Simple Summary
Microvesicles (MVs) are essential for inter-cellular signaling in health and disease. We analyzed MV levels in colorectal cancer patients and assessed their release in early-stage colorectal cancer and survival. Considering that all types of MV were elevated beginning in the very early stages of the disease, we believe that the study of circulating MV levels could provide evidence for their use in the early detection of colon cancer in patients.
Abstract
Background: The release of microvesicles (MVs) is an essential phenomenon for inter-cellular signaling in health and disease. The role of MVs in cancer is multidimensional and includes cancer cell survival, proliferation, and invasion. In this prospective study, we analyzed MV levels in colorectal cancer patients and assessed the importance of MV release in early-stage colorectal cancer and survival. Methods: This study included 98 patients and 15 controls. The characterization of MVs from human plasma was performed by flow cytometry using monoclonal antibodies. Results: The levels of total MVs and MUC-1-positive, tissue factor (TF)-positive, and endothelial cell-derived MVs (EMVs) were statistically significantly higher in the colon cancer patients than in the controls (p < 0.001). Furthermore, the subgroup of patients with very early-stage colorectal cancer also had statistically significant differences in the levels of the abovementioned MVs compared to the controls (p < 0.01). Highly differentiated tumors had lower levels of MUC-1-positive MVs (p < 0.02), EMVs (p < 0.002), and EMV/TF combinations (p < 0.001) versus those with tumors with low/intermediate differentiation. Conclusions: Our data demonstrate that the analysis of circulating MV levels in plasma could possibly become a tool for the early diagnosis of colon cancer at a very early stage of the disease.
Keywords:
circulating microvesicles; microparticles; biomarkers; cancer; colorectal cancer; survival 1. Introduction
Microvesicles are a type of extracellular vesicle that include exosomes and apoptotic bodies [1]. Exosomes are formed by the inward budding of the plasma membrane, and their size is between 40 and 150 nm [2]. Apoptotic bodies arise from the fragmentation of apoptotic cells, and their size is greater than 1000 nm. MVs are between 150 and 1000 nm and are formed by the outward blebbing of the cell membrane [3]. Recent studies suggest a multidimensional role of microvesicles (MVs) in cancer cell survival, proliferation, and invasion [4,5]. MVs derived from tumor cells affect the surrounding microenvironment and distal organs through a variety of mechanisms including immune system inhibition, angiogenesis induction, oncogene transfer, and chemotherapy resistance [6,7].
MVs have garnered significant interest as potential biomarkers due to their ability to reflect the molecular profiles of their parent cells [8], including cancer cells [9], and their presence in various biological fluids, such as blood and urine [10]. Recent studies have shown promising results regarding the diagnostic and prognostic utility of microvesicles in colon cancer, with their cargo mirroring the aberrant signaling pathways and genetic alterations characteristic of the disease [11,12]. MVs can be easily obtained by liquid biopsies, namely blood samples, and provide more information about tumor characteristics, prognosis, metastatic potential and targetable pathways for treatment than a solid biopsy [13,14,15]. In addition, they can provide tumor-specific details such as the status of mutations, gene amplifications, and the transcriptome [16,17,18]. Moreover, advancements in isolation and characterization techniques, such as flow cytometry, nanoparticle tracking analysis, and mass spectrometry, have enhanced our ability to accurately detect and analyze microvesicles, further facilitating their clinical translation [19].
The prevention and treatment of colorectal cancer (CRC) is a global health challenge since CRC ranks as the second leading cause of cancer-related mortality [20]. Recent studies highlight the importance of microvesicles as biomarkers for the early detection of CRC [21], prognosis, and the prediction of response to therapy [22,23].
We performed a prospective study to assess the levels of different types of MVs in patients newly diagnosed with colorectal cancer and correlate them with disease and patient characteristics. As cancer treatment (surgery, chemotherapy, and radiotherapy) can induce the release of MVs from tumor and normal cells [24], all patients were included in our study prior to any treatment.
2. Materials and Methods
2.1. Study Population
The present study included 98 patients diagnosed with CRC between March 2014 and December 2016 at participating centers. Blood samples were collected on the day before an elective surgery. Patients who received chemotherapy or radiotherapy before surgery and patients on anticoagulants and/or those with a history of thrombosis were not included in this study. A cancer-free control group matched for sex and age distribution was also evaluated. Demographic and medical history data were available for both groups. In addition, clinical and laboratory data, tumor location, staging, histological analysis data, outcome, and follow-up data were assessed.
This study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Institutional Review Board of Aretaieio Hospital, the Institutional Review Board of Laiko Hospital, and the Research Ethics Committee, all belonging to the National and Kapodistrian University of Athens. Written informed consent was obtained from all participants (patients and controls).
2.2. Blood Sampling
Whole blood samples were collected from colon cancer patients and healthy subjects via an antecubital vein before surgery using a 21 gauge catheter (BD Vacutainer) and transferred in ethylenediaminetetraacetic acid (EDTA) and 3.2% sodium citrate blood collection tubes (BD Vacutainer Blood Collection Tubes, BD Biosciences, San Jose, CA, USA).
2.3. Hematological and Screening Hemostasis Measurements
BC-3000 PLUS, MINDRAY Celltac E, MEK-7222 Κ, and NIHON KOHDEN automatic blood cell counters were used to obtain complete blood count through double measurements.
2.4. Characterization of Cell-Derived Microvesicles from Human Plasma
Citrated blood samples were processed within 30 min of venipuncture using two successive centrifugations at 2.500× g spin for 15 min at 20 °C, as previously described [25]. The final platelet-free plasma supernatant was immediately frozen and stored at −80 °C.
Megamix beads (Catalog No 7801, BioCytex, Marseille, France) measuring 0.5 μm, 0.9 μm, and 3.0 μm and a flow cytometry sub-micron particle size reference kit (Catalog No F13839, Thermo Fisher Scientific, Waltham, MA, USA) for sizes of 0.02 μm, 0.1 μm, 0.2 μm, 0.5 μm, 1.0 μm, and 2.0 μm were used to standardize the setup of the MV analysis region. To distinguish apoptotic MVs from non-apoptotic MVs, plasma was stained using AnnexinV (PerCP-CyTM5.5 Annexin V, Catalog No 561431, BD, San Jose, CA, USA). The microparticles were further stained with the following monoclonal antibodies: (a) vascular endothelial (VE) cadherin (CD144)–PE (clone 11D4.1, Catalog No 561714, BD, San Jose, CA, USA); (b) MUC1 (CD227)–FITC (clone HMPV, Catalog No 559774, BD, San Jose, CA, USA); (c) Tissue Factor (CD142)-APC (clone HTF-1 Catalog No 17-1429-42, eBioscienceTM) and (d) integrin-a2b (CD41a)–PE-Cy™7 (clone HIP8, Catalog No 561424, BD, San Jose, CA, USA). MVs positive for VE-cadherin were identified as endothelial cell-derived microvesicles (EMVs), and MVs positive for integrin-a2b were identified as platelet-derived microvesicles (PMVs). Briefly, the staining protocol was as follows: 10 μL of the patient’s plasma was re-suspended in 190 μL of diluted Annexin V binding buffer 10× concentrate (Catalog No 556454, BD), which originally contained 25 mM of a CaCL2 solution. Then, 5 μL of PerCP-CyTM5.5 -AnnV, 5 μL of (VE)-cadherin (CD144)–PE, 20 μL of MUC1 (CD227)–FITC, 5 μL of Tissue Factor (CD142)-APC, and 10 μL of integrin-a2b (CD41a)–PE-Cy™7 were added. The samples were incubated for 15 min in a dark room at room temperature, and the reaction was stopped by the addition of 400 μL of the diluted binding buffer. The samples were analyzed immediately using a FACSCanto II cytometer (BD Biosciences, San Jose, CA, USA). Data were analyzed from 100,000 events with the aid of FACSDivaTM software, version 6.1.3 (BD). TruCount beads (BDs) were used to calculate the absolute number of circulating MVs in the plasma (Catalog No 340334, BD Pharmingen, San Jose, CA, USA). The specificity of monoclonal antibodies was verified by using identical concentrations of an isotype-matched control antibody to adjust the instrument’s settings, set the fluorescence compensation, and check for instrument sensitivity.
2.5. Statistical Analysis
A statistical analysis was performed using SAS 9.4 for Windows (SAS Institute Inc., Cary, NC, USA). Comparisons of measurements between the various “groups” (patients vs. controls) were performed using the Kruskal–Wallis test to assess differences in measured medical quantities expressed in a numeric form, and to compare proportions when the quantities were expressed in a qualitative manner (i.e., for categorical data), the chi-square test was used. Additionally, wherever dichotomous categorical variables were available, odds ratios were evaluated via Wald’s p-value. For correlations, the Spearman correlation coefficient was used as non-parametric tests were preferred in this study. Finally, Kaplan–Meier survival curves were produced, and the log-rank test was used to examine the roles of individual characteristics (i.e., vesicle counts and patient age). The statistical significance level for this study was set at a p-value of 0.05.
3. Results
3.1. Baseline Characteristics
In total, 113 cases were available for this study: 98 patients and 15 controls. Demographic data, medical history data, and routine blood test values for patients and controls are presented in Supplementary Table S1. Despite the fact that the patients had more comorbidities than the controls (2 vs. 1.3, respectively, p = 0.046), there were no other significant differences in the above parameters. Therefore, the two populations can be considered matched. The patient group median age was 71 years (IQR 60–79 years) and included 62 male patients (63.3%). The median BMI was 26.6 (IQR 25–28.9), and 39 patients (47.6%) were active smokers. The most common comorbidities were hypertension (52%), heart disease (24.5%), and thyroid disease (17.3%). Blood test results revealed a median hemoglobin value of 11.9 (IQR 10.2–13.7).
3.2. Patients and Tumor Characteristics
In patients with CRC, the tumor was located in the left colon, rectum, or right colon in 30.3%, 31.4% and 37.2% of cases, respectively. One case had synchronous colon cancer in the left and right colon. All ninety-eight cases were adenocarcinomas, seven of which were characterized as mucinous adenocarcinomas according to the WHO classification [26]. The majority of the histology reports showed T3 tumors (59.1%), with 18.2% having a T2 tumor, while 5 patients (5.7%) had T in situ (Tis). Lymph node involvement was observed in 44% of patients, with 31.9% categorized as N1 and 12.1% categorized as N2. Tumor cells had intermediate differentiation in 70.9% of patients and high in 23.3% (Table 1). According to the TNM staging system, most patients were staged as stage 2 or 3 (18.0% and 59.0%, respectively), while a rather small percentage (4.0%) had metastases (stage 4). At the last follow up, 70/98 (80.2%) of patients were alive.

Table 1.
Collective presentation of patient description and tumor characteristics (%). The tumor differentiation was characterized as low, intermediate and high. The TNM classification was used for staging of the cancer.
3.3. Number of Microvesicles in Patients and Controls
The MV counts are presented in Table 2 and Figure 1A–D. The total number of MVs was found to be statistically significantly higher in the patients than in the controls. In addition, TF-positive MVs, MUC-1-positive MVs, and EMV counts were also found to be higher in the patients compared to the controls (all p < 0.001). The patients’ MUC-1-positive MVs and EMVs co-expressed TF (all p < 0.001). Notably, the counts of PMVs and PS-positive or -negative PMVs did not differ significantly between patients and controls. MVs that could not be characterized by the antibodies used in this study, termed unknown microvesicles (uMVs), were lower in patients compared to controls (p = 0.008).

Table 2.
Number of microvesicles in patients’ plasma and in controls. Numbers of MVs are presented as median values with (q1 and q3 quartiles). Statistically significant values are shown in bold.
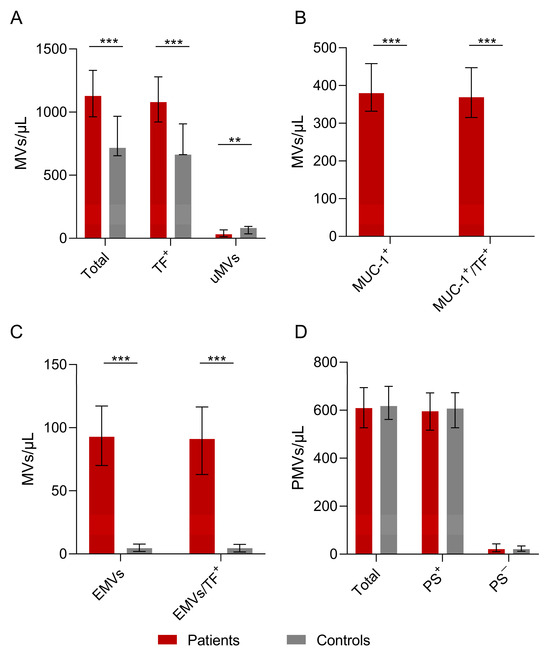
Figure 1.
The number of microvesicles in patients’ plasma versus controls. (A) The total number of patients’ MVs vs. controls. The numbers of total MVs and TF-positive MVs were higher in patients vs. controls (p = 0.000025 vs. p = 0.000068). The number of unknown microvesicles (uMVs) was higher in controls than in the patient group (p = 0.008). (B) Numbers of MUC-1-positive and MUC-1/TF combined MVs were increased in the patients compared to the controls (p = 0.000001). (C) Statistically significant differences in EMVs and TF-positive EMVs between patients and controls (D) Total PMVs, PS+ PMVs, and PS− PMVs were not significantly different between patients and controls. Values are shown are medians (bar plots), and error bars represent ± interquartile ranges. *** p < 0.001; ** p < 0.01.
3.4. Microvesicles in Tis-T1-T2 Patients
Patients with early-stage colon cancer (T1-T2) had statistically significant higher numbers of total MVs, TF-positive MVs, EMVs, combined EMV/TF MVs, MUC-1-positive MVs, and combined MUC-1/TF MVs (Figure 2A,B). Patients with a very early stage of colon cancer (Tis-T1) had also a statistically significant difference regarding total MV counts, TF-positive MVs, EMVs, combined EMV/TF MVs, MUC-1-positive MVs, and MUC-1/TF combined MVs (all p < 0.001) (Figure 2C,D).
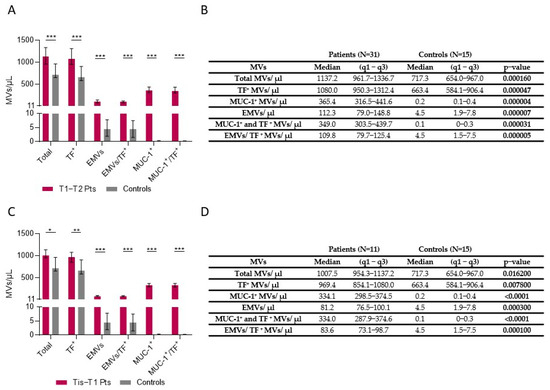
Figure 2.
Number of MVs in plasma of Tis-T1-T2 patients vs. controls. (A) Patients with early-stage CRC (T1-T2) had higher numbers of total MVs (p = 0.00016), Tissue Factor (TF)-positive MVs (p = 0.000047), endothelium-positive (p = 0.000007) MVs (EMVs), combined EMV/TF MVs (p = 0.000005), MUC-1 positive MVs (p = 0.000004), and MUC-1/TF combined MVs (p = 0.000031) vs. controls. (B) MV counts in patients with stages T1 and T2 vs. controls. (C) Patients with a very early stage (Tis) of colon cancer (Tis-T1) also had higher numbers of total MVs, TF-positive MVs, EMVs, combined EMV/TF MVs, MUC-1-positive MVs, and combined MUC-1/TF MVs compared to controls (all p < 0.05). (D) MVs in patients with Tis and stage T1 compared to controls. Values shown in graphs are medians (bar plots), and error bars represent ± interquartile ranges. *** p < 0.001, ** p < 0.01, and * p < 0.05.
3.5. Microvesicles, Cancer Differentiation, and Lymph Node Invasion
As only 5.8% of patients had a low level of tumor differentiation (Table 1), we compared cases with high differentiation with those with intermediate or low levels of differentiation (combined). According to our results, patients with highly differentiated tumors had lower counts of MUC-1-positive MVs (p = 0.027), EMVs (p = 0.0027), and combined EMV/TF MVs (p = 0.0018) compared to those with low/intermediate differentiated tumors (Figure 3A). MV levels in relation to tumor size Tis, T1-4 are shown in Figure 3B. Patients with tumor lymph node invasion had lower counts of EMV/TF MVs bearing MVs as well as uMVs (Figure 3C).
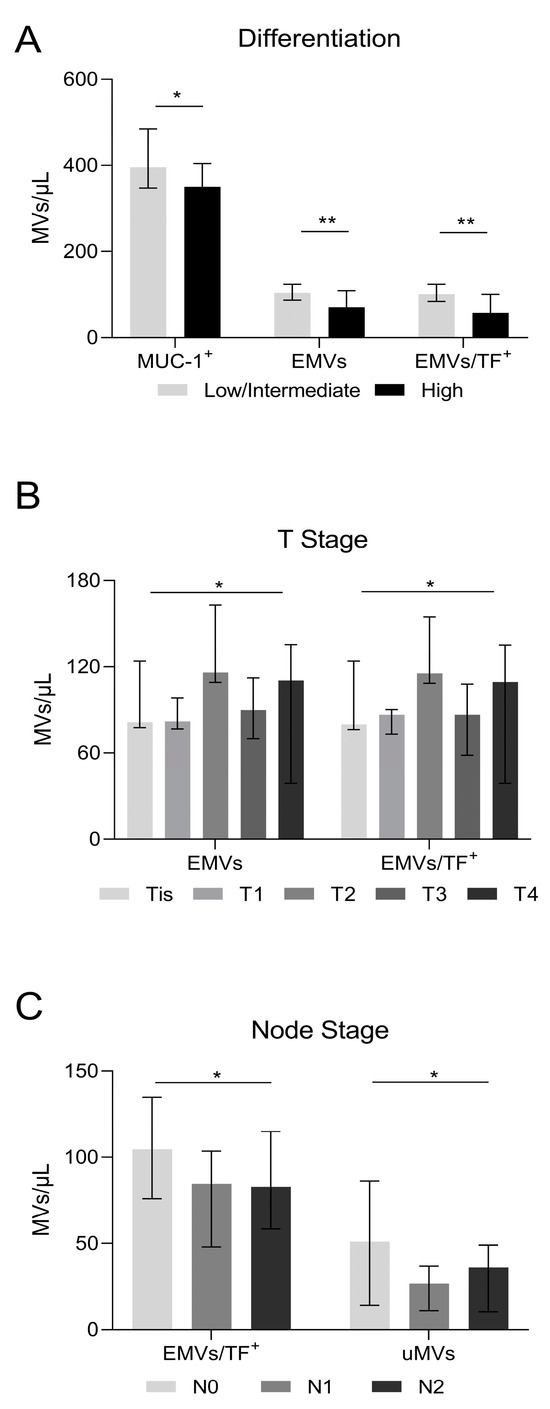
Figure 3.
Microvesicle number in relation to cancer differentiation and lymph node invasion. (A) Highly differentiated tumors had lower MUC-1-positive MVs (350/μL vs. 418/μL in low/intermediate, p = 0.027); endothelial cell-derived microvesicles (EMVs) (70/μL vs. 115/μL in low/intermediate, p = 0.0027), and combined EMV/TF MVs (57/μL vs. 110/μL in low/intermediate, p = 0.0018). (B) MVs in relation to the tumor size. EMVs detected either alone or in combination with TF-positive MVs showed differences (Tis patients: EMVs = 81.2/μL and EMV/TF MVs = 79.7/μL; T1 patients: EMVs = 81.8/μL and EMV/TF MVs = 86.4/μL; T2 patients: EMVs = 116/μL and EMV/TF MVs = 115.4/μL; T3 patients: EMVs = 90.1/μL and EMV/TF MVs = 86.4/μL; T4 patients: EMVs = 110.4/μL and EMV/TF MVs = 109.4/μL; p = 0.04 and p = 0.02, respectively). (C) Patients with lymph node invasion had lower counts of EMV/TF combined positive MVs and uMVs. (Node = 0: EMV/TF MVs = 104.7/μL and uMVs 51.1/μL; Node = 1: EMV/TF MVs = 84.7/μL and uMVs 26.8/μL; Node = 2: EMV/TF MVs = 82.9/μL and uMVs 36.2/μL; p = 0.03 and p = 0.04, respectively). Values are medians (bar plots), and error bars represent a ± interquartile range. ** p < 0.01, and * p < 0.05.
3.6. Microvesicles and Patient Survival
Regarding patient survival, 19 patients died, with a mean time to death of 4.5 years (SE: 0.1 years) and with no difference between men and women (similar survival distribution functions)(log-rank test p = 0.9669; for men: mean time to death—4.6 years and SE—0.2 years; for women: mean time to death—3.7 years and SE—0.2 years). The univariate log-rank test for the role of each type of MV identified and assessed in our study as well as patient age showed that none of these variables had a significant role in patient survival. Comparing data between the 19 patients who died and those who survived, PS-negative PMV and uMV levels were lower in patients who died (p = 0.0317 and p = 0.0254 respectively, Figure 4).
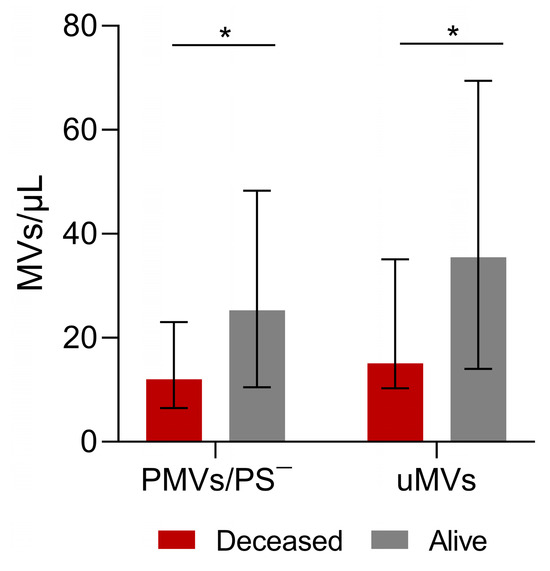
Figure 4.
Analysis of microvesicles and blood test measurements in surviving and deceased patients. Phosphatidylserine (PS)-negative platelet MVs (PMVs) and unknown MVs in patients who survived and patients who died. Patients who remained alive had more PS-negative PMVs and more uMVs. PMVs: Alive—25.2/μL; Deceased—12.0/μL; p = 0.03. unMVs: Alive—35.5/μL; Deceased—15.1/μL; p = 0.02). Values are medians (bar plots), and error bars represent ± interquartile ranges. * p < 0.05.
4. Discussion
In cancer patients, microvesicles arise from multiple cellular sources within the tumor microenvironment and play crucial roles in cancer pathogenesis and progression [27,28]. Microvesicles in cancer patients can originate from both tumor cells and non-tumor cells [29,30,31]. Tumor-derived microvesicles are directly released by cancer cells and carry specific molecular signatures reflective of the tumor’s genetic and phenotypic characteristics [29,32,33,34]. On the other hand, microvesicles released by non-tumor cells in response to the tumor microenvironment represent the body’s reaction to the tumor [32,33,35,36].
In this prospective study, we identified and assessed MV levels in the blood of CRC patients at diagnosis and compared the results with a control group. We also investigated the possible association of MVs with survival in these patients. The two populations were matched, as stated in the results section, to control for an important confounding factor, which was age-related decline in MV concentration [37].
Our study demonstrated that prior to any treatment, the CRC patients had higher plasma levels of total MVs, TF-positive MVs, MUC-1-positive MVs, and EMVs than the controls (p < 0.001). PMV levels were not significantly different between the two groups (patients vs. controls). Our results are also supported by findings from Zhao et al. [38], who showed significantly higher plasma levels of PS-positive MVs and EMVs in colon cancer patients at all stages compared to a control group. They also found statistically significantly higher levels of PS-positive PMVs in colon cancer patients, which is not supported by our data. This may be due to the different methods used to isolate PS-rich cells and MVs. In our study, we used Annexin V, whereas Zhao et al. [38] used lactadherin, which has been shown to be more effective in isolating MVs [39,40]. Eddama et al., 2022, similarly demonstrated elevated levels of MVs in CRC patients, supporting our data and further pointing out the role of MVs as a potential biomarker for early disease detection [21].
According to our study, patients with early-stage colorectal cancer (T1-T2) as well as patients with very early-stage colorectal cancer (Tis-T1) had higher total numbers of MVs, EMVs, MUC-1-positive MVs, TF-positive MVs, EMV/TF combined MVs, and MUC-1/TF combined MVs compared to controls. To the best of our knowledge, this is the first study to show statistically significant differences in MV levels in patients at a very early stage of colorectal cancer versus controls. This novel finding could indicate a possible diagnostic biomarker that may allow for the early detection of tumors.
MVs released by tumor cells (TMVs) may be of paramount importance for diagnostic and therapeutic modalities but are estimated to represent only 1% of the host’s MVs [7]. MUC-1 is a tumor marker overexpressed in colon cancer [41,42], and our data show that MUC-1 positive MVs are statistically significantly increased in CRC patients, even in the early stages of the disease. For these reasons, MUC-1 may be a promising early biomarker or therapeutic target [43,44,45]. This has also been suggested by other studies. Stec et al. [46] found that MVs from CRC patients expressed tumor markers such as HER-2/neu, MUC-1, and EGFR at much higher levels than those from healthy individuals. In addition, EGFR expression was detected by Western blotting and not by flow cytometry, probably because it is not expressed on the surface of the MV [46].
TF-positive MVs, which are associated with an increased risk of thrombosis in cancer patients due to their procoagulant properties [47], were found at significantly higher levels in CRC patients in our study as well as others [38,48,49,50]. Hisada et al. [50] also found that patients with adenocarcinoma had statistically significantly higher levels of TF-positive MVs than subjects with other cancer types, possibly due to high levels of expression of TF on the surfaces of epithelial cells. They also showed that cancer patients with high levels of TF MV activity had better prognoses than those with low levels (p < 0.0433) [50].
Our results show that highly differentiated tumors had fewer MUC-1-positive MVs, EMVs, and combined EMV/TF microvesicles than ones with intermediate/low differentiation. Furthermore, our results support the role of PS-negative PMVs (p = 0.0317) and the small count of uMVs in patient survival (p = 0.0254) (Figure 4). The significance of PS-negative MVs is still unclear; however, it is possible that they also play other roles beyond procoagulant phospholipid activity [51]. The level of circulating MVs could be used as a tumor indicator as it correlates with poor prognosis parameters and shorter survival [52]. Helley et al. [53] found that baseline levels of PMVs were significantly associated with survival in prostate cancer patients.
EMVs have been found to be elevated in metastatic colorectal cancer patients, and Nanou et al. [54] even highlighted their role as predictors of overall survival that could contribute to decision making. They are released in stages II, III, and IV of CRC during VEGF-stimulation mediated endothelial proliferation and tumor proliferation [39,55,56]. In this context, Zhao et al. [38] found increased levels of EMVs in colon cancer patients with stage II, III, or IV cancer but not in stage I. However, this finding could not be confirmed in our study due to the small number of metastatic colon cancer patients included.
The study was constrained by a relatively small number of cases, which may restrict the generalizability of our findings to broader populations. The relatively small sizes of the two groups, however well defined (patients and controls), were primarily due to logistical challenges in data recruitment and collection. Accordingly, our study predominantly focused on cases with familiar histology and specific molecular profiles, potentially limiting the applicability of our findings to other histologic subtypes or molecular subgroups of the disease.
With continued improvements in isolation techniques, biomarker discovery, and validation studies, microvesicles hold significant promise as possible non-invasive biomarkers for cancer diagnosis and prognosis in the future [30,57,58]. Overall, resolving these issues and addressing associated issues requires interdisciplinary collaboration, rigorous validation studies, and concerted efforts to standardize methodologies and promote transparency in reporting [59,60]. By overcoming these challenges, the field of MV research could realize its full potential in advancing precision medicine and improving cancer diagnosis, prognosis, and treatment [61,62,63,64].
5. Conclusions
In conclusion, our study reveals that CRC patients present at diagnosis with elevated levels of MVs, namely total MVs, MUC-1-positive MVs, TF-positive MVs, and EMVs, with statistical significance compared to controls, even in very early stages of the disease. As the identification of tumor indicators to detect the presence of disease using noninvasive diagnostic procedures plays a pivotal role in cancer research, our findings suggest that analyzing circulating MV levels in plasma could serve as a valuable tool for the early diagnosis of colorectal cancer, particularly in the initial stage of the disease. However, standardizing isolation methods, and the establishment of robust biomarker panels along with additional studies encompassing a broader spectrum of histologic and molecular phenotypes are warranted to comprehensively confirm our findings and the clinical relevance of microvesicle profiles in CRC patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16101943/s1, Table S1: Demographic data, medical history data and blood test information, for patients and controls. Numerical data are presented as median (q1 and q3 quartiles), and categorical data as corresponding percentages. p values, are presented (*). Statistically significant values are shown in bold.
Author Contributions
Conceptualization, S.V., A.G.K., M.P. and D.D.; methodology, A.G.K., A.P. (Aspasia Papailia), C.D., H.T.G., N.G. and C.S.; software, A.P. (Abraham Pouliakis), E.G.P.; validation, S.V.; investigation, L.C., S.V. and A.G.K.; resources, M.P.; data curation, I.P., D.D., G.T., T.P., S.V. and C.S.; writing—original draft preparation, L.C., S.P.F., S.V. and A.G.K.; writing—review and editing, S.V., D.D., A.G.K., G.T. and T.P. visualization, S.V.; supervision, D.D., I.P., M.P. and S.V.; project administration, M.P.; funding acquisition, M.P. and D.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from National and Kapodistrian University of Athens Medical School the “MSc in Thrombosis–Bleeding-Transfusion Medicine” Director Professor Marianna Politou, and the “MSc in Minimally Invasive Surgery, Robotic Surgery and Telesurgery” Director Professor Dimitrios Dimitoulis.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board and Ethics Committee of the National and Kapodistrian University of Athens (ethic approve number: φ-28/20-06-2013, Approve date: 20 June 2013).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon reasonable request.
Acknowledgments
The authors would like to thank all blood donors, patients, and healthy subjects who participated in the present study.
Conflicts of Interest
The authors state that they have no conflicts of interest.
References
- Georgatzakou, H.T.; Fortis, S.P.; Papageorgiou, E.G.; Antonelou, M.H.; Kriebardis, A.G. Blood Cell-Derived Microvesicles in Hematological Diseases and beyond. Biomolecules 2022, 12, 803. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.F.; Li, W.J.; Hu, K.S.; Gao, J.; Zhai, W.L.; Yang, J.H.; Zhang, S.J. Exosome biogenesis: Machinery, regulation, and therapeutic implications in cancer. Mol. Cancer 2022, 21, 207. [Google Scholar] [CrossRef] [PubMed]
- Santavanond, J.P.; Rutter, S.F.; Atkin-Smith, G.K.; Poon, I.K.H. Apoptotic Bodies: Mechanism of Formation, Isolation and Functional Relevance. Sub-Cell Biochem. 2021, 97, 61–88. [Google Scholar] [CrossRef] [PubMed]
- Trifylli, E.M.; Kriebardis, A.G.; Koustas, E.; Papadopoulos, N.; Fortis, S.P.; Tzounakas, V.L.; Anastasiadi, A.T.; Sarantis, P.; Vasileiadi, S.; Tsagarakis, A.; et al. A Current Synopsis of the Emerging Role of Extracellular Vesicles and Micro-RNAs in Pancreatic Cancer: A Forward-Looking Plan for Diagnosis and Treatment. Int. J. Mol. Sci. 2024, 25, 3406. [Google Scholar] [CrossRef] [PubMed]
- Trifylli, E.M.; Kriebardis, A.G.; Koustas, E.; Papadopoulos, N.; Vasileiadi, S.; Fortis, S.P.; Tzounakas, V.L.; Anastasiadi, A.T.; Sarantis, P.; Papageorgiou, E.G.; et al. The Arising Role of Extracellular Vesicles in Cholangiocarcinoma: A Rundown of the Current Knowledge Regarding Diagnostic and Therapeutic Approaches. Int. J. Mol. Sci. 2023, 24, 15563. [Google Scholar] [CrossRef] [PubMed]
- Ender, F.; Von Bubnoff, N.; Gieseler, F. Extracellular Vesicles: Subcellular Organelles with the Potential to Spread Cancer Resistance. Anticancer. Res. 2019, 39, 3395–3404. [Google Scholar] [CrossRef] [PubMed]
- Rak, J. Extracellular vesicles—Biomarkers and effectors of the cellular interactome in cancer. Front. Pharmacol. 2013, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Abdouh, M.; Floris, M.; Gao, Z.H.; Arena, V.; Arena, M.; Arena, G.O. Colorectal cancer-derived extracellular vesicles induce transformation of fibroblasts into colon carcinoma cells. J. Exp. Clin. Cancer Res. 2019, 38, 257. [Google Scholar] [CrossRef] [PubMed]
- Shegekar, T.; Vodithala, S.; Juganavar, A. The Emerging Role of Liquid Biopsies in Revolutionising Cancer Diagnosis and Therapy. Cureus 2023, 15, e43650. [Google Scholar] [CrossRef]
- Palanca-Ballester, C.; Rodriguez-Casanova, A.; Torres, S.; Calabuig-Farinas, S.; Exposito, F.; Serrano, D.; Redin, E.; Valencia, K.; Jantus-Lewintre, E.; Diaz-Lagares, A.; et al. Cancer Epigenetic Biomarkers in Liquid Biopsy for High Incidence Malignancies. Cancers 2021, 13, 3016. [Google Scholar] [CrossRef]
- Gao, T.; Wen, T.; Ge, Y.; Liu, J.; Yang, L.; Jiang, Y.; Dong, X.; Liu, H.; Yao, J.; An, G. Disruption of Core 1-mediated O-glycosylation oppositely regulates CD44 expression in human colon cancer cells and tumor-derived exosomes. Biochem. Biophys. Res. Commun. 2020, 521, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Soldevilla, B.; Rodriguez, M.; San Millan, C.; Garcia, V.; Fernandez-Perianez, R.; Gil-Calderon, B.; Martin, P.; Garcia-Grande, A.; Silva, J.; Bonilla, F.; et al. Tumor-derived exosomes are enriched in DeltaNp73, which promotes oncogenic potential in acceptor cells and correlates with patient survival. Hum. Mol. Genet. 2014, 23, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Menck, K.; Sivaloganathan, S.; Bleckmann, A.; Binder, C. Microvesicles in Cancer: Small Size, Large Potential. Int. J. Mol. Sci. 2020, 21, 5373. [Google Scholar] [CrossRef] [PubMed]
- Logozzi, M.; Orefice, N.S.; Di Raimo, R.; Mizzoni, D.; Fais, S. The Importance of Detecting, Quantifying, and Characterizing Exosomes as a New Diagnostic/Prognostic Approach for Tumor Patients. Cancers 2023, 15, 2878. [Google Scholar] [CrossRef]
- Irmer, B.; Chandrabalan, S.; Maas, L.; Bleckmann, A.; Menck, K. Extracellular Vesicles in Liquid Biopsies as Biomarkers for Solid Tumors. Cancers 2023, 15, 1307. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Batagov, A.O.; Schinelli, S.; Wang, J.; Wang, Y.; El Fatimy, R.; Rabinovsky, R.; Balaj, L.; Chen, C.C.; Hochberg, F.; et al. Coding and noncoding landscape of extracellular RNA released by human glioma stem cells. Nat. Commun. 2017, 8, 1145. [Google Scholar] [CrossRef] [PubMed]
- Schmidtmann, M.; D’Souza-Schorey, C. Extracellular Vesicles: Biological Packages That Modulate Tumor Cell Invasion. Cancers 2023, 15, 5617. [Google Scholar] [CrossRef] [PubMed]
- Chitoiu, L.; Dobranici, A.; Gherghiceanu, M.; Dinescu, S. Costache, MMulti-Omics Data Integration in Extracellular Vesicle Biology-Utopia or Future Reality? Int. J. Mol. Sci. 2020, 21, 8550. [Google Scholar] [CrossRef] [PubMed]
- Pinzani, P.; D’Argenio, V.; Del Re, M.; Pellegrini, C.; Cucchiara, F.; Salvianti, F.; Galbiati, S. Updates on liquid biopsy: Current trends and future perspectives for clinical application in solid tumors. Clin. Chem. Lab. Med. 2021, 59, 1181–1200. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Eddama, M.M.R.; Gurung, R.; Fragkos, K.; Lorgelly, P.; Cohen, R.; Loizidou, M.; Clapp, L. The role of microvesicles as biomarkers in the screening of colorectal neoplasm. Cancer Med. 2022, 11, 2957–2968. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Wei, Y.; Jia, Q.; Chen, J.; Chen, T.; Yuan, J.; Pi, C.; Liu, H.; Tang, J.; Yin, S.; et al. The application of extracellular vesicles in colorectal cancer metastasis and drug resistance: Recent advances and trends. J. Nanobiotechnol. 2023, 21, 143. [Google Scholar] [CrossRef]
- Kotelevets, L.; Chastre, E. Extracellular Vesicles in Colorectal Cancer: From Tumor Growth and Metastasis to Biomarkers and Nanomedications. Cancers 2023, 15, 1107. [Google Scholar] [CrossRef] [PubMed]
- Hargett, L.A.; Bauer, N.N. On the origin of microparticles: From “platelet dust” to mediators of intercellular communication. Pulm. Circ. 2013, 3, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Kriebardis, A.G.; Antonelou, M.H.; Georgatzakou, H.T.; Tzounakas, V.L.; Stamoulis, K.E.; Papassideri, I.S. Microparticles variability in fresh frozen plasma: Preparation protocol and storage time effects. Blood Transfus. 2016, 14, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Bosman, F.; Carneiro, F.; Hruban, R.; Theise, N. WHO Classification of Tumours of the Digestive System, 4th ed.; International Agency for Research on Cancer (IARC Publications): Lyon, France, 2010. [Google Scholar]
- Yamada, N.; Nakagawa, Y.; Tsujimura, N.; Kumazaki, M.; Noguchi, S.; Mori, T.; Hirata, I.; Maruo, K.; Akao, Y. Role of Intracellular and Extracellular MicroRNA-92a in Colorectal Cancer. Transl. Oncol. 2013, 6, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, Y.; Wang, Y.; Xu, S.; Jiang, F.; Han, Y.; Hu, M.; Liu, Z. Platelet-derived microvesicles (PMVs) in cancer progression and clinical applications. Clin. Transl. Oncol. 2023, 25, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.G.; Grizzle, W.E. Exosomes: A novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am. J. Pathol. 2014, 184, 28–41. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef]
- Tkach, M.; Thery, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef]
- Peinado, H.; Aleckovic, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; Garcia-Santos, G.; Ghajar, C.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef]
- Galander, S.; Barton, R.E.; Borek, W.E.; Spanos, C.; Kelly, D.A.; Robertson, D.; Rappsilber, J.; Marston, A.L. Reductional Meiosis I Chromosome Segregation Is Established by Coordination of Key Meiotic Kinases. Dev. Cell 2019, 49, 526–541.e525. [Google Scholar] [CrossRef]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H.; et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015, 17, 816–826. [Google Scholar] [CrossRef]
- Eitan, E.; Green, J.; Bodogai, M.; Mode, N.A.; Baek, R.; Jorgensen, M.M.; Freeman, D.W.; Witwer, K.W.; Zonderman, A.B.; Biragyn, A.; et al. Age-Related Changes in Plasma Extracellular Vesicle Characteristics and Internalization by Leukocytes. Sci. Rep. 2017, 7, 1342. [Google Scholar] [CrossRef]
- Zhao, L.; Bi, Y.; Kou, J.; Shi, J.; Piao, D. Phosphatidylserine exposing-platelets and microparticles promote procoagulant activity in colon cancer patients. J. Exp. Clin. Cancer Res. 2016, 35, 54. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, C.; Simiantonaki, N.; Habedank, S.; Kirkpatrick, C.J. The relevance of cell type- and tumor zone-specific VEGFR-2 activation in locally advanced colon cancer. J. Exp. Clin. Cancer Res. 2015, 34, 42. [Google Scholar] [CrossRef][Green Version]
- Dasgupta, S.K.; Guchhait, P.; Thiagarajan, P. Lactadherin binding and phosphatidylserine expression on cell surface-comparison with annexin A5. Transl. Res. 2006, 148, 19–25. [Google Scholar] [CrossRef]
- Labouvie, C.; Machado, J.; Carneiro, F.; Seitz, G.; Blin, N. Expression pattern of gastrointestinal markers in native colorectal epithelium, lesions, and carcinomas. Oncol. Rep. 1997, 4, 1367–1371. [Google Scholar] [CrossRef]
- Li, C.; Liu, T.; Yin, L.; Zuo, D.; Lin, Y.; Wang, L. Prognostic and clinicopathological value of MUC1 expression in colorectal cancer: A meta-analysis. Medicine 2019, 98, e14659. [Google Scholar] [CrossRef] [PubMed]
- Bose, M.; Mukherjee, P. Potential of Anti-MUC1 Antibodies as a Targeted Therapy for Gastrointestinal Cancers. Vaccines 2020, 8, 659. [Google Scholar] [CrossRef]
- Qing, L.; Li, Q.; Dong, Z. MUC1: An emerging target in cancer treatment and diagnosis. Bull. Du Cancer 2022, 109, 1202–1216. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Z.; Zhang, S.; Zhu, P.; Ko, J.K.; Yung, K.K. MUC1: Structure, Function, and Clinic Application in Epithelial Cancers. Int. J. Mol. Sci. 2021, 22, 6567. [Google Scholar] [CrossRef]
- Stec, M.; Baj-Krzyworzeka, M.; Baran, J.; Weglarczyk, K.; Zembala, M.; Barbasz, J.; Szczepanik, A.; Zembala, M. Isolation and characterization of circulating micro(nano)vesicles in the plasma of colorectal cancer patients and their interactions with tumor cells. Oncol. Rep. 2015, 34, 2768–2775. [Google Scholar] [CrossRef]
- Manly, D.A.; Wang, J.; Glover, S.L.; Kasthuri, R.; Liebman, H.A.; Key, N.S.; Mackman, N. Increased microparticle tissue factor activity in cancer patients with Venous Thromboembolism. Thromb. Res. 2010, 125, 511–512. [Google Scholar] [CrossRef] [PubMed]
- Hron, G.; Kollars, M.; Weber, H.; Sagaster, V.; Quehenberger, P.; Eichinger, S.; Kyrle, P.A.; Weltermann, A. Tissue factor-positive microparticles: Cellular origin and association with coagulation activation in patients with colorectal cancer. Thromb. Haemost. 2007, 97, 119–123. [Google Scholar] [CrossRef]
- Ender, F.; Freund, A.; Quecke, T.; Steidel, C.; Zamzow, P.; von Bubnoff, N.; Gieseler, F. Tissue factor activity on microvesicles from cancer patients. J. Cancer Res. Clin. Oncol. 2020, 146, 467–475. [Google Scholar] [CrossRef]
- Hisada, Y.; Thalin, C.; Lundstrom, S.; Wallen, H.; Mackman, N. Comparison of microvesicle tissue factor activity in non-cancer severely ill patients and cancer patients. Thromb. Res. 2018, 165, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Connor, D.E.; Exner, T.; Ma, D.D.; Joseph, J.E. The majority of circulating platelet-derived microparticles fail to bind annexin V, lack phospholipid-dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thromb. Haemost. 2010, 103, 1044–1052. [Google Scholar] [CrossRef]
- Silva, J.; Garcia, V.; Rodriguez, M.; Compte, M.; Cisneros, E.; Veguillas, P.; Garcia, J.M.; Dominguez, G.; Campos-Martin, Y.; Cuevas, J.; et al. Analysis of exosome release and its prognostic value in human colorectal cancer. Genes. Chromosomes Cancer 2012, 51, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Helley, D.; Banu, E.; Bouziane, A.; Banu, A.; Scotte, F.; Fischer, A.M.; Oudard, S. Platelet microparticles: A potential predictive factor of survival in hormone-refractory prostate cancer patients treated with docetaxel-based chemotherapy. Eur. Urol. 2009, 56, 479–484. [Google Scholar] [CrossRef]
- Nanou, A.; Miller, M.C.; Zeune, L.L.; de Wit, S.; Punt, C.J.A.; Groen, H.J.M.; Hayes, D.F.; de Bono, J.S.; Terstappen, L. Tumour-derived extracellular vesicles in blood of metastatic cancer patients associate with overall survival. Br. J. Cancer 2020, 122, 801–811. [Google Scholar] [CrossRef]
- Thomaidis, T.; Maderer, A.; Formentini, A.; Bauer, S.; Trautmann, M.; Schwarz, M.; Neumann, W.; Kittner, J.M.; Schad, A.; Link, K.H.; et al. Proteins of the VEGFR and EGFR pathway as predictive markers for adjuvant treatment in patients with stage II/III colorectal cancer: Results of the FOGT-4 trial. J. Exp. Clin. Cancer Res. 2014, 33, 83. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.; Febbraro, A.; Tomaselli, E.; Sarnicola, M.L.; Parcesepe, P.; Parente, D.; Forte, N.; Fabozzi, A.; Remo, A.; Bonetti, A.; et al. Cancer-related CD15/FUT4 overexpression decreases benefit to agents targeting EGFR or VEGF acting as a novel RAF-MEK-ERK kinase downstream regulator in metastatic colorectal cancer. J. Exp. Clin. Cancer Res. 2015, 34, 108. [Google Scholar] [CrossRef]
- Lotvall, J.; Hill, A.F.; Hochberg, F.; Buzas, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef]
- Raimondo, F.; Morosi, L.; Chinello, C.; Magni, F.; Pitto, M. Advances in membranous vesicle and exosome proteomics improving biological understanding and biomarker discovery. Proteomics 2011, 11, 709–720. [Google Scholar] [CrossRef]
- Glasziou, P.; Altman, D.G.; Bossuyt, P.; Boutron, I.; Clarke, M.; Julious, S.; Michie, S.; Moher, D.; Wager, E. Reducing waste from incomplete or unusable reports of biomedical research. Lancet 2014, 383, 267–276. [Google Scholar] [CrossRef]
- Nosek, B.A.; Ebersole, C.R.; DeHaven, A.C.; Mellor, D.T. The preregistration revolution. Proc. Natl. Acad. Sci. USA 2018, 115, 2600–2606. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Vader, P.; Breakefield, X.O.; Wood, M.J. Extracellular vesicles: Emerging targets for cancer therapy. Trends Mol. Med. 2014, 20, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Ihlamur, M.; Kelleci, K.; Zengin, Y.; Allahverdiyev, M.A.; Abamor, E. Applications of Exosome Vesicles in Different Cancer Types as Biomarkers. Curr. Mol. Med. 2024, 24, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Wang, Z.; Ye, Z.; Wang, Y.; Cai, X. Cancer-derived exosomes as novel biomarkers in metastatic gastrointestinal cancer. Mol. Cancer 2024, 23, 67. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).