A Novel Positive-Contrast Magnetic Resonance Imaging Line Marker for High-Dose-Rate (HDR) MRI-Assisted Radiosurgery (MARS)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Test Solutions
2.2. MRI Scan Acquisition Parameters and Image Analysis
2.3. Spin–Lattice (Longitudinal) Relaxation Time (T1) Measurements
2.4. Spin–Spin (Transverse) Relaxation Time (T2) Measurement
2.5. Relaxivity Calculation
2.6. C4:S Marker Implantation and MRI Scanning
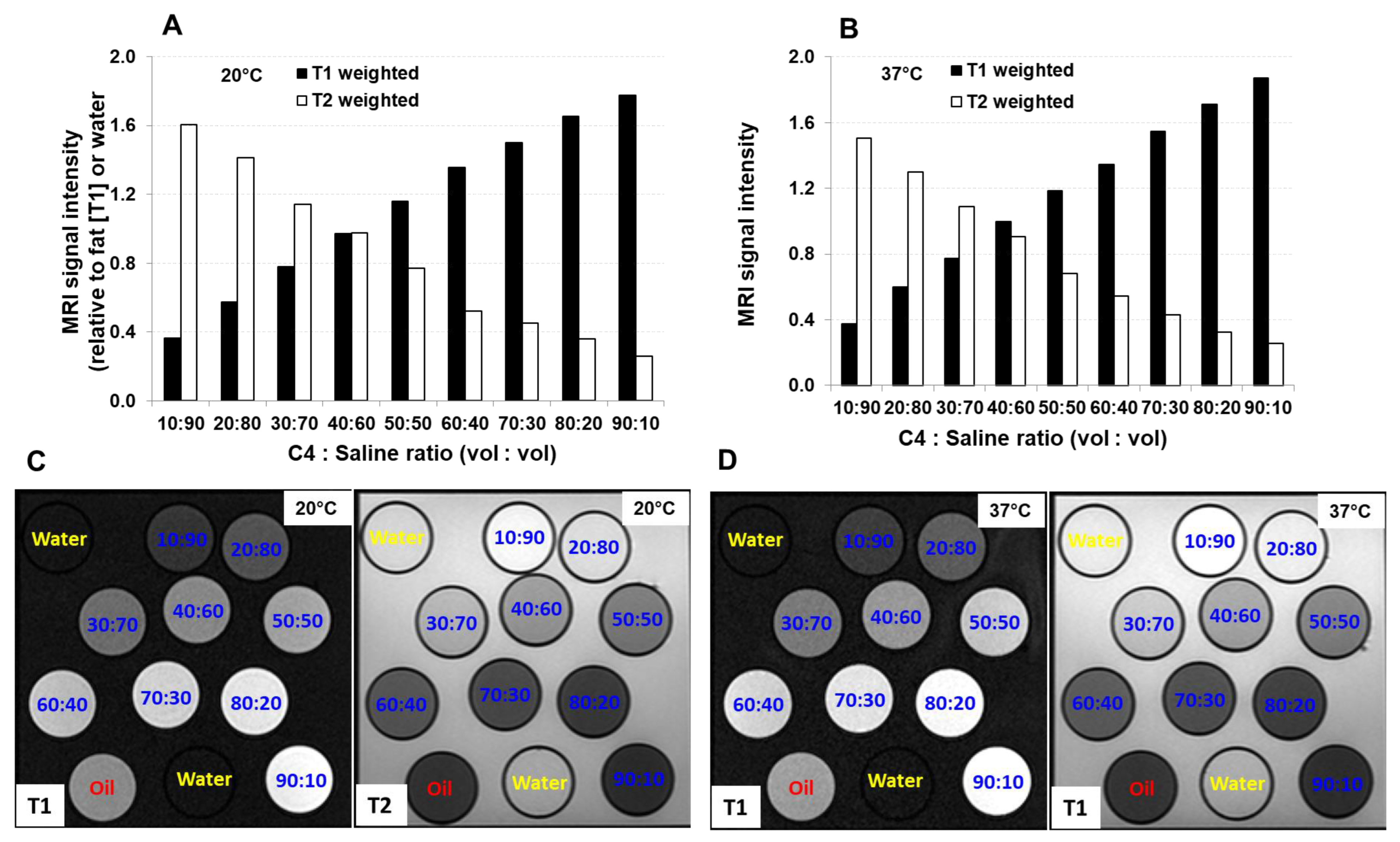
3. Results
3.1. Relaxation Time and Relaxivity of C4:S at Different Temperatures
3.2. Relaxation Time of C4:S at Different MRI Scanning Orientations
3.3. Positive-Contrast Visualization of C4:S Line Marker under MRI Scanning in a Phantom
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watanabe, Y.; Muraishi, H.; Takei, H.; Hara, H.; Terazaki, T.; Shuto, N.; Shimo, T.; Nozawa, M.; Ishiyama, H.; Hayakawa, K.; et al. Automated source tracking with a pinhole imaging system during high-dose-rate brachytherapy treatment. Phys. Med. Biol. 2018, 63, 145002. [Google Scholar] [CrossRef] [PubMed]
- Skowronek, J. Current status of brachytherapy in cancer treatment—Short overview. J. Contemp. Brachytherapy 2017, 9, 581–589. [Google Scholar] [CrossRef]
- Viswanathan, A.N.; Dimopoulos, J.; Kirisits, C.; Berger, D.; Pötter, R. Computed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy: Results of a prospective trial and preliminary guidelines for standardized contours. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 491–498. [Google Scholar] [CrossRef]
- Van den Bos, W.; Beriwal, S.; Velema, L.; de Leeuw, A.A.; Nomden, C.N.; Jürgenliemk-Schulz, I.-M. Image guided adaptive brachytherapy for cervical cancer: Dose contribution to involved pelvic nodes in two cancer centers. J. Contemp. Brachytherapy 2014, 6, 21–27. [Google Scholar] [CrossRef]
- Schindel, J.; Zhang, W.; Bhatia, S.K.; Sun, W.; Kim, Y. Dosimetric impacts of applicator displacements and applicator reconstruction-uncertainties on 3D image-guided brachytherapy for cervical cancer. J. Contemp. Brachytherapy 2013, 5, 250–257. [Google Scholar] [CrossRef]
- Wiercińska, J.; Wronczewska, A.; Kabacińska, R.; Makarewicz, R. Transition from Paris dosimetry system to 3D image-guided planning in interstitial breast brachytherapy. J. Contemp. Brachytherapy 2015, 7, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Poder, J.; Brown, R.; Howie, A.; Yuen, J.; Ralston, A.; Schreiber, K.; Bece, A.; Bucci, J. A risk-based approach to development of ultrasound-based high-dose-rate prostatebrachytherapy quality management. Brachytherapy 2018, 17, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, P.J.; Rojas, A.M.; Ostler, P.J.; Hughes, R.; Lowe, G.J.; Bryant, L. Quality of life after radical radiotherapy for prostate cancer: Longitudinal study from a randomized trial of external beam radiotherapy alone or in combination with high dose rate brachytherapy. Clin. Oncol. 2013, 25, 321–327. [Google Scholar] [CrossRef]
- Erickson, B.A.; Bittner, N.H.; Chadha, M.; Mourtada, F.; Demanes, D.J. The American College of Radiology and the American Brachytherapy Society practice parameter for the performance of radionuclide-based high-dose-rate brachytherapy. Brachytherapy 2017, 16, 75–84. [Google Scholar] [CrossRef]
- Hoskin, P.J.; Motohashi, K.; Bownes, P.; Bryant, L.; Ostler, P. High dose rate brachytherapy in combination with external beam radiotherapy in the radical treatment of prostate cancer: Initial results of a randomized phase three trial. Radiother. Oncol. 2007, 84, 114–120. [Google Scholar] [CrossRef]
- Hoskin, P.J.; Rojas, A.M.; Bownes, P.J.; Lowe, G.J.; Ostler, P.J.; Bryant, L. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for randomize prostate cancer. Radiother. Oncol. 2012, 103, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Jawad, M.S.; Dilworth, J.T.; Gustafson, G.S.; Ye, H.; Wallace, M.; Martinez, A.; Chen, P.Y.; Krauss, D.J. Outcomes associated with 3 treatment schedules of high-dose-rate brachytherapy monotherapy for favorable-risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Barkati, M.; Williams, S.G.; Foroudi, F.; Tai, K.H.; Chander, S.; van Dyk, S.; See, A.; Duchesne, G.M. High-dose-rate brachytherapy as a monotherapy for favorable-risk prostate cancer: A phase II trial. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, M.; Pharand-Charbonneau, M.; Desrosiers, M.-P.; Wright, D.; Haddad, A. Early toxicity and health-related quality of life results of high-dose-rate brachytherapy as monotherapy for low and intermediate-risk prostate cancer. Brachytherapy 2018, 17, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Skowronek, J. Low-dose-rate or high-dose-rate brachytherapy in treatment of prostate cancer—Between options. J. Contemp. Brachytherapy 2013, 5, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Jaberi, R.; Siavashpour, Z.; Aghamiri, M.R.; Kirisits, C.; Ghaderi, R. Artificial neural network based gynaecological image-guided adaptive brachytherapy treatment planning correction of intra-fractional organs at risk dose variation. J. Contemp. Brachytherapy 2017, 9, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, J.; Wang, P.; Yuan, K.; Yin, G.; Wan, B. Image-guided radiation therapy boost in combination with high-dose-rate intracavitary brachytherapy for the treatment of cervical cancer. J. Contemp. Brachytherapy 2016, 8, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Otter, S.; Franklin, A.; Ajaz, M.; Stewart, A. Improving the efficiency of image guided brachytherapy in cervical cancer. J. Contemp. Brachytherapy 2016, 8, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Skliarenko, J.; Carlone, M.; Tanderup, K.; Han, K.; Beiki-Ardakani, A.; Borg, J.; Chan, K.; Croke, J.; Rink, A.; Simeonov, A.; et al. Technique adaptation, strategic replanning, and team learning during implementation of MR-guided brachytherapy for cervical cancer. Brachytherapy 2018, 17, 86–93. [Google Scholar] [CrossRef]
- Han, K.; Milosevic, M.; Fyles, A.; Pintilie, M.; Viswanathan, A.N. Trends in the utilization of brachytherapy in cervical cancer in the United States. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 111–119. [Google Scholar] [CrossRef]
- Otter, S.; Coates, A.; Franklin, A.; Cunningham, M.; Stewart, A. Improving dose delivery by adding interstitial catheters to fixed geometry applicators in high-dose-rate brachytherapy for cervical cancer. Brachytherapy 2018, 17, 580–586. [Google Scholar] [CrossRef]
- Ko, H.C.; Huang, J.Y.; Miller, J.R.; Das, R.K.; Wallace, C.R.; De Costa, A.-M.A.; Francis, D.M.; Straub, M.R.; Anderson, B.M.; Bradley, K.A. Novel use of ViewRay MRI guidance for high-dose-rate brachytherapy in the treatment of cervical cancer. Brachytherapy 2018, 17, 680–688. [Google Scholar] [CrossRef]
- Polgár, C.; Ott, O.J.; Hildebrandt, G.; Kauer-Dorner, D.; Knauerhase, H.; Major, T.; Lyczek, J.; Guinot, J.L.; Dunst, J.; Miguelez, C.G.; et al. Late side-effects and cosmetic results of accelerated partial breast irradiation with interstitial brachytherapy versus whole-breast irradiation after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: 5-year results of a randomized, controlled, phase 3 trial. Lancet Oncol. 2017, 18, 259–268. [Google Scholar] [PubMed]
- Strnad, V.; Ott, O.J.; Hildebrandt, G.; Kauer-Dorner, D.; Knauerhase, H.; Major, T.; Lyczek, J.; Guinot, J.L.; Dunst, J.; Miguelez, C.G.; et al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: A randomized, phase 3, non-inferiority trial. Lancet 2016, 387, 229–238. [Google Scholar] [PubMed]
- Götz, T.I.; Lahmer, G.; Strnad, V.; Bert, C.; Hensel, B.; Tomé, A.M.; Lang, E.W. A tool to automatically analyze electromagnetic tracking data from high dose rate brachytherapy of breast cancer patients. PLoS ONE 2017, 12, e0183608. [Google Scholar] [CrossRef] [PubMed]
- Beld, E.; Seevinck, P.R.; Schuurman, J.; Viergever, M.A.; Lagendijk, J.J.; Moerland, M.A. Development and Testing of a Magnetic Resonance (MR) Conditional Afterloader for Source Tracking in Magnetic Resonance Imaging-Guided High-Dose-Rate (HDR) Brachytherapy. Int. J. Radiat. Oncol. 2018, 102, 960–968. [Google Scholar] [CrossRef]
- Thomadsen, B.; Lin, S.-W.; Laemmrich, P.; Waller, T.; Cheng, A.; Caldwell, B.; Rankin, R.; Stitt, J. Analysis of treatment delivery errors in brachytherapy using formal risk analysis techniques. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 1492–1508. [Google Scholar] [CrossRef]
- Wilkinson, D.A.; Kolar, M.D. Failure modes and effects analysis applied to high-dose-rate brachytherapy treatment planning. Brachytherapy 2013, 12, 382–386. [Google Scholar] [CrossRef]
- Williamson, J.F. Current brachytherapy quality assurance guidance: Does it meet the challenges of emerging image-guided technologies? Int. J. Radiat. Oncol. Biol. Phys. 2008, 71 (Suppl. 1), S18–S22. [Google Scholar] [CrossRef]
- Wadi-Ramahi, S.; Alnajjar, W.; Mahmood, R.; Jastaniyah, N.; Moftah, B. Failure modes and effects analysis in image-guided high-dose-rate brachytherapy: Quality control optimization to reduce errors in treatment volume. Brachytherapy 2016, 15, 669–678. [Google Scholar] [CrossRef]
- Beld, E.; Moerland, M.A.; Zijlstra, F.; Viergever, M.A.; Lagendijk, J.J.W.; Seevinck, P.R. MR-based source localization for MR-guided HDR brachytherapy. Phys. Med. Biol. 2018, 63, 085002. [Google Scholar] [CrossRef] [PubMed]
- Maenhout, M.; de Battisti, M.B.; Peters, M.; van Vulpen, M.; van den Bosch, M.; Moerland, M.A. The effect of catheter displacement and anatomical variations on the dose distribution in MRI-guided focal HDR brachytherapy for prostate cancer. Brachytherapy 2018, 17, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Hellebust, T. Place of modern imaging in brachytherapy planning. Cancer/Radiothérapie 2018, 22, 326–333. [Google Scholar] [CrossRef]
- Pötter, R.; Georg, P.; Dimopoulos, J.C.; Grimm, M.; Berger, D.; Nesvacil, N.; Georg, D.; Schmid, M.P.; Reinthaller, A.; Sturdza, A.; et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapycombined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother. Oncol. 2011, 100, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Lindegaard, J.; Fokdal, L.U.; Nielsen, S.K.; Juul-Christensen, J.; Tanderup, K. MRI-guidedadaptive radiotherapy in locally advanced cervical cancer from a Nordic per-spective. Acta Oncol. 2013, 52, 1510–1519. [Google Scholar] [CrossRef]
- Petric, P.; Kirisits, C. Potential role of TRAns Cervical Endosonography (TRACE) in brachytherapy of cervical cancer: Proof of concept. J. Contemp. Brachytherapy 2016, 8, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Hellebust, T.P.; Kirisits, C.; Berger, D.; Pérez-Calatayud, J.; De Brabandere, M.; De Leeuw, A.; Dumas, I.; Hudej, R.; Lowe, G.; Wills, R.; et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group: Considerations and pitfalls in commissioning and applicator reconstruction in 3D image-based treatment planning of cervix cancer brachytherapy. Radiother. Oncol. 2010, 96, 153–160. [Google Scholar] [CrossRef] [PubMed]
- De Brabandere, M.; Al-Qaisieh, B.; De Wever, L.; Haustermans, K.; Kirisits, C.; Moerland, M.A.; Oyen, R.; Rijnders, A.; Heuvel, F.V.D.; Siebert, F.-A. CT- and MRI-based seed localization in postimplant evaluation after prostate brachytherapy. Brachytherapy 2013, 12, 580–588. [Google Scholar] [CrossRef]
- Hegazy, N.; Pötter, R.; Kirisits, C.; Berger, D.; Federico, M.; Sturdza, A.; Nesvacil, N. High-risk clinical target volume delineation in CT-guided cervical cancer brachytherapy: Impact of information from FIGO stage with or without systematic inclusion of 3D documentation of clinical gynecological examination. Acta Oncol. 2013, 52, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- De Brabandere, M.; Hoskin, P.; Haustermans, K.; Van den Heuvel, F.; Siebert, F.A. Prostate post-implant dosimetry: Interobserver variability in seed location, con-touring and fusion. Radiother. Oncol. 2012, 104, 192–198. [Google Scholar] [CrossRef]
- Kim, H.; Houser, C.J.; Kalash, R.; Maceil, C.A.; Palestra, B.; Malush, D.; Beriwal, S. Workflow and efficiency in MRI-based high-dose-rate brachytherapy for cervical cancer in a high-volume brachytherapy center. Brachytherapy 2018, 17, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Harkenrider, M.M.; Alite, F.; Silva, S.R.; Small, W. Image-based brachytherapy for the treatment of cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Owrangi, A.M.; Prisciandaro, J.I.; Soliman, A.; Ravi, A.; Song, W.Y. Magnetic resonance imaging-guided brachytherapy for cervical cancer: Initiating a program. J. Contemp. Brachytherapy 2015, 7, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Tanderup, K.; Viswanathan, A.N.; Kirisits, C.; Frank, S.J. MRI-guided brachytherapy. Semin. Radiat. Oncol. 2014, 24, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, A.N.; Beriwal, S.; Santos, J.F.D.L.; Demanes, D.J.; Gaffney, D.; Hansen, J.; Jones, E.; Kirisits, C.; Thomadsen, B.; Erickson, B. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part II: High-dose-rate brachytherapy. Brachytherapy 2012, 11, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Schindel, J.; Muruganandham, M.; Pigge, F.C.; Anderson, J.; Kim, Y. Magnetic resonance imaging (MRI) Markers for MRI-guided high-dose-rate brachytherapy: Novel marker-flange for cervical cancer and marker catheters for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 387–393. [Google Scholar] [CrossRef]
- Pötter, R.; Haie-Meder, C.; Van Limbergen, E.; Barillot, I.; De Brabandere, M.; Dimopoulos, J.; Dumas, I.; Erickson, B.; Lang, S.; Nulens, A.; et al. Recommendations from the Gynaecological (GYN) GECESTRO Working Group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy–3D volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother. Oncol. 2006, 78, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Campbell-Washburn, A.E.; Faranesh, A.Z.; Lederman, R.J.; Hansen, M.S. Magnetic resonance sequences and rapid acquisition for MR-guided interventions. Magn. Reson. Imaging Clin. N. Am. 2015, 23, 669–679. [Google Scholar] [CrossRef]
- Wang, W.; Viswanathan, A.N.; Damato, A.L.; Chen, Y.; Tse, Z.; Pan, L.; Tokuda, J.; Seethamraju, R.T.; Dumoulin, C.L.; Schmidt, E.J.; et al. Evaluation of an active magnetic resonance tracking system for interstitial brachytherapy. Med. Phys. 2015, 42, 7114–7121. [Google Scholar] [CrossRef]
- Atalar, E.; Ménard, C. MR-guided interventions for prostate cancer. Magn. Reson. Imaging Clin. N. Am. 2005, 13, 491–504. [Google Scholar] [CrossRef][Green Version]
- Zangos, S.; Eichler, K.; Thalhammer, A.; Schoepf, J.U.; Costello, P.; Herzog, C.; Mack, M.G.; Vogl, T.J. MR-guided interventions of the prostate gland. Minim. Invasive Ther. Allied Technol. 2007, 16, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.P.; Pugh, T.J.; Swanson, D.A.; Kudchadker, R.J.; Bruno, T.L.; Christensen, E.N.; van Vulpen, M.; Frank, S.J. Improving prostate brachytherapy quality assurance with MRI–CT fusion–based sector analysis in a phase II prospective trial of men with intermediate-risk prostate cancer. Brachytherapy 2013, 12, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Tanderup, K.; Nielsen, S.K.; Nyvang, G.-B.; Pedersen, E.M.; Røhl, L.; Aagaard, T.; Fokdal, L.; Lindegaard, J.C. From point A to the sculpted pear: MR image guidance significantly improves tumour dose and sparing of organs at risk in brachytherapy of cervical cancer. Radiother. Oncol. 2010, 94, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Gill, B.S.; Kim, H.; Houser, C.J.; Kelley, J.L.; Sukumvanich, P.; Edwards, R.P.; Comerci, J.T.; Olawaiye, A.B.; Huang, M.; Courtney-Brooks, M.; et al. MRI-guided high–dose-rate intracavitary brachytherapy for treatment of cervical cancer: The University of Pittsburgh experience. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Lakosi, F.; de Cuypere, M.; Viet Nguyen, P.; Jansen, N.; Warlimont, B.; Gulyban, A.; Gennigens, C.; Seidel, L.; Delbecque, K.; Coucke, P.; et al. Clinical efficacy and toxicity of radio-chemotherapy and magnetic resonance imaging-guided brachytherapy for locally advanced cervical cancer patients: A mono-institutional experience. Acta Oncol. 2015, 54, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Nomden, C.N.; de Leeuw, A.A.; Roesink, J.M.; Tersteeg, R.J.; Moerland, M.A.; Witteveen, P.O.; Schreuder, H.W.; van Dorst, E.B.; Jürgenliemk-Schulz, I.M. Clinical outcome and dosimetric parameters of chemo-radiation including MRI guided adaptive brachytherapy with tandem-ovoid applicators for cervical cancer patients: A single institution experience. Radiother. Oncol. 2013, 107, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, I.; Janssen, H.; De Brabandere, M.; Nulens, A.; De Bal, D.; Vergote, I.; Van Limbergen, E. Long term experience with 3D image guided brachytherapy and clinical outcome in cervical cancer patients. Radiother. Oncol. 2016, 120, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Rijkmans, E.C.; Nout, R.A.; Rutten, I.H.H.; Ketelaars, M.; Neelis, K.J.; Laman, M.S.; Coen, V.L.; Gaarenstroom, K.N.; Kroep, J.R.; Creutzberg, C.L. Improved survival of patients with cervical cancer treated with image-guided brachytherapy compared with conventional brachytherapy. Gynecol. Oncol. 2014, 135, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Sturdza, A.; Pötter, R.; Fokdal, L.U.; Haie-Meder, C.; Tan, L.T.; Mazeron, R.; Petric, P.; Šegedin, B.; Jurgenliemk-Schulz, I.M.; Nomden, C.; et al. Image guided brachytherapy in locally advanced cervical cancer: Improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother. Oncol. 2016, 120, 428–433. [Google Scholar] [CrossRef]
- Tanderup, K.; Hellebust, T.P.; Lang, S.; Granfeldt, J.; Pötter, R.; Lindegaard, J.C.; Kirisits, C. Consequences of random and systematic reconstruction uncertainties in 3D image based brachytherapy in cervical cancer. Radiother. Oncol. 2008, 89, 156–163. [Google Scholar] [CrossRef]
- Haack, S.; Nielsen, S.K.; Lindegaard, J.C.; Gelineck, J.; Tanderup, K. Applicator reconstruction in MRI 3D image-based dose planning of brachytherapy for cervical cancer. Radiother. Oncol. 2009, 91, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Perez-Calatayud, J.; Kuipers, F.; Ballester, F.; Granero, D.; Richart, J.; Rodriguez, S.; Tormo, A.; Santos, M. Exclusive MRI-based tandem and colpostats reconstruction in gynaecological brachytherapy treatment planning. Radiother. Oncol. 2009, 91, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.J.; Stafford, R.J.; Bankson, J.A.; Li, C.; Swanson, D.A.; Kudchadker, R.J.; Martirosyan, K.S. A novel MRI marker for prostate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.Y.; Stafford, R.J.; Kudchadker, R.J.; Sankaranarayanapillai, M.; Ibbott, G.; Rao, A.; Martirosyan, K.S.; Frank, S.J. MRI characterization of cobalt dichloride-N-acetyl cysteine (C4) contrast agent marker for prostate brachytherapy. Phys. Med. Biol. 2014, 59, 2505–2516. [Google Scholar] [CrossRef] [PubMed]
- Levenberg, K. A method for the solution of certain non-linear problems in least squares. Quart. Appl. Math. 1944, 2, 164–168. Available online: https://www.ams.org/journals/qam/1944-02-02/S0033-569X-1944-10666-0/S0033-569X-1944-10666-0.pdf (accessed on 17 May 2024). [CrossRef]
- Marquardt, D.W. An algorithm for least-squares estimation of nonlinear parameters. J. Soc. Ind. Appl. Math. 1963, 11, 431–441. Available online: https://www.jstor.org/stable/2098941?seq=1#metadata (accessed on 17 May 2024). [CrossRef]
- Nelson, K.L.; Runge, V.M. Basic principles of MR contrast. Top. Magn. Reson. Imaging 1995, 7, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Henkelman, R.M.; Stanisz, G.J.; Kim, J.K.; Bronskill, M.J. Anisotropy of NMR properties of tissues. Magn. Reson. Med. 1994, 32, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Hänninen, N.; Rautiainen, J.; Rieppo, L.; Saarakkala, S.; Nissi, M.J. Orientation anisotropy of quantitative MRI relaxation parameters in ordered tissue. Sci. Rep. 2017, 7, 9606. [Google Scholar] [CrossRef]
- Thomas, S.D.; Wachowicz, K.; Fallone, B.G. MRI of prostate brachytherapy seeds at high field: A study in phantom. Med. Phys. 2009, 36, 5228–5234. [Google Scholar] [CrossRef]
- Nelson, T.; Tung, S. Temperature dependence of proton relaxation times in vitro. Magn. Reson. Imaging 1987, 5, 189–199. [Google Scholar] [CrossRef]
- Graham, S.J.; Bronskill, M.J.; Henkelman, R.M. Time and temperature dependence of MR parameters during thermal coagulation of ex vivo rabbit muscle. Magn. Reson. Med. 1998, 39, 198–203. [Google Scholar] [CrossRef]
- Reichenbach, J.R.; Hackländer, T.; Harth, T.; Hofer, M.; Rassek, M.; Mödder, U. 1H T1 and T2 measurements of the MR imaging contrast agents Gd-DTPA and Gd-DTPA BMA at 1.5T. Eur. Radiol. 1997, 7, 264–274. [Google Scholar] [CrossRef]
- Mahajan, M.; Kuber, R.; Chaudhari, K.R.; Chaudhari, P.; Ghadage, P.; Naik, R. MR imaging of carcinoma cervix. Indian J. Radiol. Imaging 2013, 23, 247–252. [Google Scholar] [CrossRef]
- Camisão, C.C.; Brenna, S.M.F.; Lombardelli, K.V.P.; Djahjah, M.C.R.; Zeferino, L.C. Magnetic resonance imaging in the staging of cervical cancer. Radiol. Bras. 2007, 40, 207–215. [Google Scholar] [CrossRef]
- Nicolet, V.; Carignan, L.; Bourdon, F.; Prosmanne, O. MR imaging of cervical carcinoma: A practical staging approach. RadioGraphics 2000, 20, 1539–1549. [Google Scholar] [CrossRef]
- Sala, E.; Wakely, S.; Senior, E.; Lomas, D. MRI of malignant neoplasms of the uterine corpus and cervix. Am. J. Roentgenol. 2007, 188, 1577–1587. [Google Scholar] [CrossRef]
- Bourgioti, C.; Chatoupis, K.; Moulopoulos, L.A. Current imaging strategies for the evaluation of uterine cervical cancer. World J. Radiol. 2016, 8, 342–354. [Google Scholar] [CrossRef]
- Abdellaoui, A.; Iyengar, S.; Freeman, S. Imaging in prostate cancer. Future Oncol. 2011, 7, 679–691. [Google Scholar] [CrossRef]
- Kuperman, V.Y.; Alley, M.T. Differentiation between the effects of T1 and T2* shortening in contrast-enhanced MRI of the breast. J. Magn. Reson. Imaging 1999, 9, 172–176. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Ding, Y.; Bruno, T.L.; Stafford, R.J.; Lin, E.; Bathala, T.K.; Sanders, J.W.; Ning, M.S.; Ma, J.; Klopp, A.H.; et al. A Novel Positive-Contrast Magnetic Resonance Imaging Line Marker for High-Dose-Rate (HDR) MRI-Assisted Radiosurgery (MARS). Cancers 2024, 16, 1922. https://doi.org/10.3390/cancers16101922
Wang L, Ding Y, Bruno TL, Stafford RJ, Lin E, Bathala TK, Sanders JW, Ning MS, Ma J, Klopp AH, et al. A Novel Positive-Contrast Magnetic Resonance Imaging Line Marker for High-Dose-Rate (HDR) MRI-Assisted Radiosurgery (MARS). Cancers. 2024; 16(10):1922. https://doi.org/10.3390/cancers16101922
Chicago/Turabian StyleWang, Li, Yao Ding, Teresa L. Bruno, R. Jason Stafford, Eric Lin, Tharakeswara K. Bathala, Jeremiah W. Sanders, Matthew S. Ning, Jingfei Ma, Ann H. Klopp, and et al. 2024. "A Novel Positive-Contrast Magnetic Resonance Imaging Line Marker for High-Dose-Rate (HDR) MRI-Assisted Radiosurgery (MARS)" Cancers 16, no. 10: 1922. https://doi.org/10.3390/cancers16101922
APA StyleWang, L., Ding, Y., Bruno, T. L., Stafford, R. J., Lin, E., Bathala, T. K., Sanders, J. W., Ning, M. S., Ma, J., Klopp, A. H., Venkatesan, A., Wang, J., Martirosyan, K. S., & Frank, S. J. (2024). A Novel Positive-Contrast Magnetic Resonance Imaging Line Marker for High-Dose-Rate (HDR) MRI-Assisted Radiosurgery (MARS). Cancers, 16(10), 1922. https://doi.org/10.3390/cancers16101922





