Exploring the Link between Head and Neck Cancer and the Elevated Risk of Acute Myocardial Infarction: A National Population-Based Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
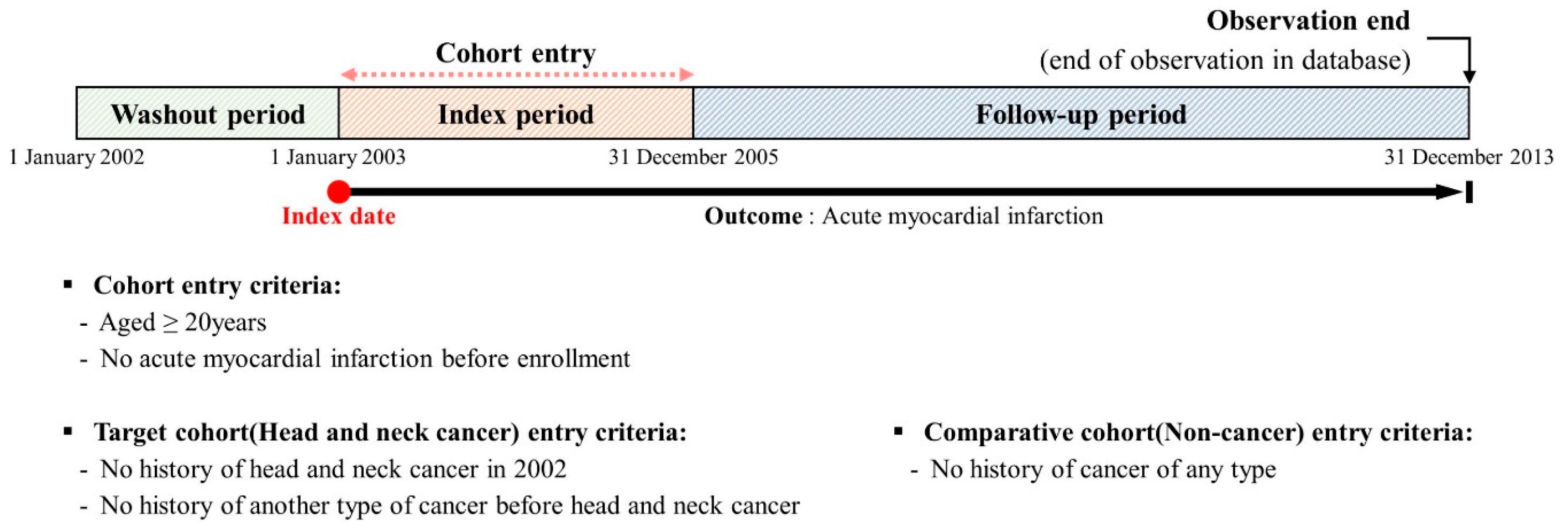
2. Materials and Methods
2.1. Characteristics of the Nationwide Population-Based Cohort
2.2. Retrospective Cohort Strategy
2.3. Ethical Considerations and Data Availability
3. Results
3.1. Effect of HNC on Subsequent Development of AMI
3.2. Evaluation of the Risk for AMI According to the Subgroups
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Owens, D.; Paleri, V.; Jones, A.V. Head and neck cancer explained: An overview of management pathways. Br. Dent. J. 2022, 233, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Amaral, M.N.; Faísca, P.; Ferreira, H.A.; Gaspar, M.M.; Reis, C.P. Current insights and progress in the clinical management of head and neck cancer. Cancers 2022, 14, 6079. [Google Scholar] [CrossRef] [PubMed]
- Vigneswaran, N.; Williams, M.D. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral. Maxillofac. Surg. Clin. N. Am. 2014, 26, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, risk factors, and prevention of head and neck squamous cell carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.; Chen, L.; Geng, Y.; Yu, S.; Chen, Z. Long-term cardiovascular disease mortality among 160 834 5-year survivors of adolescent and young adult cancer: An American population-based cohort study. Eur. Heart J. 2021, 42, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Galassi, A.; Reynolds, K.; He, J. Metabolic syndrome and risk of cardiovascular disease: A meta-analysis. Am. J. Med. 2006, 119, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Guzik, A.; Bushnell, C. Stroke epidemiology and risk factor management. Continuum 2017, 23, 15–39. [Google Scholar] [CrossRef]
- Chu, C.N.; Chen, S.W.; Bai, L.Y.; Mou, C.H.; Hsu, C.Y.; Sung, F.C. Increase in stroke risk in patients with head and neck cancer: A retrospective cohort study. Br. J. Cancer 2011, 105, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, Y.A.; Kim, H.S.; Back, J.H.; Jung, Y.H.; Lee, D.H.; Kim, S. Radiotherapy can increase the risk of ischemic cerebrovascular disease in head and neck cancer patients: A Korean population-based cohort study. Radiother. Oncol. 2020, 142, 85–91. [Google Scholar] [CrossRef]
- Kwon, H.K.; Han, K.D.; Cheon, Y.I.; Shin, S.C.; Lee, M.; Sung, E.S.; Lee, J.C.; Lee, B.J. The incidence of myocardial infarction and stroke in head and neck cancer patients. Sci. Rep. 2021, 11, 4174. [Google Scholar] [CrossRef]
- Xu, J.; Cao, Y. Radiation-induced carotid artery stenosis: A comprehensive review of the literature. Interv. Neurol. 2014, 2, 183–192. [Google Scholar] [CrossRef]
- Fernández-Alvarez, V.; López, F.; Suárez, C.; Strojan, P.; Eisbruch, A.; Silver, C.E.; Mendenhall, W.M.; Langendijk, J.A.; Rinaldo, A.; Lee, A.W.M.; et al. Radiation-induced carotid artery lesions. Strahlenther. Onkol. 2018, 194, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.L.; Smith, B.D.; Buchholz, T.A.; Giordano, S.H.; Garden, A.S.; Woodward, W.A.; Krumholz, H.M.; Weber, R.S.; Ang, K.K.; Rosenthal, D.I. Cerebrovascular disease risk in older head and neck cancer patients after radiotherapy. J. Clin. Oncol. 2008, 26, 5119–5125. [Google Scholar] [CrossRef] [PubMed]
- Wall, J.G.; Weiss, R.B.; Norton, L.; Perloff, M.; Rice, M.A.; Korzun, A.H.; Wood, W.C. Arterial thrombosis associated with adjuvant chemotherapy for breast carcinoma: A Cancer and Leukemia Group B Study. Am. J. Med. 1989, 87, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.Y.; Cheng, P.C.; Hsu, W.L.; Lo, W.C.; Hsieh, C.H.; Shueng, P.W.; Liao, L.J. Risk of CVD following radiotherapy for head and neck cancer: An updated systematic review and meta-analysis. Front. Oncol. 2022, 12, 820808. [Google Scholar] [CrossRef] [PubMed]
- Salz, T.; Zabor, E.C.; Brown, P.N.; Dalton, S.O.; Raghunathan, N.J.; Matasar, M.J.; Steingart, R.; Hjalgrim, H.; Specht, L.; Vickers, A.J.; et al. Cardiovascular risk factors, radiation therapy, and myocardial infarction among lymphoma survivors. Acta Oncol. 2022, 61, 1064–1068. [Google Scholar] [CrossRef]
- Seong, S.C.; Kim, Y.Y.; Park, S.K.; Khang, Y.H.; Kim, H.C.; Park, J.H.; Kang, H.J.; Do, C.H.; Song, J.S.; Lee, E.J.; et al. Cohort profile: The National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open 2017, 7, e016640. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J.S.; Park, S.H.; Shin, S.A.; Kim, K. Cohort profile: The National Health Insurance Service-national sample cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017, 46, e15. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Gearing, R.E.; Mian, I.A.; Barber, J.; Ickowicz, A. A methodology for conducting retrospective chart review research in child and adolescent psychiatry. J. Can. Acad. Child Adolesc. Psychiatry 2006, 15, 126–134. [Google Scholar]
- Mukherjee, A.; Wiener, H.W.; Griffin, R.L.; Lenneman, C.; Chatterjee, A.; Nabell, L.M.; Lewis, C.E.; Shrestha, S. Traditional risk factors and cancer-related factors associated with cardiovascular disease risk in head and neck cancer patients. Front. Cardiovasc. Med. 2022, 9, 1024846. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.J.; Lee, Y.G.; Koo, M.; Lee, K.; Park, I.H.; Kim, J.S.; Choi, Y.J. The risk of cardiovascular disease and stroke in survivors of head and neck cancer in Korea. Health Sci. Rep. 2022, 5, e517. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.C.; Fang, K.M.; Hsu, W.L.; Liao, L.J. It is time to take action to prevent cardiovascular disease in postirradiation head and neck cancer patients. Eur. Arch. Otorhinolaryngol. 2019, 276, 2361–2362. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, D.J.; Patel, P.; Niedzwiecki, D.; Dillon, M.; Diaz, A.K.; Kumar, A.; Mowery, Y.M.; Crowell, K.A.; D’Anna, R.; Wu, Q.; et al. Long-term risk of carotid stenosis and cerebrovascular disease after radiation therapy for head and neck cancer. Cancer 2023. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Beltrán, D.; Osorio, J.C.; Blanco, R.; Oliva, C.; Boccardo, E.; Aguayo, F. Interaction between cigarette smoke and human papillomavirus 16 E6/E7 oncoproteins to induce SOD2 expression and DNA damage in head and neck cancer. Int. J. Mol. Sci. 2023, 24, 6907. [Google Scholar] [CrossRef]
- Dal Maso, L.; Torelli, N.; Biancotto, E.; Di Maso, M.; Gini, A.; Franchin, G.; Levi, F.; La Vecchia, C.; Serraino, D.; Polesel, J. Combined effect of tobacco smoking and alcohol drinking in the risk of head and neck cancers: A re-analysis of case-control studies using bi-dimensional spline models. Eur. J. Epidemiol. 2016, 31, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Rubenstein, L.M.; Haugen, T.H.; Hamsikova, E.; Turek, L.P. Tobacco and alcohol use increases the risk of both HPV-associated and HPV-independent head and neck cancers. Cancer Causes Control 2010, 21, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Ping, Z.; Peng, Y.; Lang, H.; Xinyong, C.; Zhiyi, Z.; Xiaocheng, W.; Hong, Z.; Liang, S. Oxidative stress in radiation-induced cardiotoxicity. Oxid. Med. Cell Longev. 2020, 2020, 3579143. [Google Scholar] [CrossRef]
- Virmani, R.; Farb, A.; Carter, A.J.; Jones, R.M. Pathology of radiation-induced coronary artery disease in human and pig. Cardiovasc. Radiat. Med. 1999, 1, 98–101. [Google Scholar] [CrossRef]
- Verheij, M.; Dewit, L.G.; Boomgaard, M.N.; Brinkman, H.J.; van Mourik, J.A. Ionizing radiation enhances platelet adhesion to the extracellular matrix of human endothelial cells by an increase in the release of von Willebrand factor. Radiat. Res. 1994, 137, 202–207. [Google Scholar] [CrossRef]
- Bachaud, J.M.; David, J.M.; Shubinski, R.E.; Perineau, D.; Boussin, G.; Serrano, E.; De Forni, M.; Pessey, J.J.; Daly-Schveitzer, N.J. Predictive factors of a complete response to and adverse effects of a CDDP-5FU combination as primary therapy for head and neck squamous carcinomas. J. Laryngol. Otol. 1993, 107, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Bourdeanu, L.; Luu, T. Arterial thrombosis associated with adjuvant chemotherapy for breast cancer: A case report. J. Am. Acad. Nurse Pract. 2010, 22, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Chen, W.H.; Tang, Y.; Rau, K.M.; Chen, Y.Y.; Huang, T.L.; Liu, J.S.; Huang, C.H. Incidence of ischemic stroke post-chemotherapy: A retrospective review of 10,963 patients. Clin. Neurol. Neurosurg. 2006, 108, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Weijl, N.I.; Rutten, M.F.; Zwinderman, A.H.; Keizer, H.J.; Nooy, M.A.; Rosendaal, F.R.; Cleton, F.J.; Osanto, S. Thromboembolic events during chemotherapy for germ cell cancer: A cohort study and review of the literature. J. Clin. Oncol. 2000, 18, 2169–2178. [Google Scholar] [CrossRef]
- Azak, A.; Oksüzoğlu, B.; Deren, T.; Oneç, B.M.; Zengin, N. Cerebrovascular accident during cisplatin-based combination chemotherapy of testicular germ cell tumor: An unusual case report. Anti. Cancer Drugs. 2008, 19, 97–98. [Google Scholar] [CrossRef]
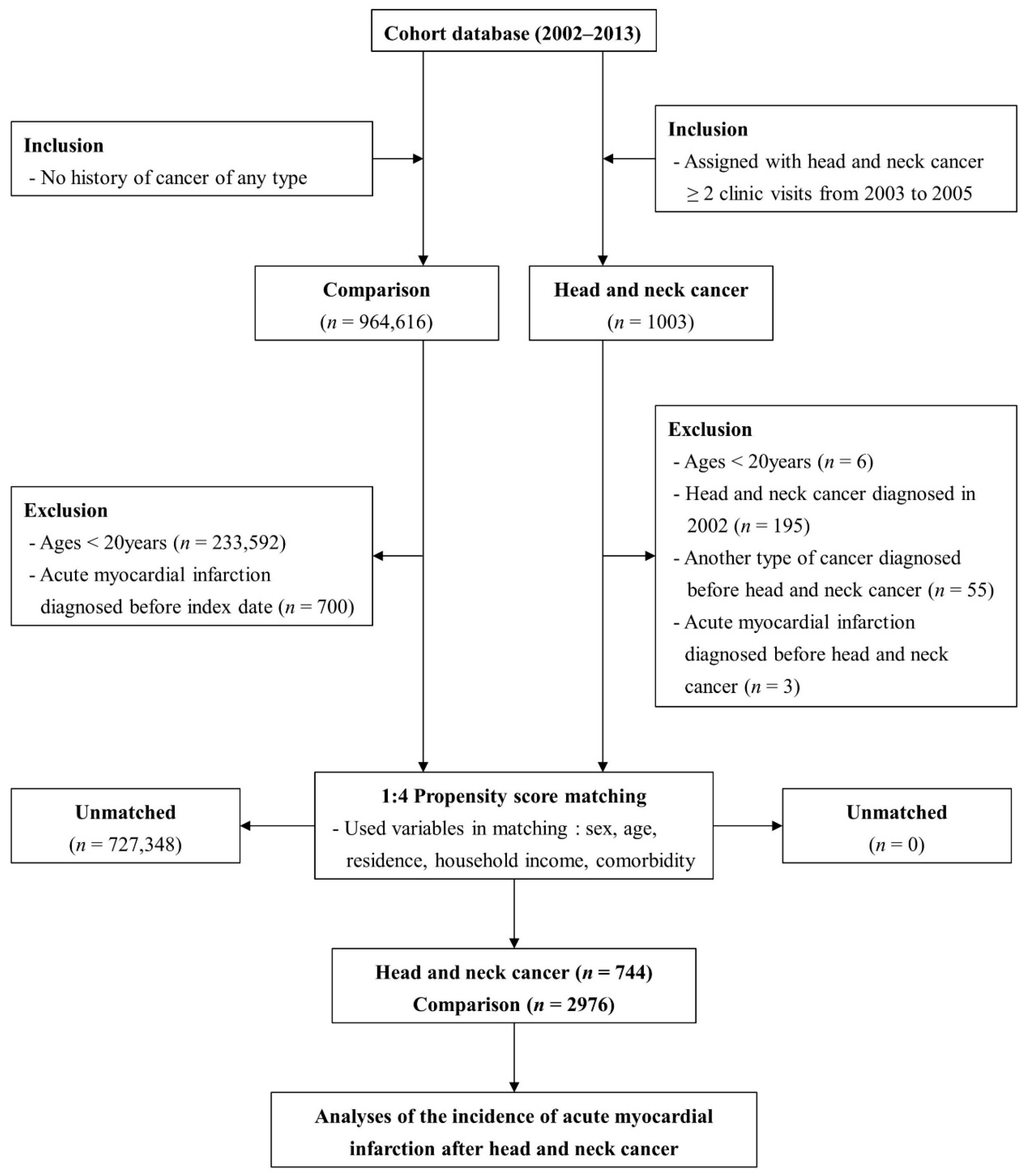
| Variables | Comparison (n = 2976) 1 | Head and Neck Cancer (n = 744) |
|---|---|---|
| Sex | ||
| Male | 1420 (47.7%) | 355 (47.7%) |
| Female | 1556 (52.3%) | 389 (52.3%) |
| Ages (years) | ||
| <45 | 316 (10.6%) | 79 (10.6%) |
| 45–64 | 1100 (37.0%) | 275 (37.0%) |
| >64 | 1560 (52.4%) | 390 (52.4%) |
| Residence | ||
| Seoul 2 | 400 (13.4%) | 100 (13.4%) |
| Second area 3 | 520 (17.5%) | 130 (17.5%) |
| Third area 4 | 2056 (69.1%) | 514 (69.1%) |
| Household income | ||
| Low (0–30%) | 776 (26.1%) | 194 (26.1%) |
| Middle (30–70%) | 940 (31.6%) | 235 (31.6%) |
| High (70–100%) | 1260 (42.3%) | 315 (42.3%) |
| CCI | ||
| 0 | 1744 (58.6%) | 436 (58.6%) |
| 1 | 668 (22.4%) | 167 (22.4%) |
| ≥2 | 564 (19.0%) | 141 (19.0%) |
| Variables | N | Case | Person-Years | Incidence Rate | Unadjusted HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|---|---|
| AMI | ||||||
| Comparison | 2976 | 61 | 26,002.8 | 2.35 | 1.00 (ref) | 1.00 (ref) |
| Head and neck Cancer | 744 | 12 | 5476.2 | 2.19 | 0.91 (0.49–1.70) | 0.93 (0.50–1.73) |
| Sex | Male | Female | ||
|---|---|---|---|---|
| Comparison | HNC | Comparison | HNC | |
| AMI | ||||
| Unadjusted HR (95% CI) | 1.00 (ref) | 0.88 (0.37–2.09) | 1.00 (ref) | 0.98 (0.40–2.38) |
| Adjusted HR (95% CI) | 1.00 (ref) | 0.89 (0.37–2.13) | 1.00 (ref) | 0.98 (0.40–2.38) |
| Ages (Years) | <45 | 45–64 | >64 | |||
|---|---|---|---|---|---|---|
| Comparison | HNC | Comparison | HNC | Comparison | HNC | |
| AMI | ||||||
| Unadjusted HR (95% CI) | 1.00 (ref) | 1.50 (0.16–14.45) | 1.00 (ref) | 1.20 (0.34–4.32) | 1.00 (ref) | 0.80 (0.38–1.70) |
| Adjusted HR (95% CI) | 1.00 (ref) | 1.52 (0.16–14.68) | 1.00 (ref) | 1.18 (0.33–4.24) | 1.00 (ref) | 0.82 (0.38–1.73) |
| CCI | 0 | 1 | ≥2 | |||
|---|---|---|---|---|---|---|
| Comparison | HNC | Comparison | HNC | Comparison | HNC | |
| AMI | ||||||
| Unadjusted HR (95% CI) | 1.00 (ref) | 1.15 (0.50–2.64) | 1.00 (ref) | 0.28 (0.04–2.10) | 1.00 (ref) | 1.13 (0.37–3.40) |
| Adjusted HR (95% CI) | 1.00 (ref) | 1.15 (0.50–2.64) | 1.00 (ref) | 0.30 (0.04–2.26) | 1.00 (ref) | 1.11 (0.37–3.35) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-K. Exploring the Link between Head and Neck Cancer and the Elevated Risk of Acute Myocardial Infarction: A National Population-Based Cohort Study. Cancers 2024, 16, 1930. https://doi.org/10.3390/cancers16101930
Kim D-K. Exploring the Link between Head and Neck Cancer and the Elevated Risk of Acute Myocardial Infarction: A National Population-Based Cohort Study. Cancers. 2024; 16(10):1930. https://doi.org/10.3390/cancers16101930
Chicago/Turabian StyleKim, Dong-Kyu. 2024. "Exploring the Link between Head and Neck Cancer and the Elevated Risk of Acute Myocardial Infarction: A National Population-Based Cohort Study" Cancers 16, no. 10: 1930. https://doi.org/10.3390/cancers16101930
APA StyleKim, D.-K. (2024). Exploring the Link between Head and Neck Cancer and the Elevated Risk of Acute Myocardial Infarction: A National Population-Based Cohort Study. Cancers, 16(10), 1930. https://doi.org/10.3390/cancers16101930





