Validation of a Blood-Based Protein Biomarker Panel for a Risk Assessment of Lethal Lung Cancer in the Physicians’ Health Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Physicians’ Health Study (PHS)
2.2. Assay of Protein Biomarkers
2.3. Statistical Analyses
3. Results
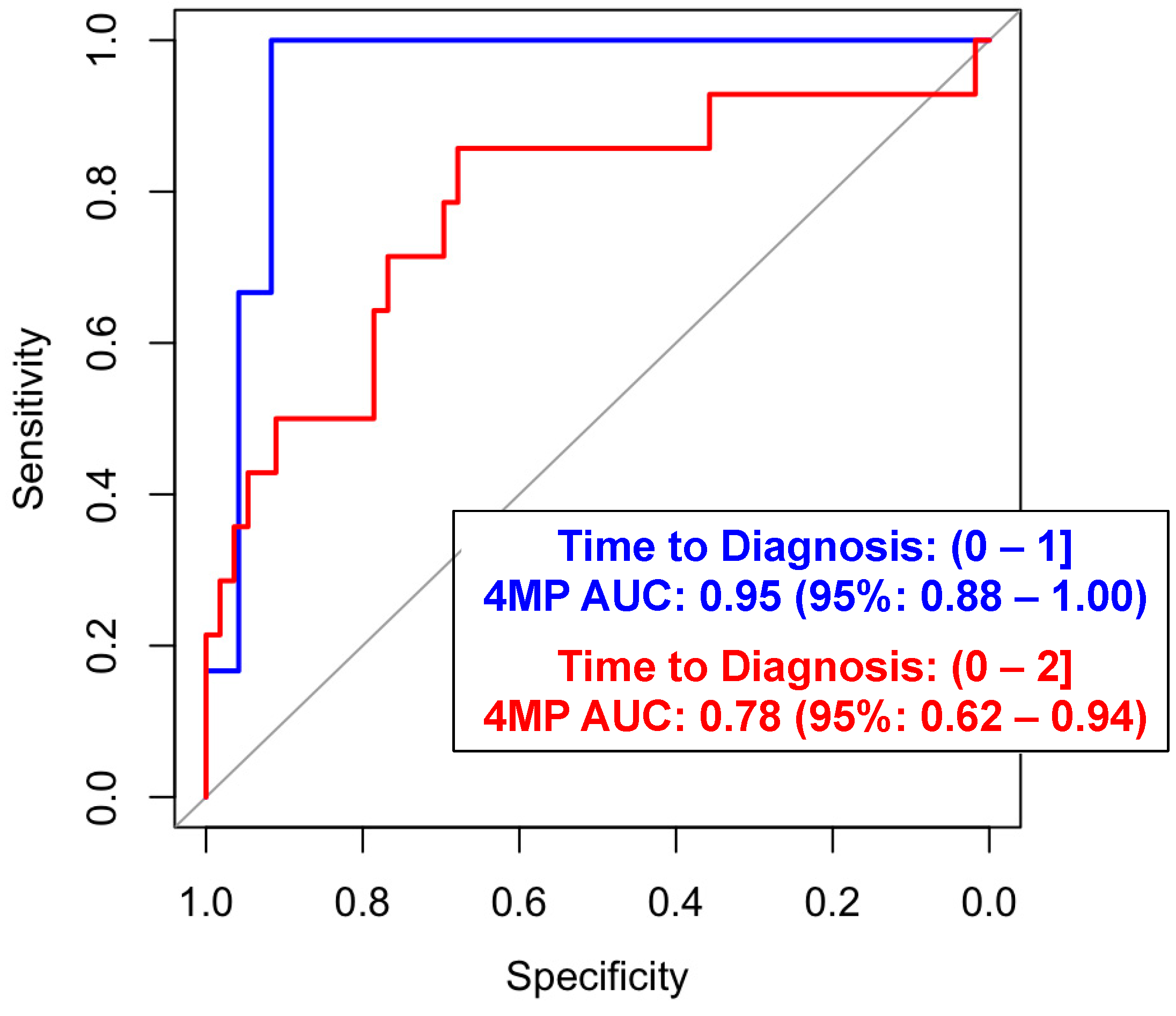
3.1. Performance Estimates of the Four-Marker Protein Panel and Additional Protein Biomarkers for Risk Assessment of Lung Cancer in the PHS Cohort
3.2. Performance Estimates of the Four-Marker Protein Panel for Metastatic Lung Cancer
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Lung Screening Trial Research Team; Aberle, D.R.; Berg, C.D.; Black, W.C.; Church, T.R.; Fagerstrom, R.M.; Galen, B.; Gareen, I.F.; Gatsonis, C.; Goldin, J.; et al. The National Lung Screening Trial: Overview and study design. Radiology 2011, 258, 243–253. [Google Scholar] [CrossRef] [PubMed]
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [PubMed]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.-W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Pastorino, U.; Silva, M.; Sestini, S.; Sabia, F.; Boeri, M.; Cantarutti, A.; Sverzellati, N.; Sozzi, G.; Corrao, G.; Marchiano, A. Prolonged lung cancer screening reduced 10-year mortality in the MILD trial: New confirmation of lung cancer screening efficacy. Ann. Oncol. 2019, 30, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.; Motsch, E.; Trotter, A.; Heussel, C.P.; Dienemann, H.; Schnabel, P.A.; Kauczor, H.-U.; Maldonado, S.G.; Miller, A.B.; Kaaks, R.; et al. Lung cancer mortality reduction by LDCT screening—Results from the randomized German LUSI trial. Int. J. Cancer 2020, 146, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Krist, A.H.; Davidson, K.W.; Mangione, C.M.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Kubik, M.; et al. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA 2021, 325, 962–970. [Google Scholar] [PubMed]
- Rendle, K.A.; Saia, C.A.; Vachani, A.; Burnett-Hartman, A.N.; Doria-Rose, V.P.; Beucker, S.; Neslund-Dudas, C.; Oshiro, C.; Kim, R.Y.; Elston-Lafata, J.; et al. Rates of Downstream Procedures and Complications Associated With Lung Cancer Screening in Routine Clinical Practice: A Retrospective Cohort Study. Ann. Intern. Med. 2024, 177, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Li, Q.; Wang, R.-T.; Zhang, L.; Zhang, W.; Deng, M.-S.; Luo, Y.-Y.; Ji, X.; Wen, Y.; Zhou, X.-R.; et al. Downregulation of pro-surfactant protein B contributes to the recurrence of early-stage non-small cell lung cancer by activating PGK1-mediated Akt signaling. Exp. Hematol. Oncol. 2023, 12, 94. [Google Scholar] [CrossRef] [PubMed]
- D’Ascanio, M.; Viccaro, F.; Pizzirusso, D.; Guerrieri, G.; Pagliuca, A.; Guerrini, S.; Innammorato, M.; De Vitis, C.; Raffa, S.; Pezzuto, A.; et al. Surfactant protein B plasma levels: Reliability as a biomarker in COPD patients. Biomedicines 2023, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Sin, D.D.; Tammemagi, C.M.; Lam, S.; Barnett, M.J.; Duan, X.; Tam, A.; Auman, H.; Feng, Z.; Goodman, G.E.; Hanash, S.; et al. Pro–surfactant protein B as a biomarker for lung cancer prediction. J. Clin. Oncol. 2013, 31, 4536. [Google Scholar] [CrossRef]
- Christen, W.G.; Gaziano, J.M.; Hennekens, C.H. Design of Physicians’ Health Study II—A Randomized Trial of Beta-Carotene, Vitamins E and C, and Multivitamins, in Prevention of Cancer, Cardiovascular Disease, and Eye Disease, and Review of Results of Completed Trials. Ann. Epidemiol. 2000, 10, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Fedewa, S.A.; Kazerooni, E.A.; Studts, J.L.; Smith, R.A.; Bandi, P.; Sauer, A.G.; Cotter, M.; Sineshaw, H.M.; Jemal, A.; Silvestri, G.A. State Variation in Low-Dose Computed Tomography Scanning for Lung Cancer Screening in the United States. J. Natl. Cancer Inst. 2021, 113, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pan, I.E.; Tak, H.J.; Vlahos, I.; Volk, R.; Shih, Y.T. Assessment of Uptake Appropriateness of Computed Tomography for Lung Cancer Screening According to Patients Meeting Eligibility Criteria of the US Preventive Services Task Force. JAMA Netw. Open 2022, 5, e2243163. [Google Scholar] [CrossRef] [PubMed]
- Dickson, J.L.; Horst, C.; Nair, A.; Tisi, S.; Prendecki, R.; Janes, S.M. Hesitancy around low-dose CT screening for lung cancer. Ann. Oncol. 2022, 33, 34–41. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, L.M.; Taylor, G.; Huxley, R.R.; Mitchell, P.; Woodward, M.; Peters, S.A.E. Smoking as a risk factor for lung cancer in women and men: A systematic review and meta-analysis. BMJ Open 2018, 8, e021611. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S. Lung carcinogenesis by tobacco smoke. Int. J. Cancer 2012, 131, 2724–2732. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.L.; O’Dowd, E. Smoking cessation and lung cancer: Never too late to quit. Lancet Public Health 2023, 8, e664–e665. [Google Scholar] [CrossRef]
- Fares, A.F.; Li, Y.; Jiang, M.; Brown, M.C.; Lam, A.C.L.; Aggarwal, R.; Schmid, S.; Leighl, N.B.; Shepherd, F.A.; Wang, Z.; et al. Association between duration of smoking abstinence before non-small-cell lung cancer diagnosis and survival: A retrospective, pooled analysis of cohort studies. Lancet Public Health 2023, 8, e691–e700. [Google Scholar] [CrossRef]
- Melzer, A.C.; Golden, S.E.; Wiener, R.S.; Iaccarino, J.M.; Slatore, C.G. A Brief Report of Smoking Behaviors in Patients with Incidental Pulmonary Nodules: Associations with Communication and Risk Perception. Tob. Use Insights 2019, 12, 1179173x19839059. [Google Scholar] [CrossRef]
- Maci, E.; Comito, F.; Frezza, A.M.; Tonini, G.; Pezzuto, A. Lung Nodule and Functional Changes in Smokers After Smoking Cessation Short-Term Treatment. Cancer Investig. 2014, 32, 388–393. [Google Scholar] [CrossRef]
- LoPiccolo, J.; Gusev, A.; Christiani, D.C.; Janne, P.A. Lung cancer in patients who have never smoked—An emerging disease. Nat. Rev. Clin. Oncol. 2024, 21, 121–146. [Google Scholar] [CrossRef] [PubMed]
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, J.; Govindan, R. Lung cancer in never smokers: A review. J. Clin. Oncol. 2007, 25, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tan, F.; Shen, S.; Wu, Z.; Cao, W.; Yu, Y.; Dong, X.; Xia, C.; Tang, W.; Xu, Y.; et al. Risk-stratified Approach for Never- and Ever-Smokers in Lung Cancer Screening: A Prospective Cohort Study in China. Am. J. Respir. Crit. Care Med. 2023, 207, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Osarogiagbon, R.U.; Yang, P.C.; Sequist, L.V. Expanding the Reach and Grasp of Lung Cancer Screening. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e389958. [Google Scholar] [CrossRef]
- Goo, J.M.; Jung, K.W.; Kim, H.Y.; Kim, Y. Potential Overdiagnosis with CT Lung Cancer Screening in Taiwanese Female: Status in South Korea. Korean J. Radiol. 2022, 23, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wen, C.P.; Wu, A.; Welch, H.G. Association of Computed Tomographic Screening Promotion With Lung Cancer Overdiagnosis Among Asian Women. JAMA Intern. Med. 2022, 182, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Kondo, R.; Yoshida, K.; Kawakami, S.; Shiina, T.; Kurai, M.; Takasuna, K.; Yamamoto, H.; Koizumi, T.; Honda, T.; Kubo, K. Efficacy of CT screening for lung cancer in never-smokers: Analysis of Japanese cases detected using a low-dose CT screen. Lung Cancer 2011, 74, 426–432. [Google Scholar] [CrossRef]
- Irajizad, E.; Fahrmann, J.F.; Marsh, T.; Vykoukal, J.; Dennison, J.B.; Long, J.P.; Do, K.-A.; Feng, Z.; Hanash, S.; Ostrin, E.J. Mortality Benefit of a Blood-Based Biomarker Panel for Lung Cancer on the Basis of the Prostate, Lung, Colorectal, and Ovarian Cohort. J. Clin. Oncol. 2023, 41, 4360–4368. [Google Scholar] [CrossRef]

| Controls (n = 100) | Lung Cancer Cases (n = 25) | ||
|---|---|---|---|
| N (%) | N (%) | ||
| Smoking Status | Never | 12 (12) | 3 (12) |
| Past | 56 (56) | 14 (56) | |
| Current | 32 (32) | 8 (32) | |
| Metastatic Status | Non-Metastatic | 11 (44) | |
| Metastatic | 14 (56) | ||
| Time to Diagnosis | 0–1 yr | 13 (52) | |
| 1–2 yr | 12 (48) | ||
| Histology | Not available | 2 (8) | |
| Neoplasm, Malignant | 1 (4) | ||
| Carcinoma, NOS | 4 (16) | ||
| Small Cell Carcinoma | 2 (8) | ||
| Squamous Cell Carcinoma | 6 (24) | ||
| Adenocarcinoma | 9 (36) | ||
| Alveolar Adenocarcinoma | 1 (4) | ||
| Mean, Median (IQR) | |||
| Age at blood draw date | 69.16, 70.08 (65.95, 74.58) | 69.43, 70.10 (66.28, 75.65) | |
| Pro-SFTPB (ng/mL) | 51.27, 35.39 (14.43, 61.63) | 98.06, 47.25 (25.44, 81.77) | |
| CEA (ng/mL) | 1.09, 0.93 (0.56, 1.44) | 4.44, 1.37 (0.58, 3.41) | |
| CA125 (U/mL) | 3.36, 2.77 (2.24, 4.04) | 5.27, 3.32 (2.36, 4.50) | |
| CYFRA-21-1 (ng/mL) | 0.20, 0.05 (0.02, 0.21) | 0.35, 0.09 (0.02, 0.60) | |
| CA15-3 (U/mL) | 17.65, 15.12 (9.31, 20.47) | 23.08, 13.94 (11.06, 24.07) | |
| OPN (ng/mL) | 26.68, 24.34 (15.73, 31.96) | 26.48, 26.18 (17.48, 33.22) | |
| HE4 (ng/mL) | 10.17, 2.53 (0.75, 7.65) | 5.15, 4.69 (2.71, 8.41) | |
| AUC Performance of 4MP (95% CI) | ||
|---|---|---|
| Time to DX | Never-Smokers | Ever-Smokers |
| [0–1) | 0.72 (0.17–1.00) | 0.77 (0.63–0.92) |
| [1–2) | - | 0.59 (0.38–0.80) |
| [0–2) | 0.72 (0.17–1.00) | 0.68 (0.54–0.82) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Irajizad, E.; Rundle, A.; Sesso, H.D.; Gaziano, J.M.; Vykoukal, J.V.; Do, K.-A.; Dennison, J.B.; Ostrin, E.J.; Fahrmann, J.F.; et al. Validation of a Blood-Based Protein Biomarker Panel for a Risk Assessment of Lethal Lung Cancer in the Physicians’ Health Study. Cancers 2024, 16, 2070. https://doi.org/10.3390/cancers16112070
Song L, Irajizad E, Rundle A, Sesso HD, Gaziano JM, Vykoukal JV, Do K-A, Dennison JB, Ostrin EJ, Fahrmann JF, et al. Validation of a Blood-Based Protein Biomarker Panel for a Risk Assessment of Lethal Lung Cancer in the Physicians’ Health Study. Cancers. 2024; 16(11):2070. https://doi.org/10.3390/cancers16112070
Chicago/Turabian StyleSong, Lulu, Ehsan Irajizad, Andrew Rundle, Howard D. Sesso, John Michael Gaziano, Jody V. Vykoukal, Kim-Anh Do, Jennifer B. Dennison, Edwin J. Ostrin, Johannes F. Fahrmann, and et al. 2024. "Validation of a Blood-Based Protein Biomarker Panel for a Risk Assessment of Lethal Lung Cancer in the Physicians’ Health Study" Cancers 16, no. 11: 2070. https://doi.org/10.3390/cancers16112070
APA StyleSong, L., Irajizad, E., Rundle, A., Sesso, H. D., Gaziano, J. M., Vykoukal, J. V., Do, K.-A., Dennison, J. B., Ostrin, E. J., Fahrmann, J. F., Perera, F., & Hanash, S. (2024). Validation of a Blood-Based Protein Biomarker Panel for a Risk Assessment of Lethal Lung Cancer in the Physicians’ Health Study. Cancers, 16(11), 2070. https://doi.org/10.3390/cancers16112070







