Surgical Treatment for Endometrial Cancer, Hysterectomy Performed via Minimally Invasive Routes Compared with Open Surgery: A Systematic Review and Network Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria, Information Sources, Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Risk of Bias of Included Studies
2.5. Synthesis of Results
3. Results
3.1. Study Characteristics
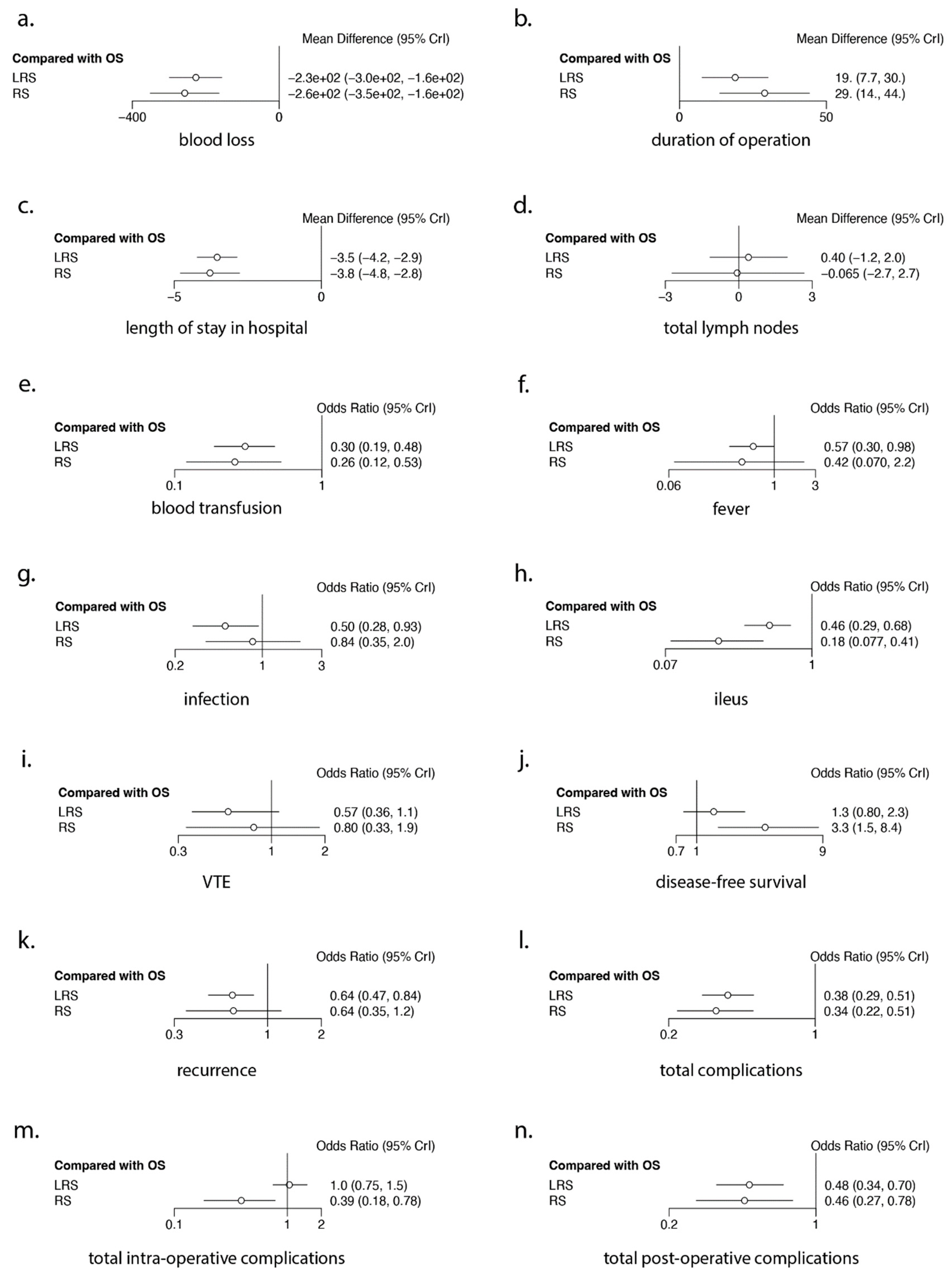
3.2. Intra-Operative Outcomes (Figure 2 and Figure 3, Table 2, Table 3 and Table 4)
3.2.1. Blood Loss
| Outcome | OS | LRS | RS |
|---|---|---|---|
| Blood Loss | 0.0000 | 0.6275 | 0.8725 |
| Duration of Operation | 0.9996 | 0.4496 | 0.0508 |
| Length of Stay in Hospital | 0.0000 | 0.6576 | 0.8424 |
| Total Lymph Nodes | 0.5924 | 0.3200 | 0.5877 |
| Blood Transfusion | 0.0002 | 0.6661 | 0.8338 |
| Fever | 0.0783 | 0.6683 | 0.7533 |
| Infection | 0.1757 | 0.9319 | 0.3925 |
| Ileus | 0.0005 | 0.5055 | 0.9941 |
| VTE | 0.1567 | 0.8688 | 0.4745 |
| Disease-free Survival | 0.0616 | 0.4509 | 0.9876 |
| Recurrence | 0.0393 | 0.7606 | 0.7001 |
| Total Complications | 0.0000 | 0.6194 | 0.8806 |
| Total Intra-operative Complications | 0.3017 | 0.2014 | 0.9969 |
| Total Post-operative Complications | 0.0014 | 0.7118 | 0.7868 |
| Outcome | p-Value | ||||
|---|---|---|---|---|---|
| Blood Loss | LRS vs. OS | 41 | 1.2784 | 9.7550 | 0.8957 |
| RS vs. OS | 16 | −6.7529 | 17.6153 | 0.7015 | |
| RS vs. LRS | 18 | 15.8981 | 4.3915 | 0.0003 | |
| Duration of Operation | LRS vs. OS | 39 | −2.3569 | 1.1987 | 0.0493 |
| RS vs. OS | 15 | −4.9162 | 3.2948 | 0.1357 | |
| RS vs. LRS | 14 | 0.6972 | 3.1650 | 0.8257 | |
| Length of Stay in Hospital | LRS vs. OS | 44 | 0.0241 | 0.0702 | 0.7317 |
| RS vs. OS | 13 | 0.0773 | 0.2957 | 0.7939 | |
| RS vs. LRS | 13 | 0.1858 | 0.1794 | 0.3003 | |
| Total Lymph Nodes | LRS vs. OS | 12 | −0.3310 | 0.2225 | 0.1368 |
| Blood Transfusion | LRS vs. OS | 19 | −0.0915 | 0.0380 | 0.0160 |
| Fever | LRS vs. OS | 10 | 0.0057 | 0.0699 | 0.9344 |
| Infection | LRS vs. OS | 16 | −0.0395 | 0.0525 | 0.4523 |
| Disease-free Survival | LRS vs. OS | 11 | 0.0098 | 0.0262 | 0.7087 |
| Recurrence | LRS vs. OS | 20 | 0.0130 | 0.0334 | 0.6983 |
| Total Complications | LRS vs. OS | 24 | 0.0414 | 0.0213 | 0.0526 |
| RS vs. LRS | 11 | 0.1415 | 0.0674 | 0.0357 | |
| Total Intra-operative Complications | LRS vs. OS | 14 | −0.0448 | 0.0378 | 0.2369 |
| Total Post-operative Complications | LRS vs. OS | 20 | −0.0058 | 0.0438 | 0.8950 |


3.2.2. Duration of Operating Time
3.2.3. Total Lymph Nodes Resected
3.3. Post-Operative Outcomes (Figure 2 and Figure 3, Table 2, Table 3 and Table 4)
Length of Hospital Stay
3.4. Oncological Outcomes (Figure 2 and Figure 3, Table 2, Table 3 and Table 4)
3.5. Ranking Analysis (Figure 2 and Table 2 and Table 3)
3.6. Meta-Regression Analysis (Figure 4 and Table 4)
4. Discussion
4.1. Principal Findings
4.1.1. Robotic-Assisted Surgery
4.1.2. Laparoscopic Surgery
4.2. Comparison with Existing Literature
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Cancer Research Fund. 2020. Available online: https://www.wcrf.org/cancer-trends/endometrial-cancer-statistics/ (accessed on 3 October 2023).
- Cancer Research UK. 2021. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/uterine-cancer/incidence#heading-One (accessed on 3 October 2023).
- Sant, M.; Chirlaque Lopez, M.D.; Agresti, R.; Sánchez Pérez, M.J.; Holleczek, B.; Bielska-Lasota, M.; Dimitrova, N.; Innos, K.; Katalinic, A.; Langseth, H.; et al. Survival of women with cancers of breast and genital organs in Europe 1999-2007: Results of the EUROCARE-5 study. Eur. J. Cancer 2015, 51, 2191–2205. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Balega, J.; Crosbie, E.; Drake, A.; Edmondson, R.; Fotopoulou, C.; Gallos, I.; Ganesan, R.; Gupta, J.; Johnson, N.; et al. BGCS uterine cancer guidelines: Recommendations for practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 213, 71–97. [Google Scholar] [CrossRef]
- Morrison, J.; Balega, J.; Buckley, L.; Clamp, A.; Crosbie, E.; Drew, Y.; Durrant, L.; Forrest, J.; Fotopoulou, C.; Gajjar, K.; et al. British Gynaecological Cancer Society (BGCS) Uterine Cancer Guidelines: Recommendations for Practice. Available online: https://www.bgcs.org.uk/wp-content/uploads/2021/11/British-Gynaecological-Cancer-Society-v13-for-website-with-figure1.pdf (accessed on 8 April 2022).
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Galaal k, D.H.; Bryant, A.; Lopes, A.D. Laparoscopy versus Laprotomy for the managment of early stage endometrial cancer. Cochrane Database Syst. Rev. 2018, 2018, CD006655. [Google Scholar] [CrossRef] [PubMed]
- Deimling, T.A.; Eldridge, J.L.; Riley, K.A.; Kunselman, A.R.; Harkins, G.J. Randomized controlled trial comparing operative times between standard and robot-assisted laparoscopic hysterectomy. Int. J. Gynaecol. Obstet. Off. Organ. Int. Fed. Gynaecol. Obstet. 2017, 136, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Mäenpää, M.M.; Nieminen, K.; Tomás, E.I.; Laurila, M.; Luukkaala, T.H.; Mäenpää, J.U. Robotic-assisted vs traditional laparoscopic surgery for endometrial cancer: A randomized controlled trial. Am. J. Obstet. Gynecol. 2016, 215, 588.e581–588.e587. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.; Åvall-Lundqvist, E.; Legerstam, B.; Carlson, J.W.; Falconer, H. Robot-assisted laparoscopy versus laparotomy for infrarenal paraaortic lymphadenectomy in women with high-risk endometrial cancer: A randomised controlled trial. Eur. J. Cancer 2017, 79, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Wijk, L.; Nilsson, K.; Ljungqvist, O. Metabolic and inflammatory responses and subsequent recovery in robotic versus abdominal hysterectomy: A randomised controlled study. Clin. Nutr. 2018, 37, 99–106. [Google Scholar] [CrossRef]
- Kelley, W.E., Jr. The evolution of laparoscopy and the revolution in surgery in the decade of the 1990s. JSLS J. Soc. Laparoendosc. Surg. 2008, 12, 351–357. [Google Scholar]
- Fram, K.M. Laparoscopically assisted vaginal hysterectomy versus abdominal hysterectomy in stage I endometrial cancer. Int. J. Gynecol. Cancer 2002, 12, 57. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Harrer, M.; Cuijpers, P.; Furukawa, T.; Ebert, D. Doing Meta-Analysis with R: A Hands-On Guide; Taylor & Francis Ltd.: London, UK, 2021. [Google Scholar] [CrossRef]
- Shim, S.R.; Kim, S.J. Intervention meta-analysis: Application and practice using R software. Epidemiol. Health 2019, 41, e2019008. [Google Scholar] [CrossRef]
- Hu, D.; O’Connor, A.M.; Wang, C.; Sargeant, J.M.; Winder, C.B. How to Conduct a Bayesian Network Meta-Analysis. Front. Vet. Sci. 2020, 7, 271. [Google Scholar] [CrossRef] [PubMed]
- van Valkenhoef, G.; Dias, S.; Ades, A.E.; Welton, N.J. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res. Synth. Methods 2016, 7, 80–93. [Google Scholar] [CrossRef]
- Salanti, G.; Ades, A.E.; Ioannidis, J.P.A. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 2011, 64, 163–171. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting Meta-Analyses in R with The metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Abel, M.K.; Chan, J.K.; Chow, S.; Darcy, K.; Tian, C.; Kapp, D.S.; Mann, A.K.; Liao, C.I. Trends and survival outcomes of robotic, laparoscopic, and open surgery for stage II uterine cancer. Int. J. Gynecol. Cancer 2020, 30, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Abitbol, J.; Cohn, R.; Hunter, S.; Rombaldi, M.; Cohen, E.; Kessous, R.; Large, N.; Reiss, A.; Lau, S.; Salvador, S.; et al. Minimizing pain medication use and its associated costs following robotic surgery. Gynecol. Oncol. 2017, 144, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Rajanbabu, A.; Unnikrishnan, U.G. A retrospective evaluation of the perioperative drug use and comparison of its cost in robotic vs open surgery for endometrial cancer. J. Robot. Surg. 2018, 12, 665–672. [Google Scholar] [CrossRef]
- Aiko, K.; Kanno, K.; Yanai, S.; Masuda, S.; Yasui, M.; Ichikawa, F.; Teishikata, Y.; Shirane, T.; Yoshino, Y.; Sakate, S.; et al. Short-term outcomes of robot-assisted versus conventional laparoscopic surgery for early-stage endometrial cancer: A retrospective, single-center study. J. Obstet. Gynaecol. Res. 2020, 46, 1157–1164. [Google Scholar] [CrossRef]
- Ansar, P.P.; Ayyappan, S.; Mahajan, V. Prospective Nonrandomized Comparative Study of Laparoscopic Versus Open Surgical Staging for Endometrial Cancer in India. Indian. J. Surg. Oncol. 2018, 9, 133–140. [Google Scholar] [CrossRef]
- Api, M.; Kayatas, S.; Boza, A.T.; Nazik, H.; Adiguzel, C.; Guzin, K.; Eroglu, M. Surgical Staging of Early Stage Endometrial Cancer: Comparison between Laparotomy and Laparoscopy. World J. Oncol. 2013, 4, 235–240. [Google Scholar] [CrossRef][Green Version]
- Armfield, N.R.; Janda, M.; Obermair, A. Obesity in total laparoscopic hysterectomy for early stage endometrial cancer: Health gain and inpatient resource use. Int. J. Qual. Health Care J. Int. Soc. Qual. Health Care 2019, 31, 283–288. [Google Scholar] [CrossRef]
- Avondstondt, A.M.; Wallenstein, M.; D’Adamo, C.R.; Ehsanipoor, R.M. Change in cost after 5 years of experience with robotic-assisted hysterectomy for the treatment of endometrial cancer. J. Robot. Surg. 2018, 12, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Backes, F.J.; Rosen, M.; Liang, M.; McCann, G.A.; Clements, A.; Cohn, D.E.; O’Malley, D.M.; Salani, R.; Fowler, J.M. Robotic Hysterectomy for Endometrial Cancer in Obese Patients with Comorbidities: Evaluating Postoperative Complications. Int. J. Gynecol. Cancer 2015, 25, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Backes, F.J.; ElNaggar, A.C.; Farrell, M.R.; Brudie, L.A.; Ahmad, S.; Salani, R.; Cohn, D.E.; Holloway, R.W.; Fowler, J.M.; O’Malley, D.M. Perioperative Outcomes for Laparotomy Compared to Robotic Surgical Staging of Endometrial Cancer in the Elderly: A Retrospective Cohort. Int. J. Gynecol. Cancer 2016, 26, 1717–1721. [Google Scholar] [CrossRef]
- Baek, M.H.; Lee, S.W.; Park, J.Y.; Kim, D.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. Feasibility and safety of laparoscopic surgery for obese Korean women with endometrial cancer: Long-term results at a single institution. J. Korean Med. Sci. 2014, 29, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, P.K.; Barnes, M.N.; Robertson, M.W.; Shah, P.; Austin, J.M., 3rd; Partridge, E.E.; Austin, J.M., Jr. Surgical management of endometrial adenocarcinoma using laparoscopically assisted staging and treatment. South. Med. J. 1999, 92, 1174–1177. [Google Scholar] [CrossRef]
- Baker, J.; Janda, M.; Belavy, D.; Obermair, A. Differences in Epidural and Analgesic Use in Patients with Apparent Stage I Endometrial Cancer Treated by Open versus Laparoscopic Surgery: Results from the Randomised LACE Trial. Minim. Invasive Surg. 2013, 2013, 764329. [Google Scholar] [CrossRef][Green Version]
- Baker, J.; Janda, M.; Gebski, V.; Forder, P.; Hogg, R.; Manolitsas, T.; Obermair, A. Lower preoperative quality of life increases postoperative risk of adverse events in women with endometrial cancer: Results from the LACE trial. Gynecol. Oncol. 2015, 137, 102–105. [Google Scholar] [CrossRef]
- Bakkum-Gamez, J.N.; Dowdy, S.C.; Borah, B.J.; Haas, L.R.; Mariani, A.; Martin, J.R.; Weaver, A.L.; McGree, M.E.; Cliby, W.A.; Podratz, K.C. Predictors and costs of surgical site infections in patients with endometrial cancer. Gynecol. Oncol. 2013, 130, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Ball, A.; Bentley, J.R.; O’Connell, C.; Kieser, K.E. Choosing the right patient: Planning for laparotomy or laparoscopy in the patient with endometrial cancer. J. Obstet. Gynaecol. Can. JOGC = J. D’obstetrique Gynecol. Can. JOGC 2011, 33, 468–474. [Google Scholar] [CrossRef]
- Barber, E.L.; Gehrig, P.A.; Clarke-Pearson, D.L. Venous Thromboembolism in Minimally Invasive Compared with Open Hysterectomy for Endometrial Cancer. Obstet. Gynecol. 2016, 128, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Barnes, H.; Harrison, R.; Huffman, L.; Medlin, E.; Spencer, R.; Al-Niaimi, A. The Adoption of Single-port Laparoscopy for Full Staging of Endometrial Cancer: Surgical and Oncology Outcomes and Evaluation of the Learning Curve. J. Minim. Invasive Gynecol. 2017, 24, 1029–1036. [Google Scholar] [CrossRef]
- Barnett, J.C.; Havrilesky, L.J.; Bondurant, A.E.; Fleming, N.D.; Lee, P.S.; Secord, A.A.; Berchuck, A.; Valea, F.A. Adverse events associated with laparoscopy vs laparotomy in the treatment of endometrial cancer. Am. J. Obstet. Gynecol. 2011, 205, 143.e141–143.e146. [Google Scholar] [CrossRef] [PubMed]
- Barnett, J.C.; Judd, J.P.; Wu, J.M.; Scales, C.D., Jr.; Myers, E.R.; Havrilesky, L.J. Cost comparison among robotic, laparoscopic, and open hysterectomy for endometrial cancer. Obstet. Gynecol. 2010, 116, 685–693. [Google Scholar] [CrossRef]
- Barraez, D.; Godoy, H.; McElrath, T.; Kredentser, D.; Timmins, P. Low incidence of port-site metastasis after robotic assisted surgery for endometrial cancer staging: Descriptive analysis. J. Robot. Surg. 2015, 9, 91–95. [Google Scholar] [CrossRef]
- Barwijuk, A.; Jankowska, S. Is laparoscopic or abdominal hysterectomy with bilateral salpingo-oophorectomy more efficient in operative treatment of endometrial cancer? J. Obstet. Gynaecol. 2005, 25, 703–705. [Google Scholar] [CrossRef]
- Bell, M.C.; Torgerson, J.; Seshadri-Kreaden, U.; Suttle, A.W.; Hunt, S. Comparison of outcomes and cost for endometrial cancer staging via traditional laparotomy, standard laparoscopy and robotic techniques. Gynecol. Oncol. 2008, 111, 407–411. [Google Scholar] [CrossRef]
- Bennich, G.; Rudnicki, M.; Lassen, P.D. Laparoscopic surgery for early endometrial cancer. Acta Obstet. Gynecol. Scand. 2016, 95, 894–900. [Google Scholar] [CrossRef]
- Beck, T.L.; Schiff, M.A.; Goff, B.A.; Urban, R.R. Robotic, Laparoscopic, or Open Hysterectomy: Surgical Outcomes by Approach in Endometrial Cancer. J. Minim. Invasive Gynecol. 2018, 25, 986–993. [Google Scholar] [CrossRef]
- Bergstrom, J.; Aloisi, A.; Armbruster, S.; Yen, T.T.; Casarin, J.; Leitao, M.M., Jr.; Tanner, E.J.; Matsuno, R.; Machado, K.K.; Dowdy, S.C.; et al. Minimally invasive hysterectomy surgery rates for endometrial cancer performed at National Comprehensive Cancer Network (NCCN) Centers. Gynecol. Oncol. 2018, 148, 480–484. [Google Scholar] [CrossRef]
- Bernardini, M.Q.; Gien, L.T.; Tipping, H.; Murphy, J.; Rosen, B.P. Surgical outcome of robotic surgery in morbidly obese patient with endometrial cancer compared to laparotomy. Int. J. Gynecol. Cancer 2012, 22, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Berretta, R.; Gizzo, S.; Noventa, M.; Marrazzo, V.; Franchi, L.; Migliavacca, C.; Michela, M.; Merisio, C.; Modena, A.B.; Patrelli, T.S. Quality of Life in Patients Affected by Endometrial Cancer: Comparison Among Laparotomy, Laparoscopy and Vaginal Approach. Pathol. Oncol. Res. 2015, 21, 811–816. [Google Scholar] [CrossRef]
- Bige, Ö.; Demir, A.; Saatli, B.; Koyuncuoğlu, M.; Saygılı, U. Laparoscopy versus laparotomy for the management of endometrial carcinoma in morbidly obese patients: A prospective study. J. Turk. Ger. Gynecol. Assoc. 2015, 16, 164–169. [Google Scholar] [CrossRef]
- Bishop, E.A.; Java, J.J.; Moore, K.N.; Spirtos, N.M.; Pearl, M.L.; Zivanovic, O.; Kushner, D.M.; Backes, F.; Hamilton, C.A.; Geller, M.A.; et al. Surgical outcomes among elderly women with endometrial cancer treated by laparoscopic hysterectomy: A NRG/Gynecologic Oncology Group study. Am. J. Obstet. Gynecol. 2018, 218, 109.e101–109.e111. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Cromi, A.; Uccella, S.; Serati, M.; Casarin, J.; Mariani, A.; Ghezzi, F. Laparoscopic staging in women older than 75 years with early-stage endometrial cancer: Comparison with open surgical operation. Menopause 2014, 21, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Multinu, F.; Dowdy, S.C.; Cliby, W.A.; Wilson, T.O.; Gostout, B.S.; Weaver, A.L.; Borah, B.J.; Killian, J.M.; Bijlani, A.; et al. Incorporating robotic-assisted surgery for endometrial cancer staging: Analysis of morbidity and costs. Gynecol. Oncol. 2016, 141, 218–224. [Google Scholar] [CrossRef]
- Boggess, J.F.; Gehrig, P.A.; Cantrell, L.; Shafer, A.; Ridgway, M.; Skinner, E.N.; Fowler, W.C. A comparative study of 3 surgical methods for hysterectomy with staging for endometrial cancer: Robotic assistance, laparoscopy, laparotomy. Am. J. Obstet. Gynecol. 2008, 199, 360.e361–360.e369. [Google Scholar] [CrossRef]
- Bourgin, C.; Lambaudie, E.; Houvenaeghel, G.; Foucher, F.; Levêque, J.; Lavoué, V. Impact of age on surgical staging and approaches (laparotomy, laparoscopy and robotic surgery) in endometrial cancer management. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2017, 43, 703–709. [Google Scholar] [CrossRef]
- Bouwman, F.; Smits, A.; Lopes, A.; Das, N.; Pollard, A.; Massuger, L.; Bekkers, R.; Galaal, K. The impact of BMI on surgical complications and outcomes in endometrial cancer surgery--an institutional study and systematic review of the literature. Gynecol. Oncol. 2015, 139, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.; Vaknin, Z.; Ramana-Kumar, A.V.; Halliday, D.; Franco, E.L.; Gotlieb, W.H. Outcomes and cost comparisons after introducing a robotics program for endometrial cancer surgery. Obstet. Gynecol. 2012, 119, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Lavoue, V.; Zeng, X.; Lau, S.; Press, J.Z.; Abitbol, J.; Gotlieb, R.; How, J.; Wang, Y.; Gotlieb, W.H. Impact of robotics on the outcome of elderly patients with endometrial cancer. Gynecol. Oncol. 2014, 133, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Frey, M.K.; Ihnow, S.B.; Worley, M.J.; Heyman, K.P.; Kessler, R.; Slomovitz, B.M.; Holcomb, K.M. Minimally Invasive Staging of Endometrial Cancer Is Feasible and Safe in Elderly Women. J. Minim. Invasive Gynecol. 2011, 18, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Seamon, L.G.; Fowler, J.M.; Richardson, D.L.; Carlson, M.J.; Valmadre, S.; Phillips, G.S.; Cohn, D.E. A detailed analysis of the learning curve: Robotic hysterectomy and pelvic-aortic lymphadenectomy for endometrial cancer. Gynecol. Oncol. 2009, 114, 162–167. [Google Scholar] [CrossRef]
- Gunderson, C.C.; Java, J.; Moore, K.N.; Walker, J.L. The impact of obesity on surgical staging, complications, and survival with uterine cancer: A Gynecologic Oncology Group LAP2 ancillary data study. Gynecol. Oncol. 2014, 133, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Rabischong, B.; Larraín, D.; Canis, M.; Le Bouëdec, G.; Pomel, C.; Jardon, K.; Kwiatkowski, F.; Bourdel, N.; Achard, J.L.; Dauplat, J.; et al. Long-term follow-up after laparoscopic management of endometrial cancer in the obese: A fifteen-year cohort study. J. Minim. Invasive Gynecol. 2011, 18, 589–596. [Google Scholar] [CrossRef]
- Eltabbakh, G.H. Effect of surgeon’s experience on the surgical outcome of laparoscopic surgery for women with endometrial cancer. Gynecol. Oncol. 2000, 78, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Eltabbakh, G.H. Analysis of survival after laparoscopy in women with endometrial carcinoma. Cancer 2002, 95, 1894–1901. [Google Scholar] [CrossRef]
- Peiretti, M.; Congiu, F.; Ricciardi, E.; Maniglio, P.; Mais, V.; Angioni, S. Conservative treatment for well-differentiated endometrial cancer: When and why it should be considered in young women. Ecancermedicalscience 2019, 13, 892. [Google Scholar] [CrossRef]
- Lee, J.; Gerber, D.; Aphinyanaphongs, Y.; Curtin, J.P.; Boyd, L.R. Laparoscopy decreases the disparity in postoperative complications between black and white women after hysterectomy for endometrial cancer. Gynecol. Oncol. 2018, 149, 22–27. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, U.S.; Kyung, M.S.; Choi, J.S. Laparoscopic-assisted staging surgery for Korean women with endometrial cancer. JSLS J. Soc. Laparoendosc. Surg. 2008, 12, 150–155. [Google Scholar]
- Fader, A.N.; Seamon, L.G.; Escobar, P.F.; Frasure, H.E.; Havrilesky, L.A.; Zanotti, K.M.; Secord, A.A.; Boggess, J.F.; Cohn, D.E.; Fowler, J.M.; et al. Minimally invasive surgery versus laparotomy in women with high grade endometrial cancer: A multi-site study performed at high volume cancer centers. Gynecol. Oncol. 2012, 126, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Fader, A.N.; Weise, R.M.; Sinno, A.K.; Tanner, E.J., 3rd; Borah, B.J.; Moriarty, J.P.; Bristow, R.E.; Makary, M.A.; Pronovost, P.J.; Hutfless, S.; et al. Utilization of Minimally Invasive Surgery in Endometrial Cancer Care: A Quality and Cost Disparity. Obstet. Gynecol. 2016, 127, 91–100. [Google Scholar] [CrossRef]
- Fagotti, A.; Boruta, D.M., 2nd; Scambia, G.; Fanfani, F.; Paglia, A.; Escobar, P.F. First 100 early endometrial cancer cases treated with laparoendoscopic single-site surgery: A multicentric retrospective study. Am. J. Obstet. Gynecol. 2012, 206, 353.e1–353.e6. [Google Scholar] [CrossRef]
- Fagotti, A.; Corrado, G.; Fanfani, F.; Mancini, M.; Paglia, A.; Vizzielli, G.; Sindico, S.; Scambia, G.; Vizza, E. Robotic single-site hysterectomy (RSS-H) vs. laparoendoscopic single-site hysterectomy (LESS-H) in early endometrial cancer: A double-institution case-control study. Gynecol. Oncol. 2013, 130, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Gagliardi, M.L.; Fanfani, F.; Salerno, M.G.; Ercoli, A.; D’Asta, M.; Tortorella, L.; Turco, L.C.; Escobar, P.; Scambia, G. Perioperative outcomes of total laparoendoscopic single-site hysterectomy versus total robotic hysterectomy in endometrial cancer patients: A multicentre study. Gynecol. Oncol. 2012, 125, 552–555. [Google Scholar] [CrossRef]
- Fanning, J.; Hossler, C. Laparoscopic conversion rate for uterine cancer surgical staging. Obstet. Gynecol. 2010, 116, 1354–1357. [Google Scholar] [CrossRef]
- Farthing, A.; Chatterjee, J.; Joglekar-Pai, P.; Dorney, E.; Ghaem-Maghami, S. Total laparoscopic hysterectomy for early stage endometrial cancer in obese and morbidly obese women. J. Obstet. Gynaecol. 2012, 32, 580–584. [Google Scholar] [CrossRef]
- Fleming, N.D.; Axtell, A.E.; Lentz, S.E. Operative and anesthetic outcomes in endometrial cancer staging via three minimally invasive methods. J. Robot. Surg. 2012, 6, 337–344. [Google Scholar] [CrossRef]
- Fleming, N.D.; Havrilesky, L.J.; Valea, F.A.; Allen, T.K.; Broadwater, G.; Bland, A.; Habib, A.S. Analgesic and antiemetic needs following minimally invasive vs open staging for endometrial cancer. Am. J. Obstet. Gynecol. 2011, 204, 65.e1–65.e6. [Google Scholar] [CrossRef] [PubMed]
- Gehrig, P.A.; Cantrell, L.A.; Shafer, A.; Abaid, L.N.; Mendivil, A.; Boggess, J.F. What is the optimal minimally invasive surgical procedure for endometrial cancer staging in the obese and morbidly obese woman? Gynecol. Oncol. 2008, 111, 41–45. [Google Scholar] [CrossRef]
- Gemignani, M.L.; Curtin, J.P.; Zelmanovich, J.; Patel, D.A.; Venkatraman, E.; Barakat, R.R. Laparoscopic-assisted vaginal hysterectomy for endometrial cancer: Clinical outcomes and hospital charges. Gynecol. Oncol. 1999, 73, 5–11. [Google Scholar] [CrossRef]
- Ghazali, W.; Jamil, S.A.; Sharin, I.A. Laparoscopic versus Laparotomy: Staging Surgery for Endometrial Cancer—Malaysia’s Early Experience. Gynecol. Minim. Invasive Ther. 2019, 8, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, F.; Cromi, A.; Bergamini, V.; Uccella, S.; Beretta, P.; Franchi, M.; Bolis, P. Laparoscopic management of endometrial cancer in nonobese and obese women: A consecutive series. J. Minim. Invasive Gynecol. 2006, 13, 269–275. [Google Scholar] [CrossRef]
- Ghezzi, F.; Cromi, A.; Bergamini, V.; Uccella, S.; Beretta, P.; Franchi, M.; Bolis, P. Laparoscopic-assisted vaginal hysterectomy versus total laparoscopic hysterectomy for the management of endometrial cancer: A randomized clinical trial. J. Minim. Invasive Gynecol. 2006, 13, 114–120. [Google Scholar] [CrossRef]
- Ghezzi, F.; Cromi, A.; Siesto, G.; Zefiro, F.; Franchi, M.; Bolis, P. Microlaparoscopy: A further development of minimally invasive surgery for endometrial cancer staging—Initial experience. Gynecol. Oncol. 2009, 113, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, F.; Cromi, A.; Uccella, S.; Siesto, G.; Giudici, S.; Serati, M.; Franchi, M. Laparoscopic Versus Open Surgery for Endometrial Cancer: A Minimum 3-Year Follow-Up Study. Ann. Surg. Oncol. 2010, 17, 271–278. [Google Scholar] [CrossRef]
- Giannini, A.; Malacarne, E.; Sergiampietri, C.; Mannella, P.; Perutelli, A.; Cela, V.; Stomati, M.; Melfi, F.; Simoncini, T. Comparison of perioperative outcomes and technical features using da Vinci Si and Xi robotic platforms for early stages of endometrial cancer. J. Robot. Surg. 2021, 15, 195–201. [Google Scholar] [CrossRef]
- Gil-Moreno, A.; Díaz-Feijoo, B.; Morchón, S.; Xercavins, J. Analysis of survival after laparoscopic-assisted vaginal hysterectomy compared with the conventional abdominal approach for early-stage endometrial carcinoma: A review of the literature. J. Minim. Invasive Gynecol. 2006, 13, 26–35. [Google Scholar] [CrossRef]
- Göçmen, A.; Şanlıkan, F.; Uçar, M.G. Comparison of robotic-assisted surgery outcomes with laparotomy for endometrial cancer staging in Turkey. Arch. Gynecol. Obstet. 2010, 282, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Gueli Alletti, S.; Rossitto, C.; Cianci, S.; Restaino, S.; Costantini, B.; Fanfani, F.; Fagotti, A.; Cosentino, F.; Scambia, G. Telelap ALF-X vs Standard Laparoscopy for the Treatment of Early-Stage Endometrial Cancer: A Single-Institution Retrospective Cohort Study. J. Minim. Invasive Gynecol. 2016, 23, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.B.; Habib, A.S.; Broadwater, G.; Valea, F.A.; Fleming, N.D.; Ehrisman, J.A.; Di Santo, N.; Havrilesky, L.J. Postoperative Pain Scores and Narcotic Use in Robotic-assisted Versus Laparoscopic Hysterectomy for Endometrial Cancer Staging. J. Minim. Invasive Gynecol. 2015, 22, 1004–1010. [Google Scholar] [CrossRef]
- Helm, C.W.; Arumugam, C.; Gordinier, M.E.; Metzinger, D.S.; Pan, J.; Rai, S.N. Laparoscopic surgery for endometrial cancer: Increasing body mass index does not impact postoperative complications. J. Gynecol. Oncol. 2011, 22, 168–176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Herling, S.F. Robotic-assisted laparoscopic hysterectomy for women with endometrial cancer—Complications, women’s experiences, quality of life and a health economic evaluation. Dan. Med. J. 2016, 63, B5262. [Google Scholar] [PubMed]
- Hinshaw, S.J.; Gunderson, S.; Eastwood, D.; Bradley, W.H. Endometrial carcinoma: The perioperative and long-term outcomes of robotic surgery in the morbidly obese. J. Surg. Oncol. 2016, 114, 884–887. [Google Scholar] [CrossRef] [PubMed]
- Holloway, R.W.; Ahmad, S.; DeNardis, S.A.; Peterson, L.B.; Sultana, N.; Bigsby, G.E.; Pikaart, D.P.; Finkler, N.J. Robotic-assisted laparoscopic hysterectomy and lymphadenectomy for endometrial cancer: Analysis of surgical performance. Gynecol. Oncol. 2009, 115, 447–452. [Google Scholar] [CrossRef]
- Holtz, D.O.; Miroshnichenko, G.; Finnegan, M.O.; Chernick, M.; Dunton, C.J. Endometrial Cancer Surgery Costs: Robot vs Laparoscopy. J. Minim. Invasive Gynecol. 2010, 17, 500–503. [Google Scholar] [CrossRef]
- Holub, Z.; Jabor, A.; Bartos, P.; Hendl, J.; Urbánek, S. Laparoscopic surgery in women with endometrial cancer: The learning curve. Eur. J. Obs. Gynecol. Reprod. Biol. 2003, 107, 195–200. [Google Scholar] [CrossRef]
- Freeman, A.H.; Barrie, A.; Lyon, L.; Littell, R.D.; Garcia, C.; Conell, C.; Powell, C.B. Venous thromboembolism following minimally invasive surgery among women with endometrial cancer. Gynecol. Oncol. 2016, 142, 267–272. [Google Scholar] [CrossRef]
- Frey, M.K.; Lin, J.F.; Stewart, L.E.; Makaroun, L.; Panico, V.J.; Holcomb, K. Comparison of two minimally invasive approaches to endometrial cancer staging: A single-surgeon experience. J. Reprod. Med. 2015, 60, 127–134. [Google Scholar] [PubMed]
- Frigerio, L.; Gallo, A.; Ghezzi, F.; Trezzi, G.; Lussana, M.; Franchi, M. Laparoscopic-assisted vaginal hysterectomy versus abdominal hysterectomy in endometrial cancer. Int. J. Gynaecol. Obstet. Off. Organ. Int. Fed. Gynaecol. Obstet. 2006, 93, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Gambacorti-Passerini, Z.M.; López-De la Manzanara Cano, C.; Pérez Parra, C.; Cespedes Casas, M.C.; Sánchez Hipólito, L.; Martín Francisco, C.; Muñoz-Rodríguez, J.R. Obesity in Patients with Endometrial Cancer: May It Affect the Surgical Outcomes of Laparoscopic Approach? Obes. Surg. 2019, 29, 3285–3290. [Google Scholar] [CrossRef] [PubMed]
- Giannini, A.; Di Donato, V.; Schiavi, M.C.; May, J.; Panici, P.B.; Congiu, M.A. Predictors of postoperative overall and severe complications after surgical treatment for endometrial cancer: The role of the fragility index. Int. J. Gynaecol. Obstet. Off. Organ. Int. Fed. Gynaecol. Obstet. 2020, 148, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Gildea, C.; Nordin, A.; Hirschowitz, L.; Poole, J. Thirty-day postoperative mortality for endometrial carcinoma in England: A population-based study. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.; Zollinger, T.W.; Moore, D.H. Surgical staging of endometrial cancer: Robotic versus open technique outcomes in a contemporary single surgeon series. J. Robot. Surg. 2011, 5, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Grabosch, S.; Xynos, F. Isolated port-site metastasis after robotic hysterectomy for stage IA endometrial adenocarcinoma. Obstet. Gynecol. 2013, 122, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Graves, N.; Janda, M.; Merollini, K.; Gebski, V.; Obermair, A. The cost-effectiveness of total laparoscopic hysterectomy compared to total abdominal hysterectomy for the treatment of early stage endometrial cancer. BMJ Open 2013, 3, e001884. [Google Scholar] [CrossRef] [PubMed]
- Graybill, W.S.; Frumovitz, M.; Nick, A.M.; Wei, C.; Mena, G.E.; Soliman, P.T.; dos Reis, R.; Schmeler, K.M.; Ramirez, P.T. Impact of smoking on perioperative pulmonary and upper respiratory complications after laparoscopic gynecologic surgery. Gynecol. Oncol. 2012, 125, 556–560. [Google Scholar] [CrossRef]
- Lee, C.L.; Han, C.M.; Su, H.; Wu, K.Y.; Wang, C.J.; Yen, C.F. Robot-assisted laparoscopic staging surgery for endometrial cancer—A preliminary report. Taiwan. J. Obstet. Gynecol. 2010, 49, 401–406. [Google Scholar] [CrossRef]
- Lee, C.L.; Huang, K.G.; Wu, P.J.; Lee, P.S.; Yen, C.F. Long-term survival outcome of laparoscopic staging surgery for endometrial cancer in Taiwanese experience. Taiwan. J. Obstet. Gynecol. 2014, 53, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Kusunoki, S.; Huang, K.G.; Wu, K.Y.; Huang, C.Y.; Yen, C.F. Long-term survival outcomes of laparoscopic staging surgery in treating endometrial cancer: 20 years of follow-up. Taiwan. J. Obstet. Gynecol. 2016, 55, 545–551. [Google Scholar] [CrossRef][Green Version]
- Lee, C.-L.; Wu, K.-Y.; Tsao, F.-Y.; Huang, C.-Y.; Han, C.-M.; Yen, C.-F.; Huang, K.-G. Natural orifice transvaginal endoscopic surgery for endometrial cancer. Gynecol. Minim. Invasive Ther. 2014, 3, 89–92. [Google Scholar] [CrossRef]
- Lee, J.; Aphinyanaphongs, Y.; Curtin, J.P.; Chern, J.Y.; Frey, M.K.; Boyd, L.R. The safety of same-day discharge after laparoscopic hysterectomy for endometrial cancer. Gynecol. Oncol. 2016, 142, 508–513. [Google Scholar] [CrossRef]
- Lee, M.; Kim, Y.T.; Kim, S.W.; Kim, S.; Kim, J.H.; Nam, E.J. Effects of uterine manipulation on surgical outcomes in laparoscopic management of endometrial cancer: A prospective randomized clinical trial. Int. J. Gynecol. Cancer 2013, 23, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Leiserowitz, G.S.; Xing, G.; Parikh-Patel, A.; Cress, R.; Abidi, A.; Rodriguez, A.O.; Dalrymple, J.L. Laparoscopic versus abdominal hysterectomy for endometrial cancer: Comparison of patient outcomes. Int. J. Gynecol. Cancer 2009, 19, 1370–1376. [Google Scholar] [CrossRef]
- Leitao, M.M.; Malhotra, V.; Briscoe, G.; Suidan, R.; Dholakiya, P.; Santos, K.; Jewell, E.L.; Brown, C.L.; Sonoda, Y.; Abu-Rustum, N.R.; et al. Postoperative Pain Medication Requirements in Patients Undergoing Computer-Assisted (“Robotic”) and Standard Laparoscopic Procedures for Newly Diagnosed Endometrial Cancer. Ann. Surg. Oncol. 2013, 20, 3561–3567. [Google Scholar] [CrossRef] [PubMed]
- Leitao, M.M., Jr.; Briscoe, G.; Santos, K.; Winder, A.; Jewell, E.L.; Hoskins, W.J.; Chi, D.S.; Abu-Rustum, N.R.; Sonoda, Y.; Brown, C.L.; et al. Introduction of a computer-based surgical platform in the surgical care of patients with newly diagnosed uterine cancer: Outcomes and impact on approach. Gynecol. Oncol. 2012, 125, 394–399. [Google Scholar] [CrossRef]
- Leitao, M.M.; Narain, W.R.; Boccamazzo, D.; Sioulas, V.; Cassella, D.; Ducie, J.A.; Eriksson, A.G.; Sonoda, Y.; Chi, D.S.; Brown, C.L.; et al. Impact of Robotic Platforms on Surgical Approach and Costs in the Management of Morbidly Obese Patients with Newly Diagnosed Uterine Cancer. Ann. Surg. Oncol. 2016, 23, 2192–2198. [Google Scholar] [CrossRef]
- Li, L.a.; Wang, X.; Zhang, Y.; Fan, W.; Li, Y.; Song, L.; Yao, Y.; Guan, Z.; Meng, Y. Laparoscopic surgery in endometrial carcinoma staging operation: Analysis of 39 cases. Chin.-Ger. J. Clin. Oncol. 2011, 10, 108–110. [Google Scholar] [CrossRef]
- Liang, M.I.; Rosen, M.A.; Rath, K.S.; Clements, A.E.; Backes, F.J.; Eisenhauer, E.L.; Salani, R.; O’Malley, D.M.; Fowler, J.M.; Cohn, D.E. Reducing readmissions after robotic surgical management of endometrial cancer: A potential for improved quality care. Gynecol. Oncol. 2013, 131, 508–511. [Google Scholar] [CrossRef]
- Liang, M.I.; Rosen, M.A.; Rath, K.S.; Hade, E.M.; Clements, A.E.; Backes, F.J.; Eisenhauer, E.L.; Salani, R.; O’Malley, D.M.; Fowler, J.M.; et al. Predicting inpatient stay lasting 2 midnights or longer after robotic surgery for endometrial cancer. J. Minim. Invasive Gynecol. 2015, 22, 583–589. [Google Scholar] [CrossRef]
- Liauw, L.; Chung, Y.N.; Tsoi, C.W.; Pang, C.P.; Cheung, K.B. Laparoscopy for the treatment of women with endometrial cancer. Hong Kong Med. J. 2003, 9, 108–112. [Google Scholar]
- Tenney, M.; Walker, J.L. Role of laparoscopic surgery in the management of endometrial cancer. J. Natl. Compr. Cancer Netw. JNCCN 2009, 7, 559–567. [Google Scholar] [CrossRef]
- Lim, B.K.; Lavie, O.; Bolger, B.; Lopes, T.; Monaghan, J.M. The role of laparoscopic surgery in the management of endometrial cancer. BJOG Int. J. Obstet. Gynaecol. 2000, 107, 24–27. [Google Scholar] [CrossRef][Green Version]
- Lim, S.; Kim, H.S.; Lee, K.B.; Yoo, C.W.; Park, S.Y.; Seo, S.S. Does the use of a uterine manipulator with an intrauterine balloon in total laparoscopic hysterectomy facilitate tumor cell spillage into the peritoneal cavity in patients with endometrial cancer? Int. J. Gynecol. Cancer 2008, 18, 1145–1149. [Google Scholar] [CrossRef]
- Limbachiya, D.J. Surgicopathological Outcomes and Survival in Carcinoma Body Uterus: A Retrospective Analysis of Cases Managed by Laparoscopic Staging Surgery in Indian Women. Gynecol. Minim. Invasive Ther. 2020, 9, 139–144. [Google Scholar] [CrossRef]
- Lindfors, A.; Åkesson, Å.; Staf, C.; Sjöli, P.; Sundfeldt, K.; Dahm-Kähler, P. Robotic vs Open Surgery for Endometrial Cancer in Elderly Patients: Surgical Outcome, Survival, and Cost Analysis. Int. J. Gynecol. Cancer 2018, 28, 692–699. [Google Scholar] [CrossRef]
- Lindfors, A.; Heshar, H.; Adok, C.; Sundfeldt, K.; Dahm-Kähler, P. Long-term survival in obese patients after robotic or open surgery for endometrial cancer. Gynecol. Oncol. 2020, 158, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.P.; Cheng, X.W.; Tian, X.; Zhang, Q.; Cui, H.Y.; Hua, K.Q. Laparoscopic surgery for endometrial cancer in aged patients: Experience from a tertiary referral center in Eastern China. J. Cancer Res. Ther. 2017, 13, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Loaec, C.; Bats, A.S.; Ngo, C.; Cornou, C.; Rossi, L.; Bensaid, C.; Nos, C.; Lecuru, F. Dual docking robotic surgical staging for high risk endometrial cancer. Eur. J. Obs. Gynecol. Reprod. Biol. 2018, 225, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Lowe, M.P.; Kumar, S.; Johnson, P.R.; Kamelle, S.A.; Chamberlain, D.H.; Tillmanns, T.D. Robotic surgical management of endometrial cancer in octogenarians and nonagenarians: Analysis of perioperative outcomes and review of the literature. J. Robot. Surg. 2010, 4, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Lowe, M.P.; Johnson, P.R.; Kamelle, S.A.; Kumar, S.; Chamberlain, D.H.; Tillmanns, T.D. A multiinstitutional experience with robotic-assisted hysterectomy with staging for endometrial cancer. Obstet. Gynecol. 2009, 114, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Liu, H.; Liu, C.; Wang, S.; Li, S.; Guo, S.; Lu, J.; Zhang, Z. Comparison of laparoscopy and laparotomy for management of endometrial carcinoma: A prospective randomized study with 11-year experience. J. Cancer Res. Clin. Oncol. 2013, 139, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Yi, X.; Feng, W.; Ding, J.; Xu, H.; Zhou, X.; Hua, K. Cost-benefit analysis of laparoscopic surgery versus laparotomy for patients with endometrioid endometrial cancer: Experience from an institute in China. J. Obstet. Gynaecol. Res. 2012, 38, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Lunde, S.; Nguyen, H.T.; Petersen, K.K.; Arendt-Nielsen, L.; Krarup, H.B.; Søgaard-Andersen, E. Chronic Postoperative Pain After Hysterectomy for Endometrial Cancer: A Metabolic Profiling Study. Mol. Pain 2020, 16, 1744806920923885. [Google Scholar] [CrossRef] [PubMed]
- Lundin, E.S.; Carlsson, P.; Wodlin, N.B.; Nilsson, L.; Kjölhede, P. Cost-effectiveness of robotic hysterectomy versus abdominal hysterectomy in early endometrial cancer. Int. J. Gynecol. Cancer 2020, 30, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Lundin, E.S.; Wodlin, N.B.; Nilsson, L.; Kjölhede, P. A prospective randomized assessment of quality of life between open and robotic hysterectomy in early endometrial cancer. Int. J. Gynecol. Cancer 2019, 29, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Machida, H.; Casey, J.P.; Garcia-Sayre, J.; Jung, C.E.; Casabar, J.K.; Moeini, A.; Kato, K.; Roman, L.D.; Matsuo, K. Timing of Intrauterine Manipulator Insertion During Minimally Invasive Surgical Staging and Results of Pelvic Cytology in Endometrial Cancer. J. Minim. Invasive Gynecol. 2016, 23, 234–241. [Google Scholar] [CrossRef]
- Machida, H.; Hom, M.S.; Adams, C.L.; Eckhardt, S.E.; Garcia-Sayre, J.; Mikami, M.; Matsuo, K. Intrauterine Manipulator Use During Minimally Invasive Hysterectomy and Risk of Lymphovascular Space Invasion in Endometrial Cancer. Int. J. Gynecol. Cancer 2018, 28, 208–219. [Google Scholar] [CrossRef]
- Kalogiannidis, I.; Lambrechts, S.; Amant, F.; Neven, P.; Van Gorp, T.; Vergote, I. Laparoscopy-assisted vaginal hysterectomy compared with abdominal hysterectomy in clinical stage I endometrial cancer: Safety, recurrence, and long-term outcome. Am. J. Obstet. Gynecol. 2007, 196, 248.e1–248.e8. [Google Scholar] [CrossRef] [PubMed]
- Corrado, G.; Cutillo, G.; Pomati, G.; Mancini, E.; Sperduti, I.; Patrizi, L.; Saltari, M.; Vincenzoni, C.; Baiocco, E.; Vizza, E. Surgical and oncological outcome of robotic surgery compared to laparoscopic and abdominal surgery in the management of endometrial cancer. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2015, 41, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Kroft, J.; Li, Q.; Saskin, R.; Elit, L.; Bernardini, M.Q.; Gien, L.T. Trends over time in the use of laparoscopic hysterectomy for the treatment of endometrial cancer. Gynecol. Oncol. 2015, 138, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Kuoppala, T.; Tomás, E.; Heinonen, P.K. Clinical outcome and complications of laparoscopic surgery compared with traditional surgery in women with endometrial cancer. Arch. Gynecol. Obs. 2004, 270, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Rajadurai, V.A.; Tan, J.; Salfinger, S.G.; Cohen, P.A. Outcomes in women undergoing robotic-assisted laparoscopic hysterectomy compared to conventional laparoscopic hysterectomy at a tertiary hospital in Western Australia. Aust. N. Z. J. Obstet. Gynaecol. 2018, 58, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Piovano, E.; Fuso, L.; Poma, C.B.; Ferrero, A.; Perotto, S.; Tripodi, E.; Volpi, E.; Zanfagnin, V.; Zola, P. Complications after the treatment of endometrial cancer: A prospective study using the French-Italian glossary. Int. J. Gynecol. Cancer 2014, 24, 418–426. [Google Scholar] [CrossRef]
- Praiss, A.M.; Chen, L.; St Clair, C.M.; Tergas, A.I.; Khoury-Collado, F.; Hou, J.Y.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L.; Wright, J.D. Safety of same-day discharge for minimally invasive hysterectomy for endometrial cancer. Am. J. Obstet. Gynecol. 2019, 221, 239.e1–239.e11. [Google Scholar] [CrossRef] [PubMed]
- Raventós-Tato, R.M.; de la Torre-Fernández de Vega, J.; Sánchez-Iglesias, J.L.; Díaz-Feijoó, B.; Sabadell, J.; Pérez-Benavente, M.A.; Gil-Moreno, A. Surgical approaches in women with endometrial cancer with a body mass index greater than 35 kg/m2. J. Obstet. Gynaecol. Res. 2019, 45, 195–202. [Google Scholar] [CrossRef]
- Roberts, M.; Rosales, C.; Ranka, P. Total laparoscopic hysterectomy for endometrial neoplasia. Gynecol. Surg. 2011, 8, 73–78. [Google Scholar] [CrossRef]
- Rocha Guevara, E.R.; Quijano-Castro, O.F.; Cortés-Martínez, G.; López-Hernández, D.; Abrego-Vásquez, J.A.; Gómez-Archila, J.D. Laparoscopic treatment of endometrial cancer. Institutional experience. Gac. Mex. Oncol. 2015, 14, 28–35. [Google Scholar]
- Safdieh, J.; Lee, Y.C.; Wong, A.; Lee, A.; Weiner, J.P.; Schwartz, D.; Schreiber, D. A Comparison of Outcomes between Open Hysterectomy and Robotic-Assisted Hysterectomy for Endometrial Cancer Using the National Cancer Database. Int. J. Gynecol. Cancer 2017, 27, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.; Brandberg, Y.; Åvall-Lundqvist, E.; Suzuki, C.; Johansson, H.; Legerstam, B.; Falconer, H. Long-term quality of life after comprehensive surgical staging of high-risk endometrial cancer—Results from the RASHEC trial. Acta Oncol. 2018, 57, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
- Sandadi, S.; Walter, A.; Lee, S.; Gardner, G.; Abu-Rustum, N.; Sonoda, Y.; Brown, C.; Jewell, E.; Leitao, M. Incidence of venous thromboembolism after minimally invasive surgery in patients with newly diagnosed endometrial cancer. Gynecol. Oncol. 2012, 125, S20. [Google Scholar] [CrossRef]
- Santi, A.; Kuhn, A.; Gyr, T.; Eberhard, M.; Johann, S.; Günthert, A.R.; Mueller, M.D. Laparoscopy or laparotomy? A comparison of 240 patients with early-stage endometrial cancer. Surg. Endosc. 2010, 24, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Scalici, J.; Laughlin, B.B.; Finan, M.A.; Wang, B.; Rocconi, R.P. The trend towards minimally invasive surgery (MIS) for endometrial cancer: An ACS-NSQIP evaluation of surgical outcomes. Gynecol. Oncol. 2015, 136, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Scribner, D.R.; Mannel, R.S.; Walker, J.L.; Johnson, G.A. Cost Analysis of Laparoscopy versus Laparotomy for Early Endometrial Cancer. Gynecol. Oncol. 1999, 75, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Scribner, D.R., Jr.; Walker, J.L.; Johnson, G.A.; McMeekin, S.D.; Gold, M.A.; Mannel, R.S. Surgical management of early-stage endometrial cancer in the elderly: Is laparoscopy feasible? Gynecol. Oncol. 2001, 83, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Seamon, L.G.; Cohn, D.E.; Henretta, M.S.; Kim, K.H.; Carlson, M.J.; Phillips, G.S.; Fowler, J.M. Minimally invasive comprehensive surgical staging for endometrial cancer: Robotics or laparoscopy? Gynecol. Oncol. 2009, 113, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Seracchioli, R.; Venturoli, S.; Ceccarin, M.; Cantarelli, M.; Ceccaroni, M.; Pignotti, E.; De Aloysio, D.; De Iaco, P. Is total laparoscopic surgery for endometrial carcinoma at risk of local recurrence? A long-term survival. Anticancer Res. 2005, 25, 2423–2428. [Google Scholar]
- Seror, J.; Bats, A.S.; Huchon, C.; Bensaïd, C.; Douay-Hauser, N.; Lécuru, F. Laparoscopy vs robotics in surgical management of endometrial cancer: Comparison of intraoperative and postoperative complications. J. Minim. Invasive Gynecol. 2014, 21, 120–125. [Google Scholar] [CrossRef]
- Siesto, G.; Uccella, S.; Ghezzi, F.; Cromi, A.; Zefiro, F.; Serati, M.; Bolis, P. Surgical and survival outcomes in older women with endometrial cancer treated by laparoscopy. Menopause 2010, 17, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.N.; Sutradhar, R.; Ferguson, S.E.; Robertson, D.; Cheng, S.Y.; Li, Q.; Baxter, N.N. Perioperative outcomes of women with and without class III obesity undergoing hysterectomy for endometrioid endometrial cancer: A population-based study. Gynecol. Oncol. 2020, 158, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Swarer, K.; Resnick, K. Longer operative time is associated with increased post-operative complications in patients undergoing minimally-invasive surgery for endometrial cancer. Gynecol. Oncol. 2017, 147, 554–557. [Google Scholar] [CrossRef]
- Slaughter, K.N.; Frumovitz, M.; Schmeler, K.M.; Nick, A.M.; Fleming, N.D.; dos Reis, R.; Munsell, M.F.; Westin, S.N.; Soliman, P.T.; Ramirez, P.T. Minimally invasive surgery for endometrial cancer: Does operative start time impact surgical and oncologic outcomes? Gynecol. Oncol. 2014, 134, 248–252. [Google Scholar] [CrossRef]
- Sofer, A.; Magnezi, R.; Eitan, R.; Raban, O.; Tal, O.; Smorgic, N.; Vaknin, Z. Robotic vs. open surgery in obese women with low-grade endometrial cancer: Comparison of costs and quality of life measures. Isr. J. Health Policy Res. 2020, 9, 60. [Google Scholar] [CrossRef]
- Soliman, H.O.; Elsebaie, H.I.; Gad, Z.S.; Iskandar, S.S.; Gareer, W.Y. Laparoscopic hysterectomy in the treatment of endometrial cancer: NCI experience. J. Egypt. Natl. Cancer Inst. 2011, 23, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Le, T.; Hopkins, L.; Fung-Kee-Fung, M.; Lupe, K.; Gaudet, M.; E, C.; Samant, R. A comparison of disease recurrence between robotic versus laparotomy approach in patients with intermediate-risk endometrial cancer. Int. J. Gynecol. Cancer 2020, 30, 160. [Google Scholar] [CrossRef]
- Sonoda, Y.; Zerbe, M.; Smith, A.; Lin, O.; Barakat, R.R.; Hoskins, W.J. High incidence of positive peritoneal cytology in low-risk endometrial cancer treated by laparoscopically assisted vaginal hysterectomy. Gynecol. Oncol. 2001, 80, 378–382. [Google Scholar] [CrossRef]
- Spencer, R.; Schorge, J.; Del Carmen, M.; Goodman, A.; Growdon, W.; Boruta, D. Laparoscopic surgery for endometrial cancer: Why don’t all patients go home the day after surgery? J. Minim. Invasive Gynecol. 2012, 19, 95–100. [Google Scholar] [CrossRef]
- Spirtos, N.M.; Schlaerth, J.B.; Gross, G.M.; Spirtos, T.W.; Schlaerth, A.C.; Ballon, S.C. Cost and quality-of-life analyses of surgery for early endometrial cancer: Laparotomy versus laparoscopy. Am. J. Obstet. Gynecol. 1996, 174, 1795–1799, discussion 1799–1800. [Google Scholar] [CrossRef]
- Subramaniam, A.; Kim, K.H.; Bryant, S.A.; Zhang, B.; Sikes, C.; Kimball, K.J.; Kilgore, L.C.; Huh, W.K.; Straughn, J.M., Jr.; Alvarez, R.D. A cohort study evaluating robotic versus laparotomy surgical outcomes of obese women with endometrial carcinoma. Gynecol. Oncol. 2011, 122, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Somashekhar, S.P.; Jaka, R.C.; Zaveri, S.S. Prospective randomized study comparing robotic-assisted hysterectomy and regional lymphadenectomy with traditional laparotomy for staging of endometrial carcinoma—Initial Indian experience. Indian J. Surg. Oncol. 2014, 5, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Ueda, S.; Miyamoto, S.; Terada, S.; Konishi, H.; Kogata, Y.; Fujiwara, S.; Tanaka, Y.; Taniguchi, K.; Komura, K.; et al. Oncologic outcomes for patients with endometrial cancer who received minimally invasive surgery: A retrospective observational study. Int. J. Clin. Oncol. 2020, 25, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.Y.; Gardiner, S.K.; Gould, C.; Osmundsen, B.; Collins, M.; Winter, W.E., 3rd. Robotic surgical staging for obese patients with endometrial cancer. Am. J. Obstet. Gynecol. 2012, 206, 513.e1–513.e6. [Google Scholar] [CrossRef] [PubMed]
- Taşkın, S.; Güngör, M.; Öztuna, D.; Ortaç, F. Comparison of laparoscopy and laparotomy in surgical staging of clinical early stage endometrial cancer: A report of early experiences from Turkey. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2012, 32, 687–690. [Google Scholar] [CrossRef]
- Malzoni, M.; Tinelli, R.; Cosentino, F.; Perone, C.; Rasile, M.; Iuzzolino, D.; Malzoni, C.; Reich, H. Total laparoscopic hysterectomy versus abdominal hysterectomy with lymphadenectomy for early-stage endometrial cancer: A prospective randomized study. Gynecol. Oncol. 2009, 112, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Tinelli, R.; Litta, P.; Meir, Y.; Surico, D.; Leo, L.; Fusco, A.; Angioni, S.; Cicinelli, E. Advantages of laparoscopy versus laparotomy in extremely obese women (BMI>35) with early-stage endometrial cancer: A multicenter study. Anticancer Res. 2014, 34, 2497–2502. [Google Scholar] [PubMed]
- Togami, S.; Kawamura, T.; Yanazume, S.; Kamio, M.; Kobayashi, H. Comparison of survival outcomes between laparoscopic and open surgery in patients with low-risk endometrial cancer. Jpn. J. Clin. Oncol. 2020, 50, 1261–1264. [Google Scholar] [CrossRef]
- Tollund, L.; Hansen, B.; Kjer, J.J. Laparoscopic-assisted vaginal vs. abdominal surgery in patients with endometrial cancer stage 1. Acta Obstet. Gynecol. Scand. 2006, 85, 1138–1141. [Google Scholar] [CrossRef]
- Tozzi, R.; Malur, S.; Koehler, C.; Schneider, A. Analysis of morbidity in patients with endometrial cancer: Is there a commitment to offer laparoscopy? Gynecol. Oncol. 2005, 97, 4–9. [Google Scholar] [CrossRef]
- Tozzi, R.; Malur, S.; Koehler, C.; Schneider, A. Laparoscopy versus laparotomy in endometrial cancer: First analysis of survival of a randomized prospective study. J. Minim. Invasive Gynecol. 2005, 12, 130–136. [Google Scholar] [CrossRef]
- Uccella, S.; Bonzini, M.; Palomba, S.; Fanfani, F.; Ceccaroni, M.; Seracchioli, R.; Vizza, E.; Ferrero, A.; Roviglione, G.; Casadio, P.; et al. Impact of Obesity on Surgical Treatment for Endometrial Cancer: A Multicenter Study Comparing Laparoscopy vs Open Surgery, with Propensity-Matched Analysis. J. Minim. Invasive Gynecol. 2016, 23, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Uccella, S.; Bonzini, M.; Palomba, S.; Fanfani, F.; Malzoni, M.; Ceccaroni, M.; Seracchioli, R.; Ferrero, A.; Berretta, R.; Vizza, E.; et al. Laparoscopic vs. open treatment of endometrial cancer in the elderly and very elderly: An age-stratified multicenter study on 1606 women. Gynecol. Oncol. 2016, 141, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Ulm, M.A.; Ginn, D.N.; ElNaggar, A.C.; Tillmanns, T.D.; Reed, K.M.; Wan, J.Y.; Watson, C.H.; Dedania, S.J.; Reed, M.E. A comparison of outcomes following robotic-assisted staging and laparotomy in patients with early stage endometrioid adenocarcinoma of the uterus with uterine weight under 480 g. Gynecol. Minim. Invasive Ther. 2016, 5, 25–29. [Google Scholar] [CrossRef]
- Vaknin, Z.; Perri, T.; Lau, S.; Deland, C.; Drummond, N.; Rosberger, Z.; Gourdji, I.; Gotlieb, W.H. Outcome and quality of life in a prospective cohort of the first 100 robotic surgeries for endometrial cancer, with focus on elderly patients. Int. J. Gynecol. Cancer 2010, 20, 1367–1373. [Google Scholar] [CrossRef]
- Vardar, M.A.; Gulec, U.K.; Guzel, A.B.; Gumurdulu, D.; Khatib, G.; Seydaoglu, G. Laparoscopic surgery for low, intermediate and high-risk endometrial cancer. J. Gynecol. Oncol. 2019, 30, e24. [Google Scholar] [CrossRef] [PubMed]
- Giray, B.; Vatansever, D.; Aboalhasan, Y. Perioperative and Postoperative Outcomes of Laparoscopy and Open Method for Surgical Staging of Endometrial Cancer. DergiPark 2019, 50, 49–53. [Google Scholar]
- Venkat, P.; Chen, L.M.; Young-Lin, N.; Kiet, T.K.; Young, G.; Amatori, D.; Dasverma, B.; Yu, X.; Kapp, D.S.; Chan, J.K. An economic analysis of robotic versus laparoscopic surgery for endometrial cancer: Costs, charges and reimbursements to hospitals and professionals. Gynecol. Oncol. 2012, 125, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.L.; Piedmonte, M.R.; Spirtos, N.M.; Eisenkop, S.M.; Schlaerth, J.B.; Mannel, R.S.; Barakat, R.; Pearl, M.L.; Sharma, S.K. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 695–700. [Google Scholar] [CrossRef]
- Wong, C.K.; Wong, Y.H.; Lo, L.S.F.; Tai, C.M.; Ng, T.K. Laparoscopy compared with laparotomy for the surgical staging of endometrial carcinoma. J. Obstet. Gynaecol. Res. 2005, 31, 286–290. [Google Scholar] [CrossRef]
- Wright, J.D.; Burke, W.M.; Tergas, A.I.; Hou, J.Y.; Huang, Y.; Hu, J.C.; Hillyer, G.C.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L. Comparative Effectiveness of Minimally Invasive Hysterectomy for Endometrial Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.D.; Neugut, A.I.; Wilde, E.T.; Buono, D.L.; Tsai, W.Y.; Hershman, D.L. Use and benefits of laparoscopic hysterectomy for stage I endometrial cancer among medicare beneficiaries. J. Oncol. Pract. 2012, 8, e89–e99. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Ling, S.; Song, Y.; Liu, J.; Xu, H.; Hu, B.; Shao, T.; Yi, B. Effects of laparoscopic surgery and laparotomy procedures on efficacy and prognosis of patients with endometrial carcinoma. Int. J. Clin. Exp. Med. 2020, 13, 3531–3539. [Google Scholar]
- Yin, X.; Shi, M.; Xu, J.; Guo, Q.; Wu, H. Perioperative and long-term outcomes of laparoscopy and laparotomy for endometrial carcinoma. Int. J. Clin. Exp. Med. 2015, 8, 19093–19099. [Google Scholar] [PubMed]
- Yu, X.; Lum, D.; Kiet, T.K.; Fuh, K.C.; Orr Jr, J.; Brooks, R.A.; Ueda, S.M.; Chen, L.-m.; Kapp, D.S.; Chan, J.K. Utilization of and charges for robotic versus laparoscopic versus open surgery for endometrial cancer. J. Surg. Oncol. 2013, 107, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Zakhari, A.; Czuzoj-Shulman, N.; Spence, A.R.; Gotlieb, W.H.; Abenhaim, H.A. Laparoscopic and robot-assisted hysterectomy for uterine cancer: A comparison of costs and complications. Am. J. Obstet. Gynecol. 2015, 213, 665.e1–665.e7. [Google Scholar] [CrossRef] [PubMed]
- Zapico, A.; Fuentes, P.; Grassa, A.; Arnanz, F.; Otazua, J.; Cortés-Prieto, J. Laparoscopic-assisted vaginal hysterectomy versus abdominal hysterectomy in stages I and II endometrial cancer. Operating data, follow up and survival. Gynecol. Oncol. 2005, 98, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Havrilesky, L.J.; Broadwater, G.; Di Santo, N.; Ehrisman, J.A.; Lee, P.S.; Berchuck, A.; Alvarez Secord, A.; Bean, S.; Bentley, R.C.; et al. Relationship between minimally invasive hysterectomy, pelvic cytology, and lymph vascular space invasion: A single institution study of 458 patients. Gynecol. Oncol. 2014, 133, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Zorlu, C.G.; Simsek, T.; Ari, E.S. Laparoscopy or laparotomy for the management of endometrial cancer. JSLS J. Soc. Laparoendosc. Surg. 2005, 9, 442–446. [Google Scholar]
- Zullo, F.; Palomba, S.; Falbo, A.; Russo, T.; Mocciaro, R.; Tartaglia, E.; Tagliaferri, P.; Mastrantonio, P. Laparoscopic surgery vs laparotomy for early stage endometrial cancer: Long-term data of a randomized controlled trial. Am. J. Obstet. Gynecol. 2009, 200, 296.e1–296.e9. [Google Scholar] [CrossRef]
- Zullo, F.; Palomba, S.; Russo, T.; Falbo, A.; Costantino, M.; Tolino, A.; Zupi, E.; Tagliaferri, P.; Venuta, S. A prospective randomized comparison between laparoscopic and laparotomic approaches in women with early stage endometrial cancer: A focus on the quality of life. Am. J. Obstet. Gynecol. 2005, 193, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Casarin, J.; Song, C.; Multinu, F.; Cappuccio, S.; Liu, E.; Butler, K.A.; Glaser, G.E.; Cliby, W.A.; Langstraat, C.L.; Ghezzi, F.; et al. Implementing robotic surgery for uterine cancer in the United States: Better outcomes without increased costs. Gynecol. Oncol. 2020, 156, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Casarin, J.; Multinu, F.; Ubl, D.S.; Dowdy, S.C.; Cliby, W.A.; Glaser, G.E.; Butler, K.A.; Ghezzi, F.; Habermann, E.B.; Mariani, A. Adoption of Minimally Invasive Surgery and Decrease in Surgical Morbidity for Endometrial Cancer Treatment in the United States. Obstet. Gynecol. 2018, 131, 304–311. [Google Scholar] [CrossRef]
- Chan, J.K.; Gardner, A.B.; Taylor, K.; Thompson, C.A.; Blansit, K.; Yu, X.; Kapp, D.S. Robotic versus laparoscopic versus open surgery in morbidly obese endometrial cancer patients—A comparative analysis of total charges and complication rates. Gynecol. Oncol. 2015, 139, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Chiou, H.-Y.; Chiu, L.-H.; Chen, C.-H.; Yen, Y.-K.; Chang, C.-W.; Liu, W.-M. Comparing robotic surgery with laparoscopy and laparotomy for endometrial cancer management: A cohort study. Int. J. Surg. 2015, 13, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Jang, T.K.; Nam, S.H.; Kwon, S.H.; Shin, S.J.; Cho, C.H. Robotic single-site staging operation for early-stage endometrial cancer: Initial experience at a single institution. Obstet. Gynecol. Sci. 2019, 62, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-H.; Kim, D.-Y.; Kim, J.-H.; Kim, Y.-M.; Kim, Y.-T.; Nam, J.-H. Laparoscopic management of early uterine cancer: 10-Year experience in Asan Medical Center. Gynecol. Oncol. 2007, 106, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.H.; Chang, W.C.; Sheu, B.C. Comparison of the laparoscopic versus conventional open method for surgical staging of endometrial carcinoma. Taiwan. J. Obstet. Gynecol. 2016, 55, 188–192. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Coronado, P.J.; Herraiz, M.A.; Magrina, J.F.; Fasero, M.; Vidart, J.A. Comparison of perioperative outcomes and cost of robotic-assisted laparoscopy, laparoscopy and laparotomy for endometrial cancer. Eur. J. Obs. Gynecol. Reprod. Biol. 2012, 165, 289–294. [Google Scholar] [CrossRef]
- Corrado, G.; Calagna, G.; Cutillo, G.; Insinga, S.; Mancini, E.; Baiocco, E.; Zampa, A.; Bufalo, A.; Perino, A.; Vizza, E. The Patient and Observer Scar Assessment Scale to Evaluate the Cosmetic Outcomes of the Robotic Single-Site Hysterectomy in Endometrial Cancer. Int. J. Gynecol. Cancer 2018, 28, 194–199. [Google Scholar] [CrossRef]
- Corrado, G.; Chiantera, V.; Fanfani, F.; Cutillo, G.; Lucidi, A.; Mancini, E.; Pedone Anchora, L.; Scambia, G.; Vizza, E. Robotic Hysterectomy in Severely Obese Patients with Endometrial Cancer: A Multicenter Study. J. Minim. Invasive Gynecol. 2016, 23, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Corrado, G.; Cutillo, G.; Pomati, G.; Mancini, E.; Baiocco, E.; Patrizi, L.; Saltari, M.; Barletta, F.; Patani, F.; Vizza, E. Single-access laparoscopic approach in the surgical treatment of endometrial cancer: A single-institution experience and review of literature. J. Minimal Access Surg. 2016, 12, 360–365. [Google Scholar] [CrossRef]
- Corrado, G.; Vizza, E.; Cela, V.; Mereu, L.; Bogliolo, S.; Legge, F.; Ciccarone, F.; Mancini, E.; Gallotta, V.; Baiocco, E.; et al. Laparoscopic versus robotic hysterectomy in obese and extremely obese patients with endometrial cancer: A multi-institutional analysis. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2018, 44, 1935–1941. [Google Scholar] [CrossRef]
- Cybulska, P.; Schiavone, M.B.; Sawyer, B.; Gardner, G.J.; Zivanovic, O.; Brown, C.L.; Jewell, E.L.; Sonoda, Y.; Barakat, R.R.; Abu-Rustum, N.R.; et al. Trocar site hernia development in patients undergoing robotically assisted or standard laparoscopic staging surgery for endometrial cancer. Gynecol. Oncol. 2017, 147, 371–374. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, Z.; Wang, J. Survival of microsatellite-stable endometrioid endometrial cancer patients after minimally invasive surgery: An analysis of the Cancer Genome Atlas data. Gynecol. Oncol. 2020, 158, 92–98. [Google Scholar] [CrossRef] [PubMed]
- DeNardis, S.A.; Holloway, R.W.; Bigsby, G.E.t.; Pikaart, D.P.; Ahmad, S.; Finkler, N.J. Robotically assisted laparoscopic hysterectomy versus total abdominal hysterectomy and lymphadenectomy for endometrial cancer. Gynecol. Oncol. 2008, 111, 412–417. [Google Scholar] [CrossRef]
- Deura, I.; Shimada, M.; Azuma, Y.; Komatsu, H.; Nagira, K.; Sawada, M.; Harada, T. Comparison of laparoscopic surgery and conventional laparotomy for surgical staging of patients with presumed low-risk endometrial cancer: The current state of Japan. Taiwan. J. Obstet. Gynecol. 2019, 58, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, M.; Schröter, V.; Stubert, J.; Reimer, T.; Gerber, B.; Stachs, A. Oncologic Outcome of Patients with (Low-Risk) Endometrial Carcinoma Undergoing Laparotomy versus Minimally Invasive Hysterectomy: A Retrospective Analysis. Oncol. Res. Treat. 2019, 42, 636–649. [Google Scholar] [CrossRef]
- Dowdy, S.C.; Borah, B.J.; Bakkum-Gamez, J.N.; Kumar, S.; Weaver, A.L.; McGree, M.E.; Haas, L.R.; Cliby, W.A.; Podratz, K.C. Factors predictive of postoperative morbidity and cost in patients with endometrial cancer. Obstet. Gynecol. 2012, 120, 1419–1427. [Google Scholar] [CrossRef]
- Turunen, H.; Pakarinen, P.; Sjöberg, J.; Loukovaara, M. Laparoscopic vs robotic-assisted surgery for endometrial carcinoma in a centre with long laparoscopic experience. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2013, 33, 720–724. [Google Scholar] [CrossRef]
- Boosz, A.; Haeberle, L.; Renner, S.P.; Thiel, F.C.; Mehlhorn, G.; Beckmann, M.W.; Mueller, A. Comparison of reoperation rates, perioperative outcomes in women with endometrial cancer when the standard of care shifts from open surgery to laparoscopy. Arch. Gynecol. Obs. 2014, 290, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Corrado, G.; Mereu, L.; Bogliolo, S.; Cela, V.; Freschi, L.; Carlin, R.; Gardella, B.; Mancini, E.; Tateo, S.; Spinillo, A.; et al. Robotic single site staging in endometrial cancer: A multi-institution study. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2016, 42, 1506–1511. [Google Scholar] [CrossRef] [PubMed]
- Tinelli, R.; Malzoni, M.; Cicinelli, E.; Fiaccavento, A.; Zaccoletti, R.; Barbieri, F.; Tinelli, A.; Perone, C.; Cosentino, F. Is early stage endometrial cancer safely treated by laparoscopy? Complications of a multicenter study and review of recent literature. Surg. Oncol. 2011, 20, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Janda, M.; Gebski, V.; Davies, L.C.; Forder, P.; Brand, A.; Hogg, R.; Jobling, T.W.; Land, R.; Manolitsas, T.; Nascimento, M.; et al. Effect of Total Laparoscopic Hysterectomy vs Total Abdominal Hysterectomy on Disease-Free Survival among Women with Stage I Endometrial Cancer: A Randomized Clinical Trial. JAMA 2017, 317, 1224–1233. [Google Scholar] [CrossRef]
- Peiretti, M.; Zanagnolo, V.; Bocciolone, L.; Landoni, F.; Colombo, N.; Minig, L.; Sanguineti, F.; Maggioni, A. Robotic Surgery: Changing the Surgical Approach for Endometrial Cancer in a Referral Cancer Center. J. Minim. Invasive Gynecol. 2009, 16, 427–431. [Google Scholar] [CrossRef]

| Author | Year of Study | Sample Size | Type of Study | Comparator |
|---|---|---|---|---|
| Abel et al. [21] | 2020 | 1805 | Retrospective Cohort Study | Laparoscopic |
| Abitbol et al. [22] | 2016 | 340 | Retrospective Cohort Study | Robotic |
| Agarwal et al. [23] | 2018 | 133 | Retrospective Cohort Study | Robotic/Open |
| Aiko et al. [24] | 2020 | 223 | Retrospective Cohort Study | Laparoscopic/Robotic |
| Ansar et al. [25] | 2018 | 60 | Prospective Non-Randomized Control Study | Laparoscopic/Open |
| Api et al. [26] | 2013 | 79 | Retrospective Cohort Study | Laparoscopic/Open |
| Armfield et al. [27] | 2018 | 404 | Randomized Controlled Trial | Laparoscopic/Open |
| Avondstond et al. [28] | 2017 | 40 | Retrospective Cohort Study | Robotic |
| Backes et al. [29] | 2015 | 543 | Retrospective Cohort Study | Robotic |
| Backes et al. [30] | 2016 | 182 | Retrospective Cohort Study | Robotic/Open |
| Baek et al. [31] | 2014 | 278 | Retrospective Cohort Study | Laparoscopic |
| Bajaj et al. [32] | 1999 | 70 | Retrospective Cohort Study | Laparoscopic/Open |
| Baker et al. [33] | 2013 | 760 | Randomized Controlled Trial | Laparoscopic/Open |
| Baker et al. [34] | 2015 | 760 | Randomized Controlled Trial | Laparoscopic/Open |
| Bakkum Gamez et al. [35] | 2013 | 1369 | Retrospective study | Laparoscopic/Robotic/Open/Vaginal |
| Ball et al. [36] | 2011 | 289 | Retrospective Cohort Study | Laparoscopic/Open |
| Barber et al. [37] | 2016 | 9948 | Retrospective Cohort Study | Minimally Invasive/Open |
| Barnes et al. [38] | 2017 | 210 | Retrospective Cohort study | Laparoscopic |
| Barnett et al. [39] | 2011 | 376 | Retrospective Cohort Study | Laparoscopic/Open |
| Barnett et al. [40] | 2010 | 0 | Decision Model Analysis | Laparoscopic/Robotic/Open |
| Barraez et al. [41] | 2014 | 446 | Retrospective Study | Robotic |
| Barwijuk et al. [42] | 2005 | 25 | Retrospective Study | Laparoscopic/Open |
| Beck et al. [45] | 2018 | 3712 | Retrospective Cohort Study | Laparoscopic/Open |
| Bell et al. [43] | 2008 | 110 | Retrospective Study | Laparoscopic/Robotic/Open |
| Bennich et al. [44] | 2016 | 227 | Retrospective Study | Laparoscopic |
| Bergstrom et al. [46] | 2018 | 1621 | Retrospective Cohort Study | Laparoscopic/Robotic/Vaginal |
| Bernardini et al. [47] | 2012 | 86 | Retrospective Cohort Study | Robotic/Open |
| Berretta et al. [48] | 2015 | 81 | Retrospective Cohort Study | Laparoscopic/Open/Vaginal |
| Bige et al. [49] | 2015 | 140 | Prospective Non-Randomized Control Study | Laparoscopic/Open |
| Bishop et al. [50] | 2018 | 1477 | Randomized Controlled Trial | Laparoscopic/Open |
| Bogani et al. [51] | 2014 | 125 | Retrospective Study | Laparoscopic/Open |
| Bogani et al. [52] | 2016 | 638 | Retrospective Cohort Study | Robotic/Open |
| Boggess et al. [53] | 2008 | 322 | Retrospective Cohort Study | Laparoscopic/Robotic/Open |
| Boosz [215] | 2014 | 267 | Retrospective Study | Laparoscopic/Open |
| Bourgin et al. [54] | 2017 | 344 | Retrospective Study | Laparoscopic/Robotic/Open/Vaginal |
| Bouwman et al. [55] | 2015 | 514 | Retrospective Study | Laparoscopic/Open |
| Casarin et al. [196] | 2020 | 35,224 | Retrospective Study | Laparoscopic/Robotic/Open |
| Casarin et al. [197] | 2018 | 12,283 | Retrospective Cohort Study | Laparoscopic/Robotic/Open |
| Chan [198] | 2015 | 1087 | Retrospective Study | Laparoscopic/Robotic/Open |
| Chiou et al. [199] | 2015 | 377 | Retrospective Study | Robotic |
| Cho et al. [201] | 2007 | 288 | Retrospective Study | Laparoscopic (LAVH)/Laparoscopic (TLH)/Open |
| Chu et al. [202] | 2016 | 151 | Retrospective Cohort Study | Laparoscopic/Open |
| Chung et al. [200] | 2019 | 15 | Retrospective Study | Robotic |
| Coronado et al. [203] | 2012 | 347 | Retrospective Study | Laparoscopic/Robotic/Open |
| Corrado et al. [205] | 2016 | 50 | Retrospective Study | Laparoscopic |
| Corrado et al. [136] | 2015 | 526 | Retrospective Study | Laparoscopic/Open |
| Corrado et al. [207] | 2018 | 655 | Retrospective Study | Laparoscopic/Robotic |
| Corrado et al. [204] | 2018 | 45 | Prospective Cohort Study | Robotic |
| Corrado et al. [216] | 2016 | 125 | Prospective Cohort Study | Robotic |
| Corrado et al. [206] | 2016 | 70 | Retrospective Study | Robotic |
| Cybulska et al. [208] | 2018 | 760 | Retrospective Study | Laparoscopic/Robotic |
| Dai et al. [209] | 2020 | 519 | Retrospective Study | Laparoscopic/Open |
| DeNardis et al. [210] | 2008 | 162 | Retrospective Study | Robotic/Open |
| Deura et al. [211] | 2019 | 120 | Retrospective Study | Laparoscopic/Open |
| Dietrich et al. [212] | 2019 | 350 | Retrospective Study | Minimally Invasive/Open |
| Dowdy et al. [213] | 2012 | 1369 (of 1415 patients identified) | Retrospective Study | Minimally Invasive/Open |
| Eltabbakh [62] | 2000 | 75 | Retrospective Study | Laparoscopic |
| Eltabbakh [63] | 2002 | 186 | Retrospective Study | Laparoscopic/Open |
| Fader et al. [68] | 2016 | 32,560 | Retrospective Study | Laparoscopic/Open |
| Fader et al. [67] | 2012 | 383 | Retrospective Cohort Study | Laparoscopic/Robotic/Open |
| Fagotti et al. [69] | 2012 | 100 | Retrospective Study | Laparoscopic |
| Fagotti et al. [70] | 2013 | 57 | Retrospective Study | Laparoscopic |
| Fagotti et al. [71] | 2012 | 150 | Retrospective Study | Laparoscopic/Robotic |
| Fanning et al. [72] | 2010 | 235 | Retrospective Study | Laparoscopic |
| Farthing et al. [73] | 2012 | 191 | Retrospective Study | Laparoscopic |
| Fleming et al. [74] | 2012 | 66 | Retrospective Cohort Study | Laparoscopic/Robotic/Open |
| Fleming et al. [75] | 2011 | 181 | Retrospective Cohort Study | Laparoscopic/Robotic/Open |
| Fram et al. [13] | 2002 | 61 | Randomized Controlled Trial | Laparoscopic (LAVH)/Open |
| Freeman et al. [94] | 2016 | 1433 | Retrospective Study | Laparoscopic/Robotic |
| Frey et al. [95] | 2015 | 122 | Retrospective Study | Laparoscopic/Robotic |
| Frey et al. [58] | 2011 | 129 | Retrospective Cohort Study | Laparoscopic/Robotic/Open |
| Frigerio et al. [96] | 2006 | 110 | Retrospective Study | Laparoscopic (LAVH)/Open |
| Gambacorti-Passerini et al. [97] | 2019 | 83 | Prospective Observational Study | Laparoscopic |
| Gehrig et al. [76] | 2008 | 79 | Retrospective Cohort Study | Laparoscopic/Robotic |
| Gemignani et al. [77] | 1999 | 320 | Retrospective Study | Laparoscopic (LAVH)/Open |
| Ghazali et al. [78] | 2019 | 40 | Retrospective Cohort Study | Laparoscopic/Open |
| Ghezzi et al. [79] | 2006 | 101 | Prospective Cohort Study | Laparoscopic |
| Ghezzi et al. [80] | 2006 | 72 | Randomized Controlled Trial | Laparoscopic (LAVH/TLH) |
| Ghezzi et al. [81] | 2009 | 103 | Retrospective Study | Laparoscopic (Microlaparoscopy/Conventional Laparoscopy) |
| Ghezzi et al. [82] | 2010 | 117 | Prospective Cohort Study | Laparoscopic/Open |
| Giannini et al. [98] | 2020 | 100 | Retrospective Study | Laparoscopic/Open |
| Giannini et al. [83] | 2021 | 147 | Retrospective Study | Robotic (DaVinci robot Si/XI) |
| Gildea et al. [99] | 2016 | 46,859 | Retrospective Study | Laparoscopic/Open |
| Gil-Moreno et al. [84] | 2006 | 370 | Retrospective Cohort Study | Laparoscopic/Open |
| Giray et al. [181] | 2019 | 121 | Retrospective Study | Laparoscopic/Open |
| Göçmen et al. [85] | 2010 | 22 | Prospective Cohort Study | Robotic/Open |
| Goel et al. [100] | 2011 | 97 | Retrospective Study | Robotic/Open |
| Grabosch et al. [101] | 2013 | 2 | Case series | Laparoscopic |
| Graves et al. [102] | 2012 | 760 | Randomized Controlled Trial | Laparoscopic/Open |
| Gueli Alletti et al. [86] | 2016 | 89 | Retrospective Cohort Study | Laparoscopic/Robotic |
| Gunderson et al. [60] | 2014 | 2596 | Randomized Controlled Trial | Laparoscopic/Open |
| Helm et al. [88] | 2011 | 168 | Retrospective Study | Laparoscopic/Open |
| Herling et al. [89] | 2016 | 360 | Retrospective Cohort Study | Robotic/Open |
| Hinshaw et al. [90] | 2016 | 136 | Retrospective Cohort Study | Robotic/Open |
| Holloway et al. [91] | 2009 | 100 | Retrospective Study | Robotic |
| Holtz et al. [92] | 2010 | 33 | Retrospective Study | Laparoscopic/Robotic |
| Holub et al. [93] | 2003 | 108 | Prospective Cohort Study | Laparoscopic |
| Kalogiannidis et al. [135] | 2007 | 169 | Prospective Cohort Study | Laparoscopic (LAVH)/Open |
| Kroft et al. [137] | 2015 | 12,104 | Retrospective Cohort Study | Laparoscopic/Open |
| Kuoppala et al. [138] | 2004 | 80 | Retrospective Study | Laparoscopic/Open |
| Lau et al. [56] | 2012 | 303 | Retrospective Cohort Study | Robotic/Open |
| lavoue et al. [57] | 2014 | 163 | Retrospective Cohort Study | Robotic/Open |
| Lee et al. [105] | 2014 | 105 | Prospective Cohort Study | Laparoscopic |
| Lee et al. [106] | 2016 | 287 | Prospective Cohort Study | Laparoscopic |
| Lee et al. [66] | 2008 | 35 | Retrospective Study | Laparoscopic |
| Lee et al. [65] | 2018 | 17,692 | Retrospective Study | Laparoscopic |
| Lee et al. [108] | 2016 | 9020 | Retrospective Study | Laparoscopic/Robotic |
| Lee et al. [109] | 2013 | 110 | Randomized Controlled Trial | Laparoscopic with/without manipulator |
| Lee et al. [107] | 2014 | 3 | Prospective Study | NOTES surgery |
| Lee et al. [104] | 2010 | 6 | Retrospective Study | Robotic |
| Leiserowtz et al. [110] | 2009 | 12,743 | Retrospective Cohort Study | Laparoscopic (LAVH)/Open |
| Leitao et al. [111] | 2013 | 475 | Prospective Cohort Study | Laparoscopic/Robotic |
| Leitao et al. [112] | 2012 | 752 | Prospective Study | Laparoscopic/Robotic/Open |
| Leitao et al. [113] | 2016 | 426 | Retrospective Study | Laparoscopic/Robotic/Open/Vaginal |
| Li et al. [114] | 2011 | 86 | Retrospective Study | Laparoscopic/Open |
| Liang et al. [115] | 2013 | 395 | Retrospective Study | Robotic |
| Liauw et al. [117] | 2003 | 30 | Retrospective Study | Laparoscopic |
| Lim et al. [119] | 2000 | 40 | Retrospective Study | Laparoscopic (LAVH)/Open |
| Lim et al. [120] | 2008 | 46 | Retrospective Study | Laparoscopic with/without manipulator |
| Limbachiya et al. [121] | 2020 | 88 | Retrospective Study | Laparoscopic |
| Lindfors et al. [122] | 2018 | 278 | Retrospective Study | Robotic/Open |
| Lindfors et al. [123] | 2020 | 217 | Retrospective Study | Robotic/Open |
| Liu et al. [124] | 2017 | 211 | Retrospective Study | Laparoscopic/Open |
| Loaec et al. [125] | 2018 | 20 | Retrospective Study | Robotic |
| Lowe et al. [126] | 2010 | 395 | Retrospective Study | Robotic |
| Lowe et al. [127] | 2009 | 405 | Retrospective Study | Robotic |
| Lu et al. [128] | 2013 | 272 | Randomized Controlled Trial | Laparoscopic/Open |
| Lu et al. [129] | 2012 | 238 | Retrospective Study | Laparoscopic/Open |
| Lunde et al. [130] | 2020 | 207 | Nested Case Control Study | Robotic |
| Lundin et al. [131] | 2020 | 49 | Randomized Controlled Trial | Robotic/Open |
| Lundin et al. [132] | 2019 | 50 | Randomized Controlled Trial | Robotic/Open |
| Machida et al. [134] | 2018 | 613 | Retrospective Study | Laparoscopic/Open |
| Machida et al. [133] | 2016 | 333 | Case Control Study | Laparoscopic with cytology before and after manipulator |
| Mäenpää et al. [9] | 2016 | 99 | Prospective Cohort Study | Laparoscopic/Robotic |
| Malzoni et al. [170] | 2009 | 159 | Randomized Controlled Trial | Laparoscopic/Open |
| Peiretti et al. [219] | 2009 | 80 | Prospective Study | Robotic |
| Piovano et al. [140] | 2014 | 271 | Prospective study | Surgery/Radiotherapy |
| Praiss et al. [141] | 2019 | 17,935 | Retrospective Study | Minimally Invasive |
| Rabischong et al. [61] | 2011 | 207 | Retrospective Study | Laparoscopic |
| Rajadurai et al. [139] | 2018 | 90 | Retrospective Study | Laparoscopic/Robotic |
| Raventos-Tato [142] | 2019 | 138 | Retrospective Study | Laparoscopic/Robotic/Open |
| Roberts et al. [143] | 2011 | 95 | Retrospective Study | Laparoscopic (LAVH)/Laparoscopic (TLH)/Open |
| Rocha-Guevara et al. [144] | 2015 | 17,935 | Retrospective Study | Minimally Invasive |
| Safdieh et al. [145] | 2017 | 43,985 | Retrospective Study | Robotic/Open |
| Salehi et al. [146] | 2018 | 120 | Randomized Controlled Trial | Laparoscopic/Robotic |
| Sandadi et al. [147] | 2012 | 573 | Retrospective Study | Laparoscopic/Robotic |
| Santi et al. [148] | 2010 | 240 | Retrospective Study | Laparoscopic/Open |
| Scalici et al. [149] | 2015 | 2076 | Retrospective Study | Laparoscopic/Robotic |
| Scribner et al. [150] | 1999 | 36 | Retrospective Study | Laparoscopic/Open |
| Scribner et al. [151] | 2001 | 125 | Retrospective Study | Laparoscopic/Open |
| Seamon [59] | 2009 | 79 | Retrospective study | Robotic |
| Seamon et al. [152] | 2009 | 181 | Prospective/ retrospective Study | Laparoscopic/Robotic |
| Seracchioli [153] | 2005 | 113 | Retrospective Study | Laparoscopic/Open |
| Seror [154] | 2014 | 146 | Retrospective Study | Laparoscopic/Robotic |
| Siesto et al. [155] | 2010 | 108 | Retrospective Study | Laparoscopic/Open |
| Simpson et al. [156] | 2020 | 4640 | Retrospective Study | Laparoscopic/Laparoscopic (LAVH)/Robotic/Open |
| Singh et al. [157] | 2017 | 9145 | Retrospective Study | Laparoscopic |
| Slaughter et al. [158] | 2014 | 380 | Retrospective Study | Laparoscopic/Robotic |
| Sofer et al. [159] | 2020 | 138 | Retrospective Study | Robotic/Open |
| Soliman et al. [160] | 2011 | 25 | Retrospective Study | Laparoscopic |
| Somashekar et al. [166] | 2014 | 50 | Randomized Controlled Trial | Robotic/Open |
| Song et al. [161] | 2020 | 135 | Retrospective Study | Robotic/Open |
| Sonoda et al. [162] | 2001 | 377 | Retrospective Study | Laparoscopic (LAVH)/Open |
| Spencer et al. [163] | 2012 | 133 | Retrospective Study | Laparoscopic |
| Spirtos et al. [164] | 1996 | 30 | Retrospective Study | Laparoscopic/Open |
| Subramania et al. [165] | 2011 | 73 | Retrospective Study | Robotic |
| Tanaka et al. [167] | 2020 | 913 | Retrospective Study | Laparoscopic/Open |
| Tang et al. [168] | 2012 | 239 | Retrospective Cohort study | Robotic/Open |
| Taşkın et al. [169] | 2012 | 153 | Retrospective Study | Laparoscopic/Robotic/Open/Vaginal |
| Tinelli [171] | 2014 | 75 | Retrospective Study | Laparoscopic/Open |
| Tinelli et al. [217] | 2011 | 226 | Retrospective Study | Laparoscopic/Open |
| Togami et al. [172] | 2020 | 155 | Retrospective Study | Laparoscopic/Open |
| Tollund et al. [173] | 2006 | 86 | Retrospective Study | Laparoscopic (LAVH)/Laparoscopic (TLH)/Open |
| Tozzi et al. [175] | 2005 | 122 | Randomized Controlled Trial | Laparoscopic/Open |
| Turner et al. [87] | 2015 | 335 | Retrospective Study | Laparoscopic/Robotic |
| Turunen et al. [214] | 2013 | 227 | Retrospective Study | Laparoscopic/Robotic |
| Uccella et al. [176] | 2016 | 1266 | Retrospective Study | Laparoscopic/Open |
| Uccella et al. [177] | 2016 | 1606 | Retrospective Study | Laparoscopic/Open |
| Ulm et al. [178] | 2016 | 325 | Retrospective Study | Robotic/Open |
| Vardar et al. [180] | 2019 | 801 | Retrospective Study | Laparoscopic/Open |
| Venkat et al. [182] | 2012 | 54 | Retrospective Study | Robotic/Open |
| Walker et al. [183] | 2012 | 2181 | Randomized Controlled Trial | Laparoscopic/Open |
| Wong et al. [184] | 2005 | 64 | Retrospective Study | Laparoscopic/Open |
| Wright et al. [186] | 2012 | 8018 | Retrospective Study | Laparoscopic/Open |
| Wright et al. [185] | 2016 | 6304 | Retrospective Study | Laparoscopic/Robotic/Open |
| Xu et al. [187] | 2020 | 81 | Prospective Observational Study | Laparoscopic/Open |
| Yin et al. [188] | 2015 | 32 | Retrospective Study | Laparoscopic |
| Yu et al. [189] | 2013 | 2247 | Retrospective Study | Laparoscopic/Robotic/Open |
| Zakhari et al. [190] | 2015 | 10,347 | Retrospective Study | Laparoscopic/Robotic |
| Zapico et al. [191] | 2005 | 90 | Retrospective Study | Laparoscopic/Open |
| Zhang et al. [192] | 2014 | 458 | Retrospective Study | Minimally Invasive/Open |
| Zorlu et al. [193] | 2005 | 52 | Randomized Controlled Trial | Laparoscopic/Open |
| Zullo et al. [194] | 2005 | 84 | Prospective long-term extension study | Laparoscopic/Open |
| Outcome | OS | LRS | RS | |
|---|---|---|---|---|
| Blood Loss | OS | 0 (0, 0) | −226.90 (−298.40, −155.90) | −257.20 (−351.20, −163.80) |
| LRS | 226.90 (155.90, 298.40) | 0 (0, 0) | −30.33 (−122.20, 61.62) | |
| RS | 257.20 (163.80, 351.20) | 30.33 (−61.62, 122.2) | 0 (0, 0) | |
| Duration of Operation | OS | 0 (0, 0) | 18.95 (7.68, 30.20) | 29.00 (13.66, 44.23) |
| LRS | −18.95 (−30.20, −7.68) | 0 (0, 0) | 10.05 (−5.60, 25.48) | |
| RS | −29.00 (−44.22, −13.66) | −10.05 (−25.48, 5.60) | 0 (0, 0) | |
| Length of Stay in Hospital | OS | 0 (0, 0) | −3.54 (−4.22, −2.87) | −3.79 (−4.79, −2.79) |
| LRS | 3.54 (2.87, 4.22) | 0 (0, 0) | −0.25 (−1.26, 0.77) | |
| RS | 3.79 (2.79, 4.79) | 0.25 (−0.77, 1.26) | 0 (0, 0) | |
| Total Lymph Nodes | OS | 0 (0, 0) | 0.40 (−1.18, 2.01) | −0.06 (−2.72, 2.69) |
| LRS | −0.40 (−2.01, 1.18) | 0 (0, 0) | −0.46 (−2.91, 2.09) | |
| RS | 0.06 (−2.69, 2.72) | 0.46 (−2.09, 2.91) | 0 (0, 0) | |
| Blood Transfusion | OS | 1 (1, 1) | 0.30 (0.19, 0.48) | 0.26 (0.12, 0.53) |
| LRS | 3.32 (2.09, 5.38) | 1 (1, 1) | 0.85 (0.4, 1.79) | |
| RS | 3.90 (1.89, 8.31) | 1.17 (0.56, 2.50) | 1 (1, 1) | |
| Fever | OS | 1 (1, 1) | 0.57 (0.30, 0.98) | 0.42 (0.07, 2.21) |
| LRS | 1.75 (1.02, 3.29) | 1 (1, 1) | 0.74 (0.11, 4.51) | |
| RS | 2.37 (0.45, 14.38) | 1.35 (0.22, 8.73) | 1 (1, 1) | |
| Infection | OS | 1 (1, 1) | 0.50 (0.28, 0.93) | 0.84 (0.35, 2.01) |
| LRS | 1.99 (1.07, 3.60) | 1 (1, 1) | 1.66 (0.69, 3.99) | |
| RS | 1.20 (0.50, 2.84) | 0.60 (0.25, 1.46) | 1 (1, 1) | |
| Ileus | OS | 1 (1, 1) | 0.46 (0.29, 0.68) | 0.18 (0.08, 0.41) |
| LRS | 2.16 (1.47, 3.40) | 1 (1, 1) | 0.40 (0.17, 0.87) | |
| RS | 5.44 (2.43, 12.93) | 2.50 (1.14, 5.74) | 1 (1, 1) | |
| VTE | OS | 1 (1, 1) | 0.57 (0.36, 1.10) | 0.80 (0.33, 1.86) |
| LRS | 1.75 (0.91, 2.79) | 1 (1, 1) | 1.39 (0.55, 2.91) | |
| RS | 1.26 (0.54, 3.01) | 0.72 (0.34, 1.83) | 1 (1, 1) | |
| Disease-free Survival | OS | 1 (1, 1) | 1.35 (0.80, 2.32) | 3.29 (1.46, 8.36) |
| LRS | 0.74 (0.43, 1.26) | 1 (1, 1) | 2.45 (1.04, 6.34) | |
| RS | 0.30 (0.12, 0.69) | 0.41 (0.16, 0.97) | 1 (1, 1) | |
| Recurrence | OS | 1 (1, 1) | 0.64 (0.47, 0.84) | 0.64 (0.35, 1.19) |
| LRS | 1.57 (1.20, 2.15) | 1 (1, 1) | 1.02 (0.55, 1.95) | |
| RS | 1.55 (0.84, 2.86) | 0.98 (0.51, 1.81) | 1 (1, 1) | |
| Total Complications | OS | 1 (1, 1) | 0.38 (0.29, 0.51) | 0.34 (0.22, 0.51) |
| LRS | 2.61 (1.97, 3.45) | 1 (1, 1) | 0.88 (0.58, 1.31) | |
| RS | 2.97 (1.98, 4.55) | 1.14 (0.76, 1.73) | 1 (1, 1) | |
| Total Intra-operative Complications | OS | 1 (1, 1) | 1.04 (0.75, 1.49) | 0.39 (0.18, 0.78) |
| LRS | 0.96 (0.67, 1.33) | 1 (1, 1) | 0.38 (0.17, 0.75) | |
| RS | 2.55 (1.28, 5.47) | 2.66 (1.34, 5.79) | 1 (1, 1) | |
| Total Post-operative Complications | OS | 1 (1, 1) | 0.48 (0.34, 0.70) | 0.46 (0.27, 0.78) |
| LRS | 2.07 (1.43, 2.98) | 1 (1, 1) | 0.95 (0.54, 1.63) | |
| RS | 2.19 (1.29, 3.71) | 1.05 (0.61, 1.84) | 1 (1, 1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natarajan, P.; Delanerolle, G.; Dobson, L.; Xu, C.; Zeng, Y.; Yu, X.; Marston, K.; Phan, T.; Choi, F.; Barzilova, V.; et al. Surgical Treatment for Endometrial Cancer, Hysterectomy Performed via Minimally Invasive Routes Compared with Open Surgery: A Systematic Review and Network Meta-Analysis. Cancers 2024, 16, 1860. https://doi.org/10.3390/cancers16101860
Natarajan P, Delanerolle G, Dobson L, Xu C, Zeng Y, Yu X, Marston K, Phan T, Choi F, Barzilova V, et al. Surgical Treatment for Endometrial Cancer, Hysterectomy Performed via Minimally Invasive Routes Compared with Open Surgery: A Systematic Review and Network Meta-Analysis. Cancers. 2024; 16(10):1860. https://doi.org/10.3390/cancers16101860
Chicago/Turabian StyleNatarajan, Purushothaman, Gayathri Delanerolle, Lucy Dobson, Cong Xu, Yutian Zeng, Xuan Yu, Kathleen Marston, Thuan Phan, Fiona Choi, Vanya Barzilova, and et al. 2024. "Surgical Treatment for Endometrial Cancer, Hysterectomy Performed via Minimally Invasive Routes Compared with Open Surgery: A Systematic Review and Network Meta-Analysis" Cancers 16, no. 10: 1860. https://doi.org/10.3390/cancers16101860
APA StyleNatarajan, P., Delanerolle, G., Dobson, L., Xu, C., Zeng, Y., Yu, X., Marston, K., Phan, T., Choi, F., Barzilova, V., Powell, S. G., Wyatt, J., Taylor, S., Shi, J. Q., & Hapangama, D. K. (2024). Surgical Treatment for Endometrial Cancer, Hysterectomy Performed via Minimally Invasive Routes Compared with Open Surgery: A Systematic Review and Network Meta-Analysis. Cancers, 16(10), 1860. https://doi.org/10.3390/cancers16101860








