [CrossRef]
- Moessinger, C.; Kuerschner, L.; Spandl, J.; Shevchenko, A.; Thiele, C. Human lysophosphatidylcholine acyltransferases 1 and 2 are located in lipid droplets where they catalyze the formation of phosphatidylcholine. J. Biol. Chem. 2011, 286, 21330–21339. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Zhen, Y.; Liu, W.; Wang, Y.; Wang, R.; Wang, N.; Huang, S.; Yan, J.; Sun, Q. LPCAT1 Facilitates Keratinocyte Hyperproliferation and Skin Inflammation in Psoriasis by Regulating GLUT3. J. Investig. Dermatol. 2024, in press. [Google Scholar] [CrossRef]
- Du, Y.; Wang, Q.; Zhang, X.; Wang, X.; Qin, C.; Sheng, Z.; Yin, H.; Jiang, C.; Li, J.; Xu, T. Lysophosphatidylcholine acyltransferase 1 upregulation and concomitant phospholipid alterations in clear cell renal cell carcinoma. J. Exp. Clin. Cancer Res. 2017, 36, 66. [Google Scholar] [CrossRef] [PubMed]
- Lebok, P.; von Hassel, A.; Meiners, J.; Hube-Magg, C.; Simon, R.; Höflmayer, D.; Hinsch, A.; Dum, D.; Fraune, C.; Göbel, C.; et al. Up-regulation of lysophosphatidylcholine acyltransferase 1 (LPCAT1) is linked to poor prognosis in breast cancer. Aging 2019, 11, 7796–7804. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Dong, X.; Lu, H.; Tong, F.; Chen, L.; Zhang, R.; Dong, J.; Hu, Y.; Wu, G.; Dong, X. LPCAT1 promotes brain metastasis of lung adenocarcinoma by up-regulating PI3K/AKT/MYC pathway. J. Exp. Clin. Cancer Res. 2019, 38, 95. [Google Scholar] [CrossRef]
- Liu, F.; Wu, Y.; Liu, J.; Ni, R.J.; Yang, A.G.; Bian, K.; Zhang, R. A miR-205-LPCAT1 axis contributes to proliferation and progression in multiple cancers. Biochem. Biophys. Res. Commun. 2020, 527, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, K.; Xiang, Q.; Zhao, L.; Tan, B.; Ju, P.; Lan, X.; Liu, Y.; Zhang, J.; Fu, Z.; et al. LPCAT1 functions as a novel prognostic molecular marker in hepatocellular carcinoma. Genes Dis. 2020, 9, 151–164. [Google Scholar] [CrossRef]
- Wang, K.; Wu, Z.; Si, Y.; Tang, W.; Xu, X.; Cheng, Y.; Lin, J. Identification of LPCAT1 expression as a potential prognostic biomarker guiding treatment choice in acute myeloid leukemia. Oncol. Lett. 2021, 21, 105. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Luo, J.; Gu, T.; Yu, X.; Song, Z.; Jun, Y.; Gu, H.; Han, K.; Huang, X.; Yu, W.; et al. LPCAT1 reprogramming cholesterol metabolism promotes the progression of esophageal squamous cell carcinoma. Cell Death Dis. 2021, 12, 845. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhang, Y.; Ma, X.; Wei, L.; Hou, Y.; Sun, R.; Jiang, J. Elevated expression of LPCAT1 predicts a poor prognosis and is correlated with the tumour microenvironment in endometrial cancer. Cancer Cell Int. 2021, 21, 269. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chen, J.; Zhang, T.; Liu, N. LPCAT1 functions as an oncogene in cervical cancer through mediating JAK2/STAT3 signaling. Exp. Cell Res. 2022, 421, 113360. [Google Scholar] [CrossRef]
- Shen, L.; Gu, P.; Qiu, C.; Ding, W.T.; Zhang, L.; Cao, W.Y.; Li, Z.Y.; Yan, B.; Sun, X. Lysophosphatidylcholine acyltransferase 1 promotes epithelial-mesenchymal transition of hepatocellular carcinoma via the Wnt/β-catenin signaling pathway. Ann. Hepatol. 2022, 27, 100680. [Google Scholar] [CrossRef]
- Zhao, T.; Sun, R.; Ma, X.; Wei, L.; Hou, Y.; Song, K.; Jiang, J. Overexpression of LPCAT1 enhances endometrial cancer stemness and metastasis by changing lipid components and activating the TGF/β-Smad2/3 signaling pathway. Acta Biochim. Biophys. Sin. 2022, 54, 904–916. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, J.; Ye, Q.; Jiang, T.; Liu, X.; Zhang, X.; Zeng, F.; Li, J.; Zheng, Y.; Han, X.; et al. LPGAT1 controls MEGDEL syndrome by coupling phosphatidylglycerol remodeling with mitochondrial transport. Cell Rep. 2023, 42, 113214. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zheng, J.; Cai, X.; Liu, L.; Jiang, S.; Liu, Q.; Sun, Y. Glycometabolism and lipid metabolism related genes predict the prognosis of endometrial carcinoma and their effects on tumor cells. BMC Cancer 2024, 24, 571. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xu, Z.; Jiang, J.; Chen, H.; Shi, R. Identification of LPCAT1 as a key biomarker for Crohn’s disease based on bioinformatics and machine learnings and experimental verification. Gene 2024, 920, 148519. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Wang, Y.; Zhang, W.; Wang, N.; Bai, R.; Luo, R.; Tuo, H.; Zheng, Y. LPCAT1 promotes melanoma cell proliferation via Akt signaling. Oncol. Rep. 2024, 51, 67. [Google Scholar] [CrossRef] [PubMed]
- Shida-Sakazume, T.; Endo-Sakamoto, Y.; Unozawa, M.; Fukumoto, C.; Shimada, K.; Kasamatsu, A.; Ogawara, K.; Yokoe, H.; Shiiba, M.; Tanzawa, H.; et al. Lysophosphatidylcholine acyltransferase1 overexpression promotes oral squamous cell carcinoma progression via enhanced biosynthesis of platelet-activating factor. PLoS ONE 2015, 10, e0120143. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.F.; Rangel, M.C.; Halman, J.R.; Chandler, M.; de Sousa Andrade, L.N.; Odete-Bustos, S.; Furuya, T.K.; Carrasco, A.G.M.; Chaves-Filho, A.B.; Yoshinaga, M.Y.; et al. Simultaneous silencing of lysophosphatidylcholine acyltransferases 1-4 by nucleic acid nanoparticles (NANPs) improves radiation response of melanoma cells. Nanomedicine 2021, 36, 102418. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Fu, C.; Liu, J.; Li, S.; Zheng, J. Knockdown of LPCAT1 Repressed Hepatocellular Carcinoma Growth and Invasion by Targeting S100A11. Ann. Clin. Lab. Sci. 2023, 53, 212–221. [Google Scholar] [PubMed]
- Huang, Y.; Wang, Y.; Wang, Y.; Wang, N.; Duan, Q.; Wang, S.; Liu, M.; Bilal, M.A.; Zheng, Y. LPCAT1 Promotes Cutaneous Squamous Cell Carcinoma via EGFR-Mediated Protein Kinase B/p38MAPK Signaling Pathways. J. Investig. Dermatol. 2022, 142, 303–313.e9. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, Y. LPCAT1 is transcriptionally regulated by FOXA1 to promote breast cancer progression and paclitaxel resistance. Oncol. Lett. 2023, 25, 134. [Google Scholar] [CrossRef]
- Ding, J.; Ding, X.; Leng, Z. LPCAT1 promotes gefitinib resistance via upregulation of the EGFR/PI3K/AKT signaling pathway in lung adenocarcinoma. J. Cancer 2022, 13, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Shindou, H.; Hishikawa, D.; Nakanishi, H.; Harayama, T.; Ishii, S.; Taguchi, R.; Shimizu, T. A single enzyme catalyzes both platelet-activating factor production and membrane biogenesis of inflammatory cells. Cloning and characterization of acetyl-CoA:LYSO-PAF acetyltransferase. J. Biol. Chem. 2007, 282, 6532–6539. [Google Scholar] [CrossRef] [PubMed]
- Abate, W.; Alrammah, H.; Kiernan, M.; Tonks, A.J.; Jackson, S.K. Lysophosphatidylcholine acyltransferase 2 (LPCAT2) co-localises with TLR4 and regulates macrophage inflammatory gene expression in response to LPS. Sci. Rep. 2020, 10, 10355. [Google Scholar] [CrossRef] [PubMed]
- Cotte, A.K.; Aires, V.; Fredon, M.; Limagne, E.; Derangère, V.; Thibaudin, M.; Humblin, E.; Scagliarini, A.; de Barros, J.P.; Hillon, P.; et al. Lysophosphatidylcholine acyltransferase 2-mediated lipid droplet production supports colorectal cancer chemoresistance. Nat. Commun. 2018, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Englinger, B.; Laemmerer, A.; Moser, P.; Kallus, S.; Röhrl, C.; Pirker, C.; Baier, D.; Mohr, T.; Niederstaetter, L.; Meier-Menches, S.M.; et al. Lipid droplet-mediated scavenging as a novel intrinsic and adaptive resistance factor against the multikinase inhibitor ponatinib. Int. J. Cancer 2020, 147, 1680–1693. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Zhang, F.; Yin, J.; Zhang, J.; Bian, X.; Zheng, G.; Li, N.; Lin, Y.; Luo, L. LPCAT2 inhibits colorectal cancer progression via the PRMT1/SLC7A11 axis. Oncogene 2024, 43, 1714–1725. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.A.; Lee, M.; Hu, Y.; Andreas, J.; Patel, S.J.; Zhang, S.; Chines, P.; Elkahloun, A.; Chandrasekharappa, S.; Gutkind, J.S.; et al. A systems genetics approach identifies CXCL14, ITGAX, and LPCAT2 as novel aggressive prostate cancer susceptibility genes. PLoS Genet. 2014, 10, e1004809. [Google Scholar] [CrossRef] [PubMed]
- Gijón, M.A.; Riekhof, W.R.; Zarini, S.; Murphy, R.C.; Voelker, D.R. Lysophospholipid acyltransferases and arachidonate recycling in human neutrophils. J. Biol. Chem. 2008, 283, 30235–30245. [Google Scholar] [CrossRef] [PubMed]
- Kazachkov, M.; Chen, Q.; Wang, L.; Zou, J. Substrate preferences of a lysophosphatidylcholine acyltransferase highlights its role in phospholipid remodeling. Lipids 2008, 43, 895–902. [Google Scholar] [CrossRef]
- Matsuda, S.; Inoue, T.; Lee, H.C.; Kono, N.; Tanaka, F.; Gengyo-Ando, K.; Mitani, S.; Arai, H. Member of the membrane-bound O-acyltransferase (MBOAT) family encodes a lysophospholipid acyltransferase with broad substrate specificity. Genes Cells 2008, 13, 879–888. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Y.Q.; Bonacci, T.M.; Bredt, D.S.; Li, S.; Bensch, W.R.; Moller, D.E.; Kowala, M.; Konrad, R.J.; Cao, G. Identification and characterization of a major liver lysophosphatidylcholine acyltransferase. J. Biol. Chem. 2008, 283, 8258–8265. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Zhang, X.; Khandelwal, P.J.; Saunders, A.J.; Cummings, B.S.; Oelkers, P. Characterization of human lysophospholipid acyltransferase 3. J. Lipid Res. 2009, 50, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Eto, M.; Shindou, H.; Koeberle, A.; Harayama, T.; Yanagida, K.; Shimizu, T. Lysophosphatidylcholine acyltransferase 3 is the key enzyme for incorporating arachidonic acid into glycerophospholipids during adipocyte differentiation. Int. J. Mol. Sci. 2012, 13, 16267–16280. [Google Scholar] [CrossRef] [PubMed]
- Hashidate-Yoshida, T.; Harayama, T.; Hishikawa, D.; Morimoto, R.; Hamano, F.; Tokuoka, S.M.; Eto, M.; Tamura-Nakano, M.; Yanobu-Takanashi, R.; Mukumoto, Y.; et al. Fatty acid remodeling by LPCAT3 enriches arachidonate in phospholipid membranes and regulates triglyceride transport. Elife 2015, 4, e06328. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.; Ichu, T.A.; Milosevich, N.; Melillo, B.; Schafroth, M.A.; Otsuka, Y.; Scampavia, L.; Spicer, T.P.; Cravatt, B.F. LPCAT3 Inhibitors Remodel the Polyunsaturated Phospholipid Content of Human Cells and Protect from Ferroptosis. ACS Chem. Biol. 2022, 17, 1607–1618. [Google Scholar] [CrossRef]
- Cui, J.; Wang, Y.; Tian, X.; Miao, Y.; Ma, L.; Zhang, C.; Xu, X.; Wang, J.; Fang, W.; Zhang, X. LPCAT3 Is Transcriptionally Regulated by YAP/ZEB/EP300 and Collaborates with ACSL4 and YAP to Determine Ferroptosis Sensitivity. Antioxid. Redox Signal. 2023, 39, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ding, T.; Pan, X.; Li, Y.; Li, R.; Sanders, P.E.; Kuo, M.S.; Hussain, M.M.; Cao, G.; Jiang, X.C. Lysophosphatidylcholine acyltransferase 3 knockdown-mediated liver lysophosphatidylcholine accumulation promotes very low density lipoprotein production by enhancing microsomal triglyceride transfer protein expression. J. Biol. Chem. 2012, 287, 20122–20131. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, P.J.; Rong, X.; Maschek, J.A.; Verkerke, A.R.; Siripoksup, P.; Song, H.; Green, T.D.; Krishnan, K.C.; Johnson, J.M.; Turk, J.; et al. Lysophospholipid acylation modulates plasma membrane lipid organization and insulin sensitivity in skeletal muscle. J. Clin. Investig. 2021, 131, e135963. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, H.; Ding, T.; Lou, C.; Bui, H.H.; Kuo, M.S.; Jiang, X.C. Deficiency in lysophosphatidylcholine acyltransferase 3 reduces plasma levels of lipids by reducing lipid absorption in mice. Gastroenterology 2015, 149, 1519–1529. [Google Scholar] [CrossRef]
- He, M.; Li, Z.; Tung, V.S.K.; Pan, M.; Han, X.; Evgrafov, O.; Jiang, X.C. Inhibiting Phosphatidylcholine Remodeling in Adipose Tissue Increases Insulin Sensitivity. Diabetes 2023, 72, 1547–1559. [Google Scholar] [CrossRef]
- Ferrara, P.J.; Verkerke, A.R.P.; Maschek, J.A.; Shahtout, J.L.; Siripoksup, P.; Eshima, H.; Johnson, J.M.; Petrocelli, J.J.; Mahmassani, Z.S.; Green, T.D.; et al. Low lysophosphatidylcholine induces skeletal muscle myopathy that is aggravated by high-fat diet feeding. FASEB J. 2021, 35, e21867. [Google Scholar] [CrossRef]
- Shahtout, J.L.; Eshima, H.; Ferrara, P.J.; Maschek, J.A.; Cox, J.E.; Drummond, M.J.; Funai, K. Inhibition of the skeletal muscle Lands cycle ameliorates weakness induced by physical inactivity. J. Cachexia Sarcopenia Muscle 2024, 15, 319–330. [Google Scholar] [CrossRef]
- Tian, Y.; Jellinek, M.J.; Mehta, K.; Seok, S.M.; Kuo, S.H.; Lu, W.; Shi, R.; Lee, R.; Lau, G.W.; Kemper, J.K.; et al. Membrane phospholipid remodeling modulates nonalcoholic steatohepatitis progression by regulating mitochondrial homeostasis. Hepatology 2023, 79, 882–897. [Google Scholar] [CrossRef]
- Hu, J.; Deng, Y.; Ding, T.; Dong, J.; Liang, Y.; Lou, B. Lpcat3 deficiency promotes palmitic acid-induced 3T3-L1 mature adipocyte inflammation through enhanced ROS generation. Acta Biochim. Biophys. Sin. 2022, 55, 117–130. [Google Scholar] [CrossRef]
- Kondreddy, V.; Banerjee, R.; Devi, B.L.A.P.; Muralidharan, K.; Piramanayagam, S. Inhibition of the MALT1-LPCAT3 axis protects cartilage degeneration and osteoarthritis. Cell Commun. Signal. 2024, 22, 189. [Google Scholar] [CrossRef]
- Ke, P.; Bao, X.; Liu, C.; Zhou, B.; Huo, M.; Chen, Y.; Wang, X.; Wu, D.; Ma, X.; Liu, D.; et al. LPCAT3 is a potential prognostic biomarker and may be correlated with immune infiltration and ferroptosis in acute myeloid leukemia: A pan-cancer analysis. Transl. Cancer Res. 2022, 11, 3491–3505. [Google Scholar] [CrossRef]
- Wang, B.; Rong, X.; Palladino, E.N.D.; Wang, J.; Fogelman, A.M.; Martín, M.G.; Alrefai, W.A.; Ford, D.A.; Tontonoz, P. Phospholipid Remodeling and Cholesterol Availability Regulate Intestinal Stemness and Tumorigenesis. Cell Stem Cell 2018, 22, 206–220.e4. [Google Scholar] [CrossRef]
- Tian, Y.; Lu, W.; Shi, R.; McGuffee, R.; Lee, R.; Ford, D.A.; Wang, B. Targeting phospholipid remodeling pathway improves insulin resistance in diabetic mouse models. FASEB J. 2023, 37, e23251. [Google Scholar] [CrossRef]
- Ye, G.M.; Chen, C.; Huang, S.; Han, D.D.; Guo, J.H.; Wan, B.; Yu, L. Cloning and characterization a novel human 1-acyl-sn-glycerol-3-phosphate acyltransferase gene AGPAT7. DNA Seq. 2005, 16, 386–390. [Google Scholar] [CrossRef]
- Eto, M.; Shindou, H.; Yamamoto, S.; Tamura-Nakano, M.; Shimizu, T. Lysophosphatidylethanolamine acyltransferase 2 (LPEAT2) incorporates DHA into phospholipids and has possible functions for fatty acid-induced cell death. Biochem. Biophys. Res. Commun. 2020, 526, 246–252. [Google Scholar] [CrossRef]
- Mason, A.S.; Varley, C.L.; Foody, O.M.; Li, X.; Skinner, K.; Walker, D.; Larson, T.R.; Wakamatsu, D.; Baker, S.C.; Southgate, J. LPCAT4 Knockdown Alters Barrier Integrity and Cellular Bioenergetics in Human Urothelium. Int. J. Mol. Sci. 2022, 23, 11871. [Google Scholar] [CrossRef]
- Lu, Y.; Liang, H.; Li, X.; Chen, H.; Yang, C. Pan-cancer analysis identifies LPCATs family as a prognostic biomarker and validation of LPCAT4/WNT/β-catenin/c-JUN/ACSL3 in hepatocellular carcinoma. Aging 2023, 15, 4699–4713. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Liu, Y.; Lockwood, J.; Burn, P.; Shi, Y. A novel cardiolipin-remodeling pathway revealed by a gene encoding an endoplasmic reticulum-associated acyl-CoA:lysocardiolipin acyltransferase (ALCAT1) in mouse. J. Biol. Chem. 2004, 279, 31727–31734. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Shen, W.; Chang, Z.; Shi, Y. ALCAT1 is a polyglycerophospholipid acyltransferase potently regulated by adenine nucleotide and thyroid status. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E647–E653. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Wang, H.; Zhang, W.; Chan, D.C.; Shi, Y. Lysocardiolipin acyltransferase 1 (ALCAT1) controls mitochondrial DNA fidelity and biogenesis through modulation of MFN2 expression. Proc. Natl. Acad. Sci. USA 2012, 109, 6975–6980. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.S.; Kotha, S.R.; Avasarala, S.; VanScoyk, M.; Winn, R.A.; Pennathur, A.; Yashaswini, P.S.; Bandela, M.; Salgia, R.; Tyurina, Y.Y.; et al. Lysocardiolipin acyltransferase regulates NSCLC cell proliferation and migration by modulating mitochondrial dynamics. J. Biol. Chem. 2020, 295, 13393–13406. [Google Scholar] [CrossRef]
- Li, J.; Romestaing, C.; Han, X.; Li, Y.; Hao, X.; Wu, Y.; Sun, C.; Liu, X.; Jefferson, L.S.; Xiong, J.; et al. Cardiolipin remodeling by ALCAT1 links oxidative stress and mitochondrial dysfunction to obesity. Cell Metab. 2010, 12, 154–165. [Google Scholar] [CrossRef]
- Huang, L.S.; Mathew, B.; Li, H.; Zhao, Y.; Ma, S.F.; Noth, I.; Reddy, S.P.; Harijith, A.; Usatyuk, P.V.; Berdyshev, E.V.; et al. The mitochondrial cardiolipin remodeling enzyme lysocardiolipin acyltransferase is a novel target in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2014, 189, 1402–1415. [Google Scholar] [CrossRef]
- Song, C.; Zhang, J.; Qi, S.; Liu, Z.; Zhang, X.; Zheng, Y.; Andersen, J.P.; Zhang, W.; Strong, R.; Martinez, P.A.; et al. Cardiolipin remodeling by ALCAT1 links mitochondrial dysfunction to Parkinson′s diseases. Aging Cell 2019, 18, e12941. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, J.; Shi, Y. Identification and characterization of a gene encoding human LPGAT1, an endoplasmic reticulum-associated lysophosphatidylglycerol acyltransferase. J. Biol. Chem. 2004, 279, 55866–55874. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Sun, H.; Liu, X.; Zheng, Y.; Xu, D.; Wang, J.; Jia, D.; Han, X.; Liu, F.; et al. Defective Phosphatidylglycerol Remodeling Causes Hepatopathy, Linking Mitochondrial Dysfunction to Hepatosteatosis. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 763–781. [Google Scholar] [CrossRef]
- Hiramine, Y.; Emoto, H.; Takasuga, S.; Hiramatsu, R. Novel acyl-coenzyme A:monoacylglycerol acyltransferase plays an important role in hepatic triacylglycerol secretion. J. Lipid Res. 2010, 51, 1424–1431. [Google Scholar] [CrossRef]
- Kawana, H.; Ozawa, M.; Shibata, T.; Onishi, H.; Sato, Y.; Kano, K.; Shindou, H.; Shimizu, T.; Kono, N.; Aoki, J. Identification and characterization of LPLAT7 as an sn-1-specific lysophospholipid acyltransferase. J. Lipid Res. 2022, 63, 100271. [Google Scholar] [CrossRef]
- Xu, Y.; Miller, P.C.; Phoon, C.K.L.; Ren, M.; Nargis, T.; Rajan, S.; Hussain, M.M.; Schlame, M. LPGAT1 controls the stearate/palmitate ratio of phosphatidylethanolamine and phosphatidylcholine in sn-1 specific remodeling. J. Biol. Chem. 2022, 298, 101685. [Google Scholar] [CrossRef]
- Sato, T.; Umebayashi, S.; Senoo, N.; Akahori, T.; Ichida, H.; Miyoshi, N.; Yoshida, T.; Sugiura, Y.; Goto-Inoue, N.; Kawana, H.; et al. LPGAT1/LPLAT7 regulates acyl chain profiles at the sn-1 position of phospholipids in murine skeletal muscles. J. Biol. Chem. 2023, 299, 104848. [Google Scholar] [CrossRef]
- Traurig, M.T.; Orczewska, J.I.; Ortiz, D.J.; Bian, L.; Marinelarena, A.M.; Kobes, S.; Malhotra, A.; Hanson, R.L.; Mason, C.C.; Knowler, W.C.; et al. Evidence for a role of LPGAT1 in influencing BMI and percent body fat in Native Americans. Obesity 2013, 21, 193–202. [Google Scholar] [CrossRef]
- Wortmann, S.B.; Vaz, F.M.; Gardeitchik, T.; Vissers, L.E.; Renkema, G.H.; Schuurs-Hoeijmakers, J.H.; Kulik, W.; Lammens, M.; Christin, C.; Kluijtmans, L.A.; et al. Mutations in the phospholipid remodeling gene SERAC1 impair mitochondrial function and intracellular cholesterol trafficking and cause dystonia and deafness. Nat. Genet. 2012, 44, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, X.; Zhang, Y. Identification of a 5-Gene Metabolic Signature for Predicting Prognosis Based on an Integrated Analysis of Tumor Microenvironment in Lung Adenocarcinoma. J. Oncol. 2020, 2020, 5310793. [Google Scholar] [CrossRef]
- Gong, H.; Ma, C.; Li, X.; Zhang, X.; Zhang, L.; Chen, P.; Wang, W.; Hu, Y.; Huang, T.; Wu, N.; et al. Upregulation of LPGAT1 Enhances Lung Adenocarcinoma Proliferation. Front. Biosci. 2023, 28, 89. [Google Scholar] [CrossRef]
- Tabe, S.; Hikiji, H.; Ariyoshi, W.; Hashidate-Yoshida, T.; Shindou, H.; Okinaga, T.; Shimizu, T.; Tominaga, K.; Nishihara, T. Lysophosphatidylethanolamine acyltransferase 1/membrane-bound O-acyltransferase 1 regulates morphology and function of P19C6 cell-derived neurons. FASEB J. 2016, 30, 2591–2601. [Google Scholar] [CrossRef]
- Liang, D.; Feng, Y.; Zandkarimi, F.; Wang, H.; Zhang, Z.; Kim, J.; Cai, Y.; Gu, W.; Stockwell, B.R.; Jiang, X. Ferroptosis surveillance independent of GPX4 and differentially regulated by sex hormones. Cell 2023, 186, 2748–2764.e22. [Google Scholar] [CrossRef] [PubMed]
- Soleimani Zakeri, N.S.; Pashazadeh, S.; MotieGhader, H. Gene biomarker discovery at different stages of Alzheimer using gene co-expression network approach. Sci. Rep. 2020, 10, 12210. [Google Scholar] [CrossRef] [PubMed]
- Dauwerse, J.G.; de Vries, B.B.; Wouters, C.H.; Bakker, E.; Rappold, G.; Mortier, G.R.; Breuning, M.H.; Peters, D.J. A t(4;6)(q12;p23) translocation disrupts a membrane-associated O-acetyl transferase gene (MBOAT1) in a patient with a novel brachydactyly-syndactyly syndrome. Eur. J. Hum. Genet. 2007, 15, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.Y.; Guo, L.; Yao, Y.; Shi, X.Y.; Jiang, H.; Xu, B.; Hua, J.; Zhang, X.S. MBOAT1 homozygous missense variant causes nonobstructive azoospermia. Asian J. Androl. 2022, 24, 186–190. [Google Scholar] [PubMed]
- Li, Z.; Zhuang, H.; Chen, X.; Zhang, Y.; Ma, Z.; Wang, S.; Yan, Q.; Zhou, Z.; Huang, S.; Zhang, C.; et al. Identification of MBOAT2 as an Unfavorable Biomarker Correlated with KRAS Activation and Reduced CD8+ T-Cell Infiltration in Pancreatic Cancer. J. Oncol. 2022, 2022, 4269733. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, A.; Hedfalk, K.; Romeo, S.; Pingitore, P. LPIAT1/MBOAT7 contains a catalytic dyad transferring polyunsaturated fatty acids to lysophosphatidylinositol. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158891. [Google Scholar] [CrossRef] [PubMed]
- Phadnis, V.V.; Snider, J.; Varadharajan, V.; Ramachandiran, I.; Deik, A.A.; Lai, Z.W.; Kunchok, T.; Eaton, E.N.; Sebastiany, C.; Lyakisheva, A.; et al. MMD collaborates with ACSL4 and MBOAT7 to promote polyunsaturated phosphatidylinositol remodeling and susceptibility to ferroptosis. Cell Rep. 2023, 42, 113023. [Google Scholar] [CrossRef] [PubMed]
- Mancina, R.M.; Dongiovanni, P.; Petta, S.; Pingitore, P.; Meroni, M.; Rametta, R.; Borén, J.; Montalcini, T.; Pujia, A.; Wiklund, O.; et al. The MBOAT7-TMC4 Variant rs641738 Increases Risk of Nonalcoholic Fatty Liver Disease in Individuals of European Descent. Gastroenterology 2016, 150, 1219–1230.e6. [Google Scholar] [CrossRef] [PubMed]
- Saliakoura, M.; Reynoso-Moreno, I.; Pozzato, C.; Rossi Sebastiano, M.; Galié, M.; Gertsch, J.; Konstantinidou, G. The ACSL3-LPIAT1 signaling drives prostaglandin synthesis in non-small cell lung cancer. Oncogene 2020, 39, 2948–2960. [Google Scholar] [CrossRef]
- Van Horn, C.G.; Caviglia, J.M.; Li, L.O.; Wang, S.; Granger, D.A.; Coleman, R.A. Characterization of recombinant long-chain rat acyl-CoA synthetase isoforms 3 and 6: Identification of a novel variant of isoform 6. Biochemistry 2005, 44, 1635–1642. [Google Scholar] [CrossRef]
- Helsley, R.N.; Varadharajan, V.; Brown, A.L.; Gromovsky, A.D.; Schugar, R.C.; Ramachandiran, I.; Fung, K.; Kabbany, M.N.; Banerjee, R.; Neumann, C.K.; et al. Obesity-linked suppression of membrane-bound O-acyltransferase 7 (MBOAT7) drives non-alcoholic fatty liver disease. Elife 2019, 8, e49882. [Google Scholar] [CrossRef]
- Meroni, M.; Dongiovanni, P.; Longo, M.; Carli, F.; Baselli, G.; Rametta, R.; Pelusi, S.; Badiali, S.; Maggioni, M.; Gaggini, M.; et al. Mboat7 down-regulation by hyper-insulinemia induces fat accumulation in hepatocytes. EBioMedicine 2020, 52, 102658. [Google Scholar] [CrossRef] [PubMed]
- Alharthi, J.; Bayoumi, A.; Thabet, K.; Pan, Z.; Gloss, B.S.; Latchoumanin, O.; Lundberg, M.; Twine, N.A.; McLeod, D.; Alenizi, S.; et al. A metabolic associated fatty liver disease risk variant in MBOAT7 regulates toll like receptor induced outcomes. Nat. Commun. 2022, 13, 7430. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, M.C.; Pyles, K.D.; Hallcox, T.; Kamm, D.R.; Piechowski, M.; Fisk, B.; Albert, C.J.; Carpenter, D.H.; Ulmasov, B.; Ford, D.A.; et al. Enhancing Hepatic MBOAT7 Expression in Mice with Nonalcoholic Steatohepatitis. Gastro Hep Adv. 2023, 2, 558–572. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Flichman, D.; Garaycoechea, M.E.; Gazzi, C.; Martino, J.S.; Castaño, G.O.; Pirola, C.J. Lack of evidence supporting a role of TMC4-rs641738 missense variant-MBOAT7- intergenic downstream variant-in the Susceptibility to Nonalcoholic Fatty Liver Disease. Sci. Rep. 2018, 8, 5097. [Google Scholar] [CrossRef]
- Ismaiel, A.; Spinu, M.; Osan, S.; Leucuta, D.C.; Popa, S.L.; Chis, B.A.; Farcas, M.; Popp, R.A.; Olinic, D.M.; Dumitrascu, D.L. MBOAT7 rs641738 variant in metabolic-dysfunction-associated fatty liver disease and cardiovascular risk. Med. Pharm. Rep. 2023, 96, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Bianco, C.; Jamialahmadi, O.; Pelusi, S.; Baselli, G.; Dongiovanni, P.; Zanoni, I.; Santoro, L.; Maier, S.; Liguori, A.; Meroni, M.; et al. Non-invasive stratification of hepatocellular carcinoma risk in non-alcoholic fatty liver using polygenic risk scores. J. Hepatol. 2021, 74, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Teo, K.; Abeysekera, K.W.M.; Adams, L.; Aigner, E.; Anstee, Q.M.; Banales, J.M.; Banerjee, R.; Basu, P.; Berg, T.; Bhatnagar, P.; et al. rs641738C>T near MBOAT7 is associated with liver fat, ALT and fibrosis in NAFLD: A meta-analysis. J. Hepatol. 2021, 74, 20–30. [Google Scholar] [CrossRef]
- Longo, M.; Meroni, M.; Paolini, E.; Erconi, V.; Carli, F.; Fortunato, F.; Ronchi, D.; Piciotti, R.; Sabatini, S.; Macchi, C.; et al. TM6SF2/PNPLA3/MBOAT7 Loss-of-Function Genetic Variants Impact on NAFLD Development and Progression Both in Patients and in In Vitro Models. Cell Mol. Gastroenterol. Hepatol. 2022, 13, 759–788. [Google Scholar] [CrossRef] [PubMed]
- Donati, B.; Dongiovanni, P.; Romeo, S.; Meroni, M.; McCain, M.; Miele, L.; Petta, S.; Maier, S.; Rosso, C.; De Luca, L.; et al. MBOAT7 rs641738 variant and hepatocellular carcinoma in non-cirrhotic individuals. Sci. Rep. 2017, 7, 4492. [Google Scholar] [CrossRef]
- Tanaka, Y.; Shimanaka, Y.; Caddeo, A.; Kubo, T.; Mao, Y.; Kubota, T.; Kubota, N.; Yamauchi, T.; Mancina, R.M.; Baselli, G.; et al. LPIAT1/MBOAT7 depletion increases triglyceride synthesis fueled by high phosphatidylinositol turnover. Gut 2021, 70, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Thangapandi, V.R.; Knittelfelder, O.; Brosch, M.; Patsenker, E.; Vvedenskaya, O.; Buch, S.; Hinz, S.; Hendricks, A.; Nati, M.; Herrmann, A.; et al. Loss of hepatic Mboat7 leads to liver fibrosis. Gut 2021, 70, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Chandrasekaran, P.; Rong, S.; Fu, X.; Mitsche, M.A. Hepatic deletion of Mboat7 (LPIAT1) causes activation of SREBP-1c and fatty liver. J. Lipid Res. 2021, 62, 100031. [Google Scholar] [CrossRef] [PubMed]
- Hatch, G.M.; Smith, A.J.; Xu, F.Y.; Hall, A.M.; Bernlohr, D.A. FATP1 channels exogenous FA into 1,2,3-triacyl-sn-glycerol and down-regulates sphingomyelin and cholesterol metabolism in growing 293 cells. J. Lipid Res. 2002, 43, 1380–1389. [Google Scholar] [CrossRef]
- Hall, A.M.; Smith, A.J.; Bernlohr, D.A. Characterization of the Acyl-CoA synthetase activity of purified murine fatty acid transport protein 1. J. Biol. Chem. 2003, 278, 43008–43013. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, M.; Bantel, H.; Rau, M.; Schattenberg, J.M.; Grünhage, F.; Pathil, A.; Demir, M.; Kluwe, J.; Boettler, T.; Weber, S.N.; et al. Could inherited predisposition drive non-obese fatty liver disease? Results from German tertiary referral centers. J. Hum. Genet. 2018, 63, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, H.; Liu, X.; Zhang, S.; Cao, Z.; Qiu, L.; Du, X.; Liu, Y.; Wang, G.; Zhang, L.; et al. MBOAT7 rs641738 (C>T) is associated with NAFLD progression in men and decreased ASCVD risk in elder Chinese population. Front. Endocrinol. 2023, 14, 1199429. [Google Scholar] [CrossRef] [PubMed]
- Basyte-Bacevice, V.; Skieceviciene, J.; Valantiene, I.; Sumskiene, J.; Petrenkiene, V.; Kondrackiene, J.; Petrauskas, D.; Lammert, F.; Kupcinskas, J. TM6SF2 and MBOAT7 Gene Variants in Liver Fibrosis and Cirrhosis. Int. J. Mol. Sci. 2019, 20, 1277. [Google Scholar] [CrossRef] [PubMed]
- Zusi, C.; Morandi, A.; Maguolo, A.; Corradi, M.; Costantini, S.; Mosca, A.; Crudele, A.; Mantovani, A.; Alisi, A.; Miraglia Del Giudice, E.; et al. Association between MBOAT7 rs641738 polymorphism and non-alcoholic fatty liver in overweight or obese children. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1548–1555. [Google Scholar] [CrossRef]
- Massey, W.J.; Varadharajan, V.; Banerjee, R.; Brown, A.L.; Horak, A.J.; Hohe, R.C.; Jung, B.M.; Qiu, Y.; Chan, E.R.; Pan, C.; et al. MBOAT7-driven lysophosphatidylinositol acylation in adipocytes contributes to systemic glucose homeostasis. J. Lipid Res. 2023, 64, 100349. [Google Scholar] [CrossRef]
- Umano, G.R.; Caprio, S.; Di Sessa, A.; Chalasani, N.; Dykas, D.J.; Pierpont, B.; Bale, A.E.; Santoro, N. The rs626283 Variant in the MBOAT7 Gene is Associated with Insulin Resistance and Fatty Liver in Caucasian Obese Youth. Am. J. Gastroenterol. 2018, 113, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Thabet, K.; Chan, H.L.Y.; Petta, S.; Mangia, A.; Berg, T.; Boonstra, A.; Brouwer, W.P.; Abate, M.L.; Wong, V.W.; Nazmy, M.; et al. The membrane-bound O-acyltransferase domain-containing 7 variant rs641738 increases inflammation and fibrosis in chronic hepatitis B. Hepatology 2017, 65, 1840–1850. [Google Scholar] [CrossRef] [PubMed]
- Thabet, K.; Asimakopoulos, A.; Shojaei, M.; Romero-Gomez, M.; Mangia, A.; Irving, W.L.; Berg, T.; Dore, G.J.; Grønbæk, H.; Sheridan, D.; et al. MBOAT7 rs641738 increases risk of liver inflammation and transition to fibrosis in chronic hepatitis C. Nat. Commun. 2016, 7, 12757. [Google Scholar] [CrossRef] [PubMed]
- Ezzikouri, S.; Elfihry, R.; Chihab, H.; Elmessaoudi-Idrissi, M.; Zaidane, I.; Jadid, F.Z.; Karami, A.; Tahiri, M.; Elhabazi, A.; Kabine, M.; et al. Effect of MBOAT7 variant on hepatitis B and C infections in Moroccan patients. Sci. Rep. 2018, 8, 12247. [Google Scholar] [CrossRef] [PubMed]
- Buch, S.; Stickel, F.; Trépo, E.; Way, M.; Herrmann, A.; Nischalke, H.D.; Brosch, M.; Rosendahl, J.; Berg, T.; Ridinger, M.; et al. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat. Genet. 2015, 47, 1443–1448. [Google Scholar] [CrossRef]
- Israelsen, M.; Juel, H.B.; Detlefsen, S.; Madsen, B.S.; Rasmussen, D.N.; Larsen, T.R.; Kjærgaard, M.; Fernandes Jensen, M.J.; Stender, S.; Hansen, T.; et al. Metabolic and Genetic Risk Factors Are the Strongest Predictors of Severity of Alcohol-Related Liver Fibrosis. Clin. Gastroenterol. Hepatol. 2022, 20, 1784–1794.e9. [Google Scholar] [CrossRef] [PubMed]
- Varadharajan, V.; Ramachandiran, I.; Massey, W.J.; Jain, R.; Banerjee, R.; Horak, A.J.; McMullen, M.R.; Huang, E.; Bellar, A.; Lorkowski, S.W.; et al. Membrane-bound O-acyltransferase 7 (MBOAT7) shapes lysosomal lipid homeostasis and function to control alcohol-associated liver injury. Elife 2024, 12, RP92243. [Google Scholar] [CrossRef] [PubMed]
- Freund, C.; Wahlers, A.; Begli, N.H.; Leopold, Y.; Klöters-Plachky, P.; Mehrabi, A.; Mohr, I.; Sander, J.; Rupp, C.; Gotthardt, D.N.; et al. The MBOAT7 rs641738 variant is associated with an improved outcome in primary sclerosing cholangitis. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Neumann, C.K.A.; Silver, D.J.; Venkateshwari, V.; Zhang, R.; Traughber, C.A.; Przybycin, C.; Bayik, D.; Smith, J.D.; Lathia, J.D.; Rini, B.I.; et al. MBOAT7-driven phosphatidylinositol remodeling promotes the progression of clear cell renal carcinoma. Mol. Metab. 2020, 34, 136–145. [Google Scholar] [CrossRef]
- Lee, H.C.; Inoue, T.; Sasaki, J.; Kubo, T.; Matsuda, S.; Nakasaki, Y.; Hattori, M.; Tanaka, F.; Udagawa, O.; Kono, N.; et al. LPIAT1 regulates arachidonic acid content in phosphatidylinositol and is required for cortical lamination in mice. Mol. Biol. Cell 2012, 23, 4689–4700. [Google Scholar] [CrossRef]
- Johansen, A.; Rosti, R.O.; Musaev, D.; Sticca, E.; Harripaul, R.; Zaki, M.; Çağlayan, A.O.; Azam, M.; Sultan, T.; Froukh, T.; et al. Mutations in MBOAT7, Encoding Lysophosphatidylinositol Acyltransferase I, Lead to Intellectual Disability Accompanied by Epilepsy and Autistic Features. Am. J. Hum. Genet. 2016, 99, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Rawlins, L.E.; Harlalka, G.V.; Umair, M.; Ullah, A.; Shahzad, S.; Javed, M.; Baple, E.L.; Crosby, A.H.; Ahmad, W.; et al. Homozygous variants in the HEXB and MBOAT7 genes underlie neurological diseases in consanguineous families. BMC Med. Genet. 2019, 20, 199. [Google Scholar] [CrossRef] [PubMed]
- Yalnızoglu, D.; Özgül, R.K.; Oguz, K.K.; Özer, B.; Yücel-Yılmaz, D.; Gürbüz, B.; Serdaroglu, E.; Erol, İ.; Topçu, M.; Dursun, A. Expanding the phenotype of phospholipid remodelling disease due to MBOAT7 gene defect. J. Inherit. Metab. Dis. 2019, 42, 381–388. [Google Scholar] [CrossRef]
- Heidari, E.; Caddeo, A.; Zarabadi, K.; Masoudi, M.; Tavasoli, A.R.; Romeo, S.; Garshasbi, M. Identification of novel loss of function variants in MBOAT7 resulting in intellectual disability. Genomics 2020, 112, 4072–4077. [Google Scholar] [CrossRef]
- Lee, J.; Shamim, A.; Park, J.; Jang, J.H.; Kim, J.H.; Kwon, J.Y.; Kim, J.W.; Kim, K.K.; Lee, J. Functional and Structural Changes in the Membrane-Bound O-Acyltransferase Family Member 7 (MBOAT7) Protein: The Pathomechanism of a Novel MBOAT7 Variant in Patients with Intellectual Disability. Front. Neurol. 2022, 13, 836954. [Google Scholar] [CrossRef]
- HUGO Gene Nomenclature Committee. Available online: https://www.genenames.org (accessed on 14 November 2023).
- Wang, K.; Lee, C.W.; Sui, X.; Kim, S.; Wang, S.; Higgs, A.B.; Baublis, A.J.; Voth, G.A.; Liao, M.; Walther, T.C.; et al. The structure of phosphatidylinositol remodeling MBOAT7 reveals its catalytic mechanism and enables inhibitor identification. Nat. Commun. 2023, 14, 3533. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. Cancer Genome Atlas Research Network; The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
| References | Involvement in Diseases | Physiological Significance | Donor Preference of the Acyl Group | Activity | Official Name of the Gene (Other Names *) |
|---|---|---|---|---|---|
| [41,53] | Myoblast differentiation | palmitoyl-CoA, oleoyl-CoA, linoleoyl-CoA, | AGPAT, much smaller LPLAT | AGPAT1 (LPAAT-α, LPLAT1) | |
| [53,54,55,56,57,58,59,60,61,62,63,64] | Cancers—breast cancer, ovarian cancer, osteosarcoma, prostate cancer (pro-tumor properties); mutations in the AGPAT2 gene cause BSCL1 | Adipogenesis | oleoyl-CoA, linoleoyl-CoA | AGPAT, much smaller LPLAT | AGPAT2 (LPAAT-β, LPLAT2) |
| [65,66,67,68,69,70,71] | Cancers—colorectal cancer, gastric cancer (anti-tumor properties); autism spectrum disorder. Mutations in the AGPAT3 gene are associated with IDRP syndrome | Spermatogenesis, skeletal muscle physiology, neuronal migration, adipogenesis | oleoyl-CoA, DHA-CoA | AGPAT, LPLAT | AGPAT3 (LPAAT3, LPAAT-γ, LPLAT3) |
| [70,72,73] | Cancers—colorectal cancer (pro-tumor properties) | Incorporation of DHA into phospholipids in the brain | DHA-CoA | AGPAT | AGPAT4 (LPAAT4, LPAAT-δ, LPLAT4) |
| [49,74] | Colorectal cancer (anti-tumor properties) | oleoyl-CoA (AGPAT), DHA-CoA (LPLAT) | AGPAT, LPLAT | AGPAT5 (LPAAT-ε, LPLAT5) | |
| [50,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102] | Cancers—breast cancer, cervical cancer, esophageal squamous cell carcinoma, endometrial cancer, head and neck squamous cell carcinoma, hepatocellular carcinoma, kidney cancer (clear cell renal cell carcinoma), leukemia (acute myeloid leukemia), lung cancer (lung squamous cell carcinoma, lung adenocarcinoma), skin cancer (cutaneous squamous cell carcinoma; melanoma) (pro-tumor properties); psoriasis | Activity decreases sensitivity to ferroptosis. Defense by PUFA influence. Increasing transcription by causing histone H4 palmitoylation. Involvement in the physiology of lipid droplets | oleoyl-CoA | AGPAT, LPCAT, LPGAT | LPCAT1 (AGPAT9, AGPAT10, LPLAT8) |
| [51,103,104,105,106,107,108] | Cancers—colorectal cancer, prostate cancer (pro-tumor properties) | Effects on TLR4 | oleoyl-CoA | AGPAT, LPCAT | LPCAT2 (AYTL1, AGPAT11, LPLAT9) |
| [109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129] | Insulin resistance in obesity; obesity-related myopathy; NASH; osteoarthritis; Cancers—brain tumor (low-grade glioma), colon cancer, leukemia (acute myeloid leukemia), ovarian cancer, melanoma (uveal melanoma) (pro-tumor properties) renal clear cell carcinoma (anti-tumor properties); muscle weakness caused by inactivity | Activity increases sensitivity to ferroptosis. Lipid absorption. LysoPC esterification in the liver. Adipocyte differentiation. | PUFA-CoA | LPCAT, LPEAT, LPSAT | LPCAT3 (MBOAT5, OACT5, LPLAT12) |
| [18,74,130,131,132,133] | Cancers—hepatocellular carcinoma (pro-tumor properties) | Urothelial barrier function | DHA-CoA | LPLAT | LPCAT4 (AYTL3, LPLAT10, AGPAT7, LPEAT2, LPAAT-η) |
| [134,135,136,137] | Cancers—lung adenocarcinoma (pro-tumor properties); insulin resistance in obesity; pulmonary fibrosis; Parkinson’s disease | CL remodeling in mitochondria | oleoyl-CoA, linoleoyl-CoA | AGPAT, LCLAT | LCLAT1 (LPLAT6, ALCAT1, AGPAT8, LYCAT) |
| [93,141,142,143,144,145,146,147,148,149,150] | Cancer—lung adenocarcinoma (pro-tumor properties); obesity; MEGDEL syndrome | PG remodeling, TAG synthesis in liver, skeletal muscle physiology | palmitoyl-CoA, stearoyl-CoA oleoyl-CoA | LPGAT, MGAT, sn-1 LPCAT, sn-1 LPEAT. | LPGAT1 (FAM34A, LPLAT7) |
| [109,151,152,153,154,155] | Alzheimer’s disease; mutations in the gene cause brachydactyly-syndactyly syndrome and nonobstructive azoospermia | Protects against ferroptosis. Physiology of nerve cells | palmitoyl-CoA, oleoyl-CoA | LPEAT, LPSAT | MBOAT1 (OACT1, LPEAT1, LPLAT14) |
| [18,109,152,156] | Cancers—pancreatic cancer (pro-tumor properties) invasive breast cancer, cholangiocarcinoma, prostate adenocarcinoma (pro-tumor properties?) leukemia (acute myeloid leukemia), kidney cancer (renal cell carcinoma), skin cancer (cutaneous melanoma) (anti-tumor properties?) | Protects against ferroptosis | MUFA-CoA linoleoyl-CoA | AGPAT, LPEAT | MBOAT2 (OACT2, LPLAT13) |
| [18,109,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196] | Reduced expression and activity are important for increased risk and worse outcomes in liver disease: NAFLD, ALD, chronic HBV or HCV infection. Lower expression associated with increased risk of hepatocellular carcinoma. Associated with insulin resistance in obesity. Involved in certain cancers—hepatocellular carcinoma, kidney cancer (clear cell renal cell carcinoma), lung cancer (non-small cell lung cancer) (pro-tumor properties) Mutations in the MBOAT7 gene cause congenital mental retardation with epilepsy. | PI remodeling, introduction of arachidonic acid into this phospholipid. Brain development | arachidonoyl-CoA, EPA-CoA | LPIAT | MBOAT7 (LPLAT, LPIAT1, LPLAT11) |
| Name of the Cancer | GPAM | GPAT2 | GPAT3 (AGPAT9) | GPAT4 (AGPAT6) | AGPAT1 | AGPAT2 | AGPAT3 | AGPAT4 | AGPAT5 |
|---|---|---|---|---|---|---|---|---|---|
| Adrenocortical carcinoma | - | - | ↑ | - | ↓ | ↓ | - | ↓ | - |
| Bladder urothelial carcinoma | - | - | ↓ | - | - | - | - | ↓ | - |
| Breast invasive carcinoma | - | ↑ p = 0.079 | - | ↓ | ↓ | ↓ p = 0.072 | - | - | ↓ p = 0.10 |
| Cervical squamous cell carcinoma and endocervical adenocarcinoma | - | ↑ p = 0.070 | - | - | - | - | ↓ | ↓ | - |
| Cholangiocarcinoma | - | - | - | - | - | - | - | - | - |
| Colon adenocarcinoma | - | - | - | ↑ | - | - | - | - | ↑ p = 0.058 |
| Lymphoid neoplasm diffuse large B-cell lymphoma | - | - | - | - | - | ↑ p = 0.079 | - | - | - |
| Esophageal carcinoma | - | - | - | - | - | - | - | - | - |
| Glioblastoma multiforme | - | - | ↓ | - | - | ↓ p = 0.075 | - | - | - |
| Head and neck squamous cell carcinoma | - | - | - | ↑ | - | ↓ | - | ↓ | - |
| Kidney chromophobe | - | - | - | - | - | ↑ | - | - | - |
| Kidney renal clear cell carcinoma | ↑ | - | ↑ | ↑ | ↑ | - | ↑ | - | ↑ |
| Kidney renal papillary cell carcinoma | - | - | - | - | - | - | - | - | - |
| Acute myeloid leukemia | - | ↓ | ↓ | - | ↓ p = 0.093 | ↓ p = 0.097 | ↓ | - | - |
| Brain lower grade glioma | - | ↓ | ↓ | - | ↑ p = 0.095 | ↓ | - | - | - |
| Liver hepatocellular carcinoma | - | - | - | - | ↓ | ↓ p = 0.10 | - | ↓ | ↓ |
| Lung adenocarcinoma | - | - | ↓ p = 0.051 | - | - | - | - | - | - |
| Lung squamous cell carcinoma | - | - | - | - | - | ↓ | - | - | - |
| Mesothelioma | - | - | ↑ | ↓ | ↑ p = 0.083 | - | - | ↓ | ↓ |
| Ovarian serous cystadenocarcinoma | - | - | - | ↓ p = 0.079 | - | - | - | ↓ p = 0.080 | - |
| Pancreatic adenocarcinoma | - | - | - | - | - | - | - | - | - |
| Pheochromocytoma and Paraganglioma | - | - | ↓ | - | - | - | - | - | - |
| Prostate adenocarcinoma | - | - | - | - | - | - | - | - | - |
| Rectum adenocarcinoma | - | - | - | - | - | - | ↑ | - | - |
| Sarcoma | - | - | - | - | - | - | - | - | ↓ p = 0.069 |
| Skin cutaneous melanoma | - | - | - | ↓ p = 0.062 | ↓ | ↓ | ↓ | ↓ p = 0.076 | - |
| Stomach adenocarcinoma | - | - | - | - | ↓ p = 0.089 | ↑ p = 0.064 | - | ↓ | - |
| Testicular germ cell tumors | - | - | - | - | - | - | - | - | - |
| Thyroid carcinoma | - | - | - | - | ↓ | - | - | ↓ | - |
| Thymoma | - | - | - | - | - | - | ↓ p = 0.085 | - | - |
| Uterine corpus endometrial carcinoma | ↑ p = 0.082 | - | - | - | - | - | - | - | - |
| Uterine carcinosarcoma | - | - | - | - | - | ↓ | - | - | - |
| Uveal Melanoma | - | - | - | ↑ | - | ↑ p = 0.061 | ↓ | ↓ | - |
| Name of the Cancer | LPCAT1 | LPCAT2 | LPCAT3 | LPCAT4 | LCLAT1 | LPGAT1 | MBOAT1 | MBOAT2 | MBOAT7 |
|---|---|---|---|---|---|---|---|---|---|
| Adrenocortical carcinoma | - | - | - | ↓ | - | ↓ | - | ↓ | - |
| Bladder urothelial carcinoma | - | ↓ p = 0.10 | - | - | - | - | - | ↓ | - |
| Breast invasive carcinoma | ↓ p = 0.051 | - | - | - | ↓ p = 0.052 | ↓ p = 0.068 | - | - | ↓ |
| Cervical squamous cell carcinoma and endocervical adenocarcinoma | ↓ | ↓ p = 0.051 | - | - | - | - | - | ↓ p = 0.077 | ↓ p = 0.076 |
| Cholangiocarcinoma | - | - | - | - | - | - | - | - | - |
| Colon adenocarcinoma | - | - | - | - | - | - | - | - | - |
| Lymphoid neoplasm diffuse large B-cell lymphoma | - | - | - | - | - | ↓ p = 0.087 | - | - | - |
| Esophageal carcinoma | - | - | - | - | - | - | - | - | - |
| Glioblastoma multiforme | - | - | - | - | - | - | - | - | - |
| Head and neck squamous cell carcinoma | - | ↓ | - | - | ↓ | - | - | - | - |
| Kidney chromophobe | - | - | - | - | - | ↓ | - | - | - |
| Kidney renal clear cell carcinoma | - | - | ↑ | ↓ | ↑ | ↑ | ↑ | ↑ | ↓ p = 0.063 |
| Kidney renal papillary cell carcinoma | ↓ p = 0.083 | - | ↑ p = 0.074 | ↓ p = 0.060 | - | ↓ | - | - | ↓ |
| Acute myeloid leukemia | ↓ p = 0.055 | ↑ p = 0.089 | ↓ | - | - | - | - | - | - |
| Brain lower grade glioma | ↓ | ↓ | ↓ | - | ↓ p = 0.063 | ↓ | ↓ | - | ↓ |
| Liver hepatocellular carcinoma | ↓ | ↓ | - | ↓ | ↓ | - | ↓ | - | ↓ |
| Lung adenocarcinoma | ↑ | - | - | - | ↓ p = 0.054 | ↓ | - | - | ↓ |
| Lung squamous cell carcinoma | ↓ p = 0.061 | - | - | - | - | - | - | - | ↓ p = 0.051 |
| Mesothelioma | - | - | - | - | ↓ | - | - | ↓ | ↓ |
| Ovarian serous cystadenocarcinoma | - | - | ↓ | - | - | - | - | - | ↓ p = 0.053 |
| Pancreatic adenocarcinoma | ↑ | ↓ | - | ↓ | - | - | ↓ | ↓ | - |
| Pheochromocytoma and Paraganglioma | - | - | - | - | - | - | - | - | - |
| Prostate adenocarcinoma | ↓ p = 0.076 | - | - | - | - | - | - | - | - |
| Rectum adenocarcinoma | - | - | - | - | - | - | ↑ | - | - |
| Sarcoma | - | - | - | - | ↓ p = 0.075 | - | - | - | - |
| Skin cutaneous melanoma | - | ↑ | - | - | - | - | - | - | - |
| Stomach adenocarcinoma | - | - | - | - | - | - | ↑ p = 0.067 | - | - |
| Testicular germ cell tumors | - | - | - | - | - | - | - | - | - |
| Thyroid carcinoma | - | - | - | - | ↓ p = 0.097 | - | - | - | ↓ |
| Thymoma | ↑ p = 0.10 | ↓ | ↑ p = 0.084 | - | - | ↓ p = 0.062 | - | - | - |
| Uterine corpus endometrial carcinoma | - | ↑ | - | - | - | - | - | - | - |
| Uterine carcinosarcoma | - | - | - | - | - | - | - | - | - |
| Uveal Melanoma | ↓ | - | - | ↓ | - | - | - | ↓ | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korbecki, J.; Bosiacki, M.; Pilarczyk, M.; Gąssowska-Dobrowolska, M.; Jarmużek, P.; Szućko-Kociuba, I.; Kulik-Sajewicz, J.; Chlubek, D.; Baranowska-Bosiacka, I. Phospholipid Acyltransferases: Characterization and Involvement of the Enzymes in Metabolic and Cancer Diseases. Cancers 2024, 16, 2115. https://doi.org/10.3390/cancers16112115
Korbecki J, Bosiacki M, Pilarczyk M, Gąssowska-Dobrowolska M, Jarmużek P, Szućko-Kociuba I, Kulik-Sajewicz J, Chlubek D, Baranowska-Bosiacka I. Phospholipid Acyltransferases: Characterization and Involvement of the Enzymes in Metabolic and Cancer Diseases. Cancers. 2024; 16(11):2115. https://doi.org/10.3390/cancers16112115
Chicago/Turabian StyleKorbecki, Jan, Mateusz Bosiacki, Maciej Pilarczyk, Magdalena Gąssowska-Dobrowolska, Paweł Jarmużek, Izabela Szućko-Kociuba, Justyna Kulik-Sajewicz, Dariusz Chlubek, and Irena Baranowska-Bosiacka. 2024. "Phospholipid Acyltransferases: Characterization and Involvement of the Enzymes in Metabolic and Cancer Diseases" Cancers 16, no. 11: 2115. https://doi.org/10.3390/cancers16112115
APA StyleKorbecki, J., Bosiacki, M., Pilarczyk, M., Gąssowska-Dobrowolska, M., Jarmużek, P., Szućko-Kociuba, I., Kulik-Sajewicz, J., Chlubek, D., & Baranowska-Bosiacka, I. (2024). Phospholipid Acyltransferases: Characterization and Involvement of the Enzymes in Metabolic and Cancer Diseases. Cancers, 16(11), 2115. https://doi.org/10.3390/cancers16112115






