Therapeutic Patterns and Clinical Outcomes in Limited Disease Small Cell Lung Cancer: A Decade of Analysis at a Tertiary Cancer Center
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Cohort Description
2.2. Radiotherapy Target Volume Delineation and Treatment Planning
2.3. Chemotherapy
2.4. Toxicity Scoring and Follow-Up
2.5. Statistical Analyses
3. Results
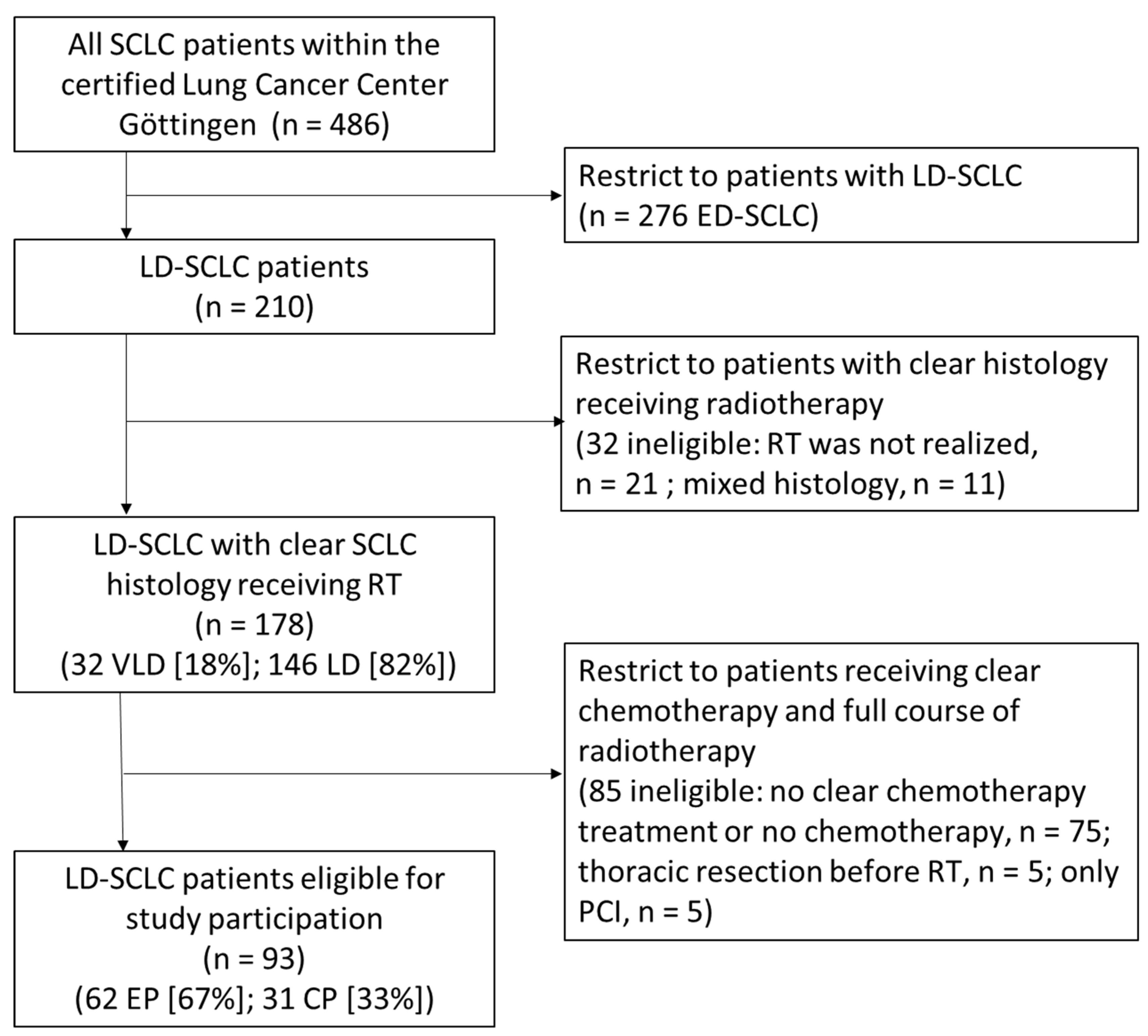
3.1. Patient Cohort and Characteristics
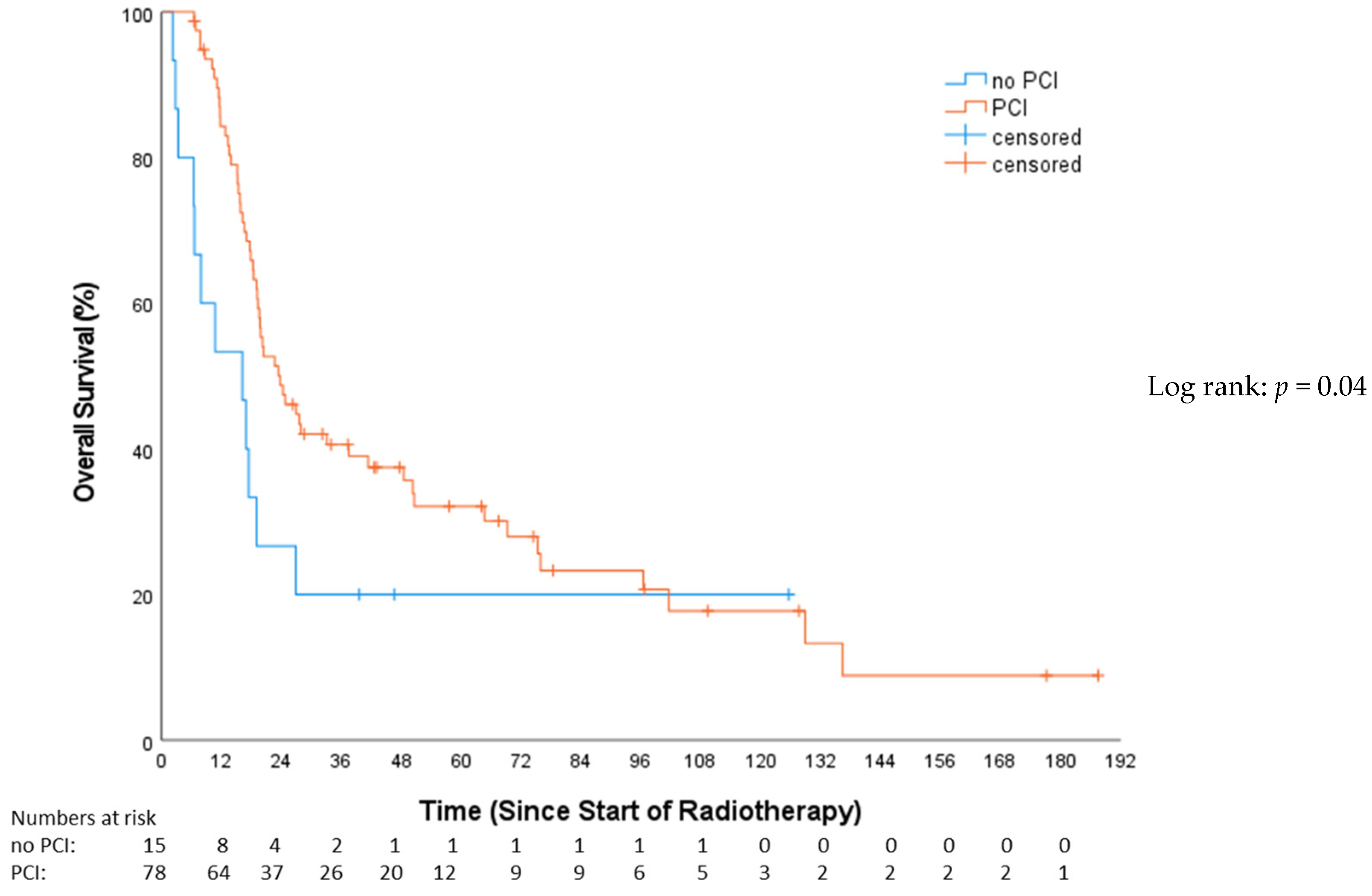
3.2. Overall Survival (OS)
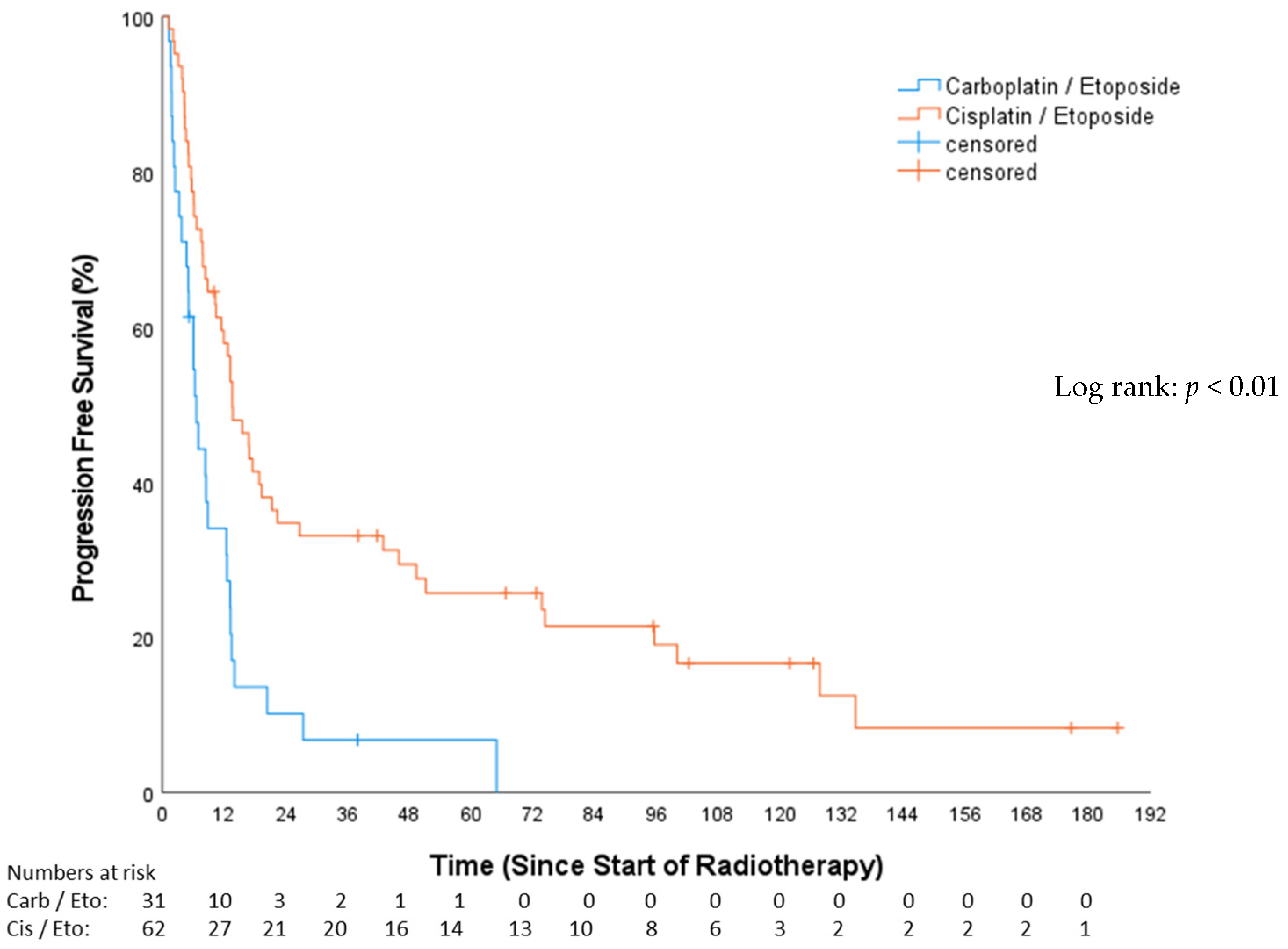
3.3. Progression-Free Survival (PFS)
4. Discussion
4.1. Treatment Patterns and Outcomes
4.2. Role of Prophylactic Cranial Irradiation (PCI)
4.3. Survival Predictors
4.4. Limitations
4.5. Strength
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petty, W.J.; Paz-Ares, L. Emerging Strategies for the Treatment of Small Cell Lung Cancer: A Review. JAMA Oncol. 2023, 9, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Stinchcombe, T.E.; Gore, E.M. Limited-stage small cell lung cancer: Current chemoradiotherapy treatment paradigms. Oncologist 2010, 15, 187–195. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Small Cell Lung Cancer (Version 03.2023). Available online: https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf (accessed on 5 July 2023).
- Turrisi, A.T., 3rd; Kim, K.; Blum, R.; Sause, W.T.; Livingston, R.B.; Komaki, R.; Wagner, H.; Aisner, S.; Johnson, D.H. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N. Engl. J. Med. 1999, 340, 265–271. [Google Scholar] [CrossRef]
- Grønberg, B.H.; Killingberg, K.T.; Fløtten, Ø.; Brustugun, O.T.; Hornslien, K.; Madebo, T.; Langer, S.W.; Schytte, T.; Nyman, J.; Risum, S.; et al. High-dose versus standard-dose twice-daily thoracic radiotherapy for patients with limited stage small-cell lung cancer: An open-label, randomised, phase 2 trial. Lancet Oncol. 2021, 22, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Faivre-Finn, C.; Snee, M.; Ashcroft, L.; Appel, W.; Barlesi, F.; Bhatnagar, A.; Bezjak, A.; Cardenal, F.; Fournel, P.; Harden, S.; et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): An open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017, 18, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Aupérin, A.; Arriagada, R.; Pignon, J.-P.; Le Péchoux, C.; Gregor, A.; Stephens, R.J.; Kristjansen, P.E.G.; Johnson, B.E.; Ueoka, H.; Wagner, H.; et al. Prophylactic Cranial Irradiation for Patients with Small-Cell Lung Cancer in Complete Remission. N. Engl. J. Med. 1999, 341, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Slotman, B.; Faivre-Finn, C.; Kramer, G.; Rankin, E.; Snee, M.; Hatton, M.; Postmus, P.; Collette, L.; Musat, E.; Senan, S.; et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N. Engl. J. Med. 2007, 357, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Yamanaka, T.; Seto, T.; Harada, H.; Nokihara, H.; Saka, H.; Nishio, M.; Kaneda, H.; Takayama, K.; Ishimoto, O.; et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 663–671. [Google Scholar] [CrossRef]
- Zelen, M. Keynote address on biostatistics and data retrieval. Cancer Chemother. Rep. Part 3 1973, 4, 31–42. [Google Scholar]
- Shepherd, F.A.; Ginsberg, R.J.; Haddad, R.; Feld, R.; Sagman, U.; Evans, W.K.; DeBoer, G.; Maki, E. Importance of clinical staging in limited small-cell lung cancer: A valuable system to separate prognostic subgroups. The University of Toronto Lung Oncology Group. J. Clin. Oncol. 1993, 11, 1592–1597. [Google Scholar] [CrossRef]
- Gatzemeier, U.; Hossfeld, D.K.; Neuhauss, R.; Reck, M.; Achterrath, W.; Lenaz, L. Phase II and III studies with carboplatin in small cell lung cancer. Semin. Oncol. 1992, 19, 28–36. [Google Scholar] [PubMed]
- Calvert, A.H.; Newell, D.R.; Gumbrell, L.A.; O’Reilly, S.; Burnell, M.; Boxall, F.E.; Siddik, Z.H.; Judson, I.R.; Gore, M.E.; Wiltshaw, E. Carboplatin dosage: Prospective evaluation of a simple formula based on renal function. J. Clin. Oncol. 1989, 7, 1748–1756. [Google Scholar] [CrossRef]
- NIH. Common Terminology Criteria for Adverse Events (CTCAE). Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed on 20 April 2023).
- Schemper, M.; Smith, T.L. A note on quantifying follow-up in studies of failure time. Control Clin. Trials 1996, 17, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Honecker, F.; Wedding, U.; Bokemeyer, C. Chemotherapy in Elderly Patients with Advanced Lung Cancer. Part I: General Aspects and Treatment of Small Cell Lung Cancer (SCLC). Onkologie 2004, 27, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Schild, S.E.; Zhao, L.; Wampfler, J.A.; Daniels, T.B.; Sio, T.; Ross, H.J.; Paripati, H.; Marks, R.S.; Yi, J.; Liu, H.; et al. Small-cell Lung Cancer in Very Elderly (≥80 Years) Patients. Clin. Lung Cancer 2019, 20, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Watanabe, K.; Kunikane, H.; Yokoyama, A.; Kudoh, S.; Asakawa, T.; Shibata, T.; Kunitoh, H.; Tamura, T.; Saijo, N. Randomised phase III trial of carboplatin plus etoposide vs split doses of cisplatin plus etoposide in elderly or poor-risk patients with extensive disease small-cell lung cancer: JCOG 9702. Br. J. Cancer 2007, 97, 162–169. [Google Scholar] [CrossRef]
- Bernhardt, D.; Hommertgen, A.; Schmitt, D.; El Shafie, R.; Paul, A.; König, L.; Mair-Walther, J.; Krisam, J.; Klose, C.; Welzel, T.; et al. Whole brain radiation therapy alone versus radiosurgery for patients with 1–10 brain metastases from small cell lung cancer (ENCEPHALON Trial): Study protocol for a randomized controlled trial. Trials 2018, 19, 388. [Google Scholar] [CrossRef]
- Rusthoven, C.G.; Yamamoto, M.; Bernhardt, D.; Smith, D.E.; Gao, D.; Serizawa, T.; Yomo, S.; Aiyama, H.; Higuchi, Y.; Shuto, T.; et al. Evaluation of First-line Radiosurgery vs Whole-Brain Radiotherapy for Small Cell Lung Cancer Brain Metastases: The FIRE-SCLC Cohort Study. JAMA Oncol. 2020, 6, 1028–1037. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Ren, F.; Huang, Z.; Tan, B.; Zhao, Z.; Yu, X.; Dong, P.; Yu, J.; Meng, X. Prophylactic cranial irradiation (PCI) versus active surveillance in patients with limited-stage small cell lung cancer: A retrospective, multicentre study. Respir. Res. 2022, 23, 274. [Google Scholar] [CrossRef]
- Lew, A.; Berghmans, T.; Andratschke, N.; Dempsey, C.; Flackett, L.; Leonetti, G.; Koller, M.; Faivre-Finn, C. 174TiP PRIMALung (EORTC-1901): Prophylactic cerebral irradiation or active brain magnetic resonance imaging surveillance in small cell lung cancer patients. J. Thorac. Oncol. 2023, 18, S136. [Google Scholar] [CrossRef]
- Chen, M.; Li, R.; Kong, Y.; Shi, L.; Wang, J.; Wang, Y.; Xu, Y.; Ji, Y.; Hu, X. Rational and design of prophylactic cranial irradiation (PCI) and brain MRI surveillance versus brain MRI surveillance alone in patients with limited-stage small cell lung cancer achieving complete remission (CR) of tumor after chemoradiotherapy: A multicenter prospective randomized study. BMC Cancer 2024, 24, 429. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Huang, L.; Zhen, H.; Jin, P.; Wang, J.; Hu, Z. Carboplatin versus cisplatin in combination with etoposide in the first-line treatment of small cell lung cancer: A pooled analysis. BMC Cancer 2021, 21, 1308. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.L.; Lippard, S.J. Binding of the antitumor drug cis-diamminedichloroplatinum(II) (cisplatin) to DNA. Biochim. Biophys. Acta (BBA)—Rev. Cancer 1985, 780, 167–180. [Google Scholar] [CrossRef]
- Forgie, B.N.; Prakash, R.; Telleria, C.M. Revisiting the Anti-Cancer Toxicity of Clinically Approved Platinating Derivatives. Int. J. Mol. Sci. 2022, 23, 15410. [Google Scholar] [CrossRef]
- Dilruba, S.; Kalayda, G.V. Platinum-based drugs: Past, present and future. Cancer Chemother. Pharmacol. 2016, 77, 1103–1124. [Google Scholar] [CrossRef] [PubMed]
- Calvert, A.H.; Harland, S.J.; Newell, D.R.; Siddik, Z.H.; Jones, A.C.; McElwain, T.J.; Raju, S.; Wiltshaw, E.; Smith, I.E.; Baker, J.M.; et al. Early clinical studies with cis-diammine-1,1-cyclobutane dicarboxylate platinum II. Cancer Chemother. Pharmacol. 1982, 9, 140–147. [Google Scholar] [CrossRef]
- Rossi, A.; Maio, M.D.; Chiodini, P.; Rudd, R.M.; Okamoto, H.; Skarlos, D.V.; Früh, M.; Qian, W.; Tamura, T.; Samantas, E.; et al. Carboplatin- or Cisplatin-Based Chemotherapy in First-Line Treatment of Small-Cell Lung Cancer: The COCIS Meta-Analysis of Individual Patient Data. J. Clin. Oncol. 2012, 30, 1692–1698. [Google Scholar] [CrossRef]


| Variable | EP Cisplatin/Etoposide (%) | CP Carboplatin/Etoposide (%) | p Value * |
|---|---|---|---|
| Sex | |||
| male | 34 (54.8) | 16 (51.6) | |
| female | 28 (45.2) | 15 (48.4) | 0.77 |
| Age, y | |||
| 46–59 | 27 (43.5) | 5 (16.1) | |
| ≥60 | 35 (56.5) | 26 (83.9) | 0.01 |
| Stage | |||
| VLD | 9 (14.5) | 6 (19.4) | |
| LD | 53 (85.5) | 25 (80.7) | 0.7 |
| Tumor status | |||
| cT1 | 4 (6.5) | 6 (19.4) | |
| cT2 | 21 (33.9) | 6 (19.4) | |
| cT3 | 10 (16.1) | 4 (12.9) | |
| cT4 | 27 (43.5) | 15 (48.4) | 0.9 |
| Nodal status | |||
| cN0 cN1 cN2 cN3 | 10 (16.1) 8 (12.9) 27 (43.5) 16 (25.8) | 9 (29.0) 6 (19.4) 7 (22.6) 9 (29.0) | 0.34 |
| CTV | |||
| Mean (SD) Min-max | 177.3 (148.8) 15.2–901.7 | 216 (120.9) 56.9–468 | 0.53 |
| RT twice daily | |||
| yes | 40 (64.5) | 8 (25.8) | |
| no | 22 (35.5) | 23 (74.2) | <0.01 |
| RT dose | |||
| 45 Gy twice daily ≤50 Gy once daily | 40 (64.5) 16 (25.8) | 8 (25.8) 21 (67.7) | |
| >50 Gy once daily | 6 (9.7) | 2 (6.4) | <0.01 |
| RT technique | |||
| 3D-conformal RT | 41 (66.1) | 22 (71) | |
| Rapid-Arc | 21 (33.9) | 9 (29) | 0.64 |
| PCI | |||
| yes no | 55 (88.7) 7 (11.3) | 27 (87.1) 4 (12.9) | 0.55 |
| Smoking status pre RT | |||
| yes | 59 (95.2) | 29 (93.5) | |
| no | 3 (4.8) | 2 (6.5) | 0.76 |
| Grade ≥III side effects/hematotoxicity | |||
| yes | 16 (25.8) | 9 (29) | |
| no | 46 (74.2) | 22 (71) | 0.74 |
| Eastern Cooperative Oncology Group performance status | |||
| 0 | 50 (80.6) | 16 (51.6) | |
| 1–4 | 12 (19.4) | 15 (48.4) | 0.04 |
| Charlson comorbidity index | |||
| 0–4 | 57 (91.9) | 25 (31) | |
| ≥5 | 5 (8.1) | 6 (19.4) | 0.11 |
| EP Cisplatin/Etoposide (%) | CP Carboplatin/Etoposide (%) | |
|---|---|---|
| Patients with pre-RT systemic therapy (sequential regimen) | 17/62 (27.4%) | 23/31 (74.2%) |
| Patients with systemic therapy concomitant to RT (simultaneous regimen) | 45/62 (72.6%) | 8/31 (25.8%) |
| Chemotherapy-cycles pre-RT | ||
| 0 cycles | 27 (43.5%) | 2 (6.5%) |
| 1 cycle | 8 (12.9%) | 6 (19.4%) |
| 2 cycles | 7 (11.3%) | 3 (9.7%) |
| 3 cycles | 3 (4.8%) | 1 (3.2%) |
| 4 cycles | 4 (6.5% | 8 (25.8%) |
| 5 cycles | 1 (1.6%) | 1 (3.2%) |
| 6 cycles | 11 (17.7%) | 10 (32.3%) |
| Chemotherapy cycles concomitant to the RT | ||
| 0 cycles | 18 (29%) | 23 (74.2%) |
| 1 cycle | 31 (50%) | 4 (12.9%) |
| 2 cycles | 13 (21%) | 4 (12.9%) |
| Variable | Overall Survival | Progression-Free Survival | ||||
|---|---|---|---|---|---|---|
| HR | 95%-CI for HR | p Value | HR | 95%-CI for HR | p Value | |
| Sex | 0.58 | 0.36–0.93 | 0.025 | 0.73 | 0.47–1.14 | 0.171 |
| Age ≥ 60 y | 1.93 | 0.71–1.93 | 0.545 | 1.40 | 0.87–2.27 | 0.167 |
| ECOG 0 vs. 1–4 | 0.45 | 0.73–2.05 | 1.223 | 1.49 | 0.93–2.38 | 0.106 |
| Charlson ≥5 | 1.00 | 0.46–2.20 | 0.998 | 1.31 | 0.67–2.57 | 0.425 |
| PCI | 0.53 | 0.28–0.98 | 0.044 | 0.56 | 0.31–1.02 | 0.057 |
| Simultaneous RCT | 0.46 | 0.28–0.75 | 0.002 | 0.49 | 0.31–0.78 | 0.003 |
| EP vs. CP | 0.49 | 0.29–0.81 | 0.006 | 0.42 | 0.26–0.67 | 0.001 |
| Hematotoxcity ≥ 3 | 0.62 | 0.35–1.08 | 0.093 | 0.74 | 0.45–1.23 | 0.249 |
| CTV | 1.05 | 0.66–1.68 | 0.835 | 1.15 | 0.74–1.79 | 0.530 |
| Tumor-Status (T) | 1.18 | 0.93–1.49 | 0.180 | 1.25 | 0.99–1.56 | 0.057 |
| Nodal-Status (N) | 1.12 | 0.89–1.40 | 0.343 | 1.16 | 0.94–1.43 | 0.176 |
| Overall Survival | |||
|---|---|---|---|
| Variable | HR | 95%-CI for HR | p Value |
| Sex | 0.49 | 0.29–0.80 | 0.004 |
| PCI | 0.27 | 0.132–0.54 | <0.001 |
| Simultaneous RCT | 0.43 | 0.244–0.77 | 0.004 |
| EP vs. CP | 0.57 | 0.319–0.99 | 0.049 |
| Progression-Free Survival | |||
| Variable | HR | 95%-CI for HR | p Value |
| Simultaneous RCT | 0.66 | 0.38–1.12 | 0.125 |
| EP vs. CP | 0.50 | 0.29–0.86 | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziegler, D.A.; Cleve, C.C.; Ziegler, S.; Schirmer, M.A.; Fischer, L.A.; Bohnenberger, H.; Overbeck, T.R.; Braulke, F.; Hammerstein-Equord, A.v.; Leu, M.; et al. Therapeutic Patterns and Clinical Outcomes in Limited Disease Small Cell Lung Cancer: A Decade of Analysis at a Tertiary Cancer Center. Cancers 2024, 16, 1953. https://doi.org/10.3390/cancers16111953
Ziegler DA, Cleve CC, Ziegler S, Schirmer MA, Fischer LA, Bohnenberger H, Overbeck TR, Braulke F, Hammerstein-Equord Av, Leu M, et al. Therapeutic Patterns and Clinical Outcomes in Limited Disease Small Cell Lung Cancer: A Decade of Analysis at a Tertiary Cancer Center. Cancers. 2024; 16(11):1953. https://doi.org/10.3390/cancers16111953
Chicago/Turabian StyleZiegler, David Alexander, Cecilia Christiane Cleve, Sonia Ziegler, Markus Anton Schirmer, Laura Anna Fischer, Hanibal Bohnenberger, Tobias Raphael Overbeck, Friederike Braulke, Alexander von Hammerstein-Equord, Martin Leu, and et al. 2024. "Therapeutic Patterns and Clinical Outcomes in Limited Disease Small Cell Lung Cancer: A Decade of Analysis at a Tertiary Cancer Center" Cancers 16, no. 11: 1953. https://doi.org/10.3390/cancers16111953
APA StyleZiegler, D. A., Cleve, C. C., Ziegler, S., Schirmer, M. A., Fischer, L. A., Bohnenberger, H., Overbeck, T. R., Braulke, F., Hammerstein-Equord, A. v., Leu, M., Guhlich, M., Dröge, L. H., Rieken, S., Rittmeyer, A., & El Shafie, R. A. (2024). Therapeutic Patterns and Clinical Outcomes in Limited Disease Small Cell Lung Cancer: A Decade of Analysis at a Tertiary Cancer Center. Cancers, 16(11), 1953. https://doi.org/10.3390/cancers16111953









