Efficacy of Pembrolizumab vs. Nivolumab Plus Ipilimumab in Metastatic NSCLC in Relation to PD-L1 and TMB Status
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population and Inclusion Criteria
2.3. Exclusion Criteria
2.4. Treatment Administered
2.4.1. Adenocarcinoma
2.4.2. Squamous Cell Carcinoma and Adeno-Squamous Cell Carcinoma
2.5. Data and Statistical Analysis
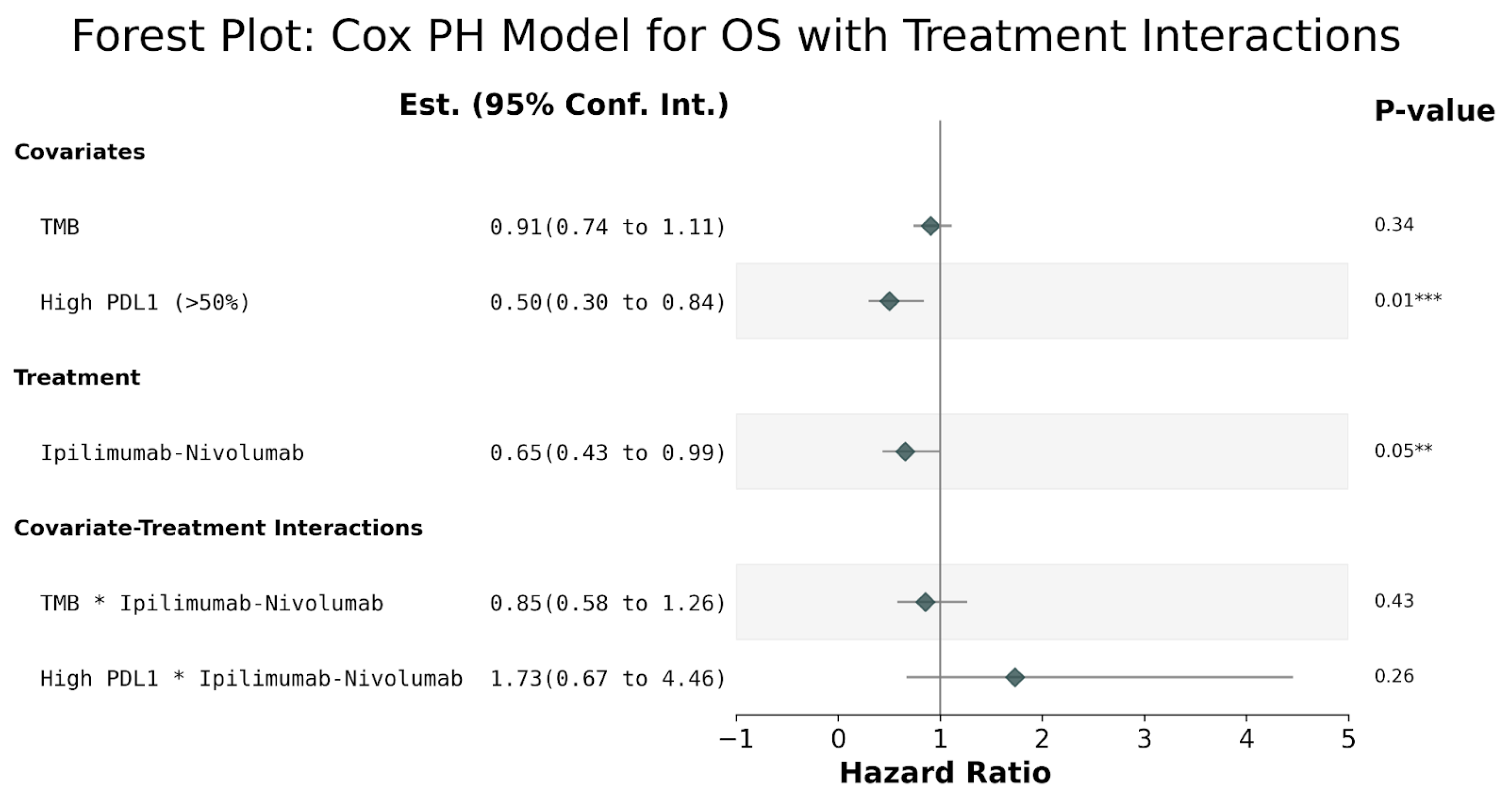
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Doi, T.; Jang, R.W.; Muro, K.; Satoh, T.; Machado, M.; Sun, W.; Jalal, S.; Shah, M.; Metges, J.; et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018, 4, e180013. [Google Scholar]
- Szeto, C.H.; Shalata, W.; Yakobson, A.; Agbarya, A. Neoadjuvant and Adjuvant Immunotherapy in Early-Stage Non-Small-Cell Lung Cancer, Past, Present, and Future. J. Clin. Med. 2021, 10, 5614. [Google Scholar] [CrossRef]
- Shalata, W.; Yakobson, A.; Dudnik, Y.; Swaid, F.; Ahmad, M.S.; Abu Jama, A.; Cohen, A.Y.; Agbarya, A. Multi-Center Real-World Outcomes of Nivolumab Plus Ipilimumab and Chemotherapy in Patients with Metastatic Non-Small-Cell Lung Cancer. Biomedicines 2023, 11, 2438. [Google Scholar] [CrossRef]
- Kian, W.; Christopoulos, P.; Remilah, A.A.; Levison, E.; Dudnik, E.; Shalata, W.; Krayim, B.; Marei, R.; Yakobson, A.; Faehling, M.; et al. Real-world efficacy and safety of mobocertinib in EGFR exon 20 insertion-mutated lung cancer. Front. Oncol. 2022, 12, 1010311. [Google Scholar] [CrossRef]
- Shalata, W.; Zolnoorian, J.; Migliozzi, G.; Abu Jama, A.; Dudnik, Y.; Cohen, A.Y.; Meirovitz, A.; Yakobson, A. Long-Lasting Therapeutic Response following Treatment with Pembrolizumab in Patients with Non-Small Cell Lung Cancer: A Real-World Experience. Int. J. Mol. Sci. 2023, 24, 5938. [Google Scholar] [CrossRef]
- Morganti, S.; Tarantino, P.; Ferraro, E.; D’Amico, P.; Duso, B.A.; Curigliano, G. Next Generation Sequencing (NGS): A Revolutionary Technology in Pharmacogenomics and Personalized Medicine in Cancer. Adv. Exp. Med. Biol. 2019, 1168, 9–30. [Google Scholar] [CrossRef]
- Janzic, U.; Shalata, W.; Szymczak, K.; Dziadziuszko, R.; Jakopovic, M.; Mountzios, G.; Płużański, A.; Araujo, A.; Charpidou, A.; Agbarya, A. Real-World Experience in Treatment of Patients with Non-Small-Cell Lung Cancer with BRAF or cMET Exon 14 Skipping Mutations. Int. J. Mol. Sci. 2023, 24, 12840. [Google Scholar] [CrossRef]
- Sha, D.; Jin, Z.; Budczies, J.; Kluck, K.; Stenzinger, A.; Sinicrope, F.A. Tumor Mutational Burden as a Predictive Biomarker in Solid Tumors. Cancer Discov. 2020, 10, 1808–1825. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H.; et al. Association of tumour mutational burden without-comes in patients with select advanced solid tumors treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Schrock, A.B.; Ouyang, C.; Sandhu, J.; Sokol, E.; Jin, D.; Ross, J.S.; Miller, V.A.; Lim, D.; Amanam, I.; Chao, J.; et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann. Oncol. 2019, 30, 1096–1103. [Google Scholar] [CrossRef]
- Fabrizio, D.A.; George, T.J., Jr.; Dunne, R.F.; Frampton, G.; Sun, J.; Gowen, K.; Kennedy, M.; Greenbowe, J.; Schrock, A.B.; Hezel, A.F.; et al. Beyond microsatellite testing: Assessment of tumor mutational burden identifies subsets of colorectal cancer who may respond to immune checkpoint inhibition. J. Gastrointest. Oncol. 2018, 9, 610–617. [Google Scholar] [CrossRef]
- Innocenti, F.; Ou, F.S.; Qu, X.; Zemla, T.J.; Niedzwiecki, D.; Tam, R.; Mahajan, S.; Goldberg, R.M.; Bertagnolli, M.M.; Blanke, C.D.; et al. Mutational analysis of patients with colorectal cancer in CALGB/SWOG 80405 identifies new roles of microsatellite instability and tumor mutational burden for patient outcome. J. Clin. Oncol. 2019, 37, 1217–1227. [Google Scholar] [CrossRef]
- Addeo, A.; Friedlaender, A.; Banna, G.L.; Weiss, G.J. TMB or not TMB as a biomarker: That is the question. Crit. Rev. Oncol. 2021, 163, 103374. [Google Scholar] [CrossRef]
- Taube, J.M.; Klein, A.; Brahmer, J.R.; Xu, H.; Pan, X.; Kim, J.H.; Chen, L.; Pardoll, D.M.; Topalian, S.L.; Anders, R.A. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 2014, 20, 5064–5074. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef]
- Yarchoan, M.; Albacker, L.A.; Hopkins, A.C.; Montesion, M.; Murugesan, K.; Vithayathil, T.T.; Zaidi, N.; Azad, N.S.; Laheru, D.A.; Frampton, G.M.; et al. PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight 2019, 4, e126908. [Google Scholar] [CrossRef]
- Chan, T.; Yarchoan, M.; Jaffee, E.; Swanton, C.; Quezada, S.; Stenzinger, A.; Peters, S. Development of tumor mutation burden as an immunotherapy biomarker: Utility for the oncology clinic. Ann. Oncol. 2018, 30, 44–56. [Google Scholar] [CrossRef]
- Alborelli, I.; Leonards, K.; Rothschild, S.I.; Leuenberger, L.P.; Prince, S.S.; Mertz, K.D.; Poechtrager, S.; Buess, M.; Zippelius, A.; Läubli, H.; et al. Tumor mutational burden assessed by targeted NGS predicts clinical benefit from immune checkpoint inhibitors in non-small cell lung cancer. J. Pathol. 2020, 250, 19–29. [Google Scholar] [CrossRef]
- Greillier, L.; Tomasini, P.; Barlesi, F. The clinical utility of tumor mutational burden in non-small cell lung cancer. Transl. Lung Cancer Res. 2018, 7, 639–646. [Google Scholar] [CrossRef]
- Yu, Y.; Zeng, D.; Ou, Q.; Liu, S.; Li, A.; Chen, Y.; Lin, D.; Gao, Q.; Zhou, H.; Liao, W.; et al. Association of Survival and Immune-Related Biomarkers with Immunotherapy in Patients With Non–Small Cell Lung Cancer: A Meta-analysis and Individual Patient-Level Analysis. JAMA Netw. Open 2019, 2, e196879. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer, Version 2.2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed on 23 March 2023).
- Paz-Ares, L.; Ciuleanu, T.-E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): An international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 198–211, Erratum in Lancet Oncol. 2021, 22, e92. [Google Scholar] [CrossRef]
- Garassino, M.C.; Gadgeel, S.; Speranza, G.; Felip, E.; Esteban, E.; Dómine, M.; Hochmair, M.J.; Powell, S.F.; Bischoff, H.G.; Peled, N.; et al. Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non–Small-Cell Lung Cancer: 5-Year Outcomes from the Phase 3 KEYNOTE-189 Study. J. Clin. Oncol. 2023, 41, 1992–1998. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Shalata, W.; Yakobson, A.; Weissmann, S.; Oscar, E.; Iraqi, M.; Kian, W.; Peled, N.; Agbarya, A. Crizotinib in MET Exon 14-Mutated or MET-Amplified in Advanced Disease Non-Small Cell Lung Cancer: A Retrospective, Single Institution Experience. Oncology 2022, 100, 467–474. [Google Scholar] [CrossRef]
- Mountzios, G.; Planchard, D.; Metro, G.; Tsiouda, D.; Prelaj, A.; Lampaki, S.; Shalata, W.; Riudavets, M.; Christopoulos, P.; Girard, N.; et al. Molecular Epidemiology and Treatment Patterns of Patients with EGFR Exon 20-Mutant NSCLC in the Precision Oncology Era: The European EXOTIC Registry. JTO Clin. Res. Rep. 2022, 4, 100433. [Google Scholar] [CrossRef]
- Shalata, W.; Iraqi, M.; Bhattacharya, B.; Fuchs, V.; Roisman, L.C.; Cohen, A.Y.; Massalha, I.; Yakobson, A.; Prasad, M.; Elkabets, M.; et al. Rapid Response to the Combination of Lenvatinib and Pembrolizumab in Patients with Advanced Carcinomas (Lung Adenocarcinoma and Malignant Pleural Mesothelioma). Cancers 2021, 13, 3630. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Langer, C.; Novello, S.; Halmos, B.; Cheng, Y.; Gadgeel, S.; Hui, R.; Sugawara, S.; Borghaei, H.; Cristescu, R.; et al. Pembrolizumab (pembro) plus platinum-based chemotherapy (chemo) for metastatic NSCLC: Tissue TMB (tTMB) and outcomes in KEYNOTE-021, 189, and 407. Ann. Oncol. 2019, 30, v917–v918. [Google Scholar] [CrossRef]
- Federico, A.D.; Gelsomino, F.; Giglio, A.D.; Sperandi, F.; Melotti, B.; Ardizzoni, A. 800 Clinicopathological and molecular predictive factors of survival in non-small cell lung cancer patients treated with first-line immunotherapy with or without chemotherapy: a systematic review and meta-analysis. J. Immuno Therapy Cancer 2022, 10, A833–A835. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Nathanson, T.; Rizvi, H.; Creelan, B.C.; Sanchez-Vega, F.; Ahuja, A.; Ni, A.; Novik, J.B.; Mangarin, L.M.; Abu-Akeel, M.; et al. Genomic Features of Response to Combination Immunotherapy in Patients with Advanced Non-Small-Cell Lung Cancer. Cancer Cell 2018, 33, 843–852. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hendriks, L.E.; Rouleau, E.; Besse, B. Clinical utility of tumor mutational burden in patients with non-small cell lung cancer treated with immunotherapy. Transl. Lung Cancer Res. 2018, 7, 647–660. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jung, J.; Heo, Y.J.; Park, S. High tumor mutational burden predicts favorable response to anti-PD-(L)1 therapy in patients with solid tumor: A real-world pan-tumor analysis. J. Immunother. Cancer 2023, 11, e006454. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiao, D.; Pan, H.; Li, F.; Wu, K.; Zhang, X.; He, J. Analysis of ultra-deep targeted sequencing reveals mutation burden is associated with gender and clinical outcome in lung adenocarcinoma. Oncotarget 2016, 7, 22857–22864. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S. Lung carcinogenesis by tobacco smoke. Int. J. Cancer 2012, 131, 2724–2732. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Ju, Y.S.; Haase, K.; Van Loo, P.; Martincorena, I.; Nik-Zainal, S.; Totoki, Y.; Fujimoto, A.; Nakagawa, H.; Shibata, T.; et al. Europe PMC Funders Group Mutational signatures associated with tobacco smoking in human cancer. Science 2018, 354, 618–622. [Google Scholar] [CrossRef]
- Sesma, A.; Pardo, J.; Cruellas, M.; Gálvez, E.M.; Gascón, M.; Isla, D.; Martínez-Lostao, L.; Ocáriz, M.; Paño, J.R.; Quílez, E.; et al. From Tumor Mutational Burden to Blood T Cell Receptor: Looking for the Best Predictive Biomarker in Lung Cancer Treated with Immunotherapy. Cancers 2020, 12, 2974. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Guo, G.; Cai, X.; Yu, H.; Cai, Y.; Zhang, B.; Hong, S.; Zhang, L. Nivolumab plus ipilimumab versus pembrolizumab as chemotherapy-free, first-line treatment for PD-L1-positive non-small cell lung cancer. Clin. Transl. Med. 2020, 10, 107–115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| Median (Range) | ||
|---|---|---|
| Age (years) | 67 (37–86) | |
| Male | 67 (37–86) | |
| Female | 66 (40–82) | |
| Gender | Frequencies (percentage-%) | |
| Male | 138 (71.13) | |
| Female | 56 (28.87) | |
| Smoking status (at diagnosis) | ||
| Current | 109 (55.61) | |
| Former | 59 (30.1) | |
| Never | 26 (13.27) | |
| ECOG status | ||
| 0 | 35 (20.12) | |
| 1 | 107 (61.49) | |
| 2 | 52 (29.89) | |
| Histology | ||
| Adenocarcinoma | 139 (71.65) | |
| SCC | 47 (24.23) | |
| Adenosquamous cell carcinoma | 5 (2.58) | |
| Others * | 3 (1.55) | |
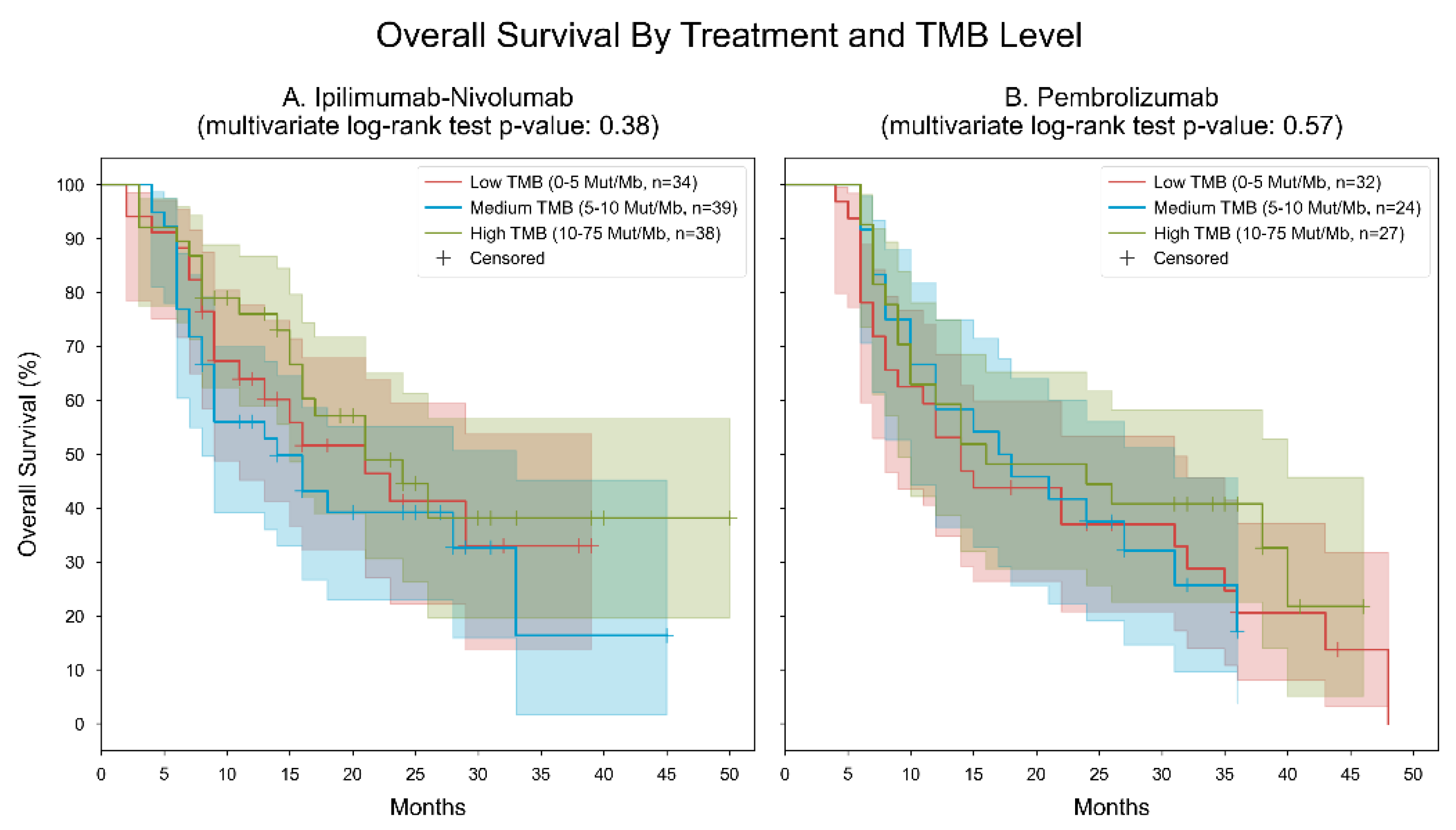
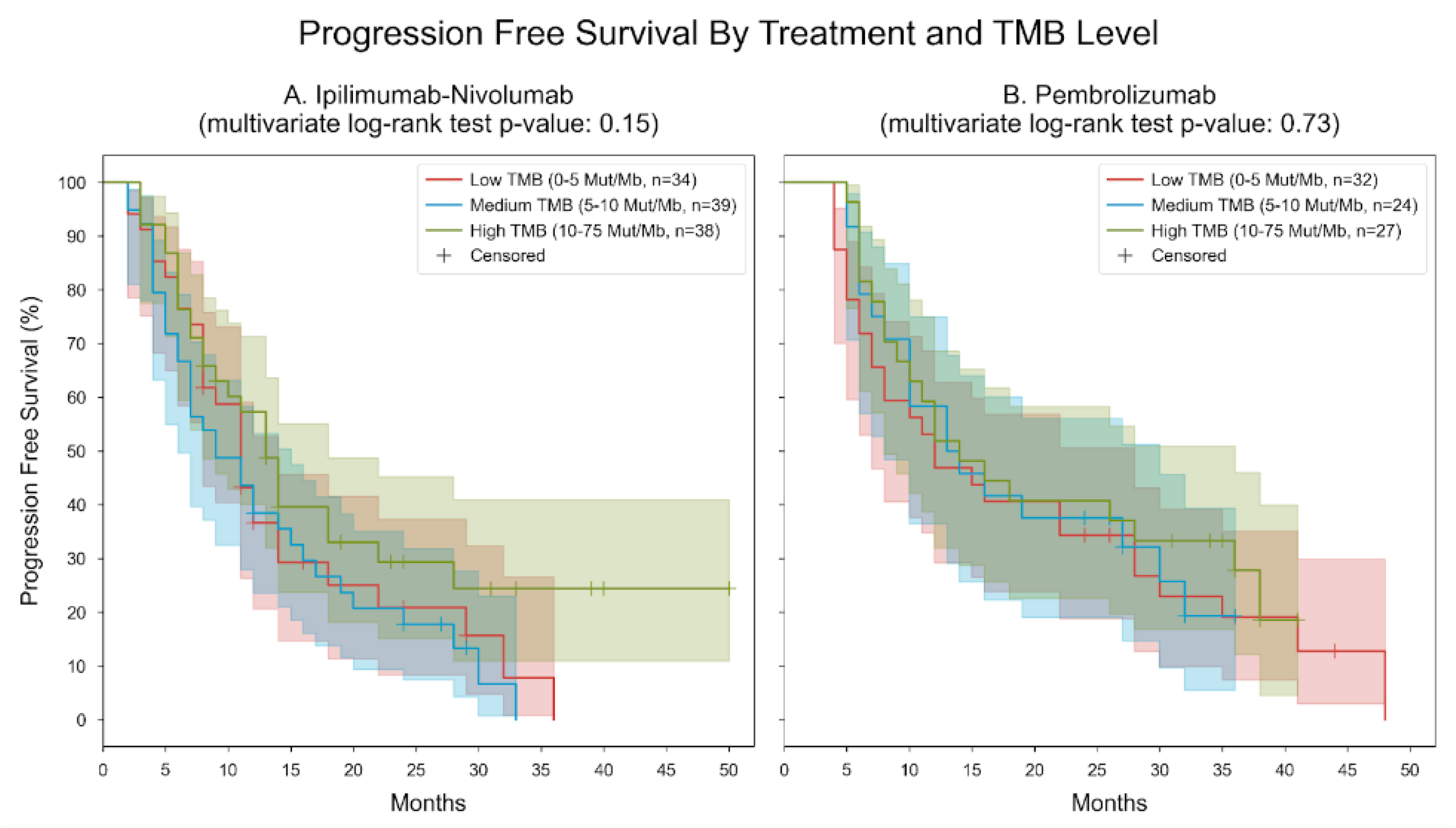
| Immunotherapy administered | ||
| nivolumab plus ipilimumab | 111 (57.22) | |
| pembrolizumab | 83 (42.78) | |
| Co-mutations | ||
| KRAS | 55 (28.35) | |
| TP53 | 52 (26.8) | |
| STK11 | 18 (9.28) | |
| CDK 4–6 | 8 (4.12) | |
| KEAP1 | 6 (3.09) | |
| CDKN2A | 6 (3.09) | |
| EGFR amplification | 5 (2.58) | |
| BRAF non V600E | 5 (2.58) | |
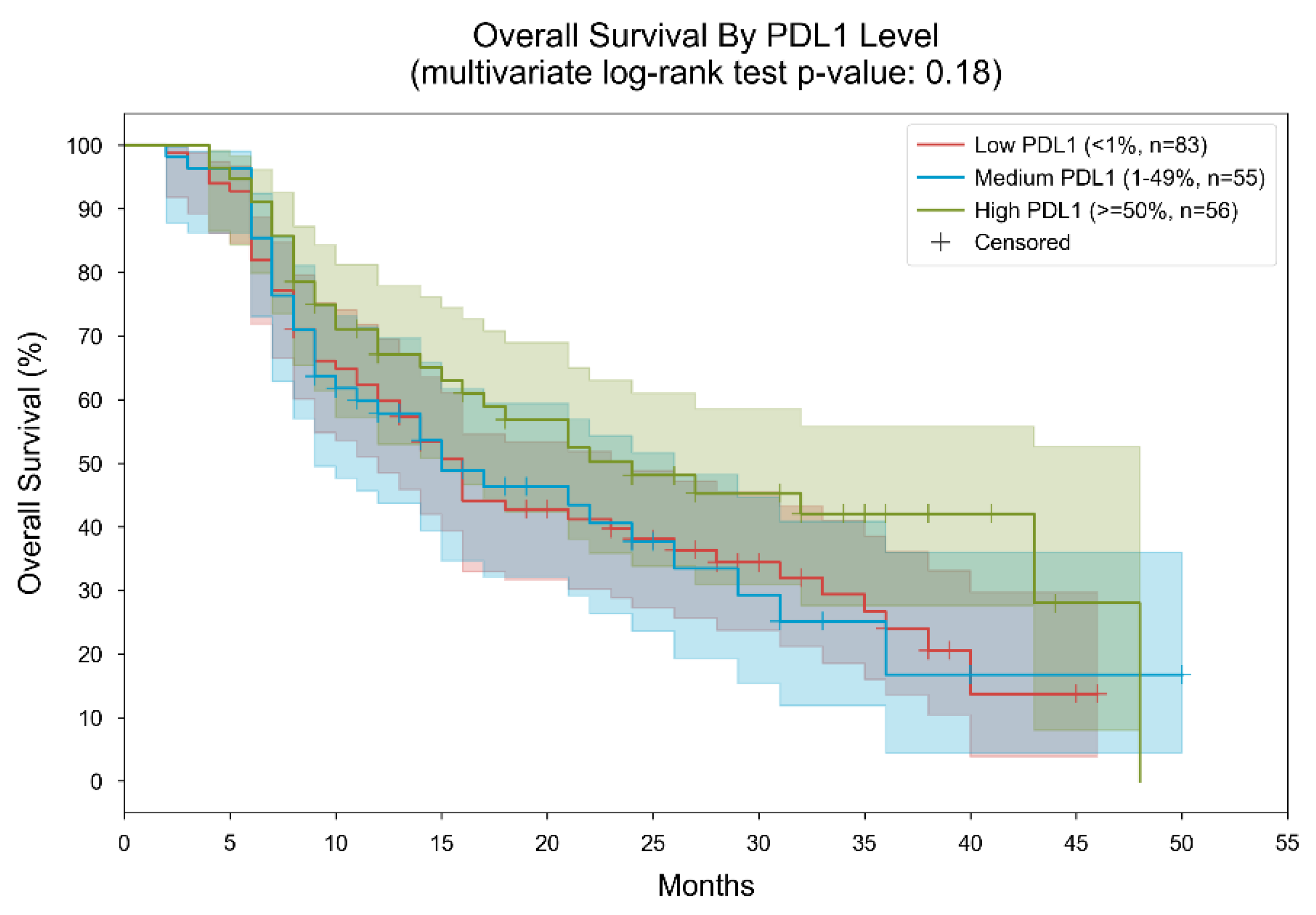
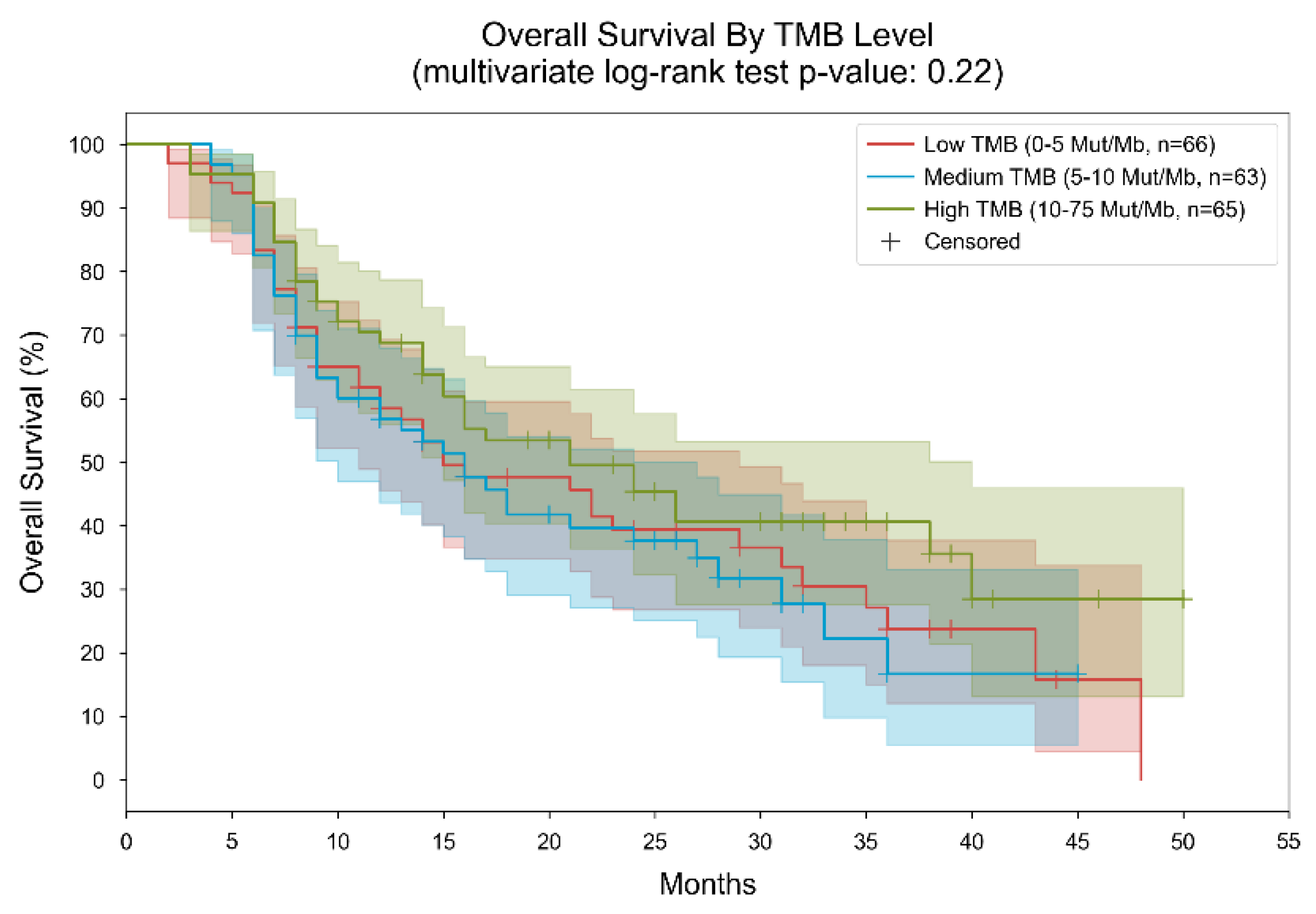
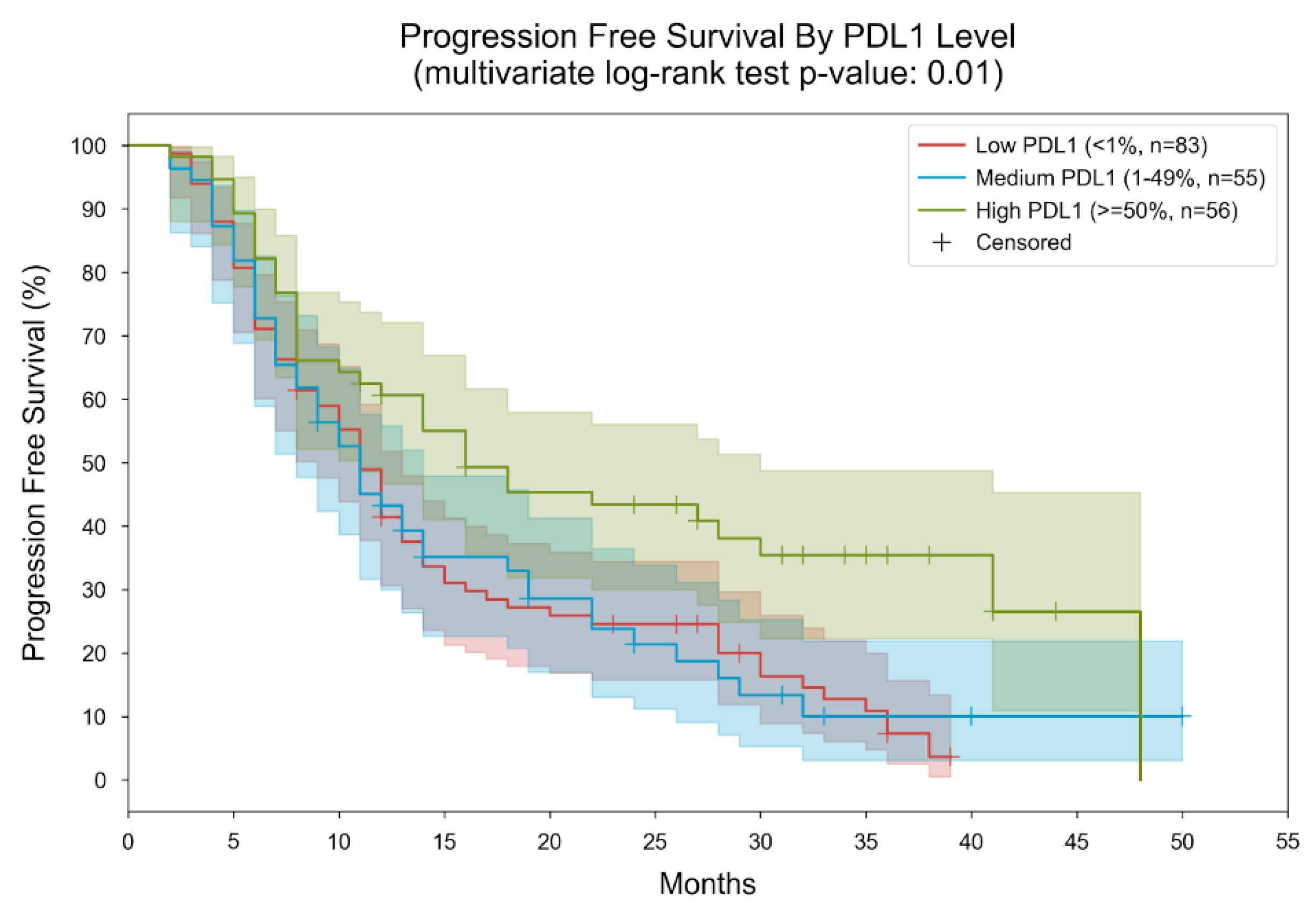
| PD-L1 status | Frequencies (percentage-%) | TMB Median (range) |
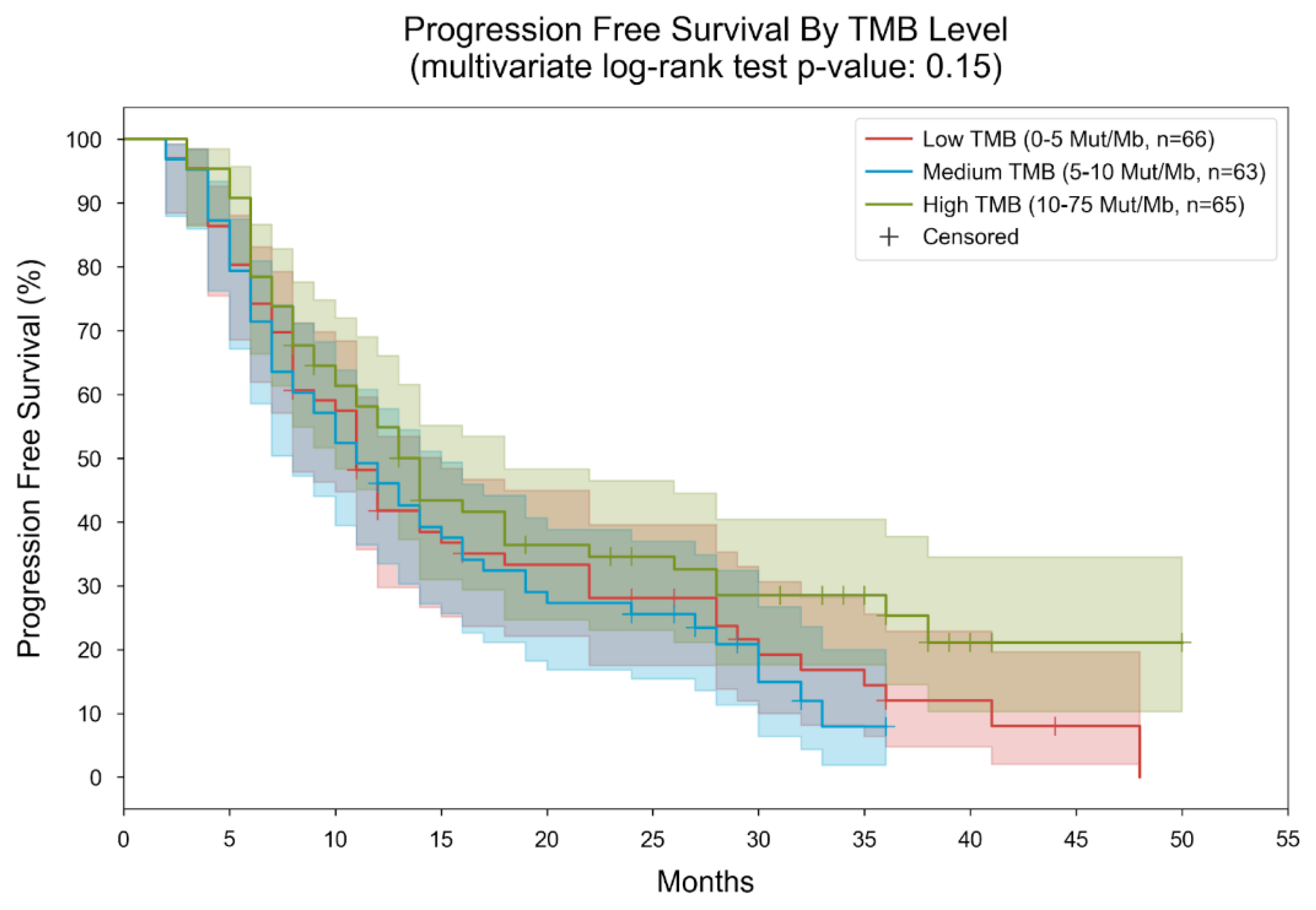
| PD-L1 expression < 1% | 83 (42.78) | 10.31 (0–75) |
| PD-L1 expression 1–49% | 55 (28.35) | 9.73 (0.95–39.63) |
| PD-L1 expression >50% | 56 (28.87) | 9.72 (0.95–48) |
| Metastasis locations | ||
| Lung | 113 (58.25) | |
| Lymph nodes involvement | 100 (51.55) | |
| Bone | 67 (34.54) | |
| Pleural effusion | 43 (22.16) | |
| Brain | 35 (18.04) | |
| Adrenal | 30 (15.46) | |
| Liver | 25 (12.89) | |
| Pericardial effusion | 4 (2.06) | |
| Spleen | 1 (0.52) |
| Type of AE (Grade 1–2) | Total Occurrences | Keytruda | Ipilimumab and Nivolumab | P, 95% CI (Difference in Proportions) |
| Anemia | 188 (97.42%) | 59 (30.41%) | 129 (66.49%) | <0.001, (0.2377, 0.4073) |
| Fatigue | 143 (73.71%) | 63 (32.47%) | 80 (41.24%) | 0.066, (0.0023, 0.3112) |
| Hypothyroidism | 65 (33.51%) | 24 (12.37%) | 41 (21.13%) | 0.008, (0.0484, 0.4136) |
| Rash | 44 (22.68%) | 13 (6.70%) | 31 (15.98%) | 0.013, (0.0451, 0.4032) |
| Diarrhea | 35 (18.04%) | 12 (6.19%) | 23 (11.86%) | 0.056, (0.0058, 0.3007) |
| Pruritus | 33 (17.01%) | 10 (5.15%) | 23 (11.86%) | 0.017, (0.0554, 0.3946) |
| Arthralgia | 24 (12.37%) | 9 (4.64%) | 15 (7.73%) | 0.199, (0.0109, 0.3609) |
| Transaminases | 24 (12.37%) | 6 (3.09%) | 18 (9.28%) | 0.174, (0.0375, 0.4135) |
| Pneumonitis | 21 (10.82%) | 6 (3.09%) | 15 (7.73%) | 0.197, (0.0284, 0.3748) |
| Hyperthyroidism | 10 (5.15%) | 0 | 10 (5.15%) | 0.003, (0.1069, 0.6531) |
| Creatinine elevation | 8 (4.12%) | 2 (1.03%) | 6 (3.09%) | <0.001, (0.2377, 0.4073) |
| Type of AE (Grade ≥ 3) | Total Occurrences | Keytruda | Ipilimumab and Nivolumab | P, 95% CI (Difference in Proportions) |
| Nausea | 5 (2.58%) | 2 (1.03%) | 3 (1.55%) | 0.564, (0.3456, 0.6456) |
| Neutropenia | 3 (1.55%) | 1 (0.52%) | 2 (1.03%) | 0.564, (0.3456, 0.6456) |
| Abdominal pain | 2 (1.03%) | 0 | 2 (1.03%) | 0.564, (0.3456, 0.6456) |
| Vomiting | 2 (1.03%) | 0 | 2 (1.03%) | 0.564, (0.3456, 0.6456) |
| Hepatitis | 2 (1.03%) | 0 | 2 (1.03%) | 0.564, (0.3456, 0.6456) |
| Diarrhea | 2 (1.03%) | 1 (0.52%) | 1 (0.52%) | 1, (0.9616, 0.9616) |
| Pneumonitis | 1 (0.52%) | 0 | 1 (0.52%) | 1, (0.9616, 0.9616) |
| Myositis | 2 (1.03%) | 2 (1.03%) | 0 | 0.564, (0.3456, 0.6456) |
| Neuropathy | 1 (0.52%) | 1 (0.52%) | 0 | 1, (0.9616, 0.9616) |
| Myocarditis | 1 (0.52%) | 1 (0.52%) | 0 | 1, (0.9616, 0.9616) |
| Polyneuropathy | 1 (0.52%) | 1 (0.52%) | 0 | 1, (0.9616, 0.9616) |
| Periorbital edema | 1 (0.52%) | 1 (0.52%) | 0 | 1, (0.9616, 0.9616) |
| Death | 1 (0.52%) | 0 | 1 (0.52%) | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shalata, W.; Maimon Rabinovich, N.; Agbarya, A.; Yakobson, A.; Dudnik, Y.; Abu Jama, A.; Cohen, A.Y.; Shalata, S.; Abu Hamed, A.; Ilan Ber, T.; et al. Efficacy of Pembrolizumab vs. Nivolumab Plus Ipilimumab in Metastatic NSCLC in Relation to PD-L1 and TMB Status. Cancers 2024, 16, 1825. https://doi.org/10.3390/cancers16101825
Shalata W, Maimon Rabinovich N, Agbarya A, Yakobson A, Dudnik Y, Abu Jama A, Cohen AY, Shalata S, Abu Hamed A, Ilan Ber T, et al. Efficacy of Pembrolizumab vs. Nivolumab Plus Ipilimumab in Metastatic NSCLC in Relation to PD-L1 and TMB Status. Cancers. 2024; 16(10):1825. https://doi.org/10.3390/cancers16101825
Chicago/Turabian StyleShalata, Walid, Natalie Maimon Rabinovich, Abed Agbarya, Alexander Yakobson, Yulia Dudnik, Ashraf Abu Jama, Ahron Yehonatan Cohen, Sondos Shalata, Ahmad Abu Hamed, Tahel Ilan Ber, and et al. 2024. "Efficacy of Pembrolizumab vs. Nivolumab Plus Ipilimumab in Metastatic NSCLC in Relation to PD-L1 and TMB Status" Cancers 16, no. 10: 1825. https://doi.org/10.3390/cancers16101825
APA StyleShalata, W., Maimon Rabinovich, N., Agbarya, A., Yakobson, A., Dudnik, Y., Abu Jama, A., Cohen, A. Y., Shalata, S., Abu Hamed, A., Ilan Ber, T., Machluf, O., Shoham Levin, G., & Meirovitz, A. (2024). Efficacy of Pembrolizumab vs. Nivolumab Plus Ipilimumab in Metastatic NSCLC in Relation to PD-L1 and TMB Status. Cancers, 16(10), 1825. https://doi.org/10.3390/cancers16101825









