Radiobiology of Proton Therapy in Human Papillomavirus-Negative and Human Papillomavirus-Positive Head and Neck Cancer Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Colony Assay
2.3. Flow Cytometry
2.4. Immunofluorescence
2.5. Western Blotting
2.6. Statistical Analysis
3. Results
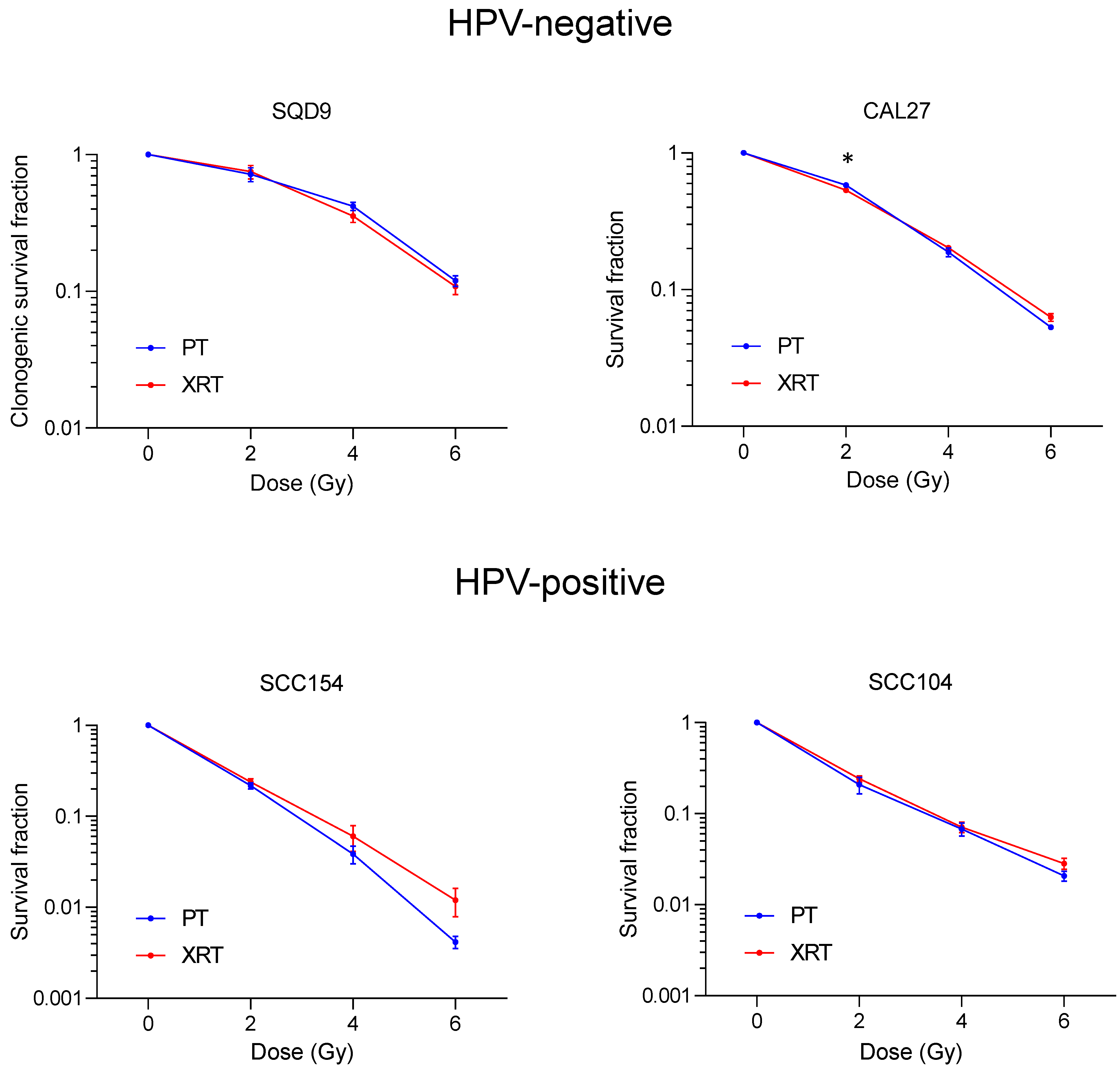
3.1. Effect of PT and XRT on Survival of HNSCC Cells
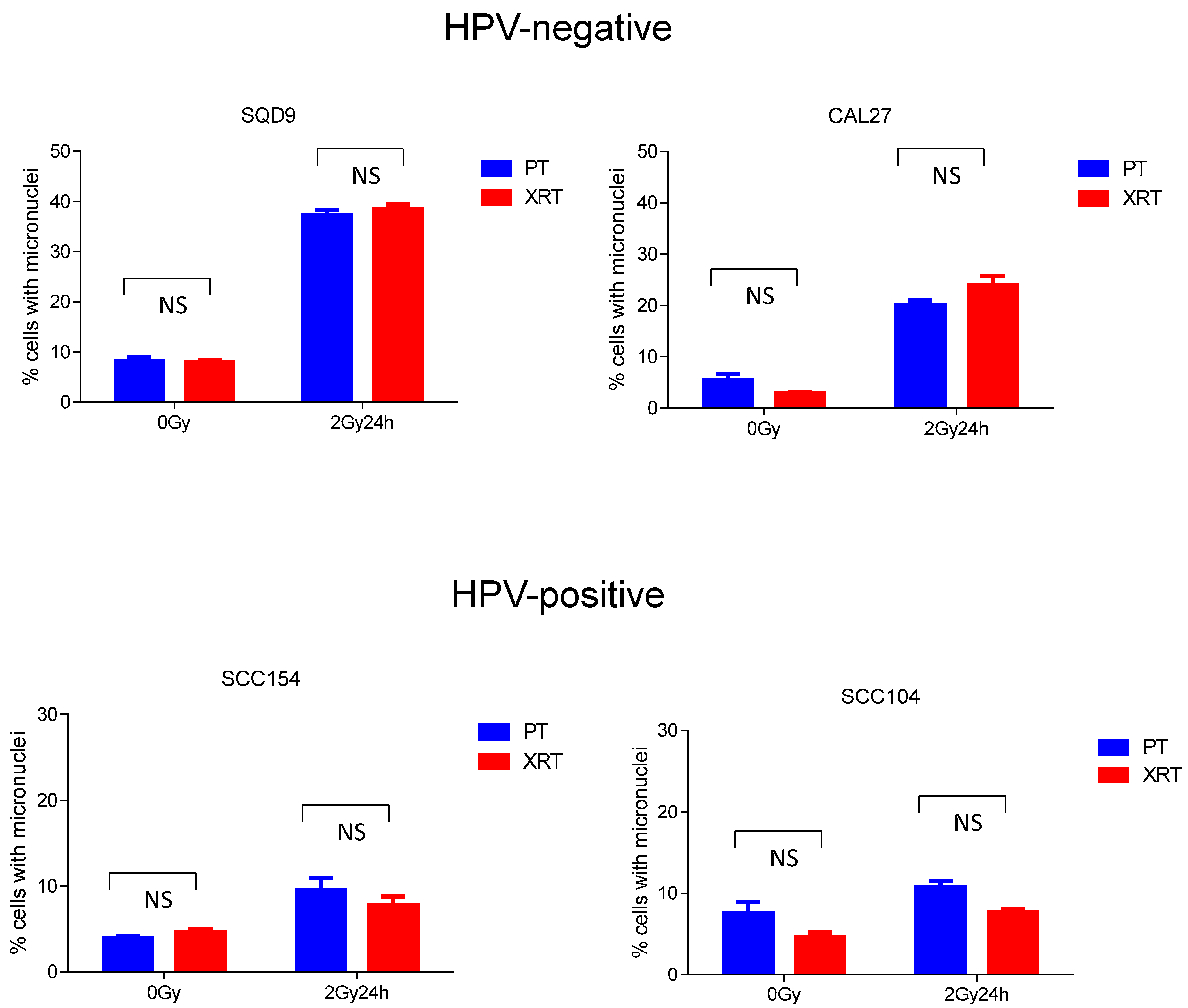
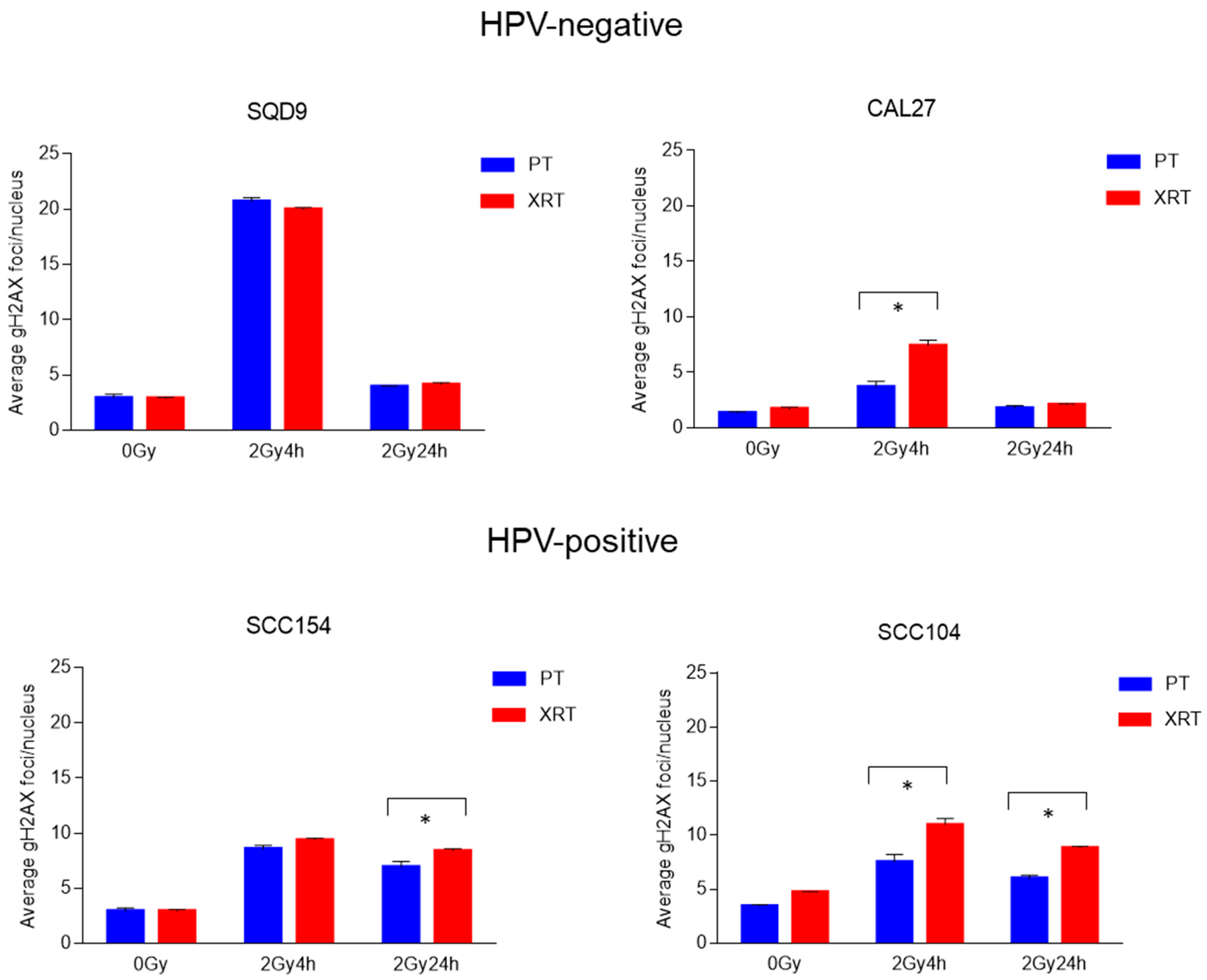
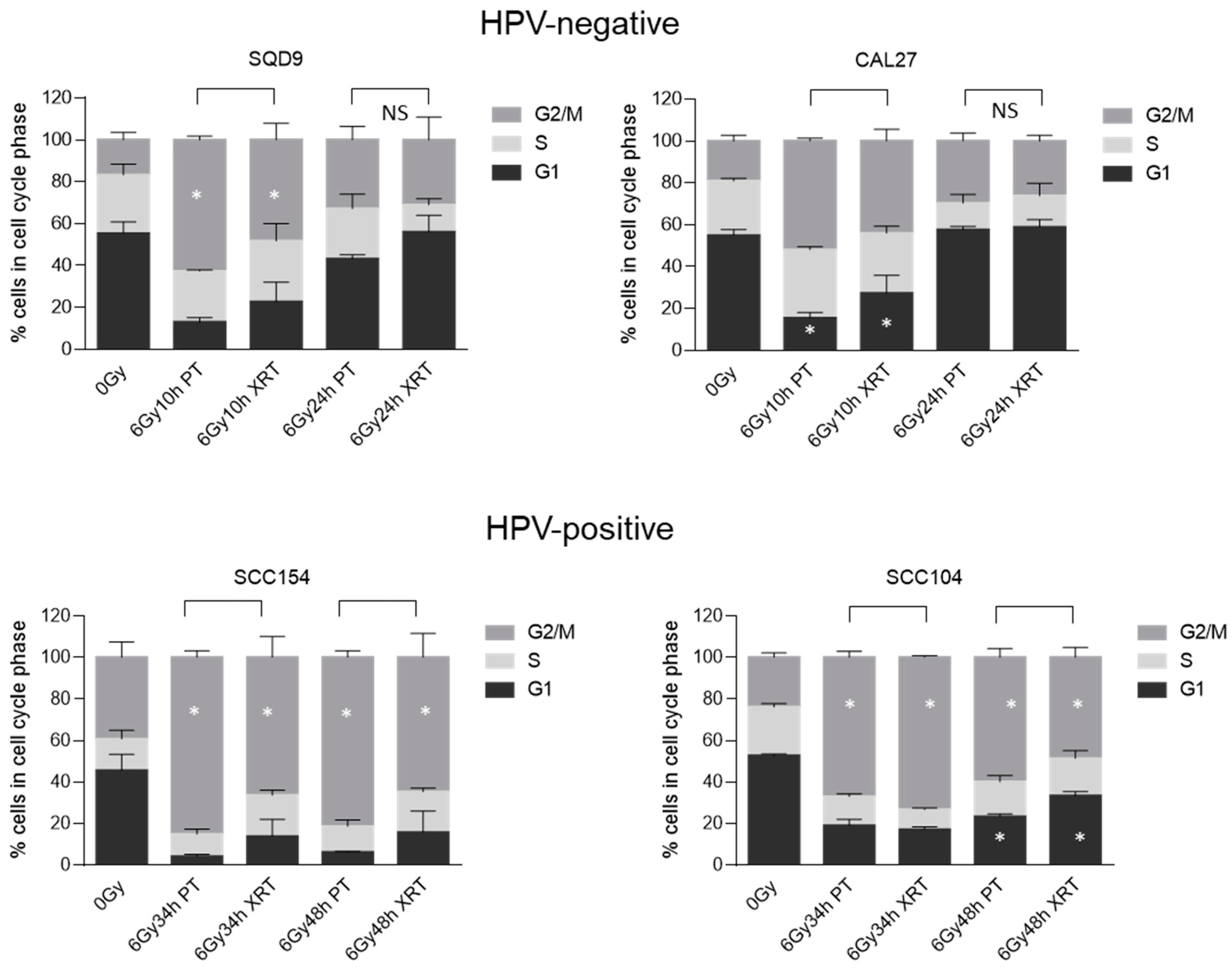
3.2. Effect of PT and XRT on DNA Damage Repair and Cell Cycle Kinetics
3.3. Effect of PT and XRT on Stress Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Algazi, A.P.; Grandis, J.R. Head and neck cancer in 2016: A watershed year for improvements in treatment? Nat. Rev. Clin. Oncol. 2017, 14, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Alsahafi, E.; Begg, K.; Amelio, I.; Raulf, N.; Lucarelli, P.; Sauter, T.; Tavassoli, M. Clinical update on head and neck cancer: Molecular biology and ongoing challenges. Cell Death Dis. 2019, 10, 540. [Google Scholar] [CrossRef]
- Cramer, J.D.; Burtness, B.; Le, Q.T.; Ferris, R.L. The changing therapeutic landscape of head and neck cancer. Nat. Rev. Clin. Oncol. 2019, 16, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Prim. 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L.; Flamand, Y.; Weinstein, G.S.; Li, S.; Quon, H.; Mehra, R.; Garcia, J.J.; Chung, C.H.; Gillison, M.L.; Duvvuri, U.; et al. Phase II Randomized Trial of Transoral Surgery and Low-Dose Intensity Modulated Radiation Therapy in Resectable p16+ Locally Advanced Oropharynx Cancer: An ECOG-ACRIN Cancer Research Group Trial (E3311). J. Clin. Oncol. 2022, 40, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, H.; Rischin, D.; Wong, S.J.; Gregoire, V.; Ferris, R.; Waldron, J.; Le, Q.-T.; Forster, M.; Gillison, M.; Laskar, S.; et al. De-Escalation After DE-ESCALATE and RTOG 1016: A Head and Neck Cancer InterGroup Framework for Future De-Escalation Studies. J. Clin. Oncol. 2020, 38, 2552–2557. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tan, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Krause, M.; Overgaard, J.; Debus, J.; Bentzen, S.M.; Daartz, J.; Richter, C.; Zips, D.; Bortfeld, T. Radiation oncology in the era of precision medicine. Nat. Rev. Cancer 2016, 16, 234–249. [Google Scholar] [CrossRef]
- Grégoire, V.; Langendijk, J.A.; Nuyts, S. Advances in Radiotherapy for Head and Neck Cancer. J. Clin. Oncol. 2015, 33, 3277–3284. [Google Scholar] [CrossRef]
- Nuyts, S.; Bollen, H.; Ng, S.P.; Corry, J.; Eisbruch, A.; Mendenhall, W.M.; Smee, R.; Strojan, P.; Ng, W.T.; Ferlito, A. Proton Therapy for Squamous Cell Carcinoma of the Head and Neck: Early Clinical Experience and Current Challenges. Cancers 2022, 14, 2587. [Google Scholar] [CrossRef]
- Vanderwaeren, L.; Dok, R.; Verstrepen, K.; Nuyts, S. Clinical Progress in Proton Radiotherapy: Biological Unknowns. Cancers 2021, 13, 604. [Google Scholar] [CrossRef] [PubMed]
- Group. PTC-O. Available online: https://www.ptcog.ch/ (accessed on 22 April 2022).
- Chaudhary, P.; Marshall, T.I.; Perozziello, F.M.; Manti, L.; Currell, F.J.; Hanton, F.; McMahon, S.J.; Kavanagh, J.N.; Cirrone, G.A.; Romano, F.; et al. Relative biological effectiveness variation along monoenergetic and modulated Bragg peaks of a 62-MeV therapeutic proton beam: A preclinical assessment. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 27–35. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Matsuura, T.; Wada, M.; Egashira, Y.; Nishio, T.; Furusawa, Y. Enhanced radiobiological effects at the distal end of a clinical proton beam: In vitro study. J. Radiat. Res. 2014, 55, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Ilicic, K.; Combs, S.E.; Schmid, T.E. New insights in the relative radiobiological effectiveness of proton irradiation. Radiat. Oncol. 2018, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Lühr, A.; von Neubeck, C.; Krause, M.; Troost, E.G.C. Relative biological effectiveness in proton beam therapy—Current knowledge and future challenges. Clin. Transl. Radiat. Oncol. 2018, 9, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Paganetti, H. Proton Relative Biological Effectiveness—Uncertainties and Opportunities. Int. J. Part. Ther. 2018, 5, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Dok, R.; Nuyts, S. HPV Positive Head and Neck Cancers: Molecular Pathogenesis and Evolving Treatment Strategies. Cancers 2016, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Gottgens, E.L.; Ostheimer, C.; Span, P.N.; Bussink, J.; Hammond, E.M. HPV, hypoxia and radiation response in head and neck cancer. Br. J. Radiol. 2018, 92, 20180047. [Google Scholar] [CrossRef] [PubMed]
- Wegge, M.; Dok, R.; Dubois, L.J.; Nuyts, S. Use of 3D Spheroid Models for the Assessment of RT Response in Head and Neck Cancer. Int. J. Mol. Sci. 2023, 24, 3763. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Han, S.; Zhu, J.; Wang, X.; Li, Y.; Wang, Z.; Lin, E.; Wang, X.; Molkentine, D.P.; Blanchard, P.; et al. Proton versus photon radiation-induced cell death in head and neck cancer cells. Head. Neck 2019, 41, 46–55. [Google Scholar] [CrossRef]
- Sia, J.; Szmyd, R.; Hau, E.; Gee, H.E. Molecular Mechanisms of Radiation-Induced Cancer Cell Death: A Primer. Front. Cell Dev. Biol. 2020, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-X.; Zhou, P.-K. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct. Target Ther. 2020, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fossati, P.; Paganetti, H.; Ma, L.; Gillison, M.; Myers, J.N.; Hug, E.; Frank, S.J. The Biological Basis for Enhanced Effects of Proton Radiation Therapy Relative to Photon Radiation Therapy for Head and Neck Squamous Cell Carcinoma. Int. J. Part. Ther. 2021, 8, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Li, Y.; Han, S.; Zhu, J.; Wang, X.; Molkentine, D.P.; Blanchard, P.; Yang, Y.; Zhang, R.; et al. Human papillomavirus status and the relative biological effectiveness of proton radiotherapy in head and neck cancer cells. Head. Neck 2017, 39, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, L.; Han, S.; Zhu, J.; Li, Y.; Wang, Z.; Fan, Y.-H.; Lin, E.; Zhang, R.; Sahoo, N.; et al. Patterns of protein expression in human head and neck cancer cell lines differ after proton vs photon radiotherapy. Head. Neck 2020, 42, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Chitsike, L.; Bertucci, A.; Vazquez, M.; Lee, S.; Unternaehrer, J.J.; Duerksen-Hughes, P.J. GA-OH enhances the cytotoxicity of photon and proton radiation in HPV(+) HNSCC cells. Front. Oncol. 2023, 13, 1070485. [Google Scholar] [CrossRef] [PubMed]
- Deycmar, S.; Faccin, E.; Kazimova, T.; Knobel, P.A.; Telarovic, I.; Tschanz, F.; Waller, V.; Winkler, R.; Yong, C.; Zingariello, D.; et al. The relative biological effectiveness of proton irradiation in dependence of DNA damage repair. Br. J. Radiol. 2020, 93, 20190494. [Google Scholar] [CrossRef] [PubMed]
- Görte, J.; Beyreuther, E.; Danen, E.H.J.; Cordes, N. Comparative Proton and Photon Irradiation Combined with Pharmacological Inhibitors in 3D Pancreatic Cancer Cultures. Cancers 2020, 12, 3216. [Google Scholar] [CrossRef]
- Vanderwaeren, L.; Dok, R.; Voordeckers, K.; Vandemaele, L.; Verstrepen, K.J.; Nuyts, S. An Integrated Approach Reveals DNA Damage and Proteotoxic Stress as Main Effects of Proton Radiation in S. cerevisiae. Int. J. Mol. Sci. 2022, 23, 5493. [Google Scholar] [CrossRef]
- Chang, C.-L.; Lin, K.-C.; Chen, W.-M.; Shia, B.-C.; Wu, S.-Y. Comparing the oncologic outcomes of proton therapy and intensity-modulated radiation therapy for head and neck squamous cell carcinoma. Radiother. Oncol. 2024, 190, 109971. [Google Scholar] [CrossRef]
- Hansen, C.R.; Jensen, K.; Smulders, B.; Holm, A.I.S.; Samsøe, E.; Nielsen, M.S.; Sibolt, P.; Skyt, P.; Elstrøm, U.V.; Nielsen, C.P.; et al. Evaluation of decentralised model-based selection of head and neck cancer patients for a proton treatment study. DAHANCA 35. Radiother. Oncol. 2024, 190, 109812. [Google Scholar] [CrossRef] [PubMed]
- Mumaw, D.A.; Hazy, A.J.; Vayntraub, A.; Quinn, T.J.; Salari, K.; Chang, J.H.; Kalman, N.; Katz, S.; Urbanic, J.; Press, R.H.; et al. Low contralateral failure rate with unilateral proton beam radiotherapy for oropharyngeal squamous cell carcinoma: A multi-institutional prospective study from the proton collaborative group. Radiother. Oncol. 2024, 190, 109977. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, E.; Fontana, G.; Licitra, L.; Tinelli, C.; Camarda, A.M.; Grau, C.; Frank, S.J. Comprehensive insights on the underlying potential and advantage of proton therapy over intensity-modulated photon radiation therapy as highlighted in a wide real world data analysis. Radiother. Oncol. 2024, 193, 110122. [Google Scholar] [CrossRef] [PubMed]
- Walser, M.A.; Bachmann, N.; Kluckert, J.; Köthe, A.; Tully, C.; Leiser, D.; Lomax, A.J.; Bizzocchi, N.; Langendijk, J.A.; Weber, D.C. Clinical outcome after pencil beam scanning proton therapy and dysphagia/xerostomia NTCP calculations of proton and photon radiotherapy delivered to patients with cancer of the major salivary glands. Br. J. Radiol. 2023, 96, 20220672. [Google Scholar] [CrossRef] [PubMed]
- Youssef, I.; Mohamed, N.; Kallini, D.; Zakeri, K.; Lin, H.; Han, D.; Qi, H.; Nosov, A.; Riaz, N.; Chen, L.; et al. An Analysis of Positron Emission Tomography Maximum Standard Uptake Value Among Patients with Head and Neck Cancer Receiving Photon and Proton Radiation. Int. J. Radiat. Oncol. Biol. Phys. 2024, 374, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Youssef, I.; Yoon, J.; Mohamed, N.; Zakeri, K.; Press, R.H.; Chen, L.; Gelblum, D.Y.; McBride, S.M.; Tsai, C.J.; Riaz, N.; et al. Toxicity Profiles and Survival Outcomes Among Patients with Nonmetastatic Oropharyngeal Carcinoma Treated with Intensity-Modulated Proton Therapy vs Intensity-Modulated Radiation Therapy. JAMA Netw. Open 2022, 5, e2241538. [Google Scholar] [CrossRef] [PubMed]
- Taku, N.; Wang, L.; Garden, A.S.; Rosenthal, D.I.; Gunn, G.B.; Morrison, W.H.; Fuller, C.D.; Phan, J.; Reddy, J.P.; Moreno, A.C.; et al. Proton Therapy for HPV-Associated Oropharyngeal Cancers of the Head and Neck: A De-Intensification Strategy. Curr. Treat. Options Oncol. 2021, 22, 54. [Google Scholar] [CrossRef] [PubMed]
- Szymonowicz, K.; Krysztofiak, A.; Linden, J.V.; Kern, A.; Deycmar, S.; Oeck, S.; Squire, A.; Koska, B.; Hlouschek, J.; Vüllings, M.; et al. Proton Irradiation Increases the Necessity for Homologous Recombination Repair Along with the Indispensability of Non-Homologous End Joining. Cells 2020, 9, 889. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.O.; Augsburger, M.A.; Grosse, N.; Guckenberger, M.; Lomax, A.J.; Sartori, A.A.; Pruschy, M.N. Differential DNA repair pathway choice in cancer cells after proton- and photon-irradiation. Radiother. Oncol. 2015, 116, 374–380. [Google Scholar] [CrossRef]
- Di Pietro, C.; Piro, S.; Tabbì, G.; Ragusa, M.; Di Pietro, V.; Zimmitti, V.; Cuda, F.; Anello, M.; Consoli, U.; Salinaro, E.T.; et al. Cellular and molecular effects of protons: Apoptosis induction and potential implications for cancer therapy. Apoptosis 2006, 11, 57–66. [Google Scholar] [CrossRef]
- Finnberg, N.; Wambi, C.; Ware, J.H.; Kennedy, A.R.; El-Deiry, W.S. Gamma-radiation (GR) triggers a unique gene expression profile associated with cell death compared to proton radiation (PR) in mice in vivo. Cancer Biol. Ther. 2008, 7, 2023–2033. [Google Scholar] [CrossRef] [PubMed]
- Narang, H.; Bhat, N.; Gupta, S.K.; Santra, S.; Choudhary, R.K.; Kailash, S.; Krishna, M. Differential activation of mitogen-activated protein kinases following high and low LET radiation in murine macrophage cell line. Mol. Cell Biochem. 2009, 324, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Lerch, S.; Berthold, S.; Ziemann, F.; Dreffke, K.; Subtil, F.S.B.; Senger, Y.; Jensen, A.; Engenhart-Cabillic, R.; Dikomey, E.; Wittig, A.; et al. HPV-positive HNSCC cell lines show strongly enhanced radiosensitivity after photon but not after carbon ion irradiation. Radiother. Oncol. 2020, 151, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Meerz, A.; Deville, S.S.; Müller, J.; Cordes, N. Comparative Therapeutic Exploitability of Acute Adaptation Mechanisms to Photon and Proton Irradiation in 3D Head and Neck Squamous Cell Carcinoma Cell Cultures. Cancers 2021, 13, 1190. [Google Scholar] [CrossRef] [PubMed]
- Vitti, E.T.; Kacperek, A.; Parsons, J.L. Targeting DNA Double-Strand Break Repair Enhances Radiosensitivity of HPV-Positive and HPV-Negative Head and Neck Squamous Cell Carcinoma to Photons and Protons. Cancers 2020, 12, 1490. [Google Scholar] [CrossRef]
- Lupu-Plesu, M.; Claren, A.; Martial, S.; N′Diaye, P.D.; Lebrigand, K.; Pons, N.; Ambrosetti, D.; Peyrottes, I.; Feuillade, J.; Hérault, J.; et al. Effects of proton versus photon irradiation on (lymph)angiogenic, inflammatory, proliferative and anti-tumor immune responses in head and neck squamous cell carcinoma. Oncogenesis 2017, 6, e354. [Google Scholar] [CrossRef] [PubMed]
- Rieckmann, T.; Tribius, S.; Grob, T.J.; Meyer, F.; Busch, C.J.; Petersen, C.; Dikomey, E.; Kriegs, M. HNSCC cell lines positive for HPV and p16 possess higher cellular radiosensitivity due to an impaired DSB repair capacity. Radiother. Oncol. 2013, 107, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Nickson, C.M.; Moori, P.; Carter, R.J.; Rubbi, C.P.; Parsons, J.L. Misregulation of DNA damage repair pathways in HPV-positive head and neck squamous cell carcinoma contributes to cellular radiosensitivity. Oncotarget 2017, 8, 29963–29975. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, B.; Wei, J.; Meng, L.; Zhang, Q.; Qu, C.; Xin, Y.; Jiang, X. Molecular mechanisms underlying increased radiosensitivity in human papillomavirus-associated oropharyngeal squamous cell carcinoma. Int. J. Biol. Sci. 2020, 16, 1035–1043. [Google Scholar] [CrossRef]
- Sorensen, B.S.; Busk, M.; Olthof, N.; Speel, E.J.; Horsman, M.R.; Alsner, J.; Overgaard, J. Radiosensitivity and effect of hypoxia in HPV positive head and neck cancer cells. Radiother. Oncol. 2013, 108, 500–505. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dok, R.; Vanderwaeren, L.; Verstrepen, K.J.; Nuyts, S. Radiobiology of Proton Therapy in Human Papillomavirus-Negative and Human Papillomavirus-Positive Head and Neck Cancer Cells. Cancers 2024, 16, 1959. https://doi.org/10.3390/cancers16111959
Dok R, Vanderwaeren L, Verstrepen KJ, Nuyts S. Radiobiology of Proton Therapy in Human Papillomavirus-Negative and Human Papillomavirus-Positive Head and Neck Cancer Cells. Cancers. 2024; 16(11):1959. https://doi.org/10.3390/cancers16111959
Chicago/Turabian StyleDok, Rüveyda, Laura Vanderwaeren, Kevin J. Verstrepen, and Sandra Nuyts. 2024. "Radiobiology of Proton Therapy in Human Papillomavirus-Negative and Human Papillomavirus-Positive Head and Neck Cancer Cells" Cancers 16, no. 11: 1959. https://doi.org/10.3390/cancers16111959
APA StyleDok, R., Vanderwaeren, L., Verstrepen, K. J., & Nuyts, S. (2024). Radiobiology of Proton Therapy in Human Papillomavirus-Negative and Human Papillomavirus-Positive Head and Neck Cancer Cells. Cancers, 16(11), 1959. https://doi.org/10.3390/cancers16111959






