Acceptance of Self-Sampling by Women Not Regularly Participating in Cervical Cancer Screening in Areas with Low Medical Density: A Qualitative Study within the French CapU4 Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Semi-Structured Interviews and Focus-Group
2.2. Population
2.3. Process and Analysis
3. Results
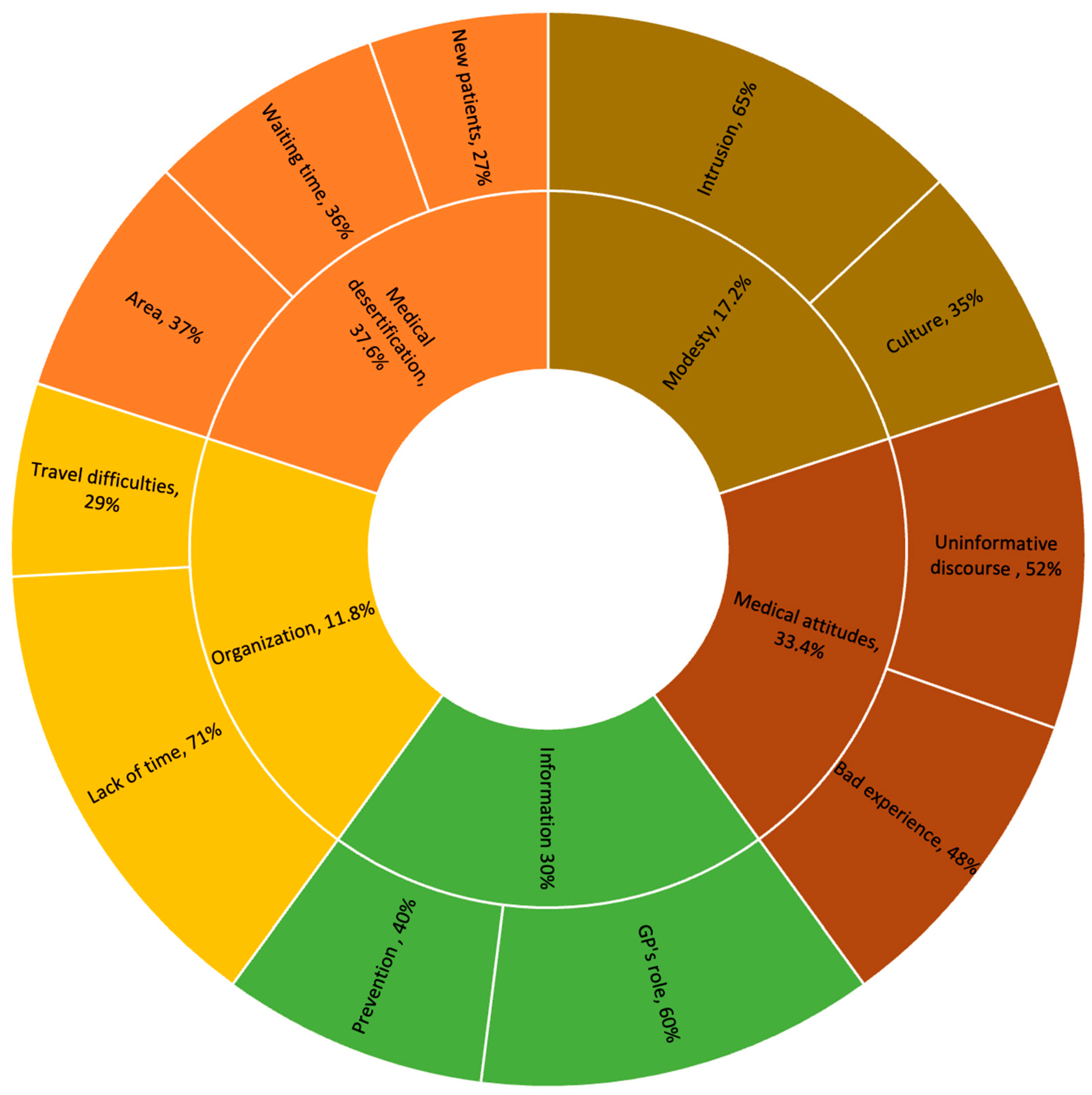
3.1. Barriers to CC Screening
3.1.1. Semi-Structured Interview Data
3.1.2. Additional Input from Focus-Groups
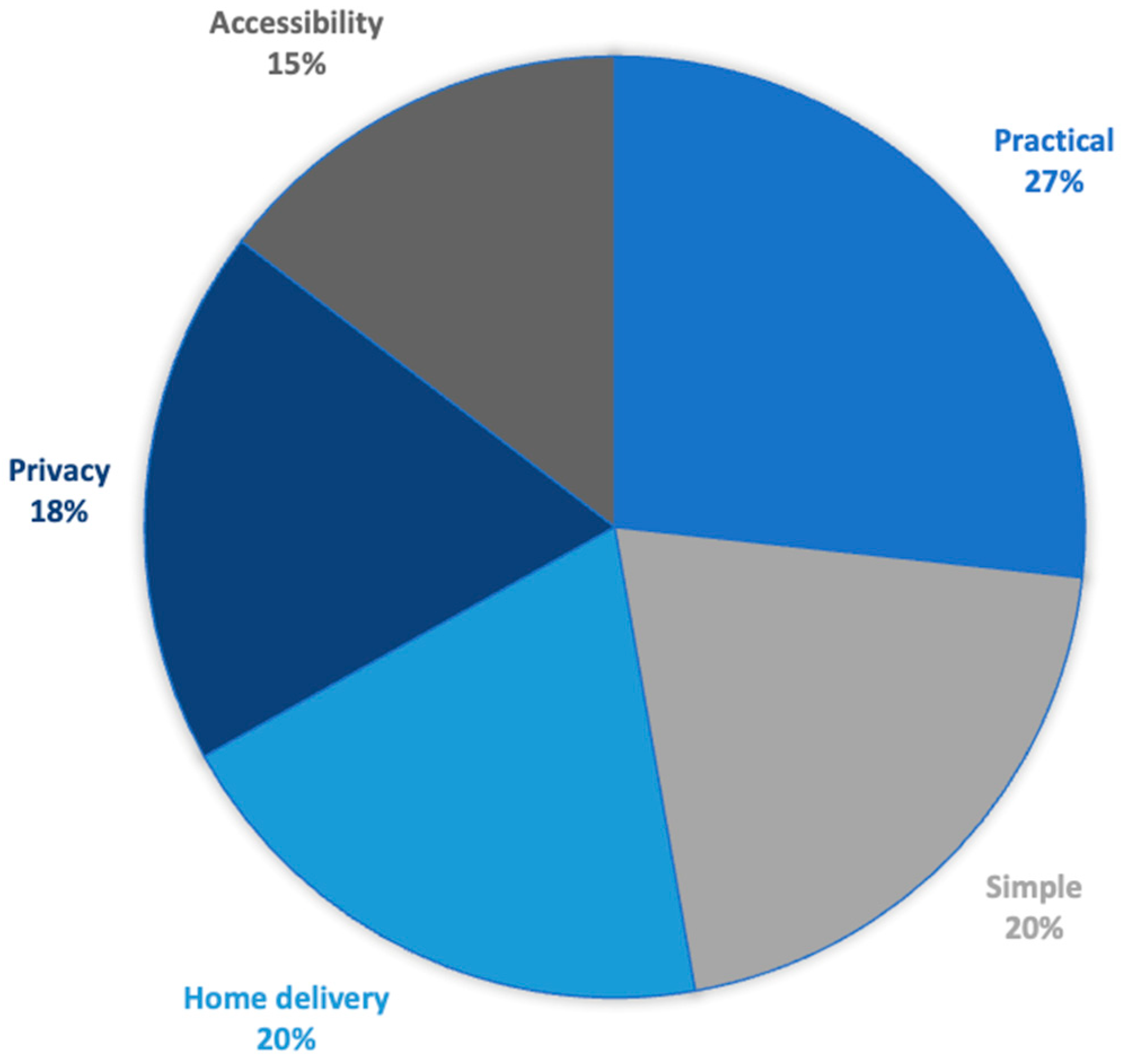
3.2. Self Screening for CC
3.2.1. Levers for Self-Sampling
3.2.2. Barriers to Self-Sampling
3.2.3. Age Group Differences
3.2.4. Adaptation of Kit Delivery to Women
4. Discussion
- -
- To strengthen information by front-line professionals, especially GPs.
- -
- To eliminate follow-up letters in priority intervention areas.
- -
- To send self-sampling kits to women who have not responded to invitations.
- -
- To deliver the kit to women’s homes address with explanatory leaflet (drawings to help you take the sample), rather than delivery to health professionals.
- -
- To inform on the accuracy of testing on self-samples (vaginal or urinary kits).
- -
- To explain in easy and understandable language how to use the self-sampling kit.
- -
- To provide the choice for a urine kit to women who do not like using a vaginal kit (in particular, due to fear of intrusion, trauma, culture).
- -
- Provide rapid medical support in the event of a positive HPV result.
- -
- Develop information campaigns accessible to all (social networks, testimonials, striking images, etc.).
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Semi-Structured Interview Guide for Women
- Screening and you:
- (1)
- What made you decide to practice self-testing?
- (2)
- Did you know about this kit before being asked?
- (3)
- Were you able to talk to anyone close to you about it? Who were they?
- (4)
- Have you read about it (on the Internet)? Listened to a radio or TV program?
- (5)
- Have you talked to your doctor or another healthcare professional about this?
- (6)
- How do you usually relate to different types of screening?
- Cervical cancer and you:
- (1)
- Do you think you have enough information about cervical cancer? If not, who should give it to you?
- (2)
- Do you feel concerned by this cancer?
- (3)
- Do you think the fact that it is located in an intimate part of your body influences your relationship with this cancer?
- (4)
- Have health professionals suggested screening during a medical consultation?
- (5)
- Are you satisfied with your gynecological care?
- (6)
- Are you afraid of treatment (laser, conization, etc.)?
- (7)
- What was your experience of having a smear test taken in the office? (Was the test intrusive? Bad experience?) Who performed it?
- (8)
- For you, is it good or bad to know the doctor who takes the smear?
- During self-sampling:
- (1)
- What did you think of the information provided in the document? Was it sufficient? What was missing?
- (2)
- Were you afraid of doing the procedure incorrectly? Of hurting yourself?
- (3)
- Were you worried about hygiene?
- (4)
- Were you embarrassed to take the sample yourself? Or even felt any pain?
- (5)
- Does the fact that the sample can be taken at home play a role in your acceptance of the procedure?
- (6)
- Do you think that home sampling is a good thing for all women? If not, what could be done?
- (7)
- Do you think it’s as reliable as a smear test?
- (8)
- Is this kit sufficiently reliable in your opinion? Has there been sufficient proof of its reliability?
- (9)
- Is this type of self-examination in line with your culture? The culture of all the women you know?
- (10)
- Does this type of self-practiced examination suit you? Why or why not?
- (11)
- How did you feel when you took the test?
- (12)
- What could be improved?
- (13)
- Is a urine sample or vaginal swab the same for you in terms of experience?
- (14)
- How would you rate your satisfaction with self-testing from 0 to 5? Why or why not?
- Before and after self-testing:
- (1)
- Do you feel that anything has changed in your relationship with your health as a result of taking this self-test? Do you feel more involved in your health? More responsible?
- (2)
- Do you feel more at ease with your intimacy?
- (3)
- Do you feel your relationship with illness is less complicated?
- (4)
- Does taking part in screening change your relationship with healthcare professionals?
- (5)
- Does it make you want to take part in other screening programs?
- (6)
- What expectations do you have of healthcare professionals in the context of this type of screening, and have they been met?
- (7)
- Did the trust you have in your healthcare professional play a role in your decision to undergo screening?
- (8)
- Between 0 and 5, how would you rate the quality of the information provided by healthcare professionals in the context of this screening? Why or why not?
- (9)
- Do you need other sources of information? Which ones?
- (10)
- What could be improved: in terms of communication? Among healthcare professionals?
- (11)
- Have you thought about the results?
- (12)
- Have you thought about follow-up after the results?
- (13)
- What would be the ideal post-test kit for you?
- (14)
- In your opinion, who would be the ideal referral professional for the screening of this cancer?
- (15)
- How much confidence do you have in your screening professional (0 to 5)? Who is this professional: midwife, general practitioner, gynecologist? Why or why not?
Appendix B. Focus-Group Interview Guide for Women
- Topic 1: Cervical cancer.
- If I say “cervical cancer,” what comes to mind?Follow-up/deepening:
- -
- How do you feel about it?
- -
- What do you know about this cancer?
- What do you know about cervical cancer screening?Follow-up/deepening:
- -
- Do you know whether this screening is financially covered or reimbursed?
- -
- Would full financial coverage be an incentive to carry out screening?
- Topic 2: The function and use of the self-testing kit.
- How did you feel when you received this kit?Follow-up/deepening:
- -
- And specifically the word cancer?
- -
- Did it concern you?
- What did you think of the instructions for use?Follow-up/deepening:
- -
- Did you encounter any difficulties?
- -
- What helped? What did you rely on? Could it be simplified (color codes, pictograms, positioning)?
- What did you think of receiving the kit at your home?Follow-up/deepening:
- -
- Do you have any other shipping/retrieval options in mind?
- Which self-test do you think is most appropriate (vaginal or urinary)?Follow-up/deepening:
- -
- In your opinion, which is the most reliable?
- Topic 3: Widespread use of self-screening.
- Do you think self-testing should be offered to all women?Follow-up/deepening:
- -
- Do you think self-testing is suitable for all ages?
- -
- For all geographical areas?
- Do you think all cultures/religions could benefit from using this kit?
- Topic 4: The role of healthcare professionals.
- In your opinion, can the self-testing system be completely separated from regular gynecological check-ups (carried out by a gynecologist, general practitioner (GP), or midwife)?Follow-up/deepening:
- -
- Would you like an explanation on how to use the kit from a healthcare pro-fessional? Why (reassuring? frightening?)
- Does certain behavior on the part of healthcare professionals discourage you from carrying out screening? Which ones?Follow-up/deepening:
- -
- Does your doctor talk to you about cervical cancer screening?
- -
- Do you feel accompanied by your doctor for CC screening? Can you de-scribe how you feel when the question of screening is raised by you or your GP? How does it make you feel?
- -
- Do you have any ideas for improving your dialogue with your doctor on this subject?
- Topic 5: Prospects and future of screening.
- Following the proposal of this self-testing kit, do you see more interest in getting tested?Follow-up/deepening:
- -
- Do you feel concerned by this cancer (more present, more real)?
- How can we make screening a priority in women’s lives?Follow-up/deepening:
- -
- Do you have any suggestions for improving the chances of women being screened?
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Papillomavirus Humain et Cancer. Available online: https://www.who.int/fr/news-room/fact-sheets/detail/human-papilloma-virus-and-cancer (accessed on 7 May 2024).
- Arbyn, M.; Ronco, G.; Anttila, A.; Meijer, C.J.; Poljak, M.; Ogilvie, G.; Koliopoulos, G.; Naucler, P.; Sankaranarayanan, R.; Peto, J. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine 2012, 30, F88–F99. [Google Scholar] [CrossRef] [PubMed]
- Das, M. WHO launches strategy to accelerate elimination of cervical cancer. Lancet Oncol. 2021, 22, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Vignat, J.; Lorenzoni, V.; Eslahi, M.; Ginsburg, O.; Lauby-Secretan, B.; Arbyn, M.; Basu, P.; Bray, F.; Vaccarella, S. Global estimates of incidence and mortality of cervical cancer in 2020: A baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 2023, 11, e197–e206. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Gultekin, M.; Morice, P.; Nieminen, P.; Cruickshank, M.; Poortmans, P.; Kelly, D.; Poljak, M.; Bergeron, C.; Ritchie, D.; et al. The European response to the WHO call to eliminate cervical cancer as a public health problem. Int. J. Cancer 2021, 148, 277–284. [Google Scholar] [CrossRef]
- Baraquin, A.; Pépin, L.; Floerchinger, P.; Lepiller, Q.; Prétet, J.L. Nouvelles recommandations pour le dépistage du cancer du col de l’utérus en France [New recommendations for cervical cancer screening in France]. Ann. Pharm. Fr. 2023, 81, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef] [PubMed]
- Hamers, F.F.; Poullié, A.I.; Arbyn, M. Updated evidence-based recommendations for cervical cancer screening in France. Eur. J. Cancer Prev. 2022, 31, 279. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Smith, S.B.; Temin, S.; Sultana, F.; Castle, P. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: Updated meta-analyses. BMJ 2018, 363, k4823. [Google Scholar] [CrossRef] [PubMed]
- Sechi, I.; Muresu, N.; Puci, M.V.; Saderi, L.; Del Rio, A.; Cossu, A.; Muroni, M.R.; Castriciano, S.; Martinelli, M.; Cocuzza, C.E.; et al. Preliminary Results of Feasibility and Acceptability of Self-Collection for Cervical Screening in Italian Women. Pathogens 2023, 12, 1169. [Google Scholar] [CrossRef]
- Martinelli, M.; Latsuzbaia, A.; Bonde, J.; Pedersen, H.; Iacobone, A.D.; Bottari, F.; Piana, A.F.; Pietri, R.; Cocuzza, C.E.; Arbyn, M. Extended Valhudes Study Group. Performance of BD Onclarity HPV assay on FLOQSwabs vaginal self-samples. Microbiol. Spectr. 2024, 12, e0287223. [Google Scholar] [CrossRef] [PubMed]
- Reques, L.; Rolland, C.; Lallemand, A.; Lahmidi, N.; Aranda-Fernández, E.; Lazzarino, A.; Bottero, J.; Hamers, F.; Bergeron, C.; Haguenoer, K.; et al. Comparison of cervical cancer screening by self-sampling papillomavirus test versus pap-smear in underprivileged women in France. BMC Women′s Health 2021, 21, 221. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Verberckmoes, B.; Castle, P.E.; Arbyn, M. Offering HPV self-sampling kits: An updated meta-analysis of the effectiveness of strategies to increase participation in cervical cancer screening. Br. J. Cancer 2023, 128, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Yeh, P.T.; Oguntade, H.; Kennedy, C.E.; Narasimhan, M. HPV self-sampling for cervical cancer screening: A systematic review of values and preferences. BMJ Glob. Health 2021, 6, e003743. [Google Scholar] [CrossRef]
- Caleia, A.I.; Pires, C.; Pereira, J.F.; Pinto-Ribeiro, F.; Longatto-Filho, A. Self-Sampling as a Plausible Alternative to Screen Cervical Cancer Precursor Lesions in a Population with Low Adherence to Screening: A Systematic Review. Acta Cytol. 2020, 64, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Serrano, B.; Ibáñez, R.; Robles, C.; Peremiquel-Trillas, P.; de Sanjosé, S.; Bruni, L. Worldwide use of HPV self-sampling for cervical cancer screening. Prev. Med. 2022, 154, 106900. [Google Scholar] [CrossRef] [PubMed]
- John, J.H.; Halder, A.; Purwar, S.; Pushpalatha, K.; Gupta, P.; Dubey, P. Study to determine efficacy of urinary HPV 16 & HPV 18 detection in predicting premalignant and malignant lesions of uterine cervix. Int. J. Gynecol. Obstet. 2023, 161, 79–85. [Google Scholar] [CrossRef]
- Arbyn, M.; Peeters, E.; Benoy, I.; Vanden Broeck, D.; Bogers, J.; De Sutter, P.; Donders, G.; Tjalma, W.; Weyers, S.; Cuschieri, K.; et al. VALHUDES: A protocol for validation of human papillomavirus assays and collection devices for HPV testing on self-samples and urine samples. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2018, 107, 52–56. [Google Scholar] [CrossRef]
- Van Keer, S.; Latsuzbaia, A.; Broeck, D.V.; De Sutter, P.; Donders, G.; Doyen, J.; Tjalma, W.A.A.; Weyers, S.; Arbyn, M.; Vorsters, A. Analytical and clinical performance of extended HPV genotyping with BD Onclarity HPV Assay in home-collected first-void urine: A diagnostic test accuracy study. J. Clin. Virol. 2022, 155, 105271. [Google Scholar] [CrossRef]
- Latsuzbaia, A.; Van Keer, S.; Vanden Broeck, D.; Weyers, S.; Donders, G.; De Sutter, P.; Tjalma, W.; Doyen, J.; Vorsters, A.; Arbyn, M. Comparison of the Accuracy of Alinity m HR HPV Assay on Self-Versus Clinician-Taken Samples Using the VALHUDES Protocol. J. Mol. Diagn. 2023, 25, 957–966. [Google Scholar] [CrossRef]
- Lefeuvre, C.; Pivert, A.; Le Guillou-Guillemette, H.; Lunel-Fabiani, F.; Veillon, P.; Le Duc-Banaszuk, A.-S.; Ducancelle, A. Urinary HPV DNA Testing as a Tool for Cervical Cancer Screening in Women Who Are Reluctant to Have a Pap Smear in France. J. Infect. 2020, 81, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Haguenoer, K.; Sengchanh, S.; Gaudy-Graffin, C.; Boyard, J.; Fontenay, R.; Marret, H.; Goudeau, A.; Pigneaux de Laroche, N.; Rusch, E.; Giraudeau, B. Vaginal self-sampling is a cost-effective way to increase participation in a cervical cancer screening programme: A randomised trial. Br. J. Cancer 2014, 111, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Santé Publique France. Couverture du Dépistage du Cancer du col de L’utérus en France. 2019. Available online: https://www.santepubliquefrance.fr/maladies-et-traumatismes/cancers/cancer-du-col-de-l-uterus/documents/couverture-du-depistage-du-cancer-du-col-de-l-uterus-en-france (accessed on 7 May 2024).
- Observatoire Géode. Santé Publique France. 2018–2020. Available online: https://geodes.santepubliquefrance.fr/#bbox=534584,6249210,886419,473249&c=indicator&i=depistage_ccu.couverture_stand&s=2018-2020&t=a01&view=map2 (accessed on 7 May 2024).
- Agence Régionale de Santé Pays de La Loire. Zonage Médecin Pour Les Pays de la Loire. 2018. Available online: https://www.pays-de-la-loire.ars.sante.fr/system/files/2018-01/carte-zonage-pays-de-la-loire-2018-medecin-departement.pdf (accessed on 7 May 2024).
- Lefeuvre, C.; De Pauw, H.; Le Duc Banaszuk, A.S.; Pivert, A.; Ducancelle, A.; Rexand-Galais, F.; Arbyn, M. Study Protocol: Randomised Controlled Trial Assessing the Efficacy of Strategies Involving Self-Sampling in Cervical Cancer Screening. Int. J. Public Health 2022, 67, 1604284. [Google Scholar] [CrossRef]
- Chatzistamatiou, K.; Vrekoussis, T.; Tsertanidou, A.; Moysiadis, T.; Mouchtaropoulou, E.; Pasentsis, K.; Kitsou, A.; Moschaki, V.; Ntoula, M.; Zempili, P.; et al. Acceptability of self-sampling for human papillomavirus-based cervical cancer screening. J. Women′s Health 2020, 29, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Fargnoli, V.; Petignat, P.; Burton-Jeangros, C. To what extent will women accept HPV self-sampling for cervical cancer screening? A qualitative study conducted in Switzerland. Int. J. Women′s Health 2015, 7, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Sultana, F.; Mullins, R.; Murphy, M.; English, D.R.; Simpson, J.A.; Drennan, K.T.; Heley, S.; Wrede, C.D.; Brotherton, J.M.; Saville, M.; et al. Women’s views on human papillomavirus self-sampling: Focus groups to assess acceptability, invitation letters and a test kit in the Australian setting. Sex. Health 2015, 12, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Institut National du Cancer. Le Programme de Dépistage Organisé du Cancer du col de L’utérus—Dépistage du Cancer du col de L’utérus. 2023. Available online: https://www.e-cancer.fr/Professionnels-de-sante/Depistage-et-detection-precoce/Depistage-du-cancer-du-col-de-l-uterus/Le-programme-de-depistage-organise (accessed on 7 May 2024).
- Hesse-Biber, S.; Leavy, P. Approaches to Qualitative Research: A Reader on Theory and Practice; Oxford University Press: Oxford, NY, USA, 2004; p. 560. [Google Scholar]
- Duggleby, W. What about focus group interaction data? Qual. Health Res. 2005, 15, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Vasileiou, K.; Barnett, J.; Thorpe, S.; Young, T. Characterising and justifying sample size sufficiency in interview-based studies: Systematic analysis of qualitative health research over a 15-year period. BMC Med. Res. Methodol. 2018, 18, 148. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.F.; Shannon, S.E. Three approaches to qualitative content analysis. Qual. Health Res. 2005, 15, 1277–1288. [Google Scholar] [CrossRef]
- Jacques, M.C.; Hébert, M.; Gallagher, F.; Tribble, D.S.C. La théorisation ancrée. Méthodes Qualitatives, Quantitatives et Mixtes. In La Recherche en Sciences Humaines, Sociales et de la Santé, 2nd ed.; Corbière, M., Larivière, N., Eds.; Presses de l’Université du Québec: Québec City, QC, Canada, 2020; pp. 97–122. Available online: https://books.google.fr/books?hl=fr&lr=&id=ngv5DwAAQBAJ&oi=fnd&pg=PT126&dq=Jacques,+M.C.%3B+H%C3%A9bert,+M.%3B+Gallagher,+F.%3B+Tribble,+D.S.C.+La+th%C3%A9orisation+ancr%C3%A9e.+M%C3%A9thodes+qualitatives,+quantitatives+et+mixtes.+In+La+Recherche+en+Sciences+Humaines,+Sociales+et+de+la+Sant%C3%A9,+2nd+ed&ots=zSUNVilKLv&sig=q56Yru_n5RyIvijXjth1GaBbvf8&redir_esc=y#v=onepage&q&f=false (accessed on 7 May 2024).
- Paillé, P.; Mucchielli, A. L’analyse thématique. In L’Analyse Qualitative en Sciences Humaines et Sociales; Paillé, P., Mucchielli, A., Eds.; Armand Colin: Paris, France, 2016; pp. 235–312. [Google Scholar] [CrossRef]
- Strauss, A.; Corbin, J. Basics of Qualitative Research Techniques; Sage Publications: Thousand Oaks, CA, USA, 1998; Available online: https://scholar.google.com/scholar_lookup?title=Basics+of+Qualitative+Research+Techniques&author=Strauss,+A.&author=Corbin,+J.&publication_year=1998 (accessed on 7 May 2024).
- DRESS. Mieux Connaitre et Evaluer la Prise en Charge des Maladies Chroniques: Lancement de L’enquête PaRIS en Septembre 2023. Available online: https://drees.solidarites-sante.gouv.fr/communique-de-presse/mieux-connaitre-et-evaluer-la-prise-en-charge-des-maladies-chroniques#:~:text=En%202021%2C%20en%20France%2C%2012,du%20vieillissement%20de%20la%20population (accessed on 7 May 2024).
- Druel, V.; Delpierre, C.; Ouanhnon, L.; Bugat, M.E.R.; Grosclaude, P. Cervical cancer screening: Inequality of screening, inequality of medical practice? BMC Public Health 2023. preprint (submitted). [Google Scholar] [CrossRef]
- Ouanhnon, L.; Bugat, M.E.R.; Lamy, S.; Druel, V.; Delpierre, C.; Grosclaude, P. Social and territorial inequalities in breast and cervical cancers screening uptake: A cross-sectional study in France. BMJ Open 2022, 12, e055363. [Google Scholar] [CrossRef] [PubMed]
- Institut National du Cancer (INCa). Synthèse—Généralisation du Dépistage du Cancer du col de L’Utérus/Étude Médico-Économique/Phase 1—Ref: APDEPCCUSYN16. 2016. Available online: https://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/Generalisation-du-depistage-du-cancer-du-col-de-l-uterus-etude-medico-economique-Phase-1 (accessed on 7 May 2024).
- Lofters, A.K.; Vahabi, M.; Kim, E.; Ellison, L.; Graves, E.; Glazier, R.H. Cervical cancer screening among women from Muslim-majority countries in Ontario, Canada. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Elmore, C.E.; Laughon, K.; Mitchell, E.M. Self-collection of samples for HPV testing to increase participation in cervical cancer screening by immigrant women: An integrative review. Public Health Nurs. 2020, 37, 677–695. [Google Scholar] [CrossRef] [PubMed]
- Chevillard, G.; Mousquès, J. Medically underserved areas: Are primary care teams efficient at attracting and retaining general practitioners? Soc. Sci. Med. 2021, 287, 114358. [Google Scholar] [CrossRef] [PubMed]
- Raginel, T.; Grandazzi, G.; Launoy, G.; Trocmé, M.; Christophe, V.; Berchi, C.; Guittet, L. Social inequalities in cervical cancer screening: A discrete choice experiment among French general practitioners and gynaecologists. BMC Health Serv. Res. 2020, 20, 693. [Google Scholar] [CrossRef] [PubMed]
- Arrivillaga, M.; Bermúdez, P.C.; García-Cifuentes, J.P.; Rodríguez-López, M.; Neira, D.; Vargas-Cardona, H.D. Women’s critical experiences with the pap smear for the development of cervical cancer screening devices. Heliyon 2023, 9, e14289. [Google Scholar] [CrossRef] [PubMed]
- Landy, R.; Hollingworth, T.; Waller, J.; Marlow, L.A.; Rigney, J.; Round, T.; Sasieni, P.D.; Lim, A.W. Non-speculum sampling approaches for cervical screening in older women: Randomised controlled trial. Br. J. Gen. Pract. 2022, 72, e26–e33. [Google Scholar] [CrossRef] [PubMed]
- Haguenoer, K.; Boyard, J.; Sengchanh, S.; Gaudy-Graffin, C.; Fontenay, R.; Marret, H. L’Auto-Prélèvement Vaginal Est Une Méthode Efficace Pour Augmenter la Participation au Dépistage du Cancer du col de L’utérus: Un Essai Randomisé en Indre-et-Loire. 2017. Available online: https://www.santepubliquefrance.fr/regions/centre-val-de-loire/documents/article/2017/l-auto-prelevement-vaginal-est-une-methode-efficace-pour-augmenter-la-participation-au-depistage-du-cancer-du-col-de-l-uterus-un-essai-randomise (accessed on 7 May 2024).
- Khoo, S.P.; Lim, W.T.; Rajasuriar, R.; Nasir, N.H.; Gravitt, P.; Woo, Y.L. The acceptability and preference of vaginal self-sampling for human papillomavirus (HPV) testing among a multi-ethnic Asian female population. Cancer Prev. Res. 2021, 14, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Megersa, B.S.; Bussmann, H.; Bärnighausen, T.; Muche, A.A.; Alemu, K.; Deckert, A. Community cervical cancer screening: Barriers to successful home-based HPV self-sampling in Dabat district, North Gondar, Ethiopia. A qualitative study. PLoS ONE 2020, 15, e0243036. [Google Scholar] [CrossRef]
- Strelow, B.; O’Laughlin, D. Barriers to cervical cancer screening among immigrants. JAAPA 2022, 35, 23–27. [Google Scholar] [CrossRef]
- Bertucci, M.; Bonnet, E.; Satger, L.; Kreiche, A.; Chappert, J.L.; Loy-Morel, S.; Segondy, M.; Daurès, J.P.; Boulle, N. Acceptability of vaginal self-sampling with high-risk human papillomavirus testing for cervical cancer screening: A French questionnaire-based study. Women Health 2021, 61, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Montealegre, J.R.; Landgren, R.M.; Anderson, M.L.; Hoxhaj, S.; Williams, S.; Robinson, D.J.; Scheurer, M.E.; Ramondetta, L.M. Acceptability of self-sample human papillomavirus testing among medically underserved women visiting the emergency department. Gynecol. Oncol. 2015, 138, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, G.; Forbat, L. Cancer as biographical disruption: Constructions of living with cancer. Support. Care Cancer 2012, 20, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Jentschke, M.; Lehmann, R.; Drews, N.; Hansel, A.; Schmitz, M.; Hillemanns, P. Psychological distress in cervical cancer screening: Results from a German online survey. Arch. Gynecol. Obstet. 2020, 302, 699–705. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Europe’s Beating Cancer Plan: A New EU Approach to Prevention, Treatment and Care. Available online: https://www.europarl.europa.eu/RegData/etudes/BRIE/2021/690526/EPRS_BRI(2021)690526_EN.pdf (accessed on 7 May 2024).
| 30–40 | 41–50 | 51–65 | Rural | Urban | LTC | |
|---|---|---|---|---|---|---|
| VSS | 6 | 15 | 7 | 19 | 9 | 5 |
| USS | 5 | 14 | 9 | 23 | 5 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Goff, J.; Le Duc-Banaszuk, A.-S.; Lefeuvre, C.; Pivert, A.; Ducancelle, A.; De Pauw, H.; Arbyn, M.; Vinay, A.; Rexand-Galais, F. Acceptance of Self-Sampling by Women Not Regularly Participating in Cervical Cancer Screening in Areas with Low Medical Density: A Qualitative Study within the French CapU4 Trial. Cancers 2024, 16, 2066. https://doi.org/10.3390/cancers16112066
Le Goff J, Le Duc-Banaszuk A-S, Lefeuvre C, Pivert A, Ducancelle A, De Pauw H, Arbyn M, Vinay A, Rexand-Galais F. Acceptance of Self-Sampling by Women Not Regularly Participating in Cervical Cancer Screening in Areas with Low Medical Density: A Qualitative Study within the French CapU4 Trial. Cancers. 2024; 16(11):2066. https://doi.org/10.3390/cancers16112066
Chicago/Turabian StyleLe Goff, Johane, Anne-Sophie Le Duc-Banaszuk, Caroline Lefeuvre, Adeline Pivert, Alexandra Ducancelle, Hélène De Pauw, Marc Arbyn, Aubeline Vinay, and Franck Rexand-Galais. 2024. "Acceptance of Self-Sampling by Women Not Regularly Participating in Cervical Cancer Screening in Areas with Low Medical Density: A Qualitative Study within the French CapU4 Trial" Cancers 16, no. 11: 2066. https://doi.org/10.3390/cancers16112066
APA StyleLe Goff, J., Le Duc-Banaszuk, A.-S., Lefeuvre, C., Pivert, A., Ducancelle, A., De Pauw, H., Arbyn, M., Vinay, A., & Rexand-Galais, F. (2024). Acceptance of Self-Sampling by Women Not Regularly Participating in Cervical Cancer Screening in Areas with Low Medical Density: A Qualitative Study within the French CapU4 Trial. Cancers, 16(11), 2066. https://doi.org/10.3390/cancers16112066






