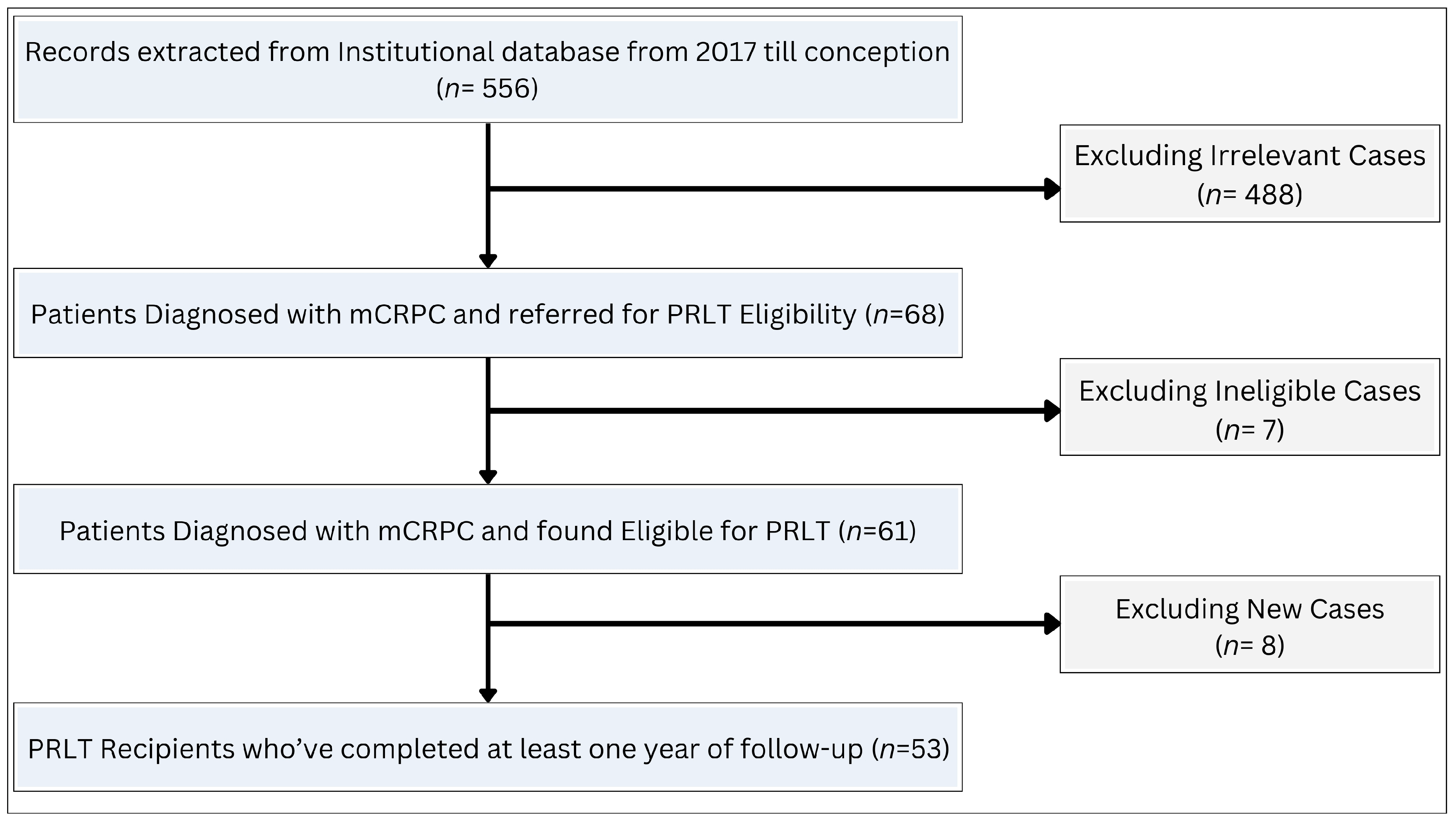
1. Introduction
The incidence of prostate cancer (PC) in the Arab world is expected to experience an increase, despite currently having a lower age-standardized rate compared to Europe [
1]. It is believed that the true incidence of PC among the Arab population is underestimated. This fact stems from a significant number of patients being diagnosed at advanced stages, coupled with underdiagnosis of early-stage disease due to inadequate screening rates and limited public awareness, rather than an accurate reflection of the disease’s prevalence. [
2,
3,
4]. Ethnic variation and tumor aggressiveness have also been proposed as factors contributing to this divergent incidence pattern [
2,
3,
4,
5]. Recent studies have shown a continued rise in new PC cases in Jordan [
6], where it is currently the fifth most common cancer among males nationally and the second most common globally [
6,
7].
Metastatic castration-resistant PC (mCRPC), a form of advanced PC, is an ongoing and serious challenge [
8]. Despite the recent increase in treatment options, it remains an invariably fatal condition, demonstrating resistance to the majority of current treatment modalities [
8]. Prostate-specific membrane antigen (PSMA) is found to be significantly upregulated in mCRPC. PSMA is highly overexpressed in more than 90% of mCRPC lesions [
9]. When patients with mCRPC experience disease progression, they are considered for radioligand therapy (RLT) as a treatment option after exhausting other conventional hormonal and chemotherapy approaches [
9]. Numerous studies have shown that PSMA RLT effectively improves biochemical control, improves quality of life, and alleviates pain [
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22]. Furthermore, the current body of evidence indicates that PSMA RLT leads to higher progression-free survival (PFS) and overall survival (OS) in mCRPC patients [
10,
23,
24,
25,
26,
27]. The observed low toxicity further supports the safety of [
177Lu]Lu-PSMA RLT in this patient population.
The labeling of PSMA with the beta-particle-emitting radionuclide [
177Lu]Lu has been approved for the treatment of mCRPC by regulatory agencies in the United States and Europe since 2022 [
28]. Nevertheless, this agent was incorporated into the theranostic institutional practice at King Hussein Cancer Center (KHCC) in Amman, Jordan, in 2017, making it one of the pioneering institutions in the Arab World to adopt this targeted treatment [
28,
29,
30]. In addition to its therapeutic benefits, treatment with beta-emitting [
177Lu]Lu facilitates post-therapeutic scintigraphic imaging to assess the localization of the radiotracer [
31]. This theranostic approach can be complemented with a positron-emitting [
68Ga]Ga-PSMA diagnostic counterpart for diagnostic and monitoring purposes [
32]. Another beta radiotracer, namely the [
161Tb]Tb-PSMA radioligand, possesses physical and biological distribution comparable to [
177Lu]Lu-PSMA (
Table 1), which has been recently used at KHCC (
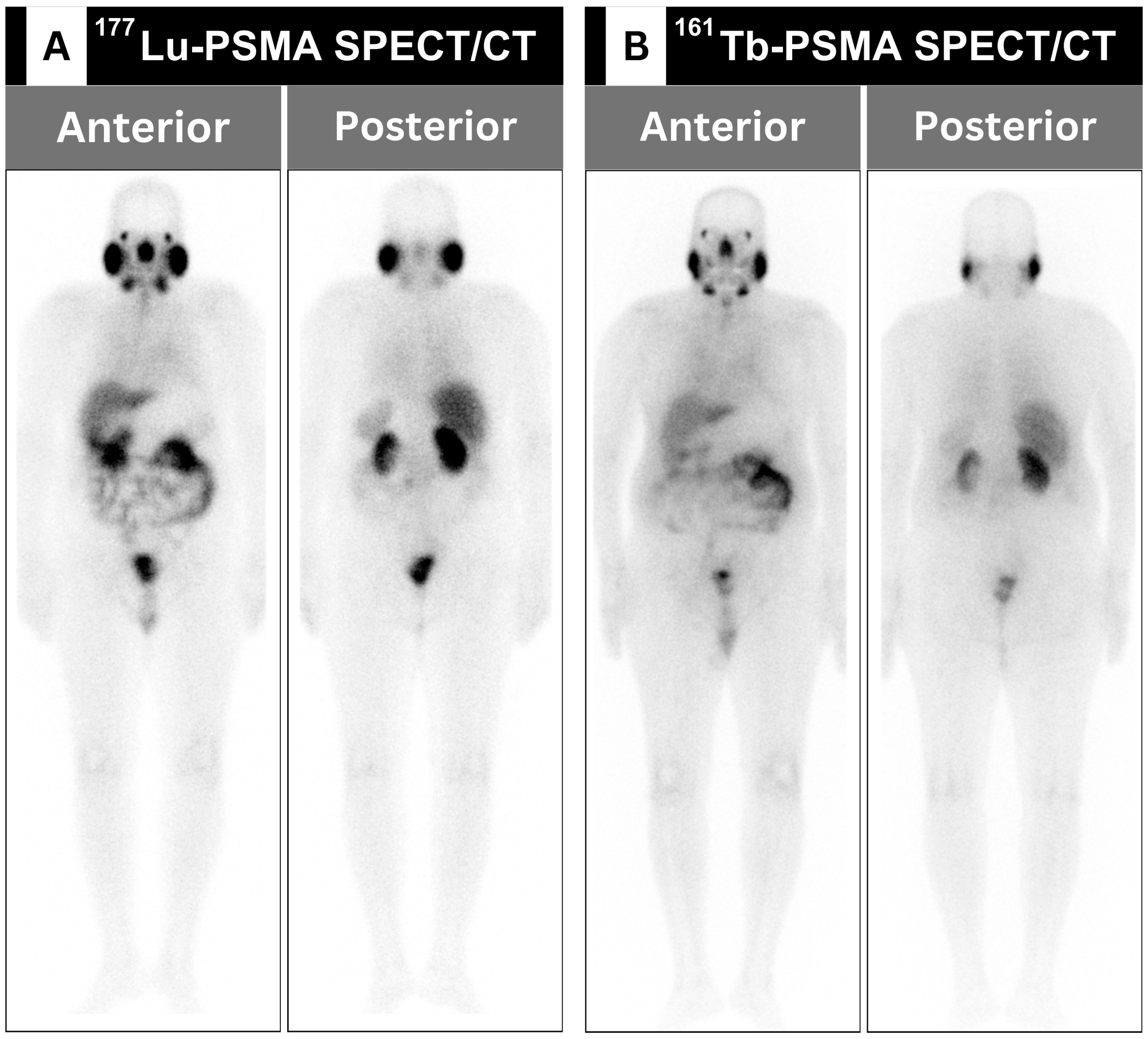
Figure 1) [
33,
34].
As the use of PSMA radioligand therapy continues to expand through promising clinical trials [
35], we conducted this study to provide evidence from real-world experience with beta-emitting PSMA therapeutic agents in mCRPC patients of Jordanian and Arab descent.
4. Discussion
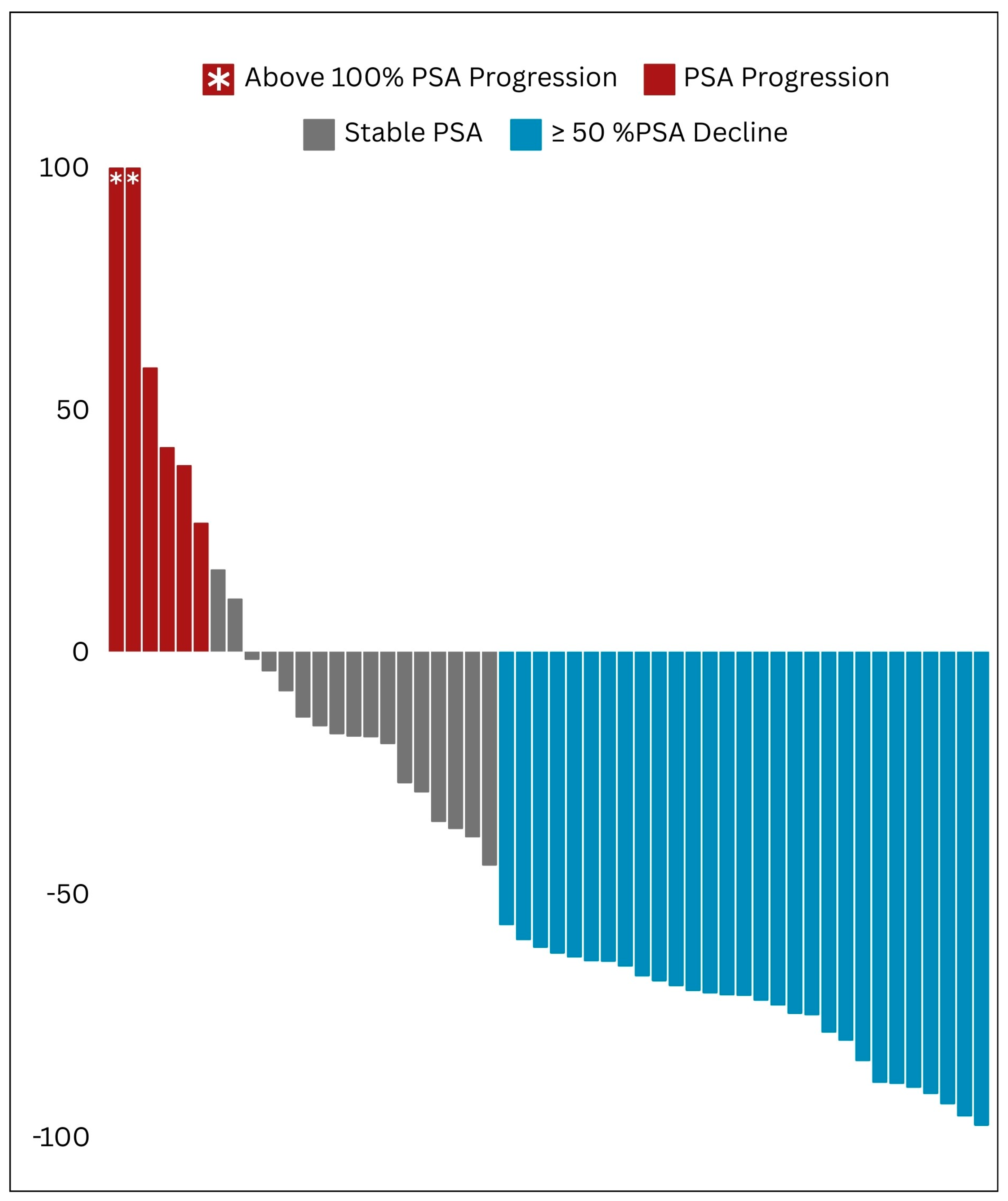
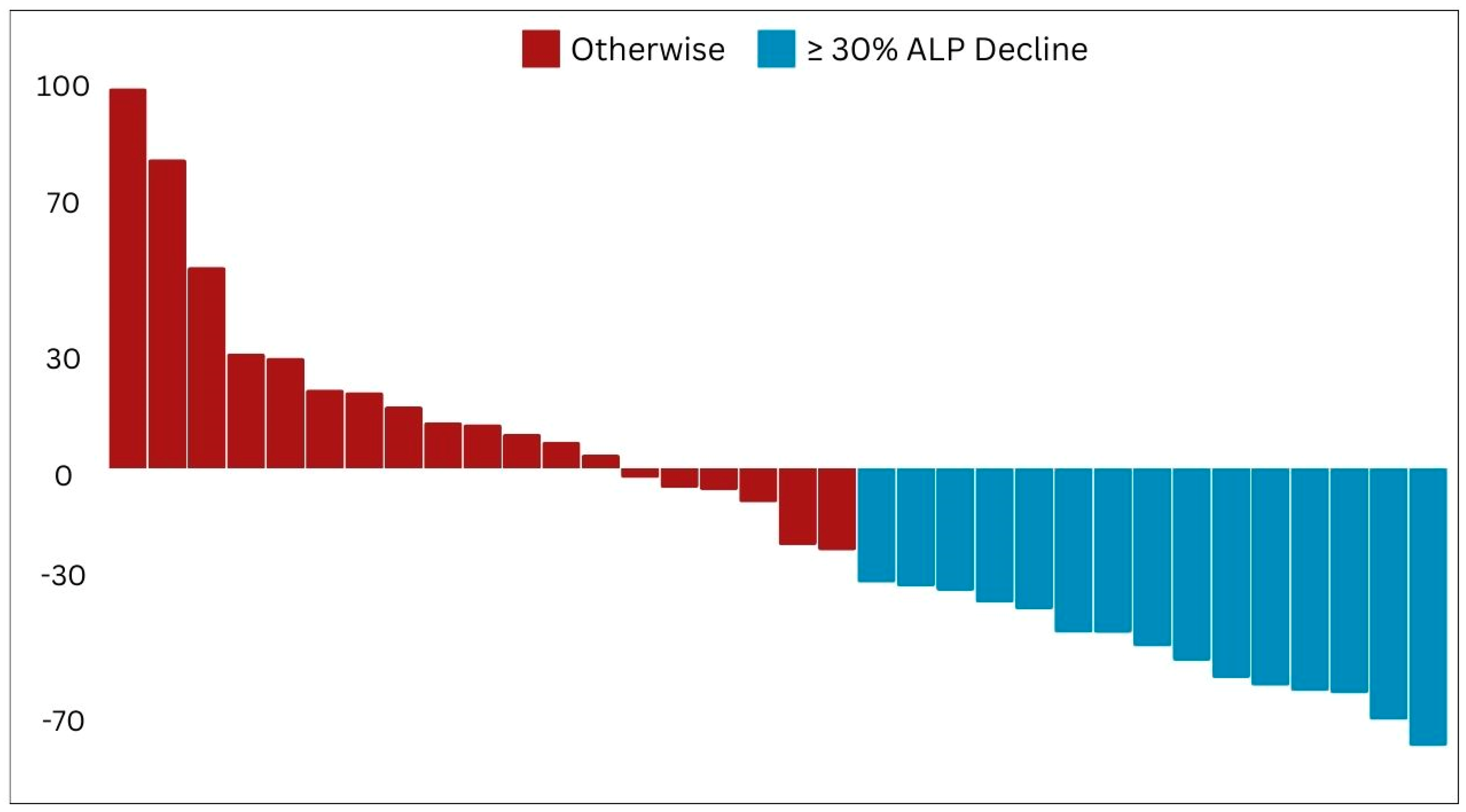
This retrospective study confirmed the therapeutic efficacy of beta-emitting PSMA RLTs, namely [
177Lu]Lu and [
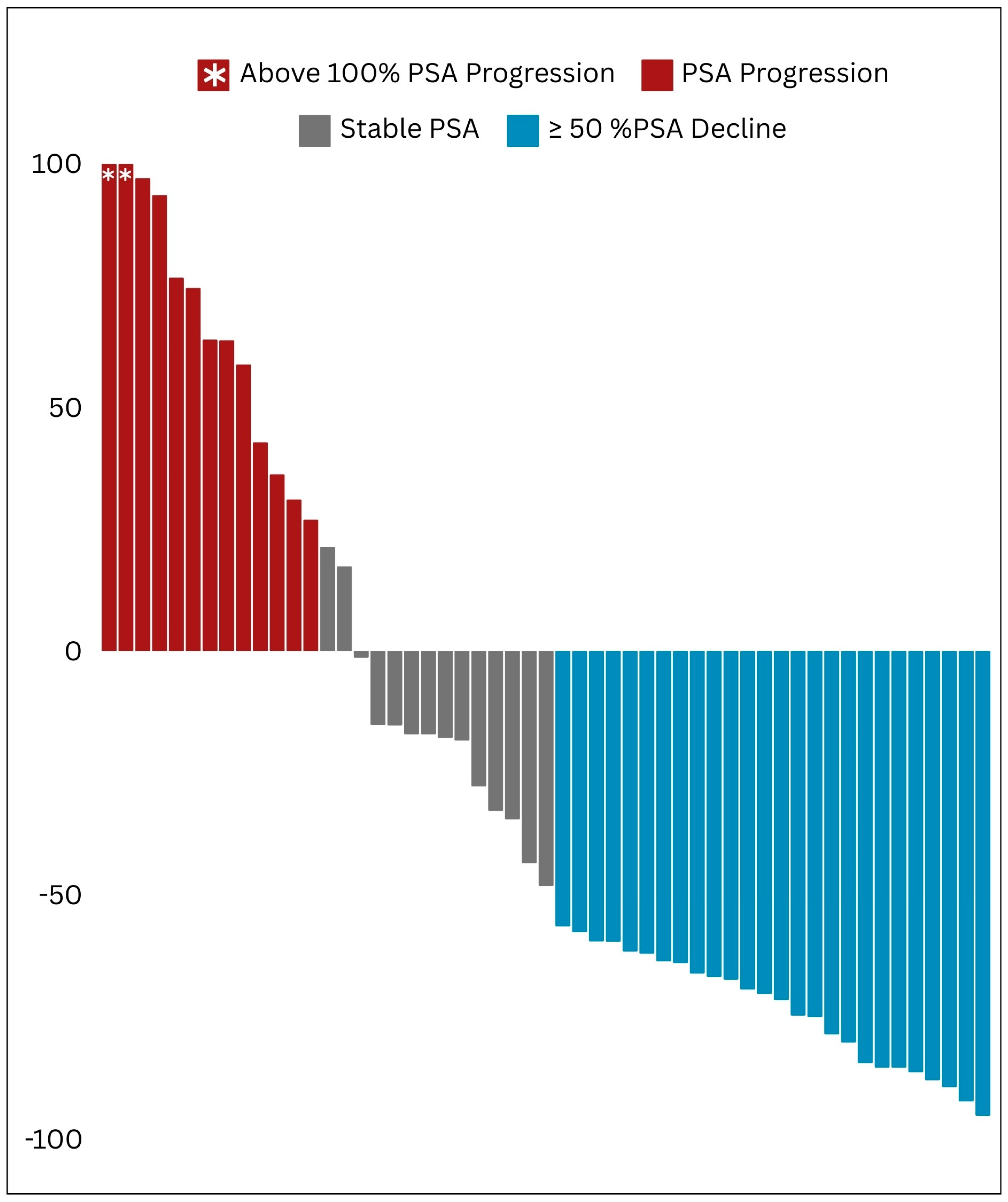
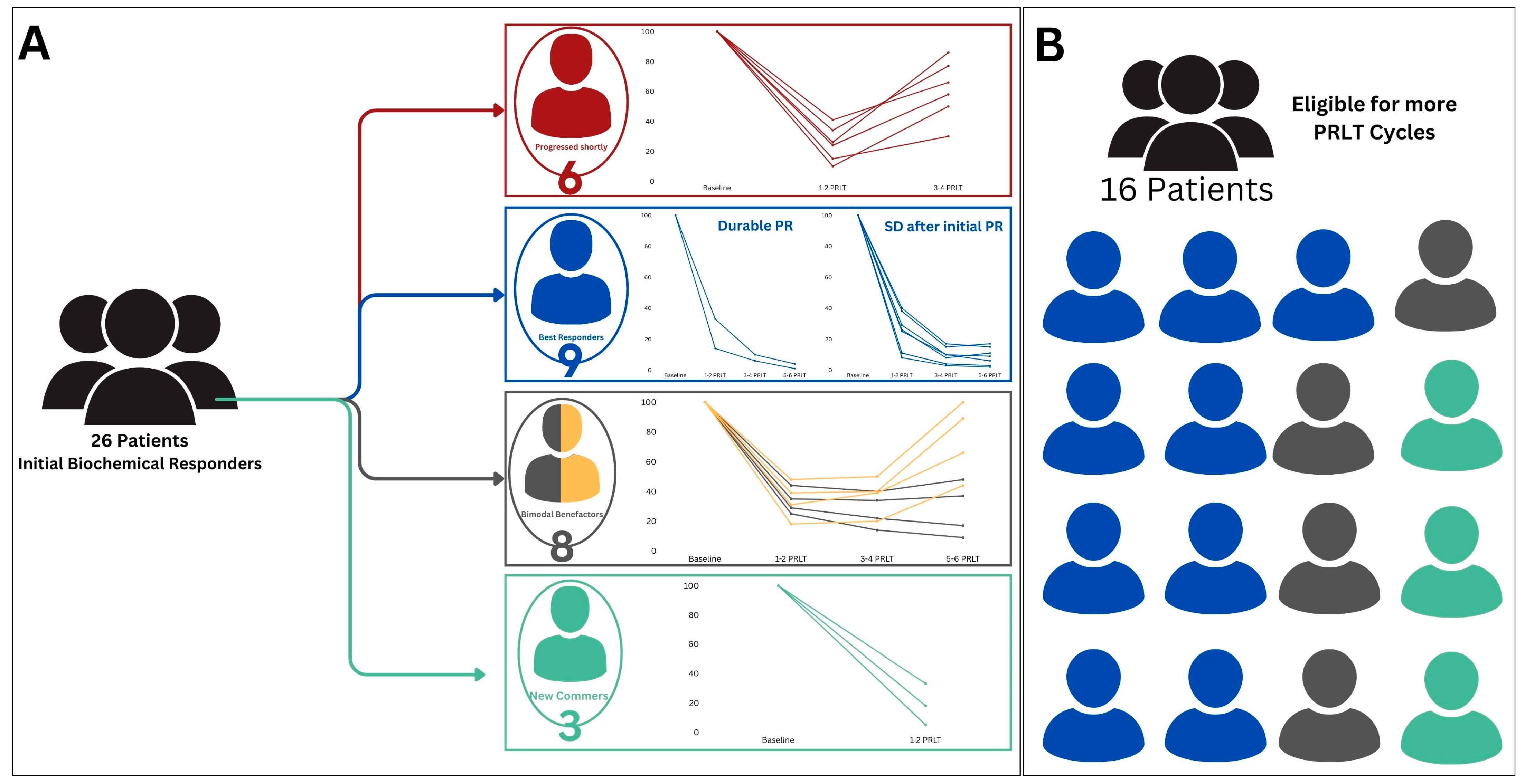
161Tb]Tb, in managing mCRPC patients. Pioneering the Arab region, our study offers robust real-world evidence supporting the clinical safety and effectiveness of these agents. Our findings demonstrate an initial significant positive response in the majority of patients, as shown by substantial decreases in PSA and ALP levels, as well as improvements in imaging results. Additionally, our study highlights the favorable safety profile of PSMA RLT, with manageable side effects observed in a small percentage of patients, further confirming the safety and effectiveness of this nuclear medicine therapy. This paper presents the initial clinical experience of PSMA RLT in the Arabic context, aiming to highlight its potential and raise awareness regarding the significance of adopting this innovative technique in both the fields of nuclear medicine and oncologic theranostics [
41].
For metastatic PC, the primary treatment option is hormonal therapy [
42]. This involves removing androgens through the use of androgen deprivation therapy, which typically can prevent disease progression for up to 18–24 months [
42]. However, if the disease progresses despite this therapy, mCRPC can develop [
43]. The use of theranostic agents such as [
177Lu]Lu-PSMA is an emerging therapy for mCRPC that is available in many countries worldwide [
44,
45]. In parallel, [
68Ga]Ga-PSMA imaging can provide high diagnostic accuracy for disease staging, monitoring response, restaging, and assessing eligibility for PSMA RLT [
46,
47,
48].
Recently, there are numerous clinical trials that have been conducted to evaluate the efficacy of [
177Lu]Lu-PSMA in the treatment of advanced mCRPC [
14,
49,
50,
51]. In a phase II trial conducted by Tagawa et al., 47 patients were treated with [
177Lu]Lu-PSMA, and 55.3% of those patients had also received prior chemotherapy [
50]. The results showed that 10.6%, 36.2%, and 59.6% of patients, respectively, had a ≥50%, ≥30%, or any decrease in PSA after a single dose of 177Lu-PSMA therapy [
50]. In a similar previous attempt, Hofman et al. recruited 30 patients with mCRPC who had previously used standard therapy options [
49]. Patients received 1 to 4 cycles of intravenous [
177Lu]Lu-PSMA at six-week intervals [
49]. Disease progression status and adverse reactions were assessed. In addition to the primary study endpoint of the PSA response rate (a 50% decrease from baseline), other endpoints, such as PFS, OS, imaging responses and quality of life (QOL), were also evaluated [
49]. Briefly, 17 (57%) patients met the PSA response criteria, and 29 (97%) of the patients had some degree of PSA response [
49]. Moreover, 3 months after the last injection, [
68Ga]Ga-PSMA imaging demonstrated that 10%, 53%, and 30% exhibited nonprogressive disease, encompassing complete response, partial response, and stable disease, respectively [
49]. The authors also reported a PSA regression in 27 (90%) patients, and the median PFS and OS were 7.6 and 13.5 months, respectively [
49]. The study also revealed that [
177Lu]Lu-PSMA therapy was well tolerated with minimal adverse events, such as dry mouth, in 87% of the patients [
49]. Moreover, the pain level of the study participants (27 patients, or 90%) improved at all study time points, which significantly contributed to quality of life [
49]. More recently, Yadav et al. investigated the efficacy of [
177Lu]Lu-PSMA therapy in a cohort of 90 patients with mCRPC [
14]. They found that after the first cycle of therapy, 32.2% of patients experienced a greater than 50% decline in PSA levels and that this percentage increased to 45.5% by the end of the study (after up to seven cycles) [
14]. Additionally, 27.5% of patients achieved partial remission, 43.5% had stable disease, and 29% had progressive disease [
14]. Furthermore, 54% of patients reported an improvement in pain score after the first cycle of therapy [
14]. In 2018, Kim et al. conducted a meta-analysis and gathered available data from 10 retrospective series including 455 patients who reported favorable outcomes [
52]. It revealed that approximately 2/3 of any decrease in PSA and 1/3 of any decrease in PSA greater than 50% can be expected after the first cycle of [
177Lu]Lu-PSMA therapy [
52]. Subsequently, in a recent systematic review conducted by Patell et al., the authors performed a comprehensive qualitative analysis of the existing literature [
53]. The authors determined that [
177Lu]Lu-PSMA had shown a minimal toxicity profile and was generally well tolerated in male patients with advanced metastatic disease that is unresponsive to conventional treatments [
53]. Both retrospective analyses and prospective clinical trials have indicated that PSMA RLT is a viable treatment option for individuals with mCRPC who have not responded to traditional hormonal therapies and chemotherapy [
53].
Table 8 summarizes the key similarities and difference between our study results and results of above discussed studies in this paragraph.
PSMA ligand therapy delivers radiation to non-target tissues that express PSMA. However, the side effects of this therapy are generally mild and related to the absorbed dose [
18,
54,
55]. The most exposed organs are the kidneys, salivary glands, lacrimal glands, and bone marrow. However, hematotoxicity is the most common serious adverse event, occurring in 12% of patients with a high tumor burden in bone [
54]. Xerostomia is currently the second most common side effect reported [
14,
54]. This adverse effect is most frequently encountered in patients who do not perform any protective measures, e.g., salivary gland cooling [
49]. Regarding the toxicity profile, [
177Lu]Lu-PSMA is generally recognized for its high safety standards, with only minimal self-limiting toxicities reported thus far. A recent meta-analysis reviewed all prior studies to affirm the safety of [
177Lu]Lu-PSMA. This analysis included 23 clinical investigations, all of which aligned with the specified research objectives. Notably, the majority of published studies reported only low-grade toxicities. Hemoglobin-related toxicities were the most prevalent, affecting 23% of patients. Additionally, severe thrombocytopenia occurred in 15% of patients, while leukopenia was noted in 14.2% of patients. Nephrotoxicity was observed in 9.5% of patients, and 14.5% reported salivary gland toxicities, including pain, swelling, and dry mouth. Other less common toxicities included fatigue, nausea, and loss of appetite.
Currently, PSMA RLT is used as a last resort in the treatment of mCRPC. However, it is unclear whether this therapy would be more effective if it was used in first-line or earlier settings. Comparative studies are needed to investigate this issue. Combination strategies are also emerging as interesting alternative options. Both the STAMPEDE and CHAARTED studies revealed that combination therapies can be effective in treating mCRPC [
56,
57]. Basu et al. reported that adding [
177Lu]Lu-PSMA to abiraterone therapy can be effective at mitigating side effects [
58]. Crumbaker et al. reported that [
177Lu]Lu-PSMA plus idronoxil is a safe and effective treatment option [
59]. Enzalutamide has been demonstrated to increase the expression of PSMA, a key factor in the effectiveness of PSMA RLT [
60]. Research has shown that treatment with enzalutamide can lead to a significant rise in PSMA uptake in cancer cells, potentially enhancing the therapeutic outcomes of subsequent Lu-PSMA treatments [
61]. Clinical studies have observed increased levels of PSMA uptake following treatment with enzalutamide, indicating a potential for improved treatment efficacy. Additionally, combining enzalutamide with Lu-PSMA has shown promising results in terms of prostate-specific antigen progression-free survival, suggesting a synergistic effect between the two treatments [
62]. This combination therapy may offer benefits in terms of overall survival and disease progression, potentially making it a valuable approach in the management of prostate cancer. Overall, [
177Lu]Lu-PSMA shows great promise as part of a combination therapy.
The exploration of [
161Tb]Tb-PSMA RLT in mCRPC is a promising avenue of investigation, with ongoing clinical trials focused on elucidating its safety and efficacy profile [
35]. Early findings suggest that the [
161Tb]Tb-PSMA may offer a favorable therapeutic index and demonstrate potential efficacy in terms of PSA decline and tumor burden reduction. However, a more comprehensive understanding of its safety and efficacy in mCRPC patients awaits further data from ongoing trials. Preliminary dosimetry assessments revealed that [
161Tb]Tb-PSMA had a slightly higher absorbed doses to organs at risk than [
177Lu]Lu-PSMA RLT but notably higher doses to tumor lesions, suggesting a potentially superior therapeutic index [
63]. A recent case study illustrated significant partial remission and PSA decline in an advanced mCRPC patient post-[
161Tb]Tb-PSMA RLT, suggesting therapeutic benefits even in patients who progressed following extensive [
177Lu]Lu-PSMA RLT [
64]. The efficacy of [
161Tb]Tb-PSMA is under investigation, with a focus on PSA response rates, radiological PFS, OS, and quality of life [
35]. Ongoing trials such as the VIOLET study and the REALITY trial in Germany aim to evaluate the efficacy and safety of [
161Tb]Tb-PSMA RLT in mCRPC patients, with anticipated results to provide further insights into its clinical utility [
35]. These trials represent significant endeavors in advancing the understanding and potential application of [
161Tb]Tb-PSMA RLT in the management of mCRPC, contributing to the evolving landscape of PC therapeutics.
Our research aligns with the existing literature on the subject, demonstrating the initial effectiveness of [
177Lu]Lu-PSMA RLT while also confirming its safety. Additionally, we provide a comprehensive analysis of the safety and efficacy of [
161Tb]Tb-PSMA, a novel treatment option [
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22]. Despite the inherent limitations of a single-center, retrospective design, and a small cohort size, our investigation stands as a pioneering contribution, offering the first documented real-world experience of RLT in metastatic castration-resistant prostate cancer (mCRPC) patients on both national and regional scales.