Systematic Review—Role of MRI in Cervical Cancer Staging
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Histological Subtypes
1.2. Clinical Management—Surgery, Radiation and Chemotherapy
1.3. Decision for Upfront Radiation Therapy
2. Materials and Methods
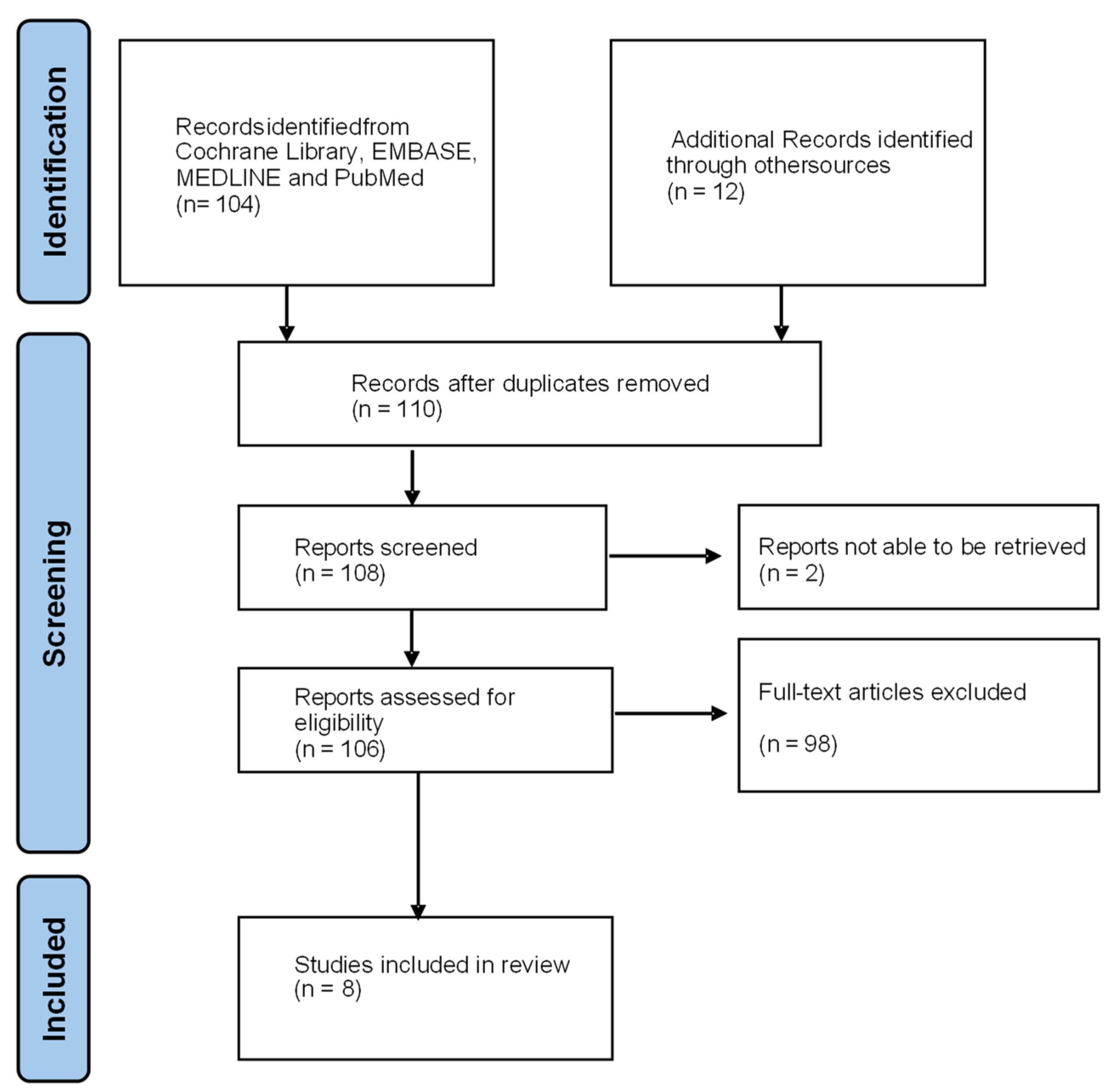
2.1. Literature Search
2.2. Inclusion Criteria
- Only original articles with reported data were included.
- The studies were limited to adult females with a histopathological diagnosis of cervical cancer.
- Preoperative MRI imaging compared to preoperative clinical assessment or postoperative histopathological diagnosis.
- Retrospective and prospective studies were included.
2.3. Exclusion Criteria
- Reviews and conference articles were excluded.
- Studies of patients who have had preoperative radiotherapy and chemotherapy after their initial MRI were excluded.
- Studies that focused on recurrent cervical cancer or cervical cancer post treatment follow up were excluded.
- Studies on MRI radiomics were excluded.
2.4. Data Evaluation
3. Results
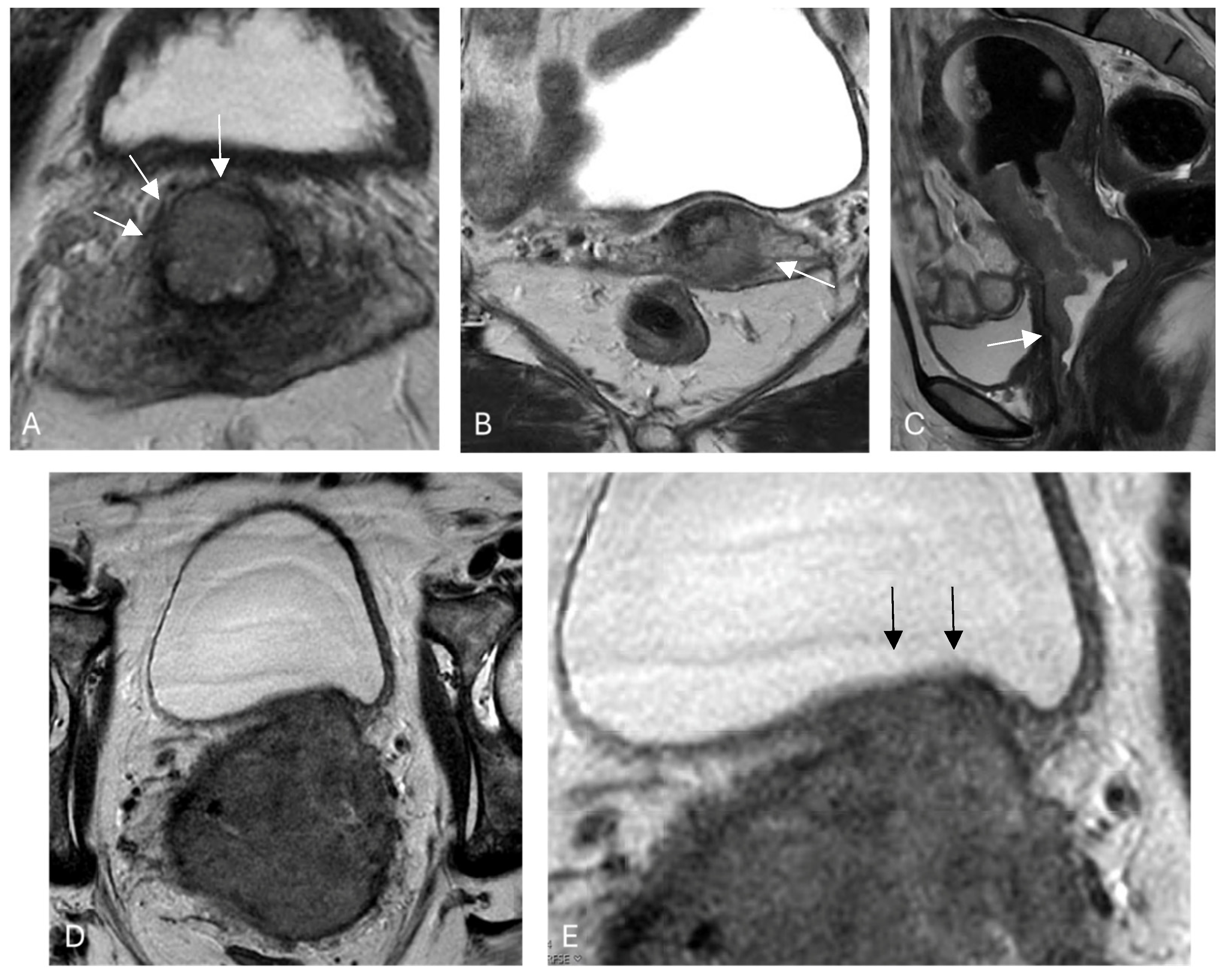
3.1. Stromal Invasion
3.2. Parametrial Invasion
3.3. Vaginal Involvement
3.4. Pelvic Side Wall
3.5. Lymph Node Metastasis
3.6. Bladder and Rectal Involvement
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; von Knebel Doeberitz, M. Harald Zur Hausen, Virologist Who Linked Viruses to Cancer (1936–2023). Nature 2023, 619, 693. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical Cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Re, G.L.; Cucinella, G.; Zaccaria, G.; Crapanzano, A.; Salerno, S.; Pinto, A.; Casto, A.L.; Chiantera, V. Role of MRI in the Assessment of Cervical Cancer. Semin. Ultrasound CT MRI 2023, 44, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Lakhman, Y.; Aherne, E.A.; Jayaprakasam, V.S.; Nougaret, S.; Reinhold, C. Staging of Cervical Cancer: A Practical Approach Using MRI and FDG PET. Am. J. Roentgenol. 2023, 221, 633–648. [Google Scholar] [CrossRef] [PubMed]
- Steiner, A.; Narva, S.; Rinta-Kiikka, I.; Hietanen, S.; Hynninen, J.; Virtanen, J. Diagnostic Efficiency of Whole-Body 18F-FDG PET/MRI, MRI Alone, and SUV and ADC Values in Staging of Primary Uterine Cervical Cancer. Cancer Imaging 2021, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Knoth, J.; Pötter, R.; Jürgenliemk-Schulz, I.M.; Haie-Meder, C.; Fokdal, L.; Sturdza, A.; Hoskin, P.; Mahantshetty, U.; Segedin, B.; Bruheim, K.; et al. Clinical and Imaging Findings in Cervical Cancer and Their Impact on FIGO and TNM Staging—An Analysis from the EMBRACE Study. Gynecol. Oncol. 2020, 159, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the Cervix Uteri. Int. J. Gynecol. Obstet. 2018, 143, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Pak, T.; Sadowski, E.A.; Patel-Lippmann, K. MR Imaging in Cervical Cancer Initial Staging and Treatment. Radiol. Clin. N. Am. 2023, 61, 639–649. [Google Scholar] [CrossRef]
- Fischerova, D.; Frühauf, F.; Burgetova, A.; Haldorsen, I.S.; Gatti, E.; Cibula, D. The Role of Imaging in Cervical Cancer Staging: ESGO/ESTRO/ESP Guidelines (Update 2023). Cancers 2024, 16, 775. [Google Scholar] [CrossRef] [PubMed]
- Lura, N.; Wagner-Larsen, K.S.; Forsse, D.; Trovik, J.; Halle, M.K.; Bertelsen, B.I.; Salvesen, Ø.; Woie, K.; Krakstad, C.; Haldorsen, I.S. What MRI-Based Tumor Size Measurement Is Best for Predicting Long-Term Survival in Uterine Cervical Cancer? Insights Imaging 2022, 13, 105. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Croke, J.; Foltz, W.; Metser, U.; Xie, J.; Shek, T.; Driscoll, B.; Ménard, C.; Vines, D.; Coolens, C.; et al. A Prospective Study of DWI, DCE-MRI and FDG PET Imaging for Target Delineation in Brachytherapy for Cervical Cancer. Radiother. Oncol. 2016, 120, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.-J.; Abu-Rustum, N.R.; Bean, S.; Bradley, K.; Campos, S.M.; Cho, K.R.; Chon, H.S.; Chu, C.; Clark, R.; Cohn, D.; et al. Cervical Cancer, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 64–84. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.B.M.; Lee, J.M.; Park, C.Y.; Lee, K.B.; Cho, H.Y.; Ha, S.Y. What Is the Difference between Squamous Cell Carcinoma and Adenocarcinoma of the Cervix? A Matched Case–Control Study. Int. J. Gynecol. Cancer 2006, 16, 1569–1573. [Google Scholar] [CrossRef]
- Giannella, L.; Giuseppe, J.D.; Carpini, G.D.; Grelloni, C.; Fichera, M.; Sartini, G.; Caimmi, S.; Natalini, L.; Ciavattini, A. HPV-Negative Adenocarcinomas of the Uterine Cervix: From Molecular Characterization to Clinical Implications. Int. J. Mol. Sci. 2022, 23, 15022. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Atri, M. 2018 FIGO Staging System for Uterine Cervical Cancer: Enter Cross-Sectional Imaging. Radiology 2019, 292, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Delgado, G.; Bundy, B.; Zaino, R.; Sevin, B.-U.; Creasman, W.T.; Major, F. Prospective Surgical-Pathological Study of Disease-Free Interval in Patients with Stage IB Squamous Cell Carcinoma of the Cervix: A Gynecologic Oncology Group Study. Gynecol. Oncol. 1990, 38, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Kinjo, Y.; Matsuura, Y.; Ohguri, T.; Aoyama, Y.; Murakami, M.; Hoshino, K.; Harada, H.; Ueda, T.; Kurita, T.; Kagami, S.; et al. Retrospective Analysis of Prognosis Using the Gynecology Oncology Group Score of Stage IB-IIA Node Negative Uterine Cervical Cancer after Radical Hysterectomy and Trachelectomy. Mol. Clin. Oncol. 2022, 16, 105. [Google Scholar] [CrossRef] [PubMed]
- Kridelka, F.J.; Berg, D.O.; Neuman, M.; Edwards, L.S.; Robertson, G.; Grant, P.T.; Hacker, N.F. Adjuvant Small Field Pelvic Radiation for Patients with High Risk, Stage IB Lymph Node Negative Cervix Carcinoma after Radical Hysterectomy and Pelvic Lymph Node Dissection. A Pilot Study. Cancer 1999, 86, 2059–2065. [Google Scholar] [CrossRef]
- Gemer, O.; Lavie, O.; Gdalevich, M.; Eitan, R.; Mamanov, E.; Piura, B.; Rabinovich, A.; Levavi, H.; Saar-Ryss, B.; Halperin, R.; et al. Evaluation of Clinical and Pathologic Risk Factors May Reduce the Rate of Multimodality Treatment of Early Cervical Cancer. Am. J. Clin. Oncol. 2016, 39, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Smits, A.; Steins, M.; van Koeverden, S.; Rundle, S.; Dekker, H.; Zusterzeel, P. Can MRI Be Used as a Sole Diagnostic Modality in Determining Clinical Stage in Cervical Cancer? Oncologist 2022, 28, e19–e25. [Google Scholar] [CrossRef] [PubMed]
- Ran, C.; Sun, J.; Qu, Y.; Long, N. Clinical Value of MRI, Serum SCCA, and CA125 Levels in the Diagnosis of Lymph Node Metastasis and Para-Uterine Infiltration in Cervical Cancer. World J. Surg. Oncol. 2021, 19, 343. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.K.; Kido, A.; Moribata, Y.; Chigusa, Y.; Himoto, Y.; Kurata, Y.; Otani, S.; Yajima, R.; Nishio, N.; Kuwahara, R.; et al. Diagnostic Accuracy of Magnetic Resonance Imaging for International Federation of Gynecology and Obstetrics 2018 IB to IIB Cervical Cancer Staging: Comparison Among Magnetic Resonance Sequences and Pathologies. J. Comput. Assist. Tomogr. 2021, 45, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Anfinan, N. Cervical Cancer Staging in Saudi Arabia Clinicoradiological Correlation. BioMed Res. Int. 2019, 2019, 8745828. [Google Scholar] [CrossRef]
- Rockall, A.G.; Barwick, T.D.; Wilson, W.; Singh, N.; Bharwani, N.; Sohaib, A.; Nobbenhuis, M.; Warbey, V.; Miquel, M.; Koh, D.-M.; et al. Diagnostic Accuracy of FEC-PET/CT, FDG-PET/CT, and Diffusion-Weighted MRI in Detection of Nodal Metastases in Surgically Treated Endometrial and Cervical Carcinoma. Clin. Cancer Res. 2021, 27, 6457–6466. [Google Scholar] [CrossRef]
- Zhu, Y.; Shen, B.; Pei, X.; Liu, H.; Li, G. CT, MRI, and PET Imaging Features in Cervical Cancer Staging and Lymph Node Metastasis. Am. J. Transl. Res. 2020, 13, 10536–10544. [Google Scholar]
- Gupta, S.; Maheshwari, A.; Parab, P.; Mahantshetty, U.; Hawaldar, R.; (Chopra), S.S.; Kerkar, R.; Engineer, R.; Tongaonkar, H.; Ghosh, J.; et al. Neoadjuvant Chemotherapy Followed by Radical Surgery Versus Concomitant Chemotherapy and Radiotherapy in Patients with Stage IB2, IIA, or IIB Squamous Cervical Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2018, 36, JCO.2017.75.998. [Google Scholar] [CrossRef] [PubMed]

| Study | Reference | Year | No. of Patients | Reference Standard | Accuracy (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Stromal Invasion | ||||||||||
| Smits et al. | [21] | 2023 | 358 | H | - | 95 | 39 | 95 | 39 | Early-stage disease |
| Ran et al. | [22] | 2021 | 200 | H | 89 | 99 | 97 | - | - | FIGO 2018 IB |
| Ran et al. | [22] | 2021 | 200 | H | 95 | 100 | 96 | - | - | FIGO 2018 IIA |
| Steiner et al. | [6] | 2021 | 20 | H | 74 | 89 | 60 | 67 | 86 | Deep stromal invasion |
| Parametrial Invasion | ||||||||||
| Smits et al. | [21] | 2023 | 167 | H | - | 33 | 96 | 25 | 98 | Parametrial |
| Ran et al. | [22] | 2021 | 200 | H | 93 | 86 | 93 | - | - | FIGO 2018 IIB |
| Ran et al. | [22] | 2021 | 200 | H | 91 | 56.9 | 74.1 | 43.5 | 92 | Para-uterine * |
| Matsumoto et al. | [23] | 2021 | 51 | H | 90 | 67–75 | 94–95 | 75–77 | 92–94 | Parametrial |
| Steiner et al. | [6] | 2021 | 33 | H | 63 | 100 | 46 | 46 | 100 | Parametrial |
| Anfinan | [24] | 2019 | 152 | CE | 74 | 72 | 82 | 96 | 33 | Parametrial |
| Study | Reference | Year | No. of Patients | Reference Standard | Accuracy | Sensitivity | Specificity | PPV | NPV | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Vaginal Involvement | ||||||||||
| Ran et al. | [22] | 2021 | 200 | H | 91 | 57 | 74 | 44 | 92 | Para-uterine * |
| Ran et al. | [22] | 2021 | 200 | H | 95 | 100 | 96 | - | - | FIGO Stage IIA |
| Steiner et al. | [6] | 2021 | 33 | H | 67 | 100 | 48 | 52 | 100 | VI |
| Knoth et al. | [7] | 2020 | 1338 | CE | 88 | 85 | 90 | 88 | 88 | VI |
| Anfinan | [24] | 2019 | 145 | CE | 62 | 67 | 60 | 47 | 77 | VI |
| Pelvic Side Wall and/or Associated Renal Complications | ||||||||||
| Ran et al. | [22] | 2021 | 200 | H | 91 | 100 | 97 | - | - | FIGO stage IIIB |
| Anfinan | [24] | 2019 | 152 | CE | 84 | 56 | 94 | 77 | 85 | Pelvic side wall |
| Lymph Node Metastases | ||||||||||
| Ran et al. | [22] | 2021 | 200 | H | 90 | 55 | 92 | 52 | 96 | LNM |
| Rockall et al. | [25] | 2021 | 40 | H | 78 | 20 | 97 | 67 | 78 | DW MRI |
| Steiner et al. | [6] | 2021 | 33 | H | 73 | 71 | 75 | 75 | 71 | LNM |
| Zhu et al. | [26] | 2021 | 196 | H | 79 | 76 | 80 | - | - | LNM |
| Bladder and Rectal Involvement | ||||||||||
| Steiner et al. | [6] | 2021 | 4 | H | 97 | - | - | - | - | BI and RI |
| Knoth et al. | [7] | 2020 | 548 | CE | 94 | 96 | 94 | 42 | 100 | BI |
| Anfinan | [24] | 2019 | 152 | CE | - | 78 | 87 | 27 | 98 | BI |
| Anfinan | [24] | 2019 | 152 | CE | - | 75 | 91 | 15 | 99 | RI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Kitzing, Y.X.; Lo, G. Systematic Review—Role of MRI in Cervical Cancer Staging. Cancers 2024, 16, 1983. https://doi.org/10.3390/cancers16111983
Chen J, Kitzing YX, Lo G. Systematic Review—Role of MRI in Cervical Cancer Staging. Cancers. 2024; 16(11):1983. https://doi.org/10.3390/cancers16111983
Chicago/Turabian StyleChen, Jason, Yu Xuan Kitzing, and Glen Lo. 2024. "Systematic Review—Role of MRI in Cervical Cancer Staging" Cancers 16, no. 11: 1983. https://doi.org/10.3390/cancers16111983
APA StyleChen, J., Kitzing, Y. X., & Lo, G. (2024). Systematic Review—Role of MRI in Cervical Cancer Staging. Cancers, 16(11), 1983. https://doi.org/10.3390/cancers16111983







