Speeding Up and Improving Image Quality in Glioblastoma MRI Protocol by Deep Learning Image Reconstruction
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
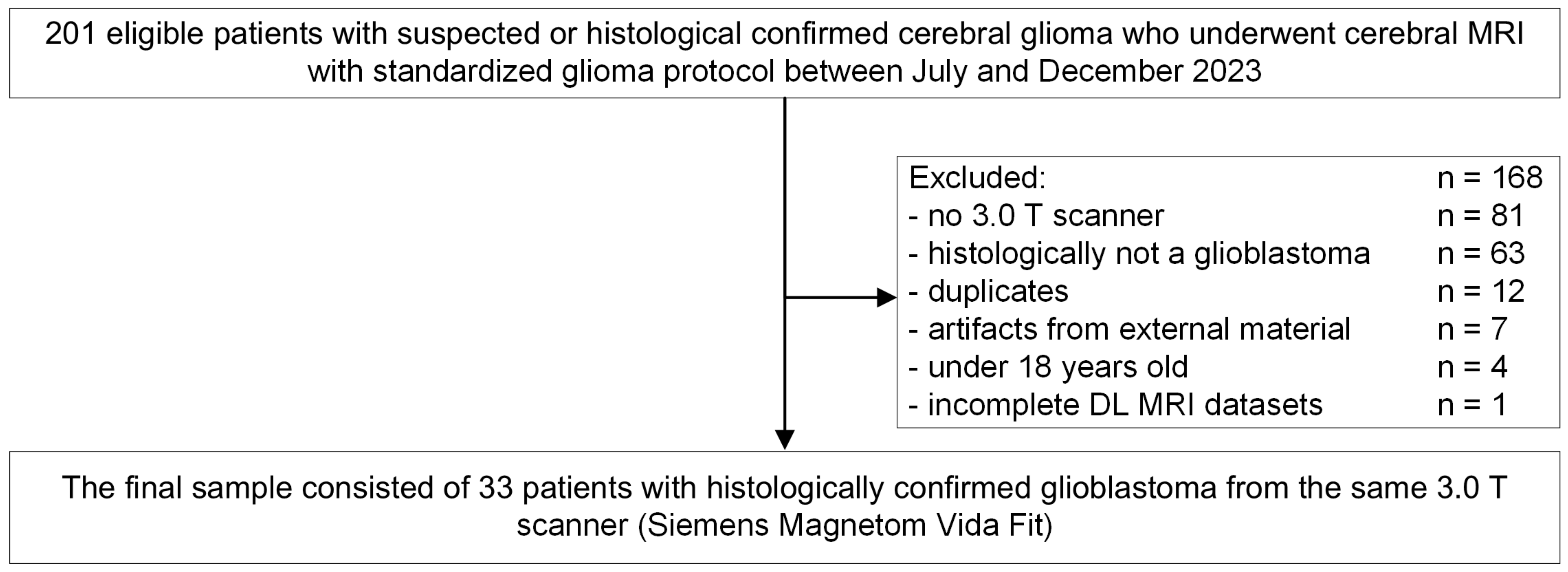
2.1. Study Design
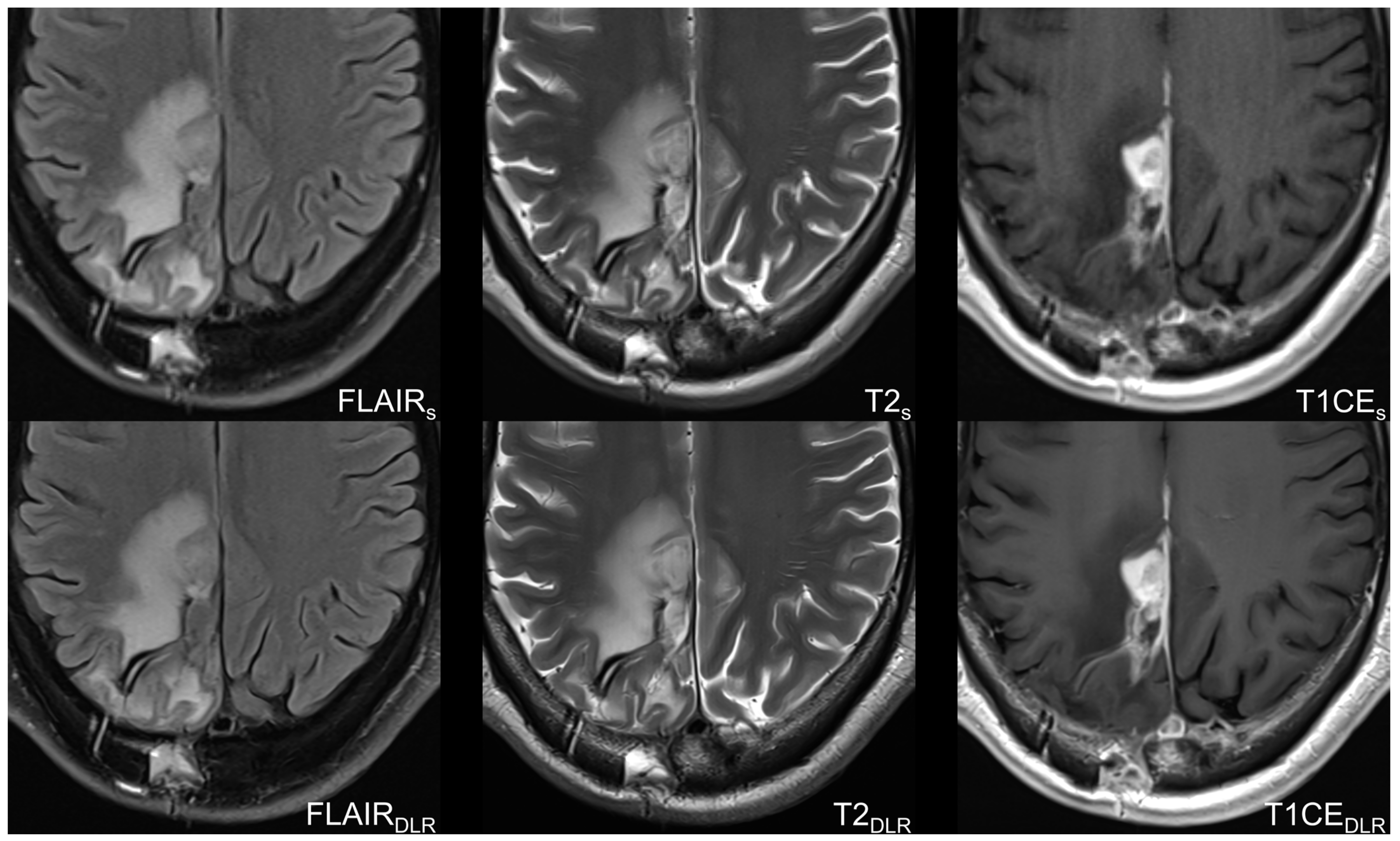
2.2. Imaging Protocol and Deep Learning Reconstruction Algorithm
2.3. Image Analysis
2.4. RANO 2.0
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Image Quality-Based Analysis
3.3. Agreement and Concordance
3.4. RANO 2.0
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme–Literature Review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef] [PubMed]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of Its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, A.; Gulani, V.; Griswold, M.A.; Seiberlich, N. Parallel MR Imaging. J. Magn. Reson. Imaging 2012, 36, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Jaspan, O.N.; Fleysher, R.; Lipton, M.L. Compressed Sensing MRI: A Review of the Clinical Literature. Br. J. Radiol. 2015, 88, 20150487. [Google Scholar] [CrossRef] [PubMed]
- Recht, M.P.; Zbontar, J.; Sodickson, D.K.; Knoll, F.; Yakubova, N.; Sriram, A.; Murrell, T.; Defazio, A.; Rabbat, M.; Rybak, L.; et al. Using Deep Learning to Accelerate Knee MRI at 3 T: Results of an Interchangeability Study. AJR Am. J. Roentgenol. 2020, 215, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Benkert, T.; Block, K.T.; Sodickson, D.K.; Otazo, R.; Chandarana, H. Compressed Sensing for Body MRI. J. Magn. Reson. Imaging 2017, 45, 966–987. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.K.; Roth, C.G.; Ward, R.J.; deJesus, J.O.; Mitchell, D.G. Optimizing Abdominal MR Imaging: Approaches to Common Problems. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2010, 30, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Armato, S.G.; Drukker, K.; Hadjiiski, L. AI in Medical Imaging Grand Challenges: Translation from Competition to Research Benefit and Patient Care. Br. J. Radiol. 2023, 96, 20221152. [Google Scholar] [CrossRef] [PubMed]
- Hokamura, M.; Uetani, H.; Nakaura, T.; Matsuo, K.; Morita, K.; Nagayama, Y.; Kidoh, M.; Yamashita, Y.; Ueda, M.; Mukasa, A.; et al. Exploring the Impact of Super-Resolution Deep Learning on MR Angiography Image Quality. Neuroradiology 2024, 66, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, M.; Liu, M.; Zhang, D. A Survey on Deep Learning for Neuroimaging-Based Brain Disorder Analysis. Front. Neurosci. 2020, 14, 779. [Google Scholar] [CrossRef] [PubMed]
- Radmanesh, A.; Muckley, M.J.; Murrell, T.; Lindsey, E.; Sriram, A.; Knoll, F.; Sodickson, D.K.; Lui, Y.W. Exploring the Acceleration Limits of Deep Learning Variational Network-Based Two-Dimensional Brain MRI. Radiol. Artif. Intell. 2022, 4, 210313. [Google Scholar] [CrossRef] [PubMed]
- Noordman, C.R.; Yakar, D.; Bosma, J.; Simonis, F.F.J.; Huisman, H. Complexities of Deep Learning-Based Undersampled MR Image Reconstruction. Eur. Radiol. Exp. 2023, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Estler, A.; Hauser, T.-K.; Mengel, A.; Brunnée, M.; Zerweck, L.; Richter, V.; Zuena, M.; Schuhholz, M.; Ernemann, U.; Gohla, G. Deep Learning Accelerated Image Reconstruction of Fluid-Attenuated Inversion Recovery Sequence in Brain Imaging: Reduction of Acquisition Time and Improvement of Image Quality. Acad. Radiol. 2024, 31, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Almansour, H.; Herrmann, J.; Gassenmaier, S.; Afat, S.; Jacoby, J.; Koerzdoerfer, G.; Nickel, D.; Mostapha, M.; Nadar, M.; Othman, A.E. Deep Learning Reconstruction for Accelerated Spine MRI: Prospective Analysis of Interchangeability. Radiology 2023, 306, 212922. [Google Scholar] [CrossRef] [PubMed]
- Gassenmaier, S.; Afat, S.; Nickel, D.; Mostapha, M.; Herrmann, J.; Othman, A.E. Deep Learning-Accelerated T2-Weighted Imaging of the Prostate: Reduction of Acquisition Time and Improvement of Image Quality. Eur. J. Radiol. 2021, 137, 109600. [Google Scholar] [CrossRef] [PubMed]
- Hokamura, M.; Uetani, H.; Hamasaki, T.; Nakaura, T.; Morita, K.; Yamashita, Y.; Kitajima, M.; Sugitani, A.; Mukasa, A.; Hirai, T. Effect of Deep Learning-Based Reconstruction on High-Resolution Three-Dimensional T2-Weighted Fast Asymmetric Spin-Echo Imaging in the Preoperative Evaluation of Cerebellopontine Angle Tumors. Neuroradiology 2024. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, B.M.; Wen, P.Y.; Cloughesy, T.F. Modified Criteria for Radiographic Response Assessment in Glioblastoma Clinical Trials. Neurotherapeutics 2017, 14, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Koerzdoerfer, G.; Nickel, D.; Mostapha, M.; Nadar, M.; Gassenmaier, S.; Kuestner, T.; Othman, A.E. Feasibility and Implementation of a Deep Learning MR Reconstruction for TSE Sequences in Musculoskeletal Imaging. Diagnostics 2021, 11, 1484. [Google Scholar] [CrossRef] [PubMed]
- Schlemper, J.; Caballero, J.; Hajnal, J.V.; Price, A.N.; Rueckert, D. A Deep Cascade of Convolutional Neural Networks for Dynamic MR Image Reconstruction. IEEE Trans. Med. Imaging 2018, 37, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Hammernik, K.; Klatzer, T.; Kobler, E.; Recht, M.P.; Sodickson, D.K.; Pock, T.; Knoll, F. Learning a Variational Network for Reconstruction of Accelerated MRI Data. Magn. Reson. Med. 2018, 79, 3055–3071. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; van den Bent, M.; Youssef, G.; Cloughesy, T.F.; Ellingson, B.M.; Weller, M.; Galanis, E.; Barboriak, D.P.; de Groot, J.; Gilbert, M.R.; et al. RANO 2.0: Update to the Response Assessment in Neuro-Oncology Criteria for High- and Low-Grade Gliomas in Adults. J. Clin. Oncol. 2023, 41, 5187–5199. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Interrater Reliability: The Kappa Statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Zou, B.; Ji, Z.; Zhu, C.; Dai, Y.; Zhang, W.; Kui, X. Multi-Scale Deformable Transformer for Multi-Contrast Knee MRI Super-Resolution. Biomed. Signal Process. Control 2023, 79, 104154. [Google Scholar] [CrossRef]
- Grigas, O.; Maskeliūnas, R.; Damaševičius, R. Improving Structural MRI Preprocessing with Hybrid Transformer GANs. Life 2023, 13, 1893. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, J.; Tomás, P.; Garcia, N.; Aidos, H. Super-Resolution of Magnetic Resonance Images Using Generative Adversarial Networks. Comput. Med. Imaging Graph. 2023, 108, 102280. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Zhou, L.; Sun, J. Deep-Learning-Based 3D Super-Resolution MRI Radiomics Model: Superior Predictive Performance in Preoperative T-Staging of Rectal Cancer. Eur. Radiol. 2023, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Monsour, R.; Dutta, M.; Mohamed, A.-Z.; Borkowski, A.; Viswanadhan, N.A. Neuroimaging in the Era of Artificial Intelligence: Current Applications. Fed. Pract. 2022, 39, S14–S20. [Google Scholar] [CrossRef] [PubMed]
- Wessling, D.; Herrmann, J.; Afat, S.; Nickel, D.; Othman, A.E.; Almansour, H.; Gassenmaier, S. Reduction in Acquisition Time and Improvement in Image Quality in T2-Weighted MR Imaging of Musculoskeletal Tumors of the Extremities Using a Novel Deep Learning-Based Reconstruction Technique in a Turbo Spin Echo (TSE) Sequence. Tomography 2022, 8, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- Estler, A.; Zerweck, L.; Brunnée, M.; Estler, B.; Richter, V.; Örgel, A.; Bürkle, E.; Becker, H.; Hurth, H.; Stahl, S.; et al. Deep Learning-Accelerated Image Reconstruction in MRI of the Orbit to Shorten Acquisition Time and Enhance Image Quality. J. Neuroimaging 2024, 34, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Estler, A.; Hauser, T.-K.; Brunnée, M.; Zerweck, L.; Richter, V.; Knoppik, J.; Örgel, A.; Bürkle, E.; Adib, S.D.; Hengel, H.; et al. Deep Learning-Accelerated Image Reconstruction in Back Pain-MRI Imaging: Reduction of Acquisition Time and Improvement of Image Quality. Radiol. Med. 2024, 129, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Wielema, M.; Cornelissen, L.J.; van Gent, M.; Iwema, W.M.; Zheng, S.; Sijens, P.E.; Oudkerk, M.; Dorrius, M.D.; van Ooijen, P.M.A. Using Deep Learning to Safely Exclude Lesions with Only Ultrafast Breast MRI to Shorten Acquisition and Reading Time. Eur. Radiol. 2022, 32, 8706–8715. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Benkert, T.; Brendlin, A.; Gassenmaier, S.; Hölldobler, T.; Maennlin, S.; Almansour, H.; Lingg, A.; Weiland, E.; Afat, S. Shortening Acquisition Time and Improving Image Quality for Pelvic MRI Using Deep Learning Reconstruction for Diffusion-Weighted Imaging at 1.5 T. Acad. Radiol. 2024, 31, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Lustig, M.; Donoho, D.; Pauly, J.M. Sparse MRI: The Application of Compressed Sensing for Rapid MR Imaging. Magn. Reson. Med. 2007, 58, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.S.; Sandino, C.M.; Cole, E.K.; Larson, D.B.; Gold, G.E.; Vasanawala, S.S.; Lungren, M.P.; Hargreaves, B.A.; Langlotz, C.P. Prospective Deployment of Deep Learning in MRI: A Framework for Important Considerations, Challenges, and Recommendations for Best Practices. J. Magn. Reson. Imaging 2021, 54, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.J.; Johnson, P.M.; Knoll, F.; Lui, Y.W. Artificial Intelligence for MR Image Reconstruction: An Overview for Clinicians. J. Magn. Reson. Imaging 2021, 53, 1015–1028. [Google Scholar] [CrossRef] [PubMed]
- Gassenmaier, S.; Afat, S.; Nickel, D.; Kannengiesser, S.; Herrmann, J.; Hoffmann, R.; Othman, A.E. Application of a Novel Iterative Denoising and Image Enhancement Technique in T1-Weighted Precontrast and Postcontrast Gradient Echo Imaging of the Abdomen: Improvement of Image Quality and Diagnostic Confidence. Investig. Radiol. 2021, 56, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Gassenmaier, S.; Herrmann, J.; Nickel, D.; Kannengiesser, S.; Afat, S.; Seith, F.; Hoffmann, R.; Othman, A.E. Image Quality Improvement of Dynamic Contrast-Enhanced Gradient Echo Magnetic Resonance Imaging by Iterative Denoising and Edge Enhancement. Investig. Radiol. 2021, 56, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Antun, V.; Renna, F.; Poon, C.; Adcock, B.; Hansen, A.C. On Instabilities of Deep Learning in Image Reconstruction and the Potential Costs of AI. Proc. Natl. Acad. Sci. USA 2020, 117, 30088–30095. [Google Scholar] [CrossRef] [PubMed]
- Gassenmaier, S.; Afat, S.; Nickel, M.D.; Mostapha, M.; Herrmann, J.; Almansour, H.; Nikolaou, K.; Othman, A.E. Accelerated T2-Weighted TSE Imaging of the Prostate Using Deep Learning Image Reconstruction: A Prospective Comparison with Standard T2-Weighted TSE Imaging. Cancers 2021, 13, 3593. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Park, J.E.; Nam, Y.K.; Lee, J.; Kim, S.; Kim, Y.-H.; Kim, H.S. Deep Learning-Based Thin-Section MRI Reconstruction Improves Tumour Detection and Delineation in Pre- and Post-Treatment Pituitary Adenoma. Sci. Rep. 2021, 11, 21302. [Google Scholar] [CrossRef]
- Knoll, F.; Hammernik, K.; Zhang, C.; Moeller, S.; Pock, T.; Sodickson, D.K.; Akçakaya, M. Deep-Learning Methods for Parallel Magnetic Resonance Imaging Reconstruction: A Survey of the Current Approaches, Trends, and Issues. IEEE Signal Process Mag. 2020, 37, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Afat, S.; Wessling, D.; Afat, C.; Nickel, D.; Arberet, S.; Herrmann, J.; Othman, A.E.; Gassenmaier, S. Analysis of a Deep Learning-Based Superresolution Algorithm Tailored to Partial Fourier Gradient Echo Sequences of the Abdomen at 1.5 T: Reduction of Breath-Hold Time and Improvement of Image Quality. Investig. Radiol. 2022, 57, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Heverhagen, J.T. Noise Measurement and Estimation in MR Imaging Experiments. Radiology 2007, 245, 638–639. [Google Scholar] [CrossRef] [PubMed]


| Parameters | FLAIRS | FLAIRDLR | T2S | T2DLR | T1CES | T1CEDLR |
|---|---|---|---|---|---|---|
| Field of view (mm) | 230 | 230 | 230 | 230 | 230 | 230 |
| Voxel size (mm) | 0.7 × 0.7 × 4.0 | 0.4 × 0.4 × 4.0 | 0.6 × 0.6 × 4.0 | 0.3 × 0.3 × 4.0 | 0.8 × 0.8 × 4.0 | 0.4 × 0.4 × 4.0 |
| Slice thickness (mm) | 4 | 4 | 4 | 4 | 4 | 4 |
| Number of slices | 40 | 40 | 40 | 40 | 40 | 40 |
| Base Resolution | 320 | 320 | 384 | 384 | 304 | 304 |
| Parallel imaging factor | 2 | 4 | 2 | 4 | 2 | 4 |
| Acceleration mode | GRAPPA | GRAPPA | GRAPPA | GRAPPA | GRAPPA | GRAPPA |
| Reference Lines | 72 | 72 | 72 | 72 | 72 | 72 |
| TR (ms) | 8800 | 8800 | 4220 | 4220 | 2170 | 2170 |
| TE (ms) | 81 | 81 | 82 | 82 | 9.7 | 9.7 |
| Averages | 1 | 1 | 1 | 1 | 1 | 1 |
| Concatenations | 2 | 2 | 2 | 2 | 2 | 2 |
| Acquisition time (min) | 2:40 | 1:57 | 1:09 | 0:51 | 2:03 | 1:19 |
| Characteristics | Values |
|---|---|
| Number of patients | n = 33 |
| Age, mean ± standard deviation (years) | 59.8 ± 10.6 |
| Sex (male vs. female) | n = 19 (57.7%) vs. n = 14 (42.4%) |
| Initial diagnosis without previous therapy | n = 2 (6%) |
| Time of imaging since first diagnosis (mean ± standard deviation (SD, months) | 23 ± 27.2 |
| Clinical Scores | |
| Karnofsky Performance Scale Index (KPS in %), median [interquartile range] | 70 [40–100] |
| ECOG Performance Status Scale, median [interquartile range] | 1 [0–2] |
| Neurologic Assessment in Neuro-Oncology (NANO), median [interquartile range] | 2 [0–4] |
| Montreal Cognitive Assessment (MoCA) *, median [interquartile range] | 26 [21.5–27.5] |
| Mini-Mental State Examination (MMSE) *, median [interquartile range] | 28.5 [26–30] |
| Distress thermometer (DT) *, median [interquartile range] | 4.5 [2–5.75] |
| Therapy | n = 31/33 |
| These 31 patients received the following therapy: | |
| Surgery | n = 31/31 (100%) |
| Radiotherapy | n = 31/31 (100%) |
| Bevacizumab | n = 7/31 (23%) |
| Immunotherapy | n = 3/31 (10%) |
| Temozolomide | n = 17/31 (54%) |
| PCV scheme | n = 2/31 (6%) |
| CeTeG | n = 2/31 (6%) |
| Lomustine | n = 6/31 (19%) |
| Parameters | Standard Acquisition Time in Min | DLR Acquisition Time in Min | Time Saving in Min |
|---|---|---|---|
| FLAIR | 2:40 | 1:57 | 0:43 (27%) |
| T2 | 1:09 | 0:51 | 0:18 (26%) |
| T1CE | 2:03 | 1:19 | 0:44 (36%) |
| Time saving (on average) | 0:35 (30%) |
| Characteristics | Rater 1 | Rater 2 | ||||
|---|---|---|---|---|---|---|
| FLAIRS | FLAIRDLR | p-Value | FLAIRS | FLAIRDLR | p-Value | |
| Image noise | 4 [4–4] | 5 [5–5] | <0.001 | 4 [4–4] | 5 [5–5] | <0.001 |
| Artifacts | 5 [5–5] | 5 [5–5] | 0.180 | 5 [5–5] | 5 [5–5] | 0.083 |
| Sharpness | 4 [3–4] | 5 [5–5] | <0.001 | 3 [3–4] | 5 [4–5] | <0.001 |
| Overall image quality | 4 [4–4] | 5 [5–5] | <0.001 | 4 [4–4] | 5 [5–5] | <0.001 |
| Tumor conspicuity | 4 [4–4] | 5 [5–5] | <0.001 | 4 [4–4] | 5 [5–5] | <0.001 |
| Diagnostic confidence | 5 [5–5] | 5 [5–5] | 0.317 | 5 [5–5] | 5 [5–5] | 0.317 |
| Characteristics | Rater 1 | Rater 2 | ||||
|---|---|---|---|---|---|---|
| T2S | T2DLR | p-Value | T2S | T2DLR | p-Value | |
| Image noise | 4 [4–4] | 5 [5–5] | <0.001 | 4 [4–4] | 5 [5–5] | <0.001 |
| Artifacts | 5 [5–5] | 5 [4–5] | 0.132 | 5 [4.5–5] | 5 [4–5] | 0.180 |
| Sharpness | 4 [4–4] | 5 [5–5] | <0.001 | 4 [4–4] | 5 [5–5] | <0.001 |
| Overall image quality | 4 [4–4] | 5 [5–5] | <0.001 | 4 [4–4] | 5 [5–5] | <0.001 |
| Tumor conspicuity | 4 [4–4] | 5 [5–5] | <0.001 | 4 [4–4] | 5 [5–5] | <0.001 |
| Diagnostic confidence | 5 [5–5] | 5 [5–5] | 0.157 | 5 [5–5] | 5 [5–5] | 0.083 |
| Characteristics | Rater 1 | Rater 2 | ||||
|---|---|---|---|---|---|---|
| T1CES | T1CEDLR | p-Value | T1CES | T1CEDLR | p-Value | |
| Image noise | 4 [4–4] | 5 [5–5] | <0.001 | 4 [3–4] | 5 [5–5] | <0.001 |
| Artifacts | 4 [4–4] | 4 [4–4] | 0.046 | 4 [4–4] | 4 [4–4] | 0.157 |
| Sharpness | 3 [3–4] | 5 [5–5] | <0.001 | 3 [3–4] | 5 [5–5] | <0.001 |
| Overall image quality | 4 [4–4] | 5 [5–5] | <0.001 | 4 [3–4] | 5 [5–5] | <0.001 |
| Tumor conspicuity | 4 [4–4] | 5 [5–5] | <0.001 | 4 [4–4] | 5 [5–5] | <0.001 |
| Diagnostic confidence | 5 [4–5] | 5 [4.5–5] | 0.414 | 5 [4–5] | 5 [4.5–5] | 0.317 |
| Cohen’s Kappa | Kendall’s Tau | |
|---|---|---|
| Overall image qualityS | 0.672 (0.577–0.787) | 0.696 (0.571–0.821) |
| Overall image qualityDLR | 0.632 (0.476–0.784) | 0.695 (0.559–0.803) |
| Diagnostic confidenceS | 0.701 (0.570–0.748) | 0.747 (0.570–0.784) |
| Diagnostic confidenceDLR | 0.632 (0.459–0.784) | 0.655 (0.459–0.803) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gohla, G.; Hauser, T.-K.; Bombach, P.; Feucht, D.; Estler, A.; Bornemann, A.; Zerweck, L.; Weinbrenner, E.; Ernemann, U.; Ruff, C. Speeding Up and Improving Image Quality in Glioblastoma MRI Protocol by Deep Learning Image Reconstruction. Cancers 2024, 16, 1827. https://doi.org/10.3390/cancers16101827
Gohla G, Hauser T-K, Bombach P, Feucht D, Estler A, Bornemann A, Zerweck L, Weinbrenner E, Ernemann U, Ruff C. Speeding Up and Improving Image Quality in Glioblastoma MRI Protocol by Deep Learning Image Reconstruction. Cancers. 2024; 16(10):1827. https://doi.org/10.3390/cancers16101827
Chicago/Turabian StyleGohla, Georg, Till-Karsten Hauser, Paula Bombach, Daniel Feucht, Arne Estler, Antje Bornemann, Leonie Zerweck, Eliane Weinbrenner, Ulrike Ernemann, and Christer Ruff. 2024. "Speeding Up and Improving Image Quality in Glioblastoma MRI Protocol by Deep Learning Image Reconstruction" Cancers 16, no. 10: 1827. https://doi.org/10.3390/cancers16101827
APA StyleGohla, G., Hauser, T.-K., Bombach, P., Feucht, D., Estler, A., Bornemann, A., Zerweck, L., Weinbrenner, E., Ernemann, U., & Ruff, C. (2024). Speeding Up and Improving Image Quality in Glioblastoma MRI Protocol by Deep Learning Image Reconstruction. Cancers, 16(10), 1827. https://doi.org/10.3390/cancers16101827







