The Association between Family History of Lung Cancer and Development of Lung Cancer: Analysis from the KoGES Data in Korea
Abstract
Simple Summary
Abstract
1. Background
2. Methods
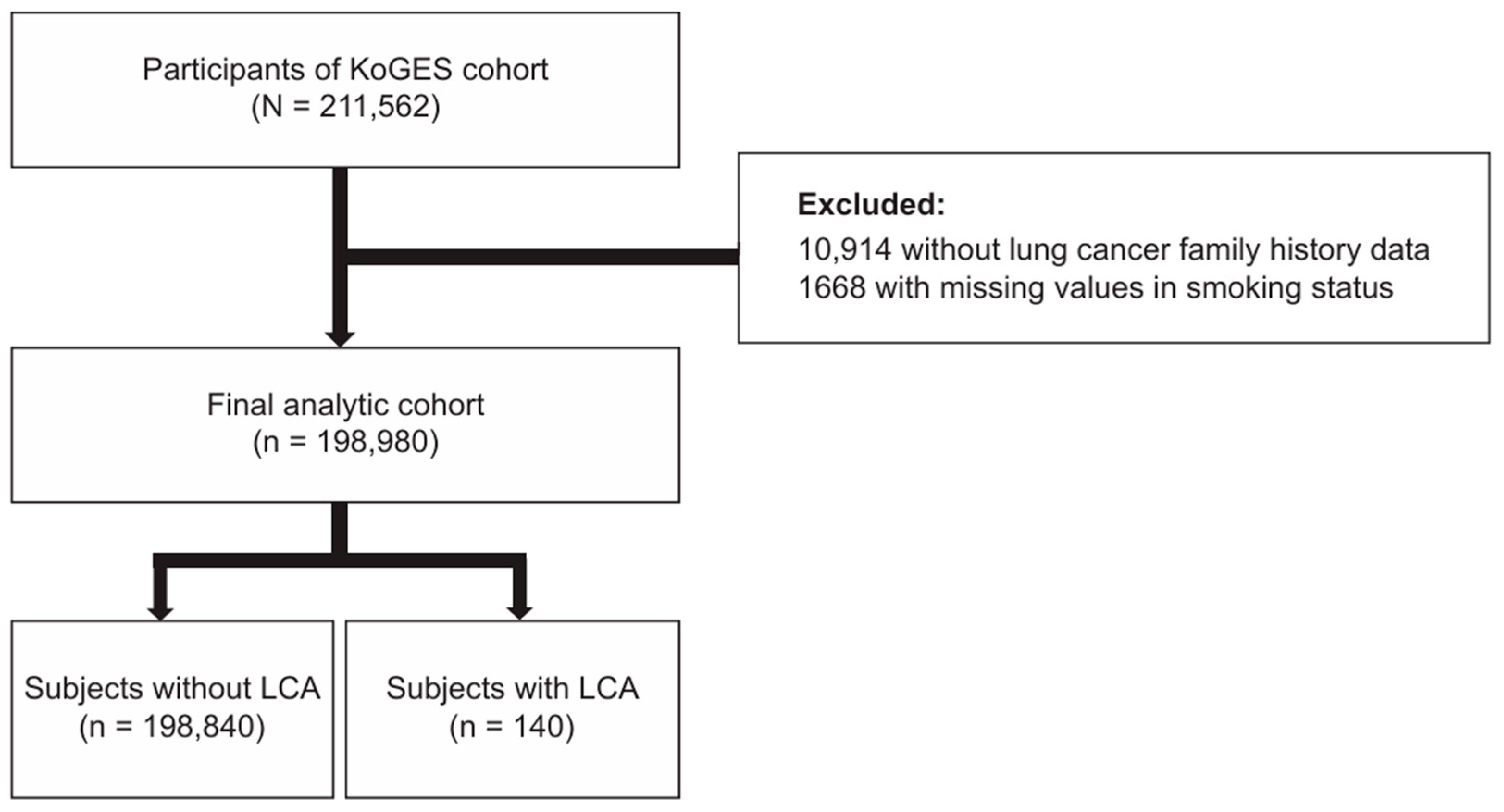
2.1. Data Source and Study Population
2.2. Family History of Lung Cancer and Lung Cancer Development
2.3. Covariates
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Family History of Lung Cancer and Lung Cancer Development
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef] [PubMed]
- Peto, R.; Darby, S.; Deo, H.; Silcocks, P.; Whitley, E.; Doll, R. Smoking, smoking cessation, and lung cancer in the UK since 1950: Combination of national statistics with two case-control studies. Bmj 2000, 321, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Gonzalez, M.; Ruano-Ravina, A.; Torres-Duran, M.; Kelsey, K.T.; Provencio, M.; Parente-Lamelas, I.; Piñeiro-Lamas, M.; Varela-Lema, L.; Perez-Rios, M.; Fernandez-Villar, A.; et al. Lung cancer risk and residential radon exposure: A pooling of case-control studies in northwestern Spain. Environ. Res. 2020, 189, 109968. [Google Scholar] [CrossRef] [PubMed]
- Kwak, K.; Kang, D.; Paek, D. Environmental exposure to asbestos and the risk of lung cancer: A systematic review and meta-analysis. Occup. Environ. Med. 2022, 79, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Matakidou, A.; Eisen, T.; Houlston, R.S. Systematic review of the relationship between family history and lung cancer risk. Br. J. Cancer 2005, 93, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Lebrett, M.B.; Crosbie, E.J.; Smith, M.J.; Woodward, E.R.; Evans, D.G.; Crosbie, P.A.J. Targeting lung cancer screening to individuals at greatest risk: The role of genetic factors. J. Med. Genet. 2021, 58, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Myles, J.P.; Duffy, S.W.; Liloglou, T.; Field, J.K. Family history and risk of lung cancer: Age-at-diagnosis in cases and first-degree relatives. Br. J. Cancer 2006, 95, 1288–1290. [Google Scholar] [CrossRef]
- Coté, M.L.; Kardia, S.L.; Wenzlaff, A.S.; Ruckdeschel, J.C.; Schwartz, A.G. Risk of lung cancer among white and black relatives of individuals with early-onset lung cancer. Jama 2005, 293, 3036–3042. [Google Scholar] [CrossRef]
- Jin, Y.; Xu, Y.; Xu, M.; Xue, S. Increased risk of cancer among relatives of patients with lung cancer in China. BMC Cancer 2005, 5, 146. [Google Scholar] [CrossRef]
- Cannon-Albright, L.A.; Carr, S.R.; Akerley, W. Population-Based Relative Risks for Lung Cancer Based on Complete Family History of Lung Cancer. J. Thorac. Oncol. 2019, 14, 1184–1191. [Google Scholar] [CrossRef]
- Lee, H.W.; Lee, H.J.; Lee, J.K.; Park, T.Y.; Heo, E.Y.; Kim, D.K. Rapid FEV(1) Decline and Lung Cancer Incidence in South Korea. Chest 2022, 162, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.S.; Lee, C.; Kim, H.W.; Jhee, J.; Yun, H.R.; Park, J.T.; Chang, T.I.; Yoo, T.H.; Kang, S.W.; Han, S.H. Association of Longitudinal Trajectories of Systolic BP with Risk of Incident CKD: Results from the Korean Genome and Epidemiology Study. J. Am. Soc. Nephrol. 2020, 31, 2133–2144. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Han, B.G. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 2017, 46, e20. [Google Scholar] [CrossRef] [PubMed]
- Ang, L.; Chan, C.P.Y.; Yau, W.P.; Seow, W.J. Association between family history of lung cancer and lung cancer risk: A systematic review and meta-analysis. Lung Cancer 2020, 148, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhong, W.; Yang, X.; Yan, H.; Wu, Y. Forecasting model of risk of cancer in lung cancer pedigree in a case-control study. Zhongguo Fei Ai Za Zhi 2011, 14, 581–587. [Google Scholar] [PubMed]
- Bromen, K.; Pohlabeln, H.; Jahn, I.; Ahrens, W.; Jöckel, K.H. Aggregation of lung cancer in families: Results from a population-based case-control study in Germany. Am. J. Epidemiol. 2000, 152, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Myles, J.P.; van Tongeren, M.; Page, R.D.; Liloglou, T.; Duffy, S.W.; Field, J.K. The LLP risk model: An individual risk prediction model for lung cancer. Br. J. Cancer 2008, 98, 270–276. [Google Scholar] [CrossRef]
- Gorlova, O.Y.; Weng, S.F.; Zhang, Y.; Amos, C.I.; Spitz, M.R. Aggregation of cancer among relatives of never-smoking lung cancer patients. Int. J. Cancer 2007, 121, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, K.; Dong, C.; Vaittinen, P. Cancer risks to spouses and offspring in the Family-Cancer Database. Genet. Epidemiol. 2001, 20, 247–257. [Google Scholar] [CrossRef]
- Leu, M.; Reilly, M.; Czene, K. Evaluation of bias in familial risk estimates: A study of common cancers using Swedish population-based registers. J. Natl. Cancer Inst. 2008, 100, 1318–1325. [Google Scholar] [CrossRef]
- Marcus, M.W.; Chen, Y.; Raji, O.Y.; Duffy, S.W.; Field, J.K. LLPi: Liverpool Lung Project Risk Prediction Model for Lung Cancer Incidence. Cancer Prev. Res. 2015, 8, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.F.; Lee, C.H.; Wang, M.J.; Goggins, W.B.; Chiang, T.A.; Huang, M.S.; Ko, Y.C. Cancer aggregation and complex segregation analysis of families with female non-smoking lung cancer probands in Taiwan. Eur. J. Cancer 2004, 40, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Chan, C.P.Y.; Seow, A.; Yau, W.P.; Seow, W.J. Association between family history and lung cancer risk among Chinese women in Singapore. Sci. Rep. 2021, 11, 21862. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Takizawa, Y.; Nishino, Y.; Takahashi, S.; Kanemura, S.; Omori, J.; Kurosawa, H.; Maemondo, M.; Minami, Y. Association between Family History of Cancer and Lung Cancer Risk among Japanese Men and Women. Tohoku J. Exp. Med. 2019, 247, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Huang, Y.S.; Yan, H.H.; Yang, X.N.; Zhong, W.Z.; Ye, H.W.; Yang, J.J.; Zhou, Q.; Wu, Y.L. A family history of cancer and lung cancer risk in never-smokers: A clinic-based case-control study. Lung Cancer 2015, 89, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Bermejo, J.L.; Sundquist, J.; Hemminki, K. Age of onset in familial cancer. Ann. Oncol. 2008, 19, 2084–2088. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, M.; Ding, X.J.; Cao, Y. Familial risk for lung cancer. Oncol. Lett. 2017, 13, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, J.; Malvezzi, M.; Negri, E.; La Vecchia, C.; Boffetta, P. Risk factors for lung cancer worldwide. Eur. Respir. J. 2016, 48, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Pesch, B.; Kendzia, B.; Gustavsson, P.; Jöckel, K.H.; Johnen, G.; Pohlabeln, H.; Olsson, A.; Ahrens, W.; Gross, I.M.; Brüske, I.; et al. Cigarette smoking and lung cancer--relative risk estimates for the major histological types from a pooled analysis of case-control studies. Int. J. Cancer 2012, 131, 1210–1219. [Google Scholar] [CrossRef]
- Meza, R.; Meernik, C.; Jeon, J.; Cote, M.L. Lung cancer incidence trends by gender, race and histology in the United States, 1973–2010. PLoS ONE 2015, 10, e0121323. [Google Scholar] [CrossRef]
- Moyer, V.A. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2014, 160, 330–338. [Google Scholar] [CrossRef] [PubMed]
| Total (N = 198,980) | Individuals without Lung Cancer (n = 198,840) | Individuals with Lung Cancer (n = 140) | p-Value | |
|---|---|---|---|---|
| Age, years | 53.9 ± 8.7 | 53.9 ± 8.7 | 62.4 ± 7.2 | <0.001 |
| 40–49 years | 68,359 (34.4) | 68,353 (34.4) | 6 (4.3) | |
| 50–59 years | 74,860 (37.6) | 74,822 (37.6) | 38 (27.1) | <0.001 |
| 60–69 years | 48,853 (24.6) | 48,774 (24.5) | 79 (56.4) | |
| 70–79 years | 6651 (3.3) | 6635 (3.3) | 16 (11.4) | |
| ≥80 years | 257 (0.1) | 256 (0.1) | 1 (0.7) | |
| Gender | <0.001 | |||
| Female | 129,649 (65.2) | 129,598 (65.2) | 51 (36.4) | |
| Male | 69,331 (34.8) | 69,242 (34.8) | 89 (63.6) | |
| Body mass index, kg/m2 (n = 198,119) | 24.0 ± 3.0 | 24.0 ± 3.0 | 23.6 ± 2.9 | 0.135 |
| <18.5 kg/m2 | 3590 (1.8) | 3586 (1.8) | 4 (2.9) | 0.627 |
| 18.5–24.9 kg/m2 | 126,973 (64.1) | 126,882 (64.1) | 91 (65.5) | |
| ≥25 kg/m2 | 67,556 (34.1) | 67,512 (34.1) | 44 (31.7) | |
| Smoking status | <0.001 | |||
| Never smoker | 144,291 (72.5) | 144,232 (72.5) | 59 (42.1) | |
| Ever smoker | 54,689 (27.5) | 54,608 (27.5) | 81 (57.9) | |
| Income (n = 159,381) | <0.001 | |||
| Lowest | 41,098 (25.8) | 41,045 (25.8) | 53 (45.7) | |
| Middle | 81,894 (51.4) | 81,842 (51.4) | 52 (44.8) | |
| Highest | 36,389 (22.8) | 36,378 (22.8) | 11 (9.5) | |
| Marital status (n = 198,011) | 0.392 | |||
| Never married | 4052 (2.0) | 4051 (2.0) | 1 (0.7) | |
| Married | 172,881 (87.3) | 172,760 (87.3) | 121 (86.4) | |
| Divorced or separated | 21,078 (10.6) | 21,060 (10.6) | 18 (12.9) | |
| Comorbidities | ||||
| Hypertension (n = 198,863) | 40,485 (20.4) | 40,448 (20.4) | 37 (26.4) | 0.093 |
| Diabetes mellitus (n = 198,812) | 14,037 (7.1) | 14,017 (7.1) | 20 (14.3) | 0.002 |
| Dyslipidemia (n = 131,188) | 17,336 (8.7) | 17,315 (8.7) | 21 (15.0) | 0.013 |
| Lung cancer family history | ||||
| Total | 6296 (3.2) | 6288 (3.2) | 8 (5.7) | 0.138 |
| Parents | 4938 (2.5) | 4937 (2.5) | 1 (0.7) | 0.283 |
| Siblings | 1436 (0.7) | 1429 (0.7) | 7 (5.0) | <0.001 |
| Individuals without a Family History of Lung Cancer (n = 192,684) | Individuals with a Family History of Lung Cancer (n = 6296) | p-Value | |
|---|---|---|---|
| Age, years | <0.001 | ||
| <60 years | 138,262 (71.8) | 4957 (78.7) | |
| ≥60 years | 54,422 (28.2) | 1339 (21.3) | |
| Gender | <0.001 | ||
| Female | 125,405 (65.1) | 4244 (67.4) | |
| Male | 67,279 (34.9) | 2052 (32.6) | |
| Body mass index, kg/m2 (n = 198,119) | |||
| <18.5 kg/m2 | 3484 (1.8) | 106 (1.7) | 0.067 |
| 18.5–24.9 kg/m2 | 122,866 (64.0) | 4107 (65.5) | |
| ≥25 kg/m2 | 65,495 (34.1) | 2061 (32.8) | |
| Smoking status | 0.546 | ||
| Never smoker | 139,747 (72.5) | 4544 (72.2) | |
| Ever smoker | 52,937 (27.5) | 1752 (27.8) | |
| Income (n = 159,381) | |||
| Lowest | 40,012 (26.0) | 1086 (20.3) | |
| Middle | 79,103 (51.4) | 2791 (52.1) | <0.001 |
| Highest | 34,911 (22.7) | 1478 (27.6) | |
| Marital status (n = 198,011) | |||
| Never married | 3919 (2.0) | 133 (2.1) | |
| Married | 167,251 (87.2) | 5630 (89.6) | <0.001 |
| Divorced or separated | 20,555 (10.7) | 523 (8.3) | |
| Comorbidities | |||
| Hypertension (n = 198,863) | 39,369 (20.4) | 1116 (17.7) | <0.001 |
| Diabetes mellitus (n = 198,812) | 13,638 (7.1) | 399 (6.3) | 0.025 |
| Dyslipidemia (n = 131,188) | 16,689 (8.7) | 647 (10.3) | <0.001 |
| Family History of Lung Cancer | Lung Cancer, n (%) | Outcome: Lung Cancer | |
|---|---|---|---|
| Unadjusted OR (95% CI, p) | Adjusted OR (95% CI, p) | ||
| Total population | |||
| No (n = 192,684) | 132 (0.07) | Ref. | Ref. |
| Yes (n = 6296) | 8 (0.13) | 1.86 (0.91–3.79, 0.090) | 2.28 (1.11–4.66, 0.024) |
| Age of affected relatives < 60 years (n = 1436) | 3 (0.21) | 3.05 (0.97–9.60,0.056) | 3.77 (1.19–11.88,0.024) |
| Age of affected relatives ≥ 60 years (n = 4860) | 5 (0.10) | 1.50 (0.61–3.67, 0.372) | 1.84 (0.75–4.50, 0.182) |
| Age < 60 years | |||
| No (n = 138,262) | 41 (0.03) | Ref. | Ref. |
| Yes (n = 4957) | 3 (0.06) | 2.04 (0.63–6.59, 0.233) | 2.03 (0.63–6.56, 0.237) |
| Age of affected relatives < 60 years (n = 1147) | 2 (0.17) | 5.89 (1.42–24.37, 0.014) | 5.89 (1.42–24.39, 0.015) |
| Age of affected relatives ≥ 60 years (n = 3810) | 1 (0.03) | 0.89 (0.12–6.44, 0.904) | 0.88 (0.12–6.39, 0.898) |
| Age ≥ 60 years | |||
| No (n = 54,422) | 91 (0.17) | Ref. | Ref. |
| Yes (n = 1339) | 5 (0.37) | 2.24 (0.91–5.51, 0.080) | 2.42 (0.98–5.98, 0.055) |
| Age of affected relatives < 60 years (n = 289) | 1 (0.35) | 2.07 (0.29–14.93, 0.469) | 2.13 (0.30–15.40, 0.453) |
| Age of affected relatives ≥ 60 years (n = 1050) | 4 (0.38) | 2.28 (0.84–6.23, 0.107) | 2.50 (0.92–6.85, 0.074) |
| p for interaction * | 0.790 | ||
| Men | |||
| No (n = 67,279) | 86 (0.13) | Ref. | Ref. |
| Yes (n = 2052) | 3 (0.15) | 1.14 (0.36–3.62, 0.819) | 1.41 (0.44–4.48, 0.560) |
| Age of affected relatives < 60 years (n = 433) | 0 | - | - |
| Age of affected relatives ≥ 60 years (n = 1619) | 3 (0.19) | 1.45 (0.46–4.59, 0.527) | 1.83 (0.57–5.81, 0.307) |
| Women | |||
| No (n = 125,405) | 46 (0.04) | Ref. | Ref. |
| Yes (n = 4244) | 5 (0.12) | 3.21 (1.28–8.09, 0.013) | 3.58 (1.42–9.02, 0.007) |
| Age of affected relatives < 60 years (n = 1003) | 3 (0.30) | 8.18 (2.54–26.33, <0.001) | 9.26 (2.87–29.91, <0.001) |
| Age of affected relatives ≥ 60 years (n = 3241) | 2 (0.06) | 1.68 (0.41–6.93, 0.471) | 1.86 (0.45–7.69, 0.390) |
| p for interaction * | 0.174 | ||
| Ever smoker | |||
| No (n = 52,937) | 78 (0.15) | Ref. | Ref. |
| Yes (n = 1752) | 3 (0.17) | 1.16 (0.37–3.69, 0.798) | 1.50 (0.47–4.78, 0.492) |
| Age of affected relatives < 60 years (n = 371) | 0 | - | - |
| Age of affected relatives ≥ 60 years (n = 1381) | 3 (0.22) | 1.48 (0.47–4.68, 0.509) | 1.95 (0.61–6.21, 0.259) |
| Never smoker | |||
| No (n = 139,747) | 54 (0.04) | Ref. | Ref. |
| Yes (n = 4544) | 5 (0.11) | 2.85 (1.14–7.13, 0.025) | 3.25 (1.30–8.16, 0.012) |
| Age of affected relatives < 60 years (n = 1065) | 3 (0.28) | 7.31 (2.28–23.41, < 0.001) | 8.52 (2.65–27.39, < 0.001) |
| Age of affected relatives ≥ 60 years (n = 3479) | 2 (0.06) | 1.49 (0.36–6.11, 0.581) | 1.69 (0.41–6.94, 0.467) |
| p for interaction * | 0.251 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.H.; Lee, H.; Kim, B.-G.; Kim, S.-H.; Sohn, J.W.; Yoon, H.J.; Jang, S.H.; Park, D.W. The Association between Family History of Lung Cancer and Development of Lung Cancer: Analysis from the KoGES Data in Korea. Cancers 2024, 16, 2063. https://doi.org/10.3390/cancers16112063
Kim SH, Lee H, Kim B-G, Kim S-H, Sohn JW, Yoon HJ, Jang SH, Park DW. The Association between Family History of Lung Cancer and Development of Lung Cancer: Analysis from the KoGES Data in Korea. Cancers. 2024; 16(11):2063. https://doi.org/10.3390/cancers16112063
Chicago/Turabian StyleKim, Sang Hyuk, Hyun Lee, Bo-Guen Kim, Sang-Heon Kim, Jang Won Sohn, Ho Joo Yoon, Seung Hun Jang, and Dong Won Park. 2024. "The Association between Family History of Lung Cancer and Development of Lung Cancer: Analysis from the KoGES Data in Korea" Cancers 16, no. 11: 2063. https://doi.org/10.3390/cancers16112063
APA StyleKim, S. H., Lee, H., Kim, B.-G., Kim, S.-H., Sohn, J. W., Yoon, H. J., Jang, S. H., & Park, D. W. (2024). The Association between Family History of Lung Cancer and Development of Lung Cancer: Analysis from the KoGES Data in Korea. Cancers, 16(11), 2063. https://doi.org/10.3390/cancers16112063







