Predictive Nomogram and Propensity Score Matching in Neuroendocrine Carcinoma of the Tubular Gastrointestinal Tract: A US Population-Based Clinical Outcome Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Demographic Characteristics
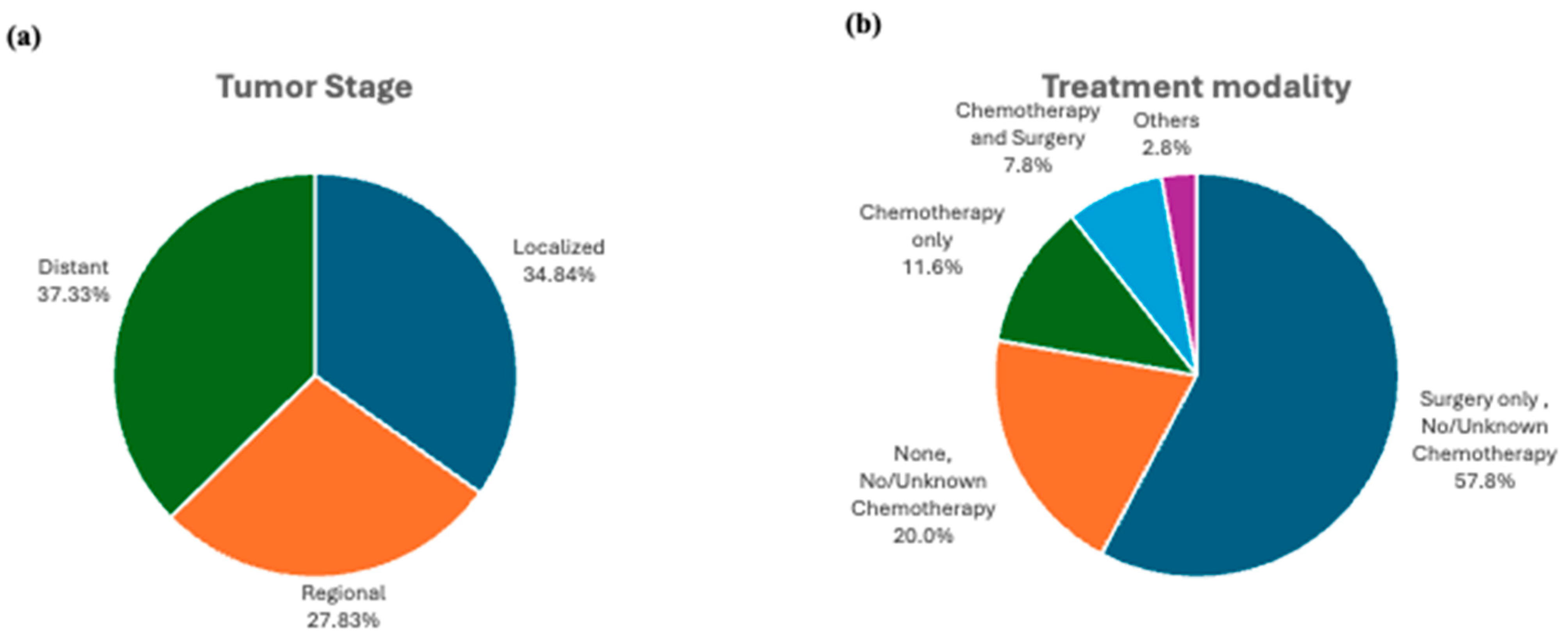
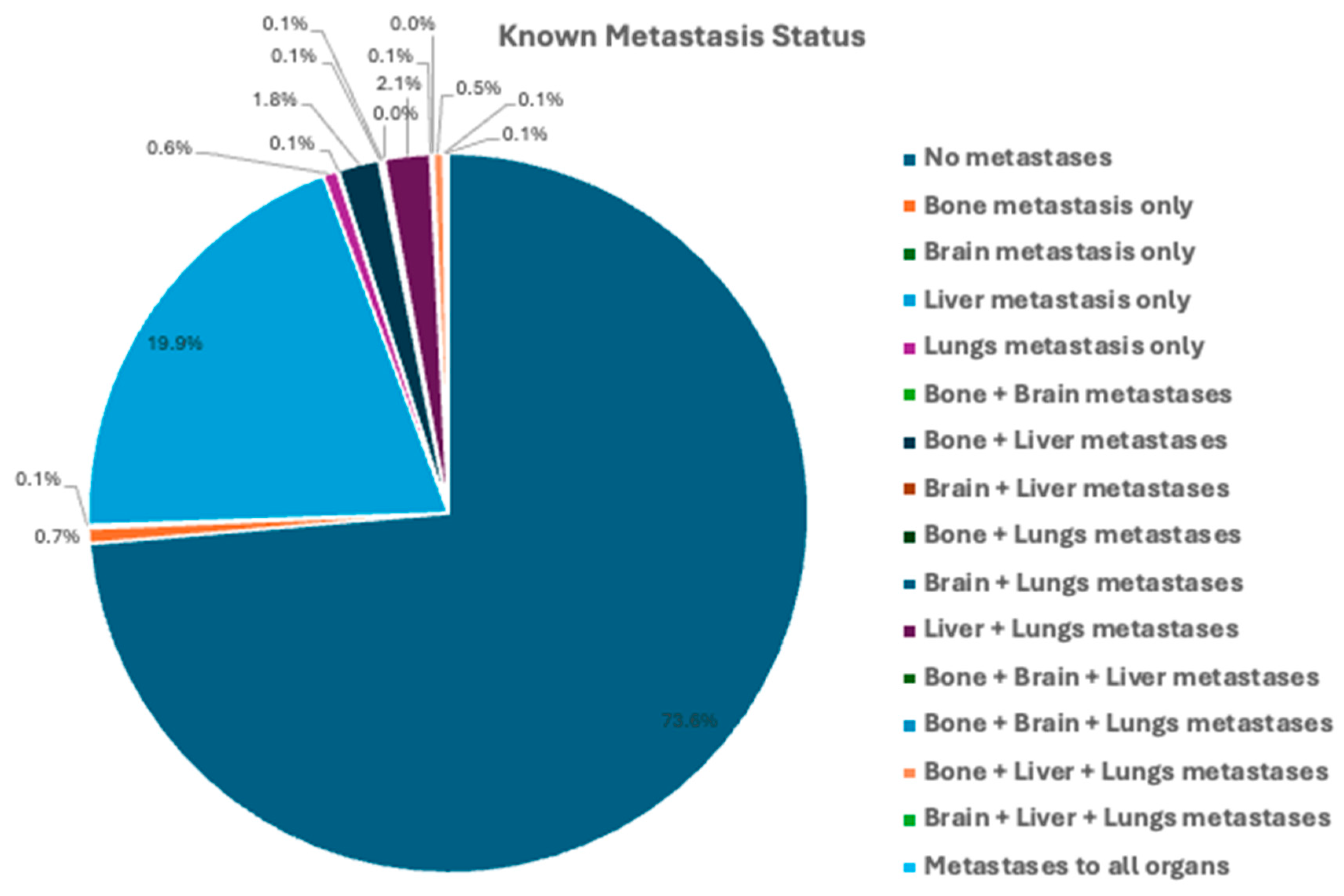
3.2. Tumor Characteristics and Treatment Modalities:
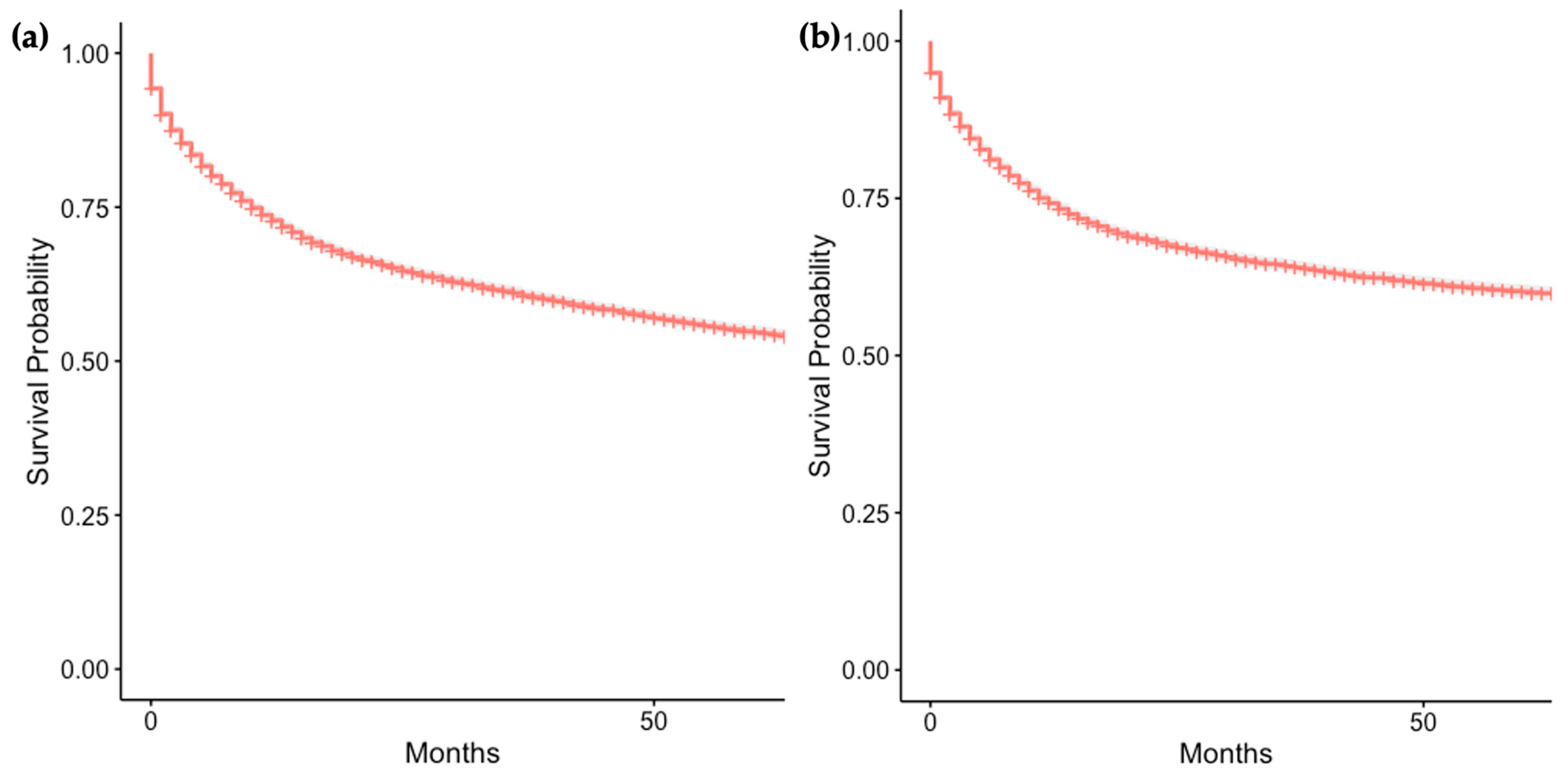
3.3. Overall and Cause-Specific Survival of Patients with GI-NEC
3.4. Survival Analysis of Demographic Factors
3.4.1. Outcomes by Age
3.4.2. Outcomes by Sex
3.4.3. Outcomes by Race
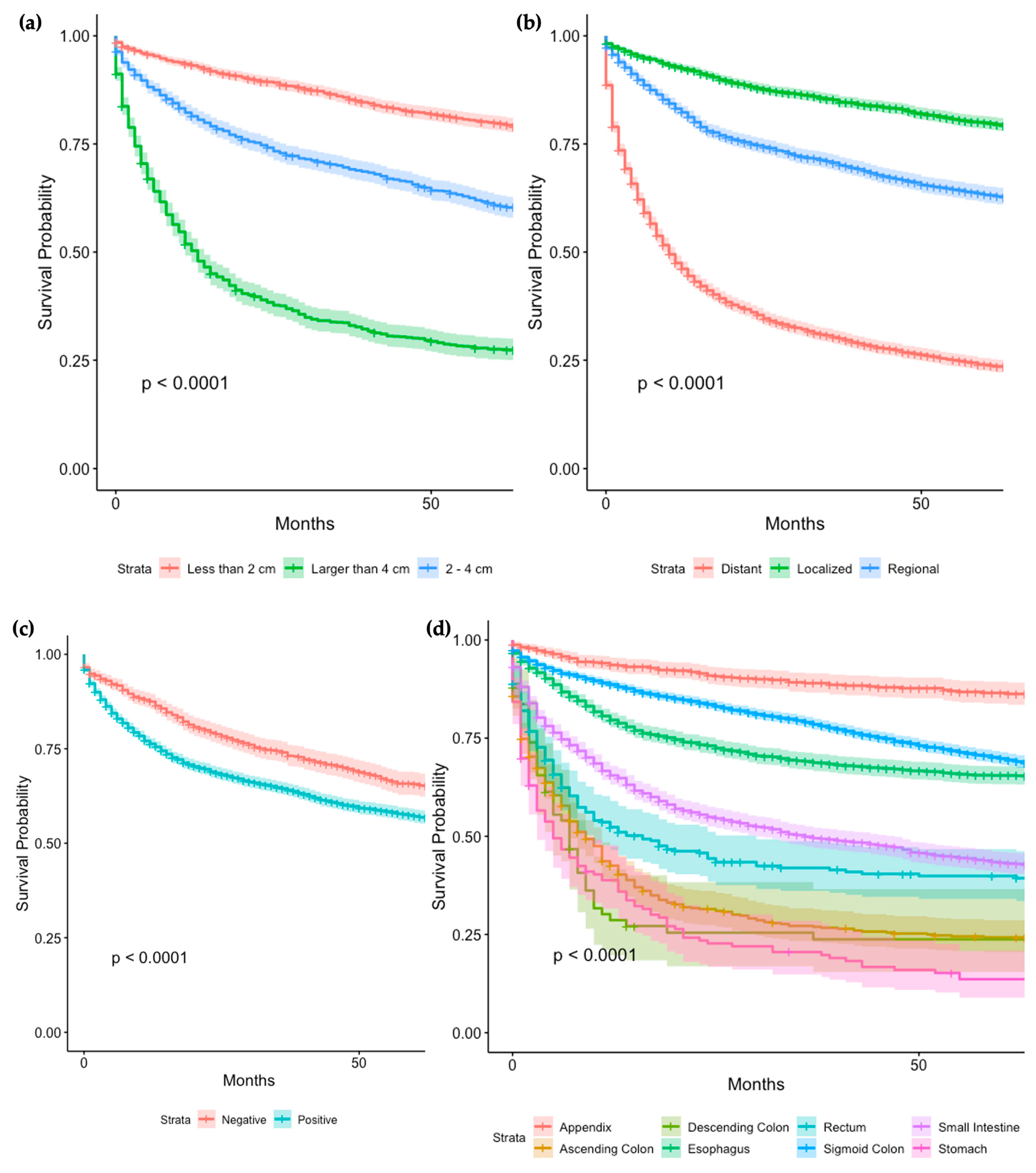
3.5. Survival Analysis of Tumor Characteristics and Treatment ModalitiesTumor Characteristics
Tumor Characteristics
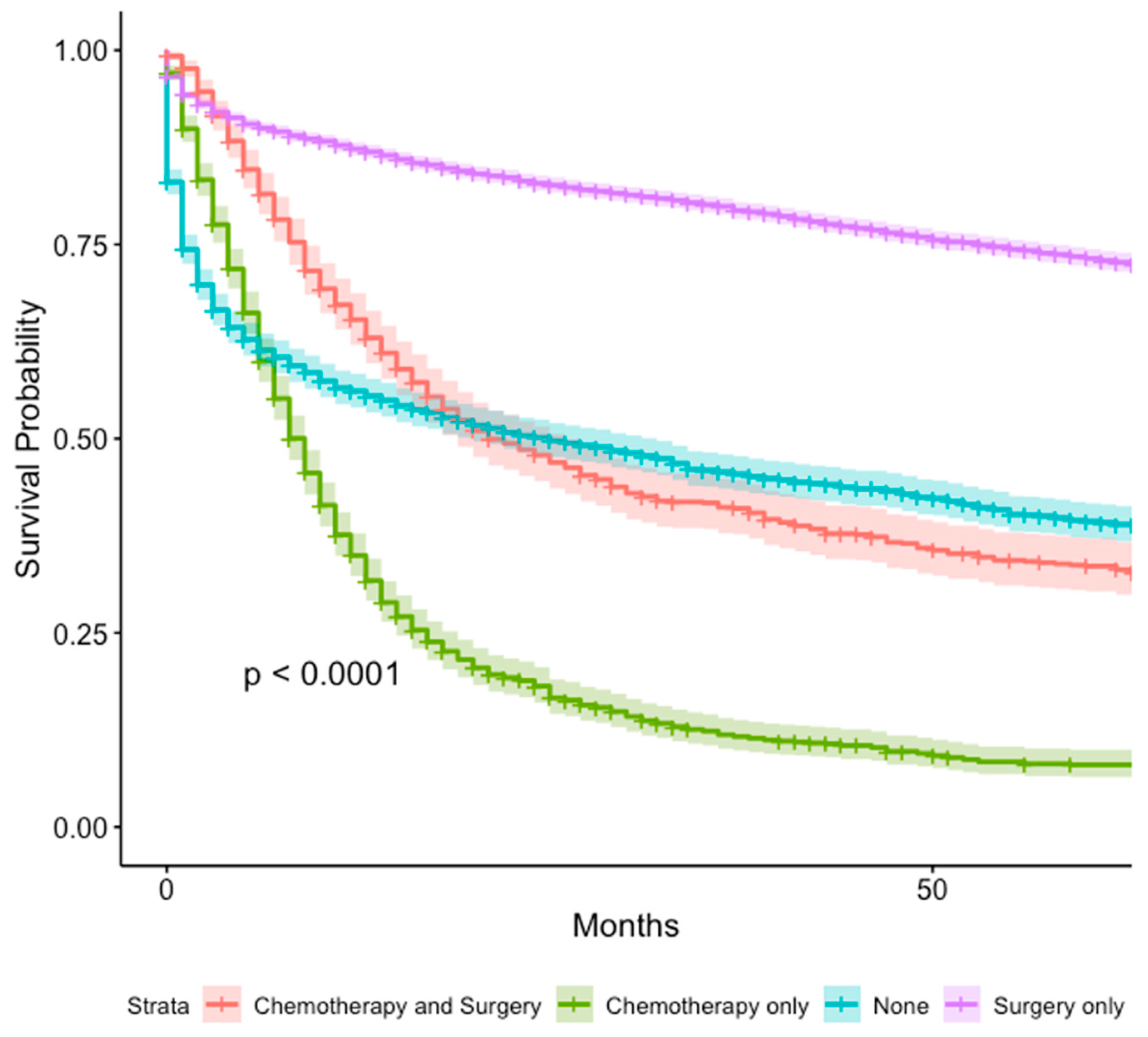
3.6. Treatment Modalities
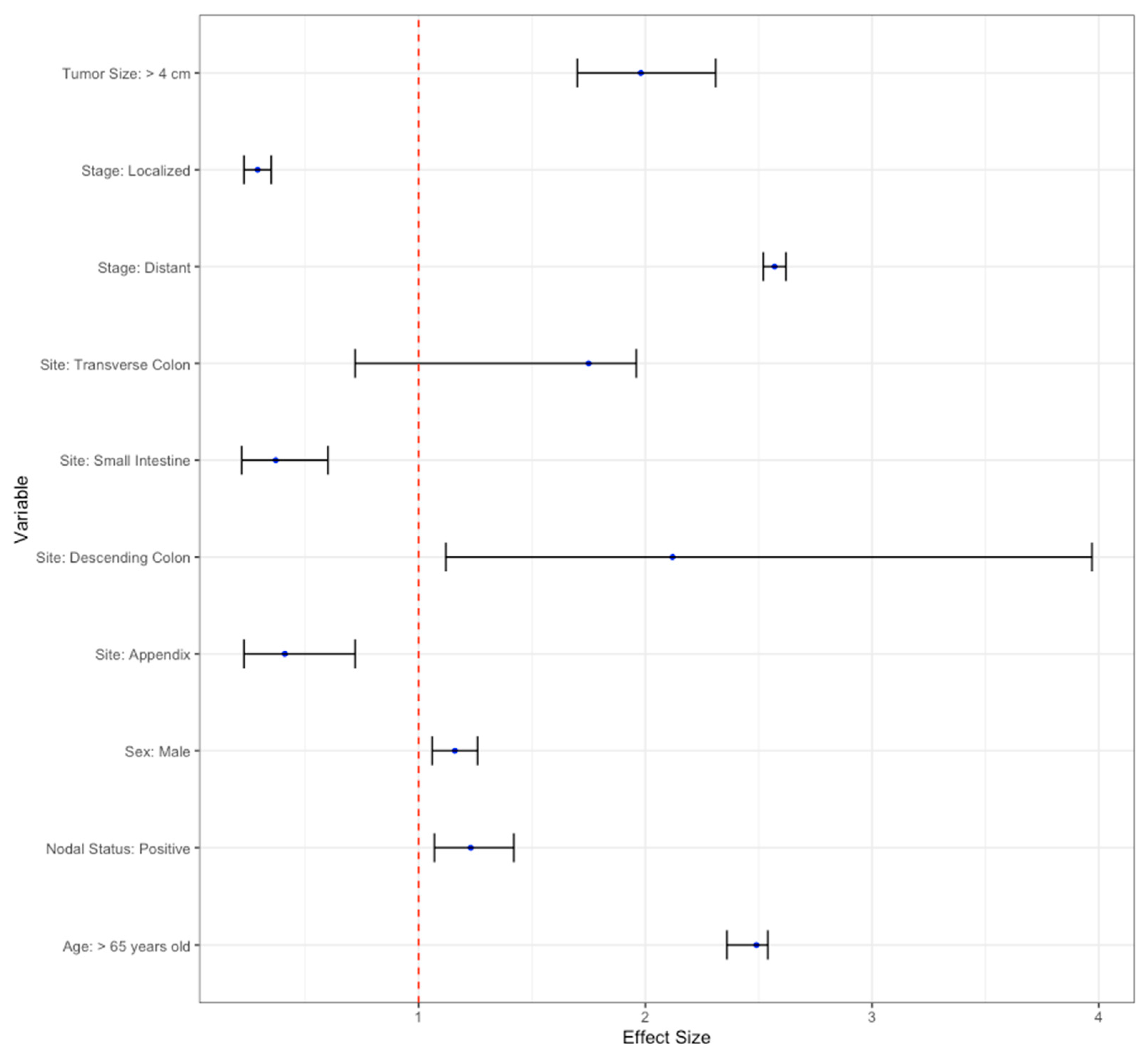
3.7. Multivariate Analysis
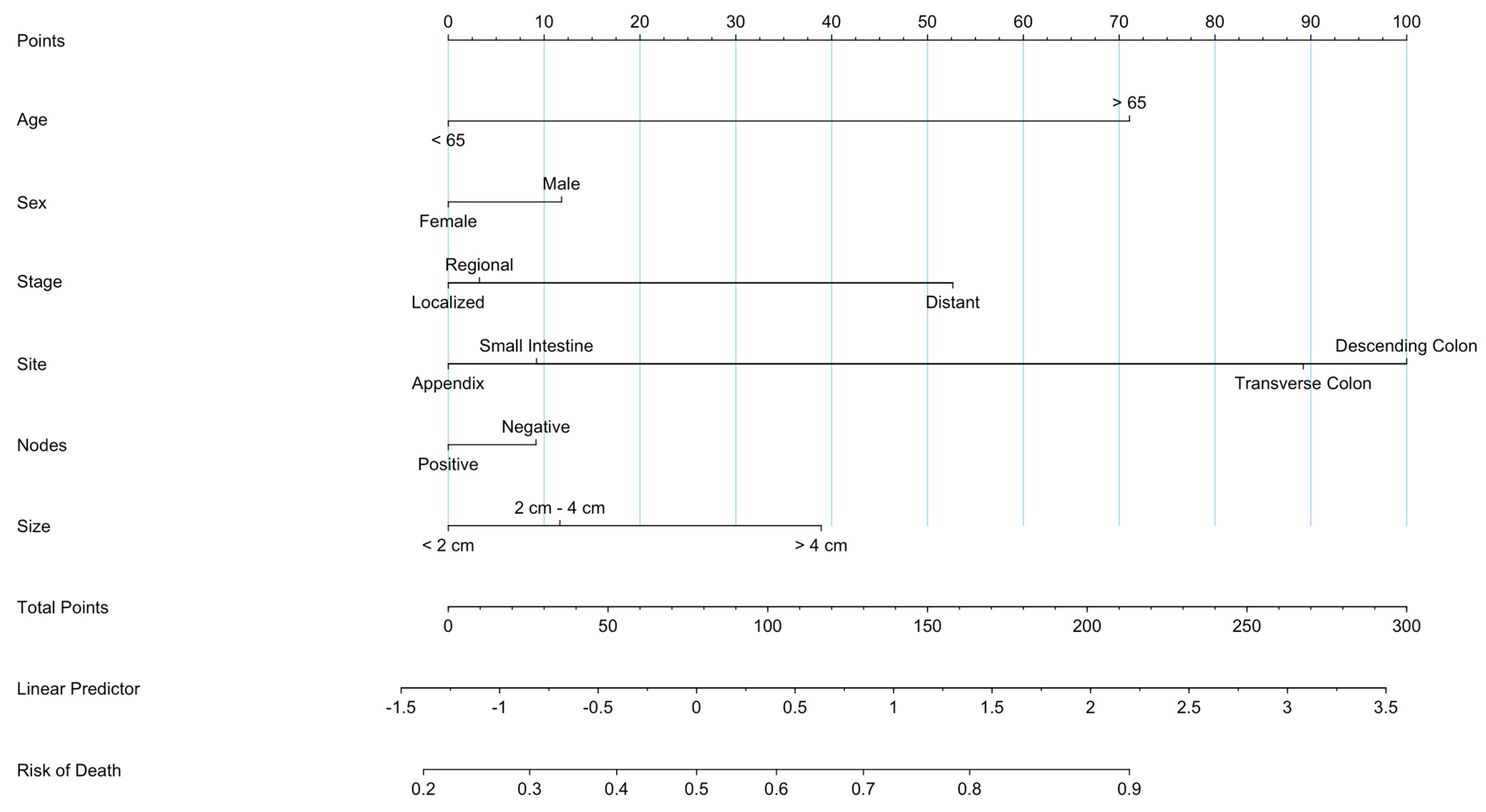
3.8. Prognostic Nomogram
3.9. Propensity Score Matching
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonds, M.; Rocha, F.G. Neuroendocrine Tumors of the Pancreatobiliary and Gastrointestinal Tracts. Surg. Clin. N. Am. 2020, 100, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Klöppel, G. Neuroendocrine Neoplasms: Dichotomy, Origin and Classifications. Visc. Med. 2017, 33, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Classification of Tumours. Digestive System Tumours: WHO Classification of Tumours, Volume 1; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Raphael, M.J.; Chan, D.L.; Law, C.; Singh, S. Principles of diagnosis and management of neuroendocrine tumours. Can. Med. Assoc. J. 2017, 189, E398–E404. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.H.; Basturk, O.; Sue, J.J.; Klimstra, D.S. A Practical Approach to the Classification of WHO Grade 3 (G3) Well-differentiated Neuroendocrine Tumor (WD-NET) and Poorly Differentiated Neuroendocrine Carcinoma (PD-NEC) of the Pancreas. Am. J. Surg. Pathol. 2016, 40, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Stawarski, A.; Maleika, P. Neuroendocrine tumors of the gastrointestinal tract and pancreas: Is it also a challenge for pediatricians? Adv. Clin. Exp. Med. 2020, 29, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Yazici, C.; Boulay, B.R. Evolving role of the endoscopist in management of gastrointestinal neuroendocrine tumors. World J. Gastroenterol. 2017, 23, 4847–4855. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Neuroendocrine Tumors of the Gastrointestinal Tract, Lung, and Thymus (Version 4.2021); National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2021. [Google Scholar]
- Garcia-Carbonero, R.; Sorbye, H.; Baudin, E.; Raymond, E.; Wiedenmann, B.; Niederle, B.; Sedlackova, E.; Toumpanakis, C.; Anlauf, M.; Cwikla, J.M.; et al. ENETS Consensus Guidelines for High-Grade Gastroenteropancreatic Neuroendocrine Tumors and Neuroendocrine Carcinomas. Neuroendocrinology 2016, 103, 186–194. [Google Scholar] [CrossRef]
- Sorbye, H.; Strosberg, J.; Baudin, E.; Klimstra, D.S.; Yao, J.C. Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer 2014, 120, 2814–2823. [Google Scholar] [CrossRef]
- Das, S.; Dasari, A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr. Oncol. Rep. 2021, 23, 43. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Walter, T.; Pavel, M.; Borbath, I.; Freis, P.; Nuñez, B.; Childs, A.; McNamara, M.G.; Hubner, R.A.; Garcia-Carbonero, R.; et al. Design and Validation of the GI-NEC Score to Prognosticate Overall Survival in Patients with High-Grade Gastrointestinal Neuroendocrine Carcinomas. J. Natl. Cancer Inst. 2017, 109, djw277. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Mehta, K.; Byers, L.A.; Sorbye, H.; Yao, J.C. Comparative study of lung and extrapulmonary poorly differentiated neuroendocrine carcinomas: A SEER database analysis of 162,983 cases. Cancer 2018, 124, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wu, S.; Han, X.; Yang, C. Effectiveness of Endoscopic Treatment for Gastrointestinal Neuroendocrine Tumors: A Retrospective Study. Medicine 2016, 95, e3308. [Google Scholar] [CrossRef] [PubMed]
- Masui, T.; Ito, T.; Komoto, I.; Uemoto, S.; JNETS Project Study Group. Recent epidemiology of patients with gastro-entero-pancreatic neuroendocrine neoplasms (GEP-NEN) in Japan: A population-based study. BMC Cancer 2020, 20, 1104. [Google Scholar] [CrossRef] [PubMed]
- Hallet, J.; Law, C.H.L.; Cukier, M.; Saskin, R.; Liu, N.; Singh, S. Exploring the rising incidence of neuroendocrine tumors: A population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015, 121, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Wyld, D.; Wan, M.H.; Moore, J.; Dunn, N.; Youl, P. Epidemiological trends of neuroendocrine tumours over three decades in Queensland, Australia. Cancer Epidemiol. 2019, 63, 101598. [Google Scholar] [CrossRef]
- Ito, T.; Sasano, H.; Tanaka, M.; Osamura, R.Y.; Sasaki, I.; Kimura, W.; Takano, K.; Obara, T.; Ishibashi, M.; Nakao, K.; et al. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J. Gastroenterol. 2010, 45, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.; Shah, A.; Wei, W.; Varadhachary, G.; Johnson, V.; Shah, V.; Kundra, V. Carcinoid tumours: Predicting the location of the primary neoplasm based on the sites of metastases. Eur. Radiol. 2013, 23, 400–407. [Google Scholar] [CrossRef]
- Elvebakken, H.; Hjortland, G.O.; Garresori, H.; Andresen, P.A.; Janssen, E.A.M.; Vintermyr, O.K.; Lothe, I.M.B.; Sorbye, H. Impact of kras and braf mutations on treatment efficacy and survival in high-grade gastroenteropancreatic neuroendocrine neoplasms. J. Neuroendocrinol. 2023, 35, e13256. [Google Scholar] [CrossRef]
- Ferlito, A.; Silver, C.E.; Bradford, C.R.; Rinaldo, A. Neuroendocrine neoplasms of the larynx: An overview. Head Neck 2009, 31, 1634–1646. [Google Scholar] [CrossRef] [PubMed]
- Alese, O.B.; Jiang, R.; Shaib, W.; Wu, C.; Akce, M.; Behera, M.; El-Rayes, B.F. High-Grade Gastrointestinal Neuroendocrine Carcinoma Management and Outcomes: A National Cancer Database Study. Oncologist 2019, 24, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Sorbye, H.; Welin, S.; Langer, S.W.; Vestermark, L.W.; Holt, N.; Osterlund, P.; Dueland, S.; Hofsli, E.; Guren, M.G.; Ohrling, K.; et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): The NORDIC NEC study. Ann. Oncol. 2013, 24, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Keller, H.R.; Senapathi, S.H.; Morada, A.; Bertsch, D.; Cagir, B. Survival in patients with neuroendocrine tumors of the colon, rectum and small intestine. Am. J. Surg. 2023, 225, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Eads, J.R.; Halfdanarson, T.R.; Asmis, T.; Bellizzi, A.M.; Bergsland, E.K.; Dasari, A.; El-Haddad, G.; Frumovittz, M.; Meyer, J.; Mittra, E.; et al. Expert Consensus Practice Recommendations of the North American Neuroendocrine Tumor Society for the management of high grade gastroenteropancreatic and gynecologic neuroendocrine neoplasms. Endocr. Relat. Cancer 2023, 30, e220206. [Google Scholar] [CrossRef]
- Efficacy of Neoadjuvant Chemotherapy in Terms of DFS in Patients with Localized Digestive Neuroendocrine Carcinomas. Available online: https://www.clinicaltrials.gov/search?term=NCT04268121 (accessed on 4 February 2024).
- Mafficini, A.; Scarpa, A. Genetics and Epigenetics of Gastroenteropancreatic Neuroendocrine Neoplasms. Endocr. Rev. 2019, 40, 506–536. [Google Scholar] [CrossRef]
- Uccella, S.; La Rosa, S.; Metovic, J.; Marchiori, D.; Scoazec, J.-Y.; Volante, M.; Mete, O.; Papotti, M. Genomics of High-Grade Neuroendocrine Neoplasms: Well-Differentiated Neuroendocrine Tumor with High-Grade Features (G3 NET) and Neuroendocrine Carcinomas (NEC) of Various Anatomic Sites. Endocr. Pathol. 2021, 32, 192–210. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, A.; Wiedmer, T.; Marinoni, I.; Perren, A. Genetic and epigenetic drivers of neuroendocrine tumours (NET). Endocr. Relat. Cancer 2017, 24, R315–R334. [Google Scholar] [CrossRef]
- Venizelos, A.; Elvebakken, H.; Perren, A.; Nikolaienko, O.; Deng, W.; Lothe, I.M.B.; Couvelard, A.; Hjortland, G.O.; Sundlöv, A.; Svensson, J.; et al. The molecular characteristics of high-grade gastroenteropancreatic neuroendocrine neoplasms. Endocr. Relat. Cancer 2021, 29, 1–14. [Google Scholar] [CrossRef]
- Sun, T.Y.; Zhao, L.; Van Hummelen, P.; Martin, B.; Hornbacker, K.; Lee, H.; Xia, L.C.; Padda, S.K.; Ji, H.P.; Kunz, P. Exploratory genomic analysis of high-grade neuroendocrine neoplasms across diverse primary sites. Endocr. Relat. Cancer 2022, 29, 665–679. [Google Scholar] [CrossRef]
- van Riet, J.; van de Werken, H.J.; Cuppen, E.; Eskens, F.A.; Tesselaar, M.; van Veenendaal, L.M.; Klümpen, H.J.; Dercksen, M.W.; Valk, G.D.; Lolkema, M.P.; et al. The genomic landscape of 85 advanced neuroendocrine neoplasms reveals subtype-heterogeneity and potential therapeutic targets. Nat. Commun. 2021, 12, 4612. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, C.; De Rycke, O.; Couvelard, A.; Turpin, A.; Cazes, A.; Hentic, O.; Gounant, V.; Zalcman, G.; Ruszniewski, P.; Cros, J.; et al. Biomarkers of Response to Etoposide-Platinum Chemotherapy in Patients with Grade 3 Neuroendocrine Neoplasms. Cancers 2021, 13, 643. [Google Scholar] [CrossRef] [PubMed]

| Variable (n = 10,387) | Frequency (%) | |
|---|---|---|
| Age | <65 years | 5652 (54.4%) |
| ≥65 years | 4735 (45.6%) | |
| Gender | Female | 5034 (48.4%) |
| Male | 5353 (51.5%) | |
| Ethnicity | White | 6832 (65.8%) |
| Black | 1500 (14.4%) | |
| Hispanic | 1276 (12.3%) | |
| Asian or Pacific Islander | 608 (5.9%) | |
| American Indian or Alaska Native | 56 (0.5%) | |
| Unknown | 115 (1.1%) | |
| Site | Esophagus | 315 (3.0%) |
| Stomach | 1599 (15.4%) | |
| Small Intestine | 3489 (33.6%) | |
| Cecum | 772 (7.4%) | |
| Anus | 137 (1.3%) | |
| Appendix | 731 (7.0%) | |
| Ascending colon | 467 (4.5%) | |
| Hepatic flexure | 109 (1.0%) | |
| Transverse colon | 146 (1.4%) | |
| Splenic flexure | 48 (0.5%) | |
| Descending colon | 74 (0.7%) | |
| Sigmoid colon | 251 (2.4%) | |
| Rectosigmoid junction | 193 (1.9%) | |
| Rectum | 1885 (18.1%) | |
| Large intestine, NOS | 171 (1.6%) | |
| Size | Known | 5111 (49.2%) |
| Unknown | 5276 (80.8%) | |
| Size where known (n = 5327) | ||
| <2 cm | 2274 (44.5%) | |
| 2–4 cm | 1590 (31.1%) | |
| >4 cm | 1247 (24.4%) | |
| Stage | Known | 9153 (88.1%) |
| Unknown | 1234 (11.9%) | |
| Stage where known (n = 9153) | ||
| Localized | 3189 (34.8%) | |
| Regional | 2547 (27.8%) | |
| Distant | 3417 (37.3%) | |
| Lymph node status | Known | 4391 (42.3%) |
| Unknown | 5996 (57.7%) | |
| Lymph node status where known (n = 4391) | ||
| Positive | 3338 (76.0%) | |
| Negative | 1053 (24.0%) | |
| Median Annual Household Income | <$70,000 | 5378 (51.8%) |
| ≥$70,000 | 5007 (48.2%) | |
| Housing Location | Metropolitan | 9032 (87.0%) |
| Rural | 1347 (13.0%) | |
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Variables | Hazard Ratio (95% C.I) | p-Value | Hazard Ratio (95% C.I) | p-Value | |
| Age | <65 years old | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| ≥65 years old | 1.62 (2.47–2.77) | <0.001 | 2.49 (2.36–2.54) | <0.001 * | |
| Sex | Male | 1.28 (1.21–1.35) | <0.001 | 1.16 (1.06–1.26) | <0.001 * |
| Female | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | |
| Race | White | 1.17 (1.07–1.28) | <0.001 | 0.99 (0.85–1.16) | 0.91 |
| Site | Esophagus | 5.98 (5.24–6.81) | <0.001 | 1.49 (0.86–2.58) | 0.15 |
| Small intestine | 0.21 (0.11–0.31) | <0.001 | 0.37 (0.22–0.60) | <0.001 ** | |
| Ascending Colon | 3.56 (3.17–4.00) | <0.001 | 1.19 (0.72–1.96) | 0.49 | |
| Transverse Colon | 4.99 (4.17–5.97) | <0.001 | 1.95 (1.15–3.33) | 0.01 * | |
| Descending Colon | 4.16 (3.20–5.42) | <0.001 | 2.12 (1.12–3.97) | 0.02 * | |
| Sigmoid Colon | 2.44 (2.07–2.86) | <0.001 | 1.41 (0.83–2.41) | 0.2 | |
| Appendix | 0.29 (0.18–0.40) | <0.001 | 0.41(0.23–0.72) | 0.002 ** | |
| Stomach | 2.02 (1.86–2.20) | <0.001 | 1.12 (0.68–1.84) | 0.39 | |
| Stage | Localized | 0.51 (0.46–0.55) | <0.001 | 0.29 (0.23–0.35) | <0.001 ** |
| Regional | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | |
| Distant | 3.05 (2.85–3.27) | <0.001 | 2.57 (2.52–2.62) | <0.001 * | |
| Nodal Status | Negative | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Positive | 1.33 (1.20–1.48) | <0.001 | 1.23 (1.07–1.42) | 0.003 * | |
| Tumor Size | <2 cm | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 2 cm–4 cm | 2.08 (1.87–2.30) | <0.001 | 1.14 (0.99–1.31) | 0.07 | |
| >4 cm | 5.20 (4.71–5.75) | <0.001 | 1.98 (1.70–2.31) | <0.001 * | |
| Treatment | Chemotherapy and Surgery | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Chemotherapy only | 6.02 (5.57–6.51) | <0.001 | 1.45 (0.75–4.19) | 0.68 | |
| None | 1.07 (0.97–1.18) | 0.2 | 0.94 (0.11–7.80) | 0.96 | |
| Surgery only | 0.36 (0.33–0.39) | <0.001 | 0.35 (0.05–2.74) | 0.32 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasinzai, A.Q.K.; Khan, M.; Chandasir, A.; Olavarria-Bernal, D.; Sohail, A.H.; Wali, A.; Tareen, B.; Nguyen, T.; Fox, A.D.; Goyal, A.; et al. Predictive Nomogram and Propensity Score Matching in Neuroendocrine Carcinoma of the Tubular Gastrointestinal Tract: A US Population-Based Clinical Outcome Study. Cancers 2024, 16, 1998. https://doi.org/10.3390/cancers16111998
Yasinzai AQK, Khan M, Chandasir A, Olavarria-Bernal D, Sohail AH, Wali A, Tareen B, Nguyen T, Fox AD, Goyal A, et al. Predictive Nomogram and Propensity Score Matching in Neuroendocrine Carcinoma of the Tubular Gastrointestinal Tract: A US Population-Based Clinical Outcome Study. Cancers. 2024; 16(11):1998. https://doi.org/10.3390/cancers16111998
Chicago/Turabian StyleYasinzai, Abdul Qahar Khan, Marjan Khan, Abdullah Chandasir, Diego Olavarria-Bernal, Amir Humza Sohail, Agha Wali, Bisma Tareen, Tena Nguyen, Ashley D. Fox, Aman Goyal, and et al. 2024. "Predictive Nomogram and Propensity Score Matching in Neuroendocrine Carcinoma of the Tubular Gastrointestinal Tract: A US Population-Based Clinical Outcome Study" Cancers 16, no. 11: 1998. https://doi.org/10.3390/cancers16111998
APA StyleYasinzai, A. Q. K., Khan, M., Chandasir, A., Olavarria-Bernal, D., Sohail, A. H., Wali, A., Tareen, B., Nguyen, T., Fox, A. D., Goyal, A., Khan, I., Waheed, A., Iqbal, A., Karki, N. R., Das, K., & Ullah, A. (2024). Predictive Nomogram and Propensity Score Matching in Neuroendocrine Carcinoma of the Tubular Gastrointestinal Tract: A US Population-Based Clinical Outcome Study. Cancers, 16(11), 1998. https://doi.org/10.3390/cancers16111998








