Efficacy of Cytoreductive Surgery (CRS) + HIPEC in Gastric Cancer with Peritoneal Metastasis: Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
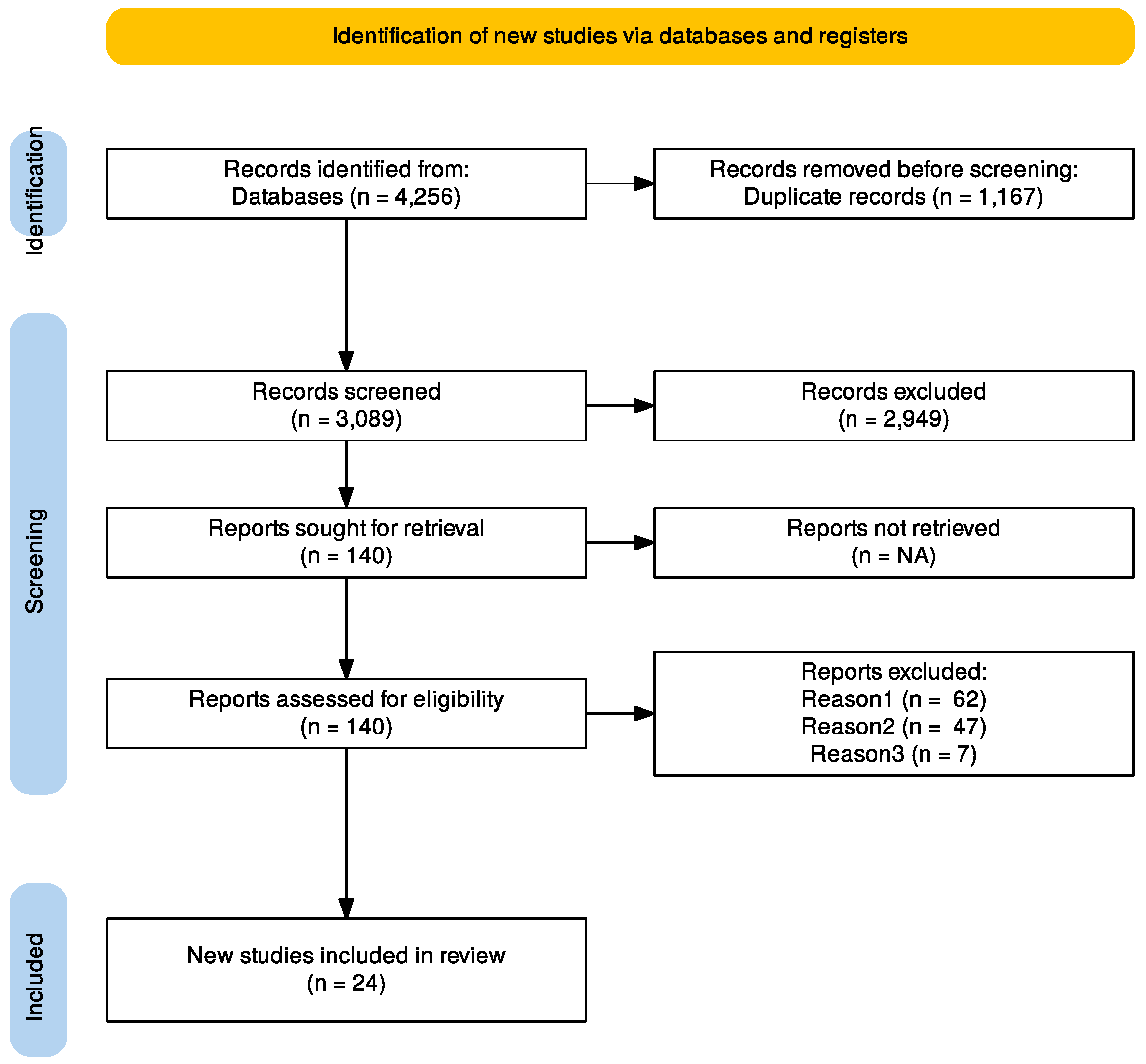
2.1. Data Sources, Search Strategy, and Selection Criteria
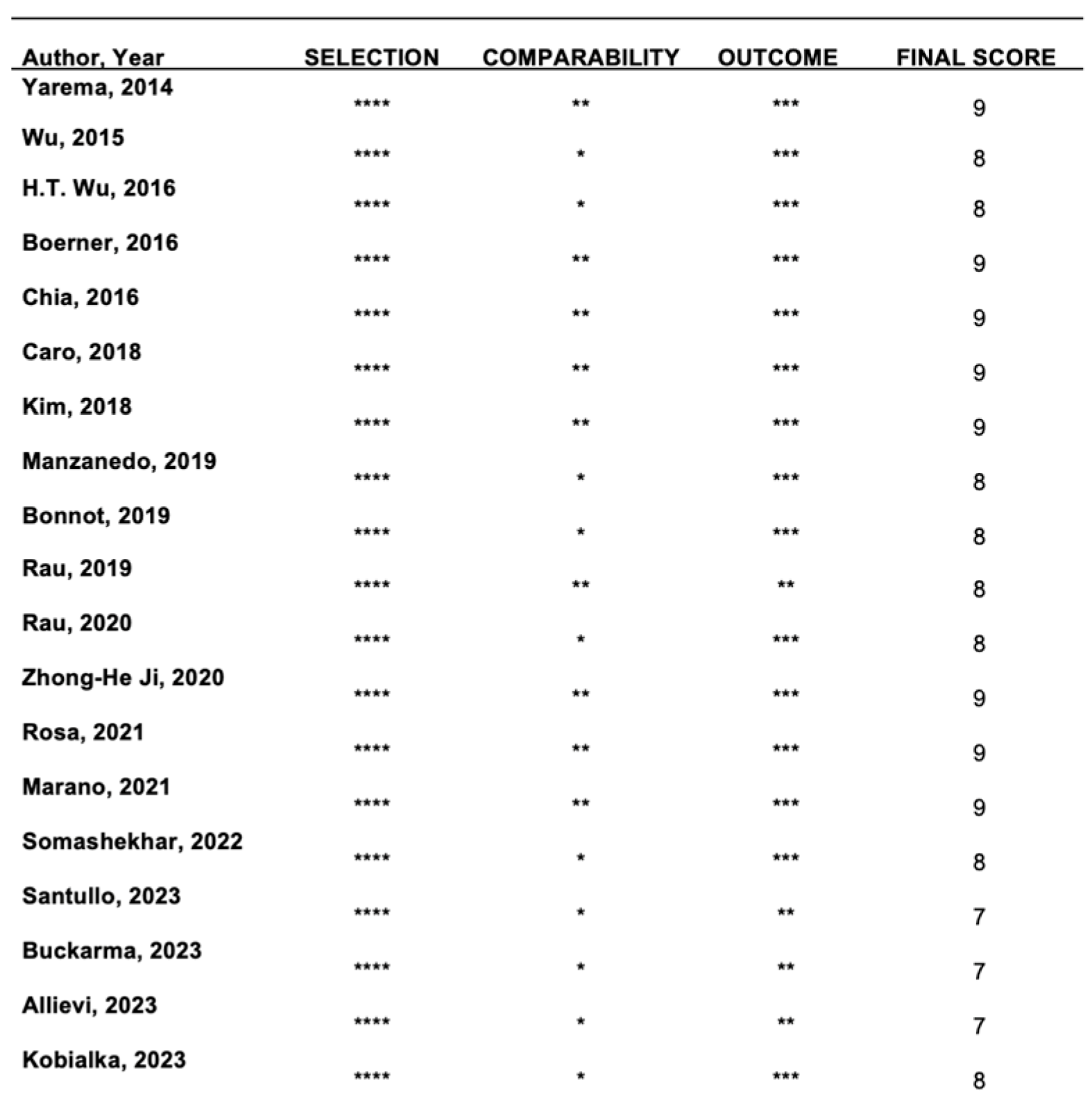
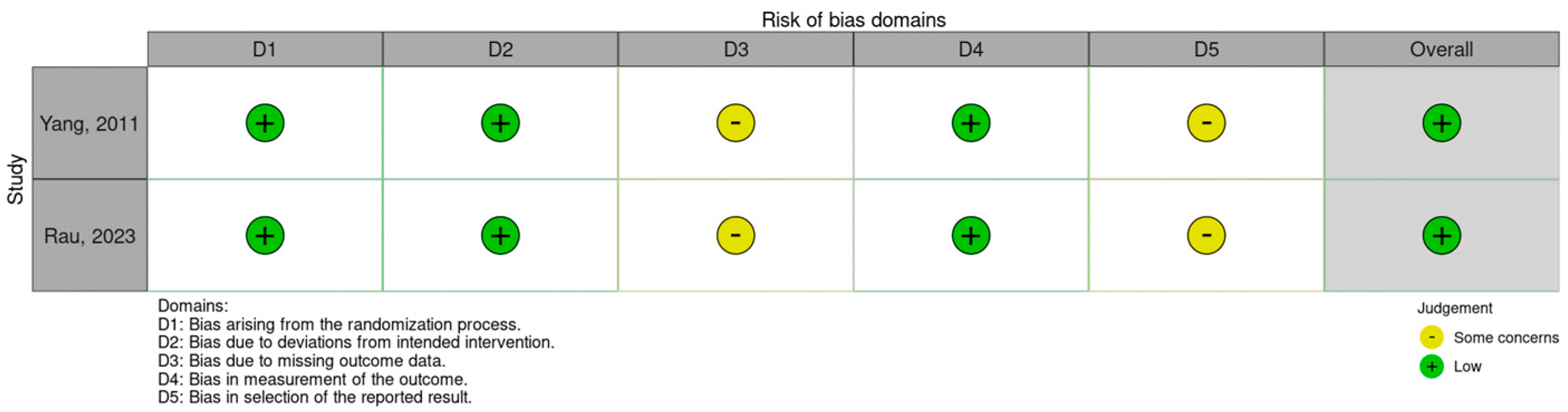
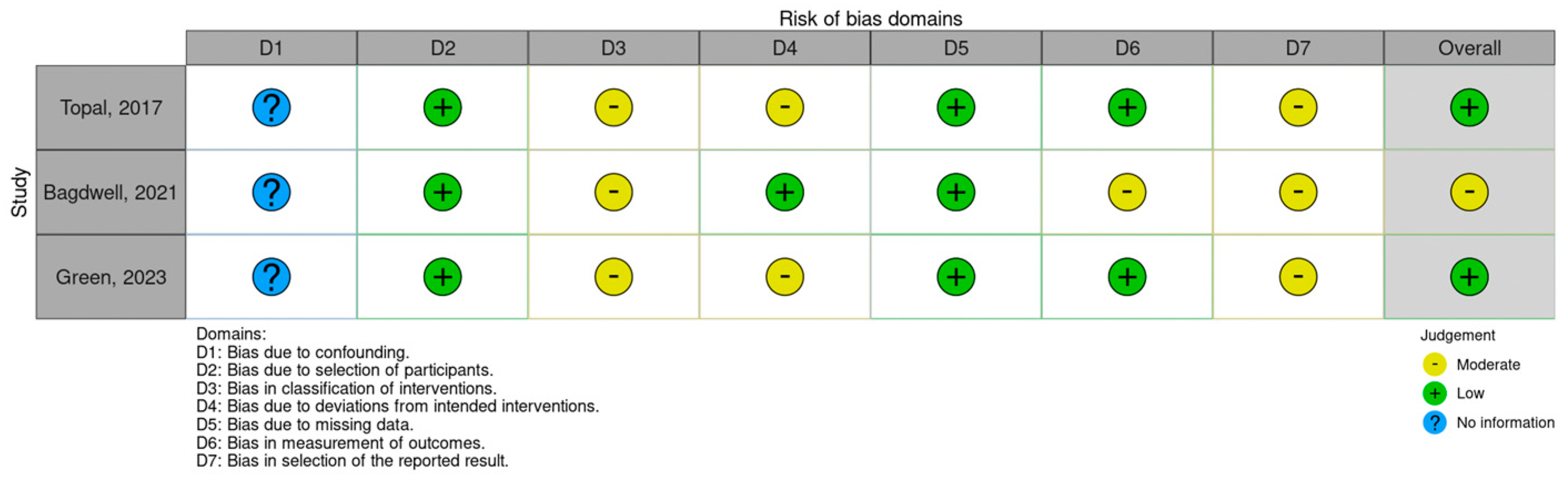
2.2. Study Risk-of-Bias Assessment
2.3. Inclusion and Exclusion Criteria
2.4. Outcomes of Interest
2.5. Statistics
3. Results
3.1. Studies Selection and Patient’s Characteristics
3.2. Studies’ Risk-of-Bias Assessment
3.3. Surgical Treatment
3.4. Survival, Recurrence, and Surgical Outcomes of CRS + HIPEC
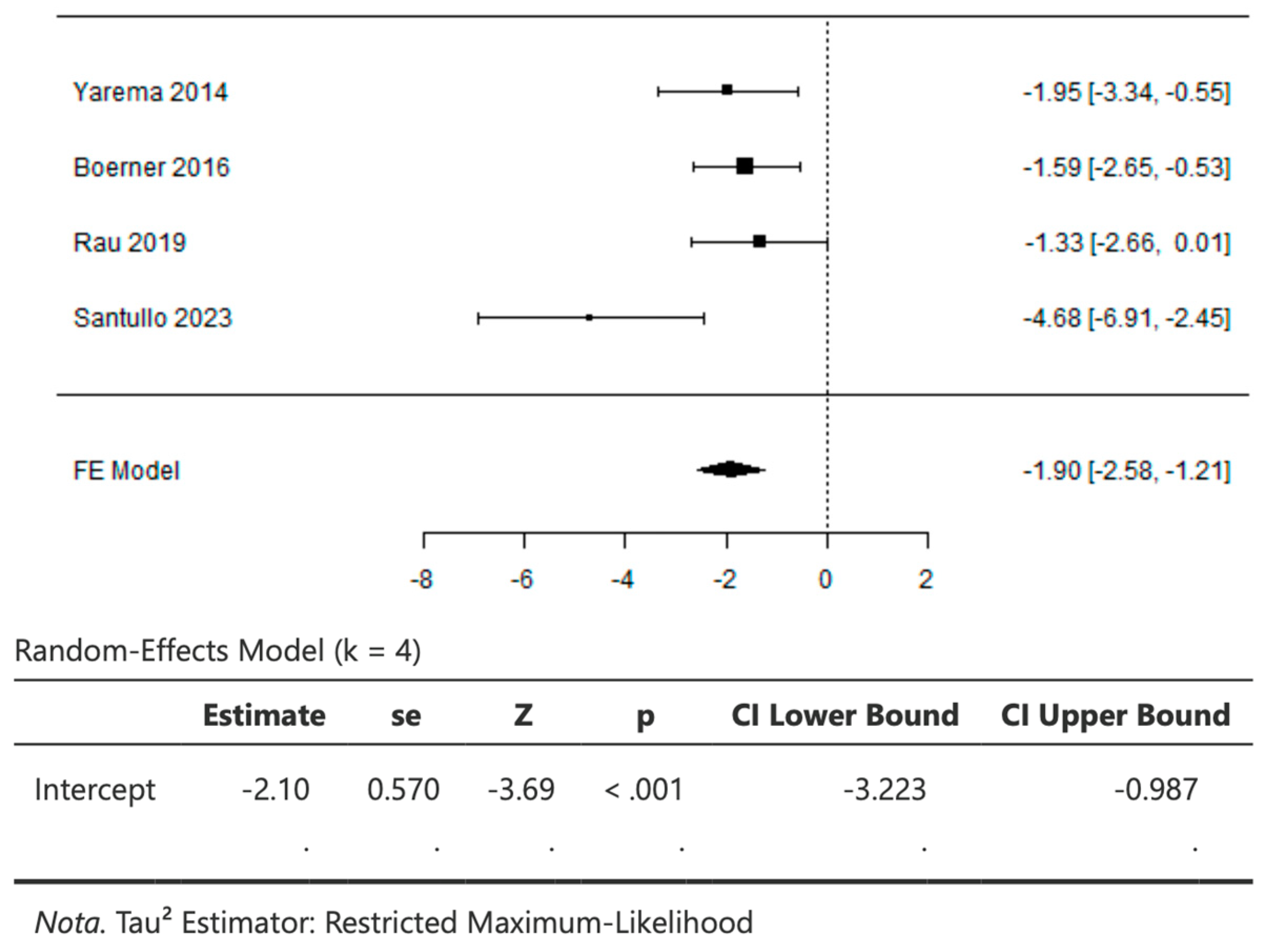
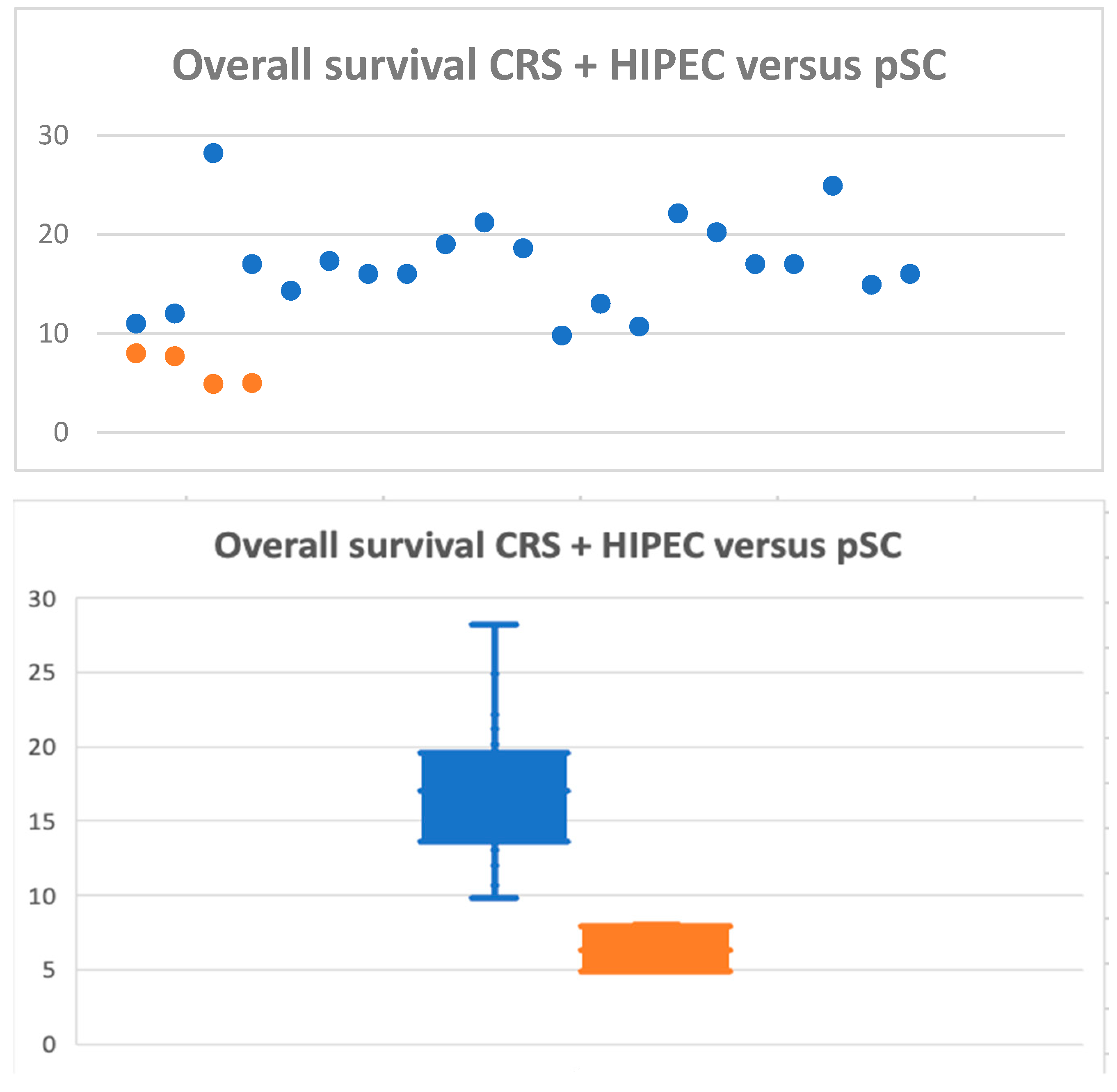
3.5. CRS + HIPEC versus Palliative Chemotherapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef]
- Thomassen, I.; van Gestel, Y.R.; van Ramshorst, B.; Luyer, M.D.; Bosscha, K.; Nienhuijs, S.W.; Lemmens, V.E.; de Hingh, I.H. Peritoneal carcinomatosis of gastric origin: A population-based study on incidence, survival and risk factors. Int. J. Cancer 2014, 134, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Linee Guida AIOM 2019 dei Tumori Peritoneali e Retroperitoneali. Available online: https://www.aiom.it/wp-content/uploads/2019/10/2019_LG_AIOM_Peritoneo.pdf (accessed on 5 March 2024).
- Al-Batran, S.E.; Hartmann, J.T.; Probst, S.; Schmalenberg, H.; Holler-bach, S.; Hofheinz, R.; Rethwisch, V.; Seipelt, G.; Homann, N.; Wilhelm, G.; et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: A study of the Arbeitsgemeinschaft Internistische Onkologie. J. Clin. Oncol. 2008, 26, 1435–1442. [Google Scholar] [CrossRef]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastu-zumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gas-tro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, A.; Santullo, F.; Attalla El Halabieh, M.; Lodoli, C.; Abatini, C.; Alessandra Calegari, M.; Martini, M.; Rotolo, S.; Pacelli, F. Clinical and molecular features in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinosis from colorectal cancer. Gastrointest. Surg. 2021, 25, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Santullo, F.; Pacelli, F.; Abatini, C.; Attalla El Halabieh, M.; Fortunato, G.; Lodoli, C.; Giovinazzo, F.; Rotolo, S.; Di Giorgio, A. Cytoreduction and hyperthermic intraperitoneal chemotherapy for pseudomyxoma peritonei of appendiceal origin: A single center experience. Front. Surg. 2021, 8, 715119. [Google Scholar] [CrossRef]
- Yang, X.J.; Huang, C.Q.; Suo, T.; Mei, L.J.; Yang, G.L.; Cheng, F.L.; Zhou, Y.F.; Xiong, B.; Yonemura, Y.; Li, Y. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: Final results of a phase III randomized clinical trial. Ann. Surg. Oncol. 2011, 18, 1575–1581. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yarema, R.R.; Ohorchak, M.A.; Zubarev, G.P.; Mylyan, Y.P.; Oliynyk, Y.Y.; Zubarev, M.G.; Gyrya, P.I.; Kovalchuk, Y.J.; Safiyan, V.I.; Fetsych, T.G. Hyperthermic intraperitoneal chemoperfusion in combined treatment of locally advanced and disseminated gastric cancer: Results of a single-centre retrospective study. Int. J. Hyperth. 2014, 30, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Z.; Li, Z.; Jia, Y.; Shan, F.; Ji, X.; Bu, Z.; Zhang, L.; Wu, A.; Ji, J. Hyperthermic intraperitoneal chemotherapy plus simultaneous versus staged cytoreductive surgery for gastric cancer with occult peritoneal metastasis. J. Surg. Oncol. 2015, 111, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.T.; Peng, K.W.; Ji, Z.H.; Sun, J.H.; Zhang, Q.; Yang, X.J.; Huang, C.Q.; Li, Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy with lobaplatin and docetaxel to treat synchronous peritoneal carcinomatosis from gastric cancer: Results from a Chinese center. Eur. J. Surg. Oncol. 2016, 42, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Boerner, T.; Graichen, A.; Jeiter, T.; Zemann, F.; Renner, P.; März, L.; Soeder, Y.; Schlitt, H.J.; Piso, P.; Dahlke, M.H. CRS-HIPEC Prolongs Survival but is Not Curative for Patients with Peritoneal Carcinomatosis of Gastric Cancer. Ann. Surg. Oncol. 2016, 23, 3972–3977. [Google Scholar] [CrossRef] [PubMed]
- Chia, C.S.; You, B.; Decullier, E.; Vaudoyer, D.; Lorimier, G.; Abboud, K.; Bereder, J.M.; Arvieux, C.; Boschetti, G.; Glehen, O.; et al. Patients with Peritoneal Carcinomatosis from Gastric Cancer Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Is Cure a Possibility? Ann. Surg. Oncol. 2016, 23, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Topal, B.; Demey, K.; Topal, H.; Jaekers, J.; Van Cutsem, E.; Vandecaveye, V.; Sagaert, X.; Prenen, H. Cytoreductive surgery and Hyperthermic intra-operative peritoneal chemotherapy with Cisplatin for gastric peritoneal Carcinomatosis Monocentric phase-2 nonrandomized prospective clinical trial. BMC Cancer 2017, 17, 771. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rihuete Caro, C.; Manzanedo, I.; Pereira, F.; Carrion-Alvarez, L.; Serrano, Á.; Pérez-Viejo, E. Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with gastric cancer and peritoneal carcinomatosis. Eur. J. Surg. Oncol. 2018, 44, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Park, D.G.; Song, S.; Jee, Y.S. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy as Treatment Options for Peritoneal Metastasis of Advanced Gastric Cancer. J. Gastric Cancer 2018, 18, 296–304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manzanedo, I.; Pereira, F.; Rihuete Caro, C.; Pérez-Viejo, E.; Serrano, Á.; Gutiérrez Calvo, A.; Regueira, F.M.; Casado-Adam, Á.; Cascales-Campos, P.A.; Arteaga, X.; et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Gastric Cancer with Peritoneal Carcinomatosis: Multicenter Study of Spanish Group of Peritoneal Oncologic Surgery (GECOP). Ann. Surg. Oncol. 2019, 26, 2615–2621. [Google Scholar] [CrossRef] [PubMed]
- Bonnot, P.E.; Piessen, G.; Kepenekian, V.; Decullier, E.; Pocard, M.; Meunier, B.; Bereder, J.M.; Abboud, K.; Marchal, F.; Quenet, F.; et al. Cytoreductive Surgery with or without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer With Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. J. Clin. Oncol. 2019, 37, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- Rau, B.; Brandl, A.; Thuss-Patience, P.; Bergner, F.; Raue, W.; Arnold, A.; Horst, D.; Pratschke, J.; Biebl, M. The efficacy of treatment options for patients with gastric cancer and peritoneal metastasis. Gastric Cancer 2019, 22, 1226–1237. [Google Scholar] [CrossRef] [PubMed]
- Rau, B.; Brandl, A.; Piso, P.; Pelz, J.; Busch, P.; Demtröder, C.; Schüle, S.; Schlitt, H.J.; Roitman, M.; Tepel, J.; et al. Peritoneal metastasis in gastric cancer: Results from the German database. Gastric Cancer 2020, 23, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.H.; Yu, Y.; Liu, G.; Zhang, Y.B.; An, S.L.; Li, B.; Li, X.B.; Yan, G.J.; Li, Y. Peritoneal cancer index (PCI) based patient selecting strategy for complete cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy in gastric cancer with peritoneal metastasis: A single-center retrospective analysis of 125 patients. Eur. J. Surg. Oncol. 2021, 47, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Rosa, F.; Galiandro, F.; Ricci, R.; Di Miceli, D.; Longo, F.; Quero, G.; Tortorelli, A.P.; Alfieri, S. Survival advantage of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for advanced gastric cancer: Experience from a Western tertiary referral center. Langenbecks Arch. Surg. 2021, 406, 1847–1857. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Badgwell, B.; Ikoma, N.; Murphy, M.B.; Wang, X.; Estrella, J.; Roy-Chowdhuri, S.; Das, P.; Minsky, B.D.; Lano, E.; Song, S.; et al. A Phase II Trial of Cytoreduction, Gastrectomy, and Hyperthermic Intraperitoneal Perfusion with Chemotherapy for Patients with Gastric Cancer and Carcinomatosis or Positive Cytology. Ann. Surg. Oncol. 2021, 28, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Marano, L.; Marrelli, D.; Sammartino, P.; Biacchi, D.; Graziosi, L.; Marino, E.; Coccolini, F.; Fugazzola, P.; Valle, M.; Federici, O.; et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer with Synchronous Peritoneal Metastases: Multicenter Study of ‘Italian Peritoneal Surface Malignancies Oncoteam-S.I.C.O.’. Ann. Surg. Oncol. 2021, 28, 9060–9070. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Somashekhar, S.P.; Karivedu, J.; Kumar, R.; Ramya, Y.; Kapoor, P.; Rauthan, A.; Ashwin, K.R. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy in Gastric Cancer with Peritoneal Metastasis-Indian Experience. S. Asian J. Cancer 2021, 11, 121–124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santullo, F.; Ferracci, F.; Abatini, C.; Halabieh, M.A.E.; Lodoli, C.; D’Annibale, G.; Di Cesare, L.; D’Agostino, L.; Pecere, S.; Di Giorgio, A.; et al. Gastric cancer with peritoneal metastases: A single center outline and comparison of different surgical and intraperitoneal treatments. Langenbecks Arch. Surg. 2023, 408, 437. [Google Scholar] [CrossRef] [PubMed]
- Buckarma, E.; Thiels, C.A.; Jin, Z.; Grotz, T.E. Cytoreduction and Hyperthermic Intraperitoneal Paclitaxel and Cisplatin for Gastric Cancer with Peritoneal Metastasis. Ann. Surg. Oncol. 2024, 31, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Green, B.L.; Blumenthaler, A.N.; Gamble, L.A.; McDonald, J.D.; Robinson, K.; Connolly, M.; Epstein, M.; Hernandez, J.M.; Blakely, A.M.; Badgwell, B.D.; et al. Cytoreduction and HIPEC for Gastric Carcinomatosis: Multi-institutional Analysis of Two Phase II Clinical Trials. Ann. Surg. Oncol. 2023, 30, 1852–1860. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rau, B.; Lang, H.; Koenigsrainer, A.; Gockel, I.; Rau, H.G.; Seeliger, H.; Lerchenmueller, C.; Reim, D.; Wahba, R.; Angele, M.; et al. Effect of Hyperthermic Intraperitoneal Chemotherapy on Cytoreductive Surgery in Gastric Cancer with Synchronous Peritoneal Metastases: The Phase III GASTRIPEC-I Trial. J. Clin. Oncol. 2024, 42, 146–156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Allievi, N.; Bianco, F.; Pisano, M.; Montori, G.; Fugazzola, P.; Coccolini, F.; Lotti, M.; Mosconi, S.; Merelli, B.; Campanati, L.; et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) as adjuvant and therapeutic options for patients with advanced gastric cancer at high risk of recurrence or established peritoneal metastases: A single-centre experience. Updates Surg. 2023, 75, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Kobiałka, S.; Sędłak, K.; Pelc, Z.; Mlak, R.; Endo, Y.; Bogacz, P.; Kurylcio, A.; Polkowski, W.P.; Pawlik, T.M.; Rawicz-Pruszyński, K. Hyperthermic Intraperitoneal Chemotherapy (HIPEC), Oncological Outcomes and Long-Term Survival among Patients with Gastric Cancer and Limited Peritoneal Disease Progression after Neoadjuvant Chemotherapy. J. Clin. Med. 2023, 13, 161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, A.; Macrì, A.; Ferracci, F.; Robella, M.; Visaloco, M.; De Manzoni, G.; Sammartino, P.; Sommariva, A.; Biacchi, D.; Roviello, F.; et al. 10 Years of pressurized intraperitoneal aerosol chemotherapy (PIPAC): A systematic review and meta-analysis. Cancers 2023, 15, 1125. [Google Scholar] [CrossRef] [PubMed]
- Chia, C.S.; Seshadri, R.A.; Kepenekian, V.; Vaudoyer, D.; Passot, G.; Glehen, O. Survival outcomes after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from gastric cancer: A systematic review. Pleura Peritoneum 2016, 1, 67–77. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martins, M.; Santos-Sousa, H.; Araújo, F.; Nogueiro, J.; Sousa-Pinto, B. Impact of Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy in the Treatment of Gastric Cancer with Peritoneal Carcinomatosis: A Systematic Review and Meta-analysis. Ann. Surg. Oncol. 2022, 29, 7528–7537. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Du, Y.; Chen, B.A. Effect of hyperthermic intraperitoneal chemotherapy for gastric cancer patients: A meta-analysis of the randomized controlled trials. J. Int. Med. Res. 2019, 47, 5926–5936. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coccolini, F.; Cotte, E.; Glehen, O.; Lotti, M.; Poiasina, E.; Catena, F.; Yonemura, Y.; Ansaloni, L. Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials. Eur. J. Surg. Oncol. 2014, 40, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, Y.; Fujimura, T.; Nishimura, G.; Falla, R.; Sawa, T.; Katayama, K.; Tsugawa, K.; Fushida, S.; Miyazaki, I.; Tanaka, M.; et al. Effects of intraoperative chemohyperthermia in patients with gastric cancer with peritoneal dissemination. Surgery 1996, 119, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Glehen, O.; Association Française de Chirurgie; Gilly, F.N.; Arvieux, C.; Cotte, E.; Boutitie, F.; Mansvelt, B.; Bereder, J.M.; Lorimier, G.; Quenet, F.; et al. Peritoneal carcinomatosis from gastric cancer: A multi-institutional study on 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann. Surg. Oncol. 2010, 17, 2370–2377. [Google Scholar] [PubMed]
- Gill, R.S.; Al-Adra, D.P.; Nagendran, J.; Campbell, S.; Shi, X.; Haase, E.; Schiller, D. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: A systematic review of survival, mortality, and morbidity. J. Surg. Oncol. 2011, 104, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Santullo, F.; Abatini, C.; Attalla El Halabieh, M.; Ferracci, F.; Lodoli, C.; Barberis, L.; Giovinazzo, F.; Di Giorgio, A.; Pacelli, F. The Road to Technical Proficiency in Cytoreductive Surgery for Peritoneal Carcinomatosis: Risk-Adjusted Cumulative Summation Analysis. Front. Surg. 2022, 9, 877970. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.E.; Goetze, T.O.; Mueller, D.W.; Vogel, A.; Winkler, M.; Lorenzen, S.; Novotny, A.; Pauligk, C.; Homann, N.; Jungbluth, T.; et al. The RENAISSANCE (AIO-FLOT5) trial: Effect of chemotherapy alone vs. chemotherapy followed by surgical resection on survival and quality of life in patients with limited-metastatic adenocarcinoma of the stomach or esophagogastric junction—A phase III trial of the German AIO/CAO-V/CAOGI. BMC Cancer 2017, 17, 893. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Author, Year | Study Design | N CRS + HIPEC | Median Age | M/F |
|---|---|---|---|---|
| Yang, 2011 [8] | RCT | 34 | 50 | 16/18 |
| Yarema, 2014 [9] | Retrospective Cohort study | 20 | NA | 10/10 |
| Wu, 2015 [10] | Retrospective Cohort study | 11 | 64 | 8/3 |
| Wu H-T, 2016 [11] | Retrospective Cohort study | 38 | NA | NA |
| Boerner, 2016 [12] | Retrospective Cohort study | 50 | NA | 22/28 |
| Chia, 2016 [13] | Retrospective Cohort study | 81 | 51 | 37/44 |
| Topal 2017 [14] | Non-randomized prospective trial | 32 | 58 | 20/12 |
| Caro, 2018 [15] | Retrospective Cohort study | 35 | 53 | 17/18 |
| Kim, 2018 [16] | Retrospective Cohort study | 38 | 45.8 | 12/26 |
| Manzanedo, 2019 [17] | Retrospective Cohort study | 88 | 53 | 43/45 |
| Bonnot, 2019 [18] | Retrospective Cohort study | 180 | 51.13 | 83/97 |
| Rau, 2019 [19] | Retrospective Cohort study | 58 | 54.6 | 38/20 |
| Rau, 2020 [20] | Retrospective Cohort study | 235 | 53.4 | 113/122 |
| Zhong-He Ji, 2020 [21] | Retrospective Cohort study | 125 | 51 | 59/66 |
| Rosa, 2021 [22] | Retrospective Cohort study | 23 | 52 | 10/13 |
| Bagdwell, 2021 [23] | Clinical trial | 20 | 58 | 13/7 |
| Marano 2021 [24] | Retrospective Cohort study | 91 | 58 | 46/45 |
| Somashekhar, 2022 [25] | Retrospective Cohort study | 16 | 55.5 | NA |
| Santullo, 2023 [26] | Retrospective Cohort study | 20 | 62 | 8/12 |
| Buckarma, 2023 [27] | Retrospective Cohort study | 22 | 56 | 17/5 |
| Green, 2023 [28] | Clinical Trial | 41 | 57 | 26/15 |
| Rau, 2023 [29] | RCT | 59 | 56 | 29 |
| Allievi, 2023 [30] | Retrospective Cohort study | 27 | 55 | 17 |
| Kobialka, 2023 [31] | Retrospective Cohort study | 25 | 51 | 12 |
| Author | N CRS + HIPEC | Median OS | 1 y Rate Survival | 3 y Rate Survival | 5 y Rate Survival | DFS | 30-Day Mortality |
|---|---|---|---|---|---|---|---|
| Yang, 2011 [8] | 34 | 11 | 41.2 | 5.9 | NA | NA | NA |
| Yarema, 2014 [9] | 20 | 12 | 68.8 | NA | NA | NA | NA |
| Wu, 2015 [10] | 11 | 28.2 | 79.5 | NA | NA | NA | 0 |
| H-T Wu, 2016 [11] | 38 | 17 | 71.1 | 35.8 | 6.4 | NA | NA |
| Boerner, 2016 [12] | 50 | 14.3 | 58 | 32 | NA | NA | NA |
| Chia, 2016 [13] | 81 | 17.3 | NA | NA | 18 | NA | 3 |
| Topal 2017 [14] | 32 | 16 | 71.9 | 14.1 | 3.5 | 7.8 | NA |
| Caro, 2018 [15] | 35 | 16 | 70.8 | 21.3 | 21.3 | 7 | NA |
| Kim, 2018 [16] | 38 | 19 | NA | NA | NA | NA | 2 |
| Manzanedo, 2019 [17] | 88 | 21.2 | 79.9 | 30.9 | 27.5 | 11.6 | NA |
| Bonnot, 2019 [18] | 180 | 18.6 | 67.9 | 27.1 | 20.21 | 11.6 | 4 |
| Rau, 2019 [19] | 58 | 9.8 | 40.9 | 12.1 | 0 | NA | NA |
| Rau, 2020 [20] | 235 | 13 | NA | NA | NA | NA | 12 |
| Zhong-He Ji, 2020 [21] | 125 | 10.7 | 43.8 | 18.6 | 15.7 | NA | 1 |
| Rosa, 2021 [22] | 23 | NA | NA | NA | 27 | NA | 1 |
| Bagdwell, 2021 [23] | 20 | 22.1 | 90 | 50 | 28 | 7 | 0 |
| Marano, 2021 [24] | 91 | 20.2 | 62 | 44 | 20.4 | 7.3 | NA |
| Somashekhar, 2022 [25] | 16 | 17 | NA | NA | NA | 12 | 0 |
| Santullo, 2023 [26] | 20 | 17 | 75 | 14.7 | 2 | 12 | 0 |
| Buckarma, 2023 [27] | 22 | NA | 96 | 78 | 55 | NA | 0 |
| Green, 2023 [28] | 41 | 24.9 | NA | 25 | NA | 7.4 | 0 |
| Rau, 2023 [29] | 59 | 14.9 | 58.2 | 13.6 | NA | 7.1 | NA |
| Allievi, 2023 [30] | 27 | NA | 60.3 | 30.1 | NA | NA | NA |
| Kobialka, 2023 [31] | 25 | 16 | NA | NA | NA | NA | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langellotti, L.; Fiorillo, C.; D’Annibale, G.; Panza, E.; Pacelli, F.; Alfieri, S.; Di Giorgio, A.; Santullo, F. Efficacy of Cytoreductive Surgery (CRS) + HIPEC in Gastric Cancer with Peritoneal Metastasis: Systematic Review and Meta-Analysis. Cancers 2024, 16, 1929. https://doi.org/10.3390/cancers16101929
Langellotti L, Fiorillo C, D’Annibale G, Panza E, Pacelli F, Alfieri S, Di Giorgio A, Santullo F. Efficacy of Cytoreductive Surgery (CRS) + HIPEC in Gastric Cancer with Peritoneal Metastasis: Systematic Review and Meta-Analysis. Cancers. 2024; 16(10):1929. https://doi.org/10.3390/cancers16101929
Chicago/Turabian StyleLangellotti, Lodovica, Claudio Fiorillo, Giorgio D’Annibale, Edoardo Panza, Fabio Pacelli, Sergio Alfieri, Andrea Di Giorgio, and Francesco Santullo. 2024. "Efficacy of Cytoreductive Surgery (CRS) + HIPEC in Gastric Cancer with Peritoneal Metastasis: Systematic Review and Meta-Analysis" Cancers 16, no. 10: 1929. https://doi.org/10.3390/cancers16101929
APA StyleLangellotti, L., Fiorillo, C., D’Annibale, G., Panza, E., Pacelli, F., Alfieri, S., Di Giorgio, A., & Santullo, F. (2024). Efficacy of Cytoreductive Surgery (CRS) + HIPEC in Gastric Cancer with Peritoneal Metastasis: Systematic Review and Meta-Analysis. Cancers, 16(10), 1929. https://doi.org/10.3390/cancers16101929