Real-World Safety and Outcome of First-Line Pembrolizumab Monotherapy for Metastatic NSCLC with PDL-1 Expression ≥ 50%: A National Italian Multicentric Cohort (“PEMBROREAL” Study)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Assessment
2.3. Statistical Analyses
3. Results
3.1. Patients and Treatment Characteristics
3.2. Reasons for Discontinuing Pembrolizumab
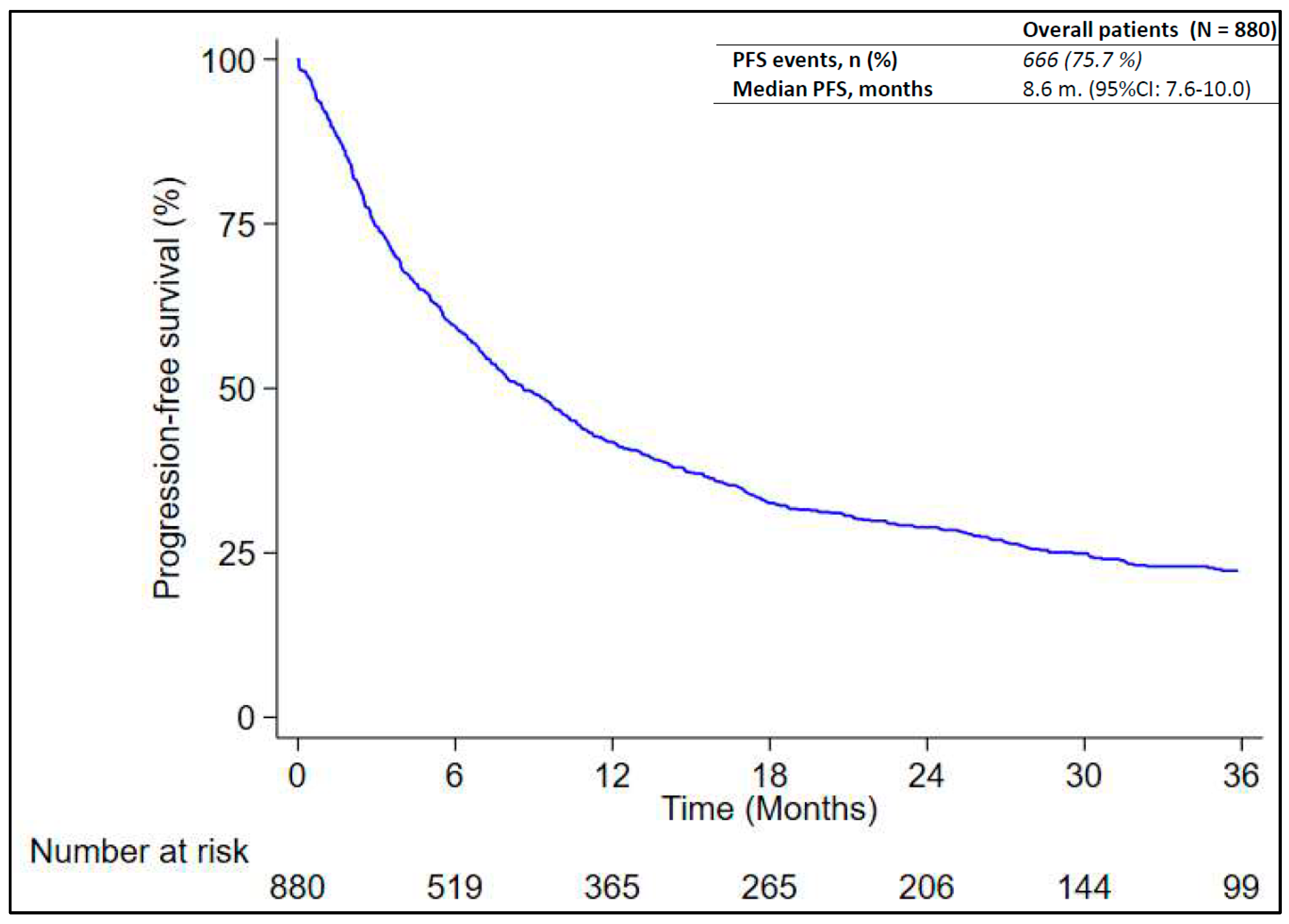
3.3. Analysis of Real-World PFS
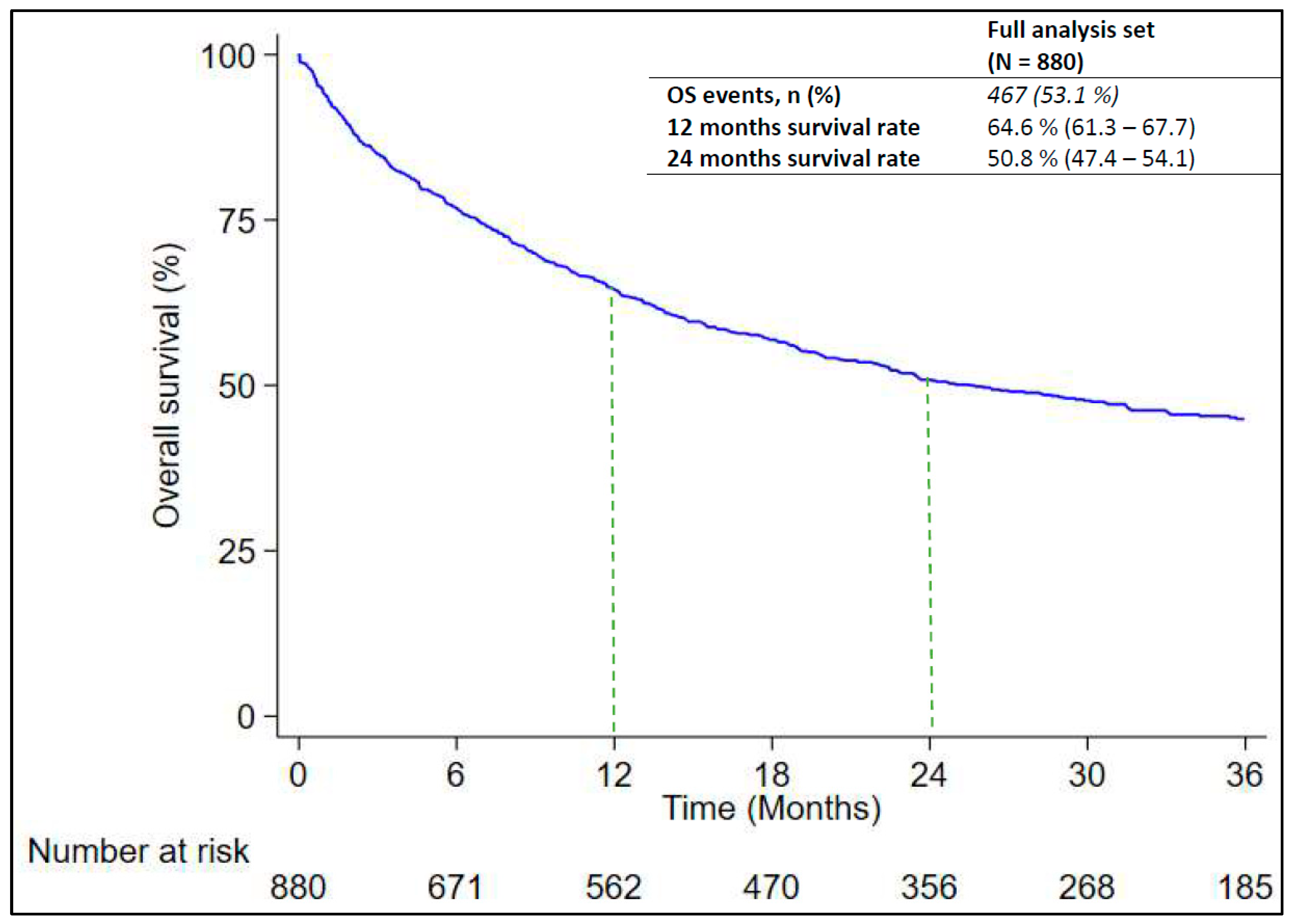
3.4. Analysis of Real-World OS
3.5. Analysis of Treatment Response
3.6. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AIOM; Airtum. I Numeri del Cancro in Italia 2023. Available online: https://www.aiom.it/wp-content/uploads/2023/12/2023_AIOM_NDC-web.pdf (accessed on 3 April 2024).
- Melosky, B.; Wheatley-Price, P.; Juergens, R.A.; Sacher, A.; Leighl, N.B.; Tsao, M.S.; Cheema, P.; Snow, S.; Liu, G.; Card, P.B.; et al. The rapidly evolving landscape of novel targeted therapies in advanced non-small cell lung cancer. Lung Cancer 2021, 160, 136–151. [Google Scholar] [CrossRef]
- Kerr, K.M.; Bibeau, F.; Thunnissen, E.; Botling, J.; Ryška, A.; Wolf, J.; Öhrling, K.; Burdon, P.; Malapelle, U.; Büttner, R. The evolving landscape of biomarker testing for non-small cell lung cancer in Europe. Lung Cancer 2021, 154, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Gregg, J.P.; Li, T.; Yoneda, K.Y. Molecular testing strategies in non-small cell lung cancer: Optimizing the diagnostic journey. Transl. Lung Cancer Res. 2019, 8, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Arbour, K.C.; Riely, G.J. Systemic therapy for locally advanced and metastatic non–small cell lung cancer: A review. JAMA 2019, 322, 764–774. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, J.; Qin, C.; Yan, H.; Liu, T.; Hu, H.; Tang, S.; Tang, S.; Zhou, H. Biomarker-Targeted Therapies in Non-Small Cell Lung Cancer: Current Status and Perspectives. Cells 2022, 11, 3200. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, L.E.; Kerr, K.M.; Menis, J.; Mok, T.S.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.F.; Solomon, B.J.; et al. Electronic address: Clinicalguidelines@esmo.org. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 339–357. [Google Scholar] [CrossRef] [PubMed]
- Bodor, J.N.; Boumber, Y.; Borghaei, H. Biomarkers for immune checkpoint inhibition in non–small cell lung cancer (NSCLC). Cancer 2020, 126, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Garassino, M.C.; Gadgeel, S.; Speranza, G.; Felip, E.; Esteban, E.; Dómine, M.; Hochmair, M.J.; Powell, S.F.; Bischoff, H.G.; Peled, N.; et al. Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non-Small-Cell Lung Cancer: 5-Year Outcomes From the Phase 3 KEYNOTE-189 Study. J. Clin. Oncol. 2023, 41, 1992–1998. [Google Scholar] [CrossRef] [PubMed]
- Novello, S.; Kowalski, D.M.; Luft, A.; Gümüş, M.; Vicente, D.; Mazières, J.; Rodríguez-Cid, J.; Tafreshi, A.; Cheng, Y.; Lee, K.H.; et al. Pembrolizumab Plus Chemotherapy in Squamous Non-Small-Cell Lung Cancer: 5-Year Update of the Phase III KEYNOTE-407 Study. J. Clin. Oncol. 2023, 41, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Cafaro, A.; Foca, F.; Nanni, O.; Chiumente, M.; Coppola, M.; Baldo, P.; Orzetti, S.; Enrico, F.; Ladisa, V.; Lerose, R.; et al. A real-world retrospective, observational study of first-line pembrolizumab plus chemotherapy for metastatic non-squamous non-small cell lung cancer with PD-L1 tumor proportion score < 50% (PEMBROREAL). Front. Oncol. 2024, 14, 1351995. [Google Scholar] [CrossRef] [PubMed]
- Velcheti, V.; Hu, X.; Li, Y.; El-Osta, H.; Pietanza, M.C.; Burke, T. Real-World Time on Treatment with First-Line Pembrolizumab Monotherapy for Advanced NSCLC with PD-L1 Expression ≥ 50%: 3-Year Follow-Up Data. Cancers 2022, 14, 1041. [Google Scholar] [CrossRef] [PubMed]
- Bérard, G.; Guévremont, C.; Marcotte, N.; Schroeder, C.; Bouchard, N.; Rajan, R.; Programme de Gestion Thérapeutique des Médicaments (PGTM). Descriptive Analysis of First-Line Non-Small Cell Lung Cancer Treatment with Pembrolizumab in Tumors Expressing PD-L1 ≥ 50% in Patients Treated in Quebec’s University Teaching Hospitals (DALP-First Study). Curr. Oncol. 2023, 30, 3251–3262. [Google Scholar] [CrossRef] [PubMed]
- Jiménez Galán, R.; Prado-Mel, E.; Pérez-Moreno, M.A.; Caballano-Infantes, E.; Flores Moreno, S. Influence of Performance Status on the Effectiveness of Pembrolizumab Monotherapy in First-Line for Advanced Non-Small-Cell Lung Cancer: Results in a Real-World Population. Biology 2021, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Pons-Tostivint, E.; Hulo, P.; Guardiolle, V.; Bodot, L.; Rabeau, A.; Porte, M.; Hiret, S.; Demontrond, P.; Curcio, H.; Boudoussier, A.; et al. Real-world multicentre cohort of first-line pembrolizumab alone or in combination with platinum-based chemotherapy in non-small cell lung cancer PD-L1 ≥ 50. Cancer Immunol. Immunother. 2023, 72, 1881–1890, Erratum in Cancer Immunol. Immunother. 2023, 72, 1891–1892. [Google Scholar] [CrossRef]
- Izano, M.A.; Sweetnam, C.; Zhang, C.; Weese, J.L.; Reding, D.; Treisman, J.; Patel, A.; Potugari, B.; Stafford, A.; Wolf, F.M.; et al. Brief Report on Use of Pembrolizumab With or Without Chemotherapy for Advanced Lung Cancer: A Real-World Analysis. Clin. Lung Cancer 2023, 24, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Descourt, R.; Chouaid, C.; Pérol, M.; Besse, B.; Greillier, L.; Bylicki, O.; Ricordel, C.; Guisier, F.; Gervais, R.; Schott, R.; et al. First-line pembrolizumab with or without platinum doublet chemotherapy in non-small-cell lung cancer patients with PD-L1 expression ≥50. Future Oncol. 2021, 17, 3007–3016. [Google Scholar] [CrossRef] [PubMed]
- Amrane, K.; Geier, M.; Corre, R.; Léna, H.; Léveiller, G.; Gadby, F.; Lamy, R.; Bizec, J.L.; Goarant, E.; Robinet, G.; et al. First-line pembrolizumab for non-small cell lung cancer patients with PD-L1 ≥50% in a multicenter real-life cohort: The PEMBREIZH study. Cancer Med. 2020, 9, 2309–2316. [Google Scholar] [CrossRef] [PubMed]
- Velcheti, V.; Hu, X.; Yang, L.; Pietanza, M.C.; Burke, T. Long-Term Real-World Outcomes of First-Line Pembrolizumab Monotherapy for Metastatic Non-Small Cell Lung Cancer With ≥50% Expression of Programmed Cell Death-Ligand 1. Front. Oncol. 2022, 12, 834761. [Google Scholar] [CrossRef] [PubMed]
- Tamiya, M.; Tamiya, A.; Hosoya, K.; Taniguchi, Y.; Yokoyama, T.; Fukuda, Y.; Hirano, K.; Matsumoto, H.; Kominami, R.; Suzuki, H.; et al. Efficacy and safety of pembrolizumab as first-line therapy in advanced non-small cell lung cancer with at least 50% PD-L1 positivity: A multicenter retrospective cohort study (HOPE-001). Investig. New Drugs 2019, 37, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Tamura, A.; Matsumoto, H.; Isobe, K.; Ozaki, T.; Santorelli, M.L.; Taniguchi, K.; Kamitani, T.; Irisawa, M.; Kanda, K.; et al. First-Line Pembrolizumab Monotherapy for Advanced NSCLC With Programmed Death-Ligand 1 Expression Greater Than or Equal to 50%: Real-World Study Including Older Patients in Japan. JTO Clin. Res. Rep. 2022, 3, 100397. [Google Scholar] [CrossRef]
- Mountzios, G.; de Toma, A.; Economopoulou, P.; Friedlaender, A.; Banini, M.; Lo Russo, G.; Baxevanos, P.; Roila, F.; Banna, G.L.; Christopoulou, A.; et al. Steroid Use Independently Predicts for Poor Outcomes in Patients With Advanced NSCLC and High PD-L1 Expression Receiving First-Line Pembrolizumab Monotherapy. Clin. Lung Cancer 2021, 22, e180–e192. [Google Scholar] [CrossRef] [PubMed]
- Dudnik, E.; Moskovitz, M.; Rottenberg, Y.; Lobachov, A.; Mandelboim, R.; Shochat, T.; Urban, D.; Wollner, M.; Nechushtan, H.; Rotem, O.; et al. Pembrolizumab as a monotherapy or in combination with platinum-based chemotherapy in advanced non-small cell lung cancer with PD-L1 tumor proportion score (TPS) ≥50%: Real-world data. Oncoimmunology 2021, 10, 1865653. [Google Scholar] [CrossRef] [PubMed]
- Tambo, Y.; Sone, T.; Shibata, K.; Nishi, K.; Shirasaki, H.; Yoneda, T.; Araya, T.; Kase, K.; Nishikawa, S.; Kimura, H.; et al. Real-World Efficacy of First-Line Pembrolizumab in Patients with Advanced or Recurrent Non-Small-Cell Lung Cancer and High PD-L1 Tumor Expression. Clin. Lung Cancer 2020, 21, e366–e379. [Google Scholar] [CrossRef] [PubMed]
- Cavaille, F.; Peretti, M.; Garcia, M.E.; Giorgi, R.; Ausias, N.; Vanelle, P.; Barlesi, F.; Montana, M. Real-world efficacy and safety of pembrolizumab in patients with non-small cell lung cancer: A retrospective observational study. Tumori 2021, 107, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Frost, N.; Kollmeier, J.; Misch, D.; Vollbrecht, C.; Grah, C.; Matthes, B.; Pultermann, D.; Olive, E.; Raspe, M.; Ochsenreither, S.; et al. Pembrolizumab as First-Line Palliative Therapy in PD-L1 Overexpressing (≥ 50%) NSCLC: Real-world Results with Special Focus on PS ≥ 2, Brain Metastases, and Steroids. Clin. Lung Cancer 2021, 22, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Tiseo, M.; Banna, G.L.; Cappuzzo, F.; Aerts, J.G.J.V.; Barbieri, F.; Giusti, R.; Bria, E.; Cortinovis, D.; Grossi, F.; et al. Clinicopathologic correlates of first-line pembrolizumab effectiveness in patients with advanced NSCLC and a PD-L1 expression of ≥ 50. Cancer Immunol. Immunother. 2020, 69, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Velcheti, V.; Chandwani, S.; Chen, X.; Pietanza, M.C.; Piperdi, B.; Burke, T. Outcomes of first-line pembrolizumab monotherapy for PD-L1-positive (TPS ≥50%) metastatic NSCLC at US oncology practices. Immunotherapy 2019, 11, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, E.J.; Ricciuti, B.; Gainor, J.F.; Kehl, K.L.; Kravets, S.; Dahlberg, S.; Nishino, M.; Sholl, L.M.; Adeni, A.; Subegdjo, S.; et al. Outcomes to first-line pembrolizumab in patients with non-small-cell lung cancer and very high PD-L1 expression. Ann. Oncol. 2019, 30, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Hubbard, R.A.; Mamtani, R.; Marmarelis, M.E.; Hennessy, S. Very high PD-L1 expression as a prognostic indicator of overall survival among patients with advanced non-small cell lung cancer receiving anti-PD-(L)1 monotherapies in routine practice. Pharmacoepidemiol. Drug Saf. 2022, 31, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Mazzaschi, G.; Barbieri, F.; Passiglia, F.; Mazzoni, F.; Berardi, R.; Proto, C.; Cecere, F.L.; Pilotto, S.; Scotti, V.; et al. First-line pembrolizumab in advanced non-small cell lung cancer patients with poor performance status. Eur. J. Cancer 2020, 130, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Gainor, J.F.; Rizvi, H.; Jimenez Aguilar, E.; Skoulidis, F.; Yeap, B.Y.; Naidoo, J.; Khosrowjerdi, S.; Mooradian, M.; Lydon, C.; Illei, P.; et al. Clinical activity of programmed cell death 1 (PD-1) blockade in never, light, and heavy smokers with non-small-cell lung cancer and PD-L1 expression ≥50. Ann. Oncol. 2020, 31, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Q.; Yap, M.L.; Cheng, E.S.; Ngo, P.J.; Vaneckova, P.; Karikios, D.; Canfell, K.; Weber, M.F. Evaluating Prognostic Factors for Sex Differences in Lung Cancer Survival: Findings From a Large Australian Cohort. J. Thorac. Oncol. 2022, 17, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Pala, L.; Pagan, E.; Bagnardi, V.; De Pas, T.; Queirolo, P.; Pennacchioli, E.; Catania, C.; Cocorocchio, E.; Ferrucci, P.F.; et al. Sex-Based Dimorphism of Anticancer Immune Response and Molecular Mechanisms of Immune Evasion. Clin. Cancer Res. 2021, 27, 4311–4324. [Google Scholar] [CrossRef] [PubMed]
- Popat, S.; Liu, S.V.; Scheuer, N.; Gupta, A.; Hsu, G.G.; Ramagopalan, S.V.; Griesinger, F.; Subbiah, V. Association between Smoking History and Overall Survival in Patients Receiving Pembrolizumab for First-Line Treatment of Advanced Non-Small Cell Lung Cancer. JAMA Netw. Open 2022, 5, e2214046. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Choi, S.M.; Lee, J.; Lee, C.H.; Lee, S.M.; Kim, D.W.; Yim, J.J.; Kim, Y.T.; Yoo, C.G.; Kim, Y.W.; et al. Proportion and clinical features of never-smokers with non-small cell lung cancer. Chin. J. Cancer 2017, 36, 20. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | N° of Patients n = 880 |
|---|---|
| Age (continuous), median (minimum-maximum) | 69.9 (37.9–90.2) |
| Age (categorical), n (%) | |
| <75 | 651 (74.0) |
| ≥75 | 229 (26.0) |
| Sex, n (%) | |
| Male | 618 (70.2) |
| Female | 262 (29.8) |
| Histology, n (%) | |
| Adenocarcinoma | 673 (76.5) |
| NOS carcinoma | 37 (4.2) |
| Large-cell lung Cancer | 4 (0.5) |
| Adenosquamous | 7 (0.8) |
| Squamous | 158 (18.0) |
| Unknown—N/D | 1 |
| EGFR status known, n (%) | |
| Yes | 788 (89.5) |
| No | 92 (10.5) |
| EGFR mutation, n (%) | |
| Negative | 788 (100.0) |
| ALK status known, n (%) | |
| Yes | 790 (89.8) |
| No | 90 (10.2) |
| ALK status known, n (%) | |
| Negative | 790 (100.0) |
| PDL1, n (%) | |
| 50–59% | 140 (26.5) |
| 60–69% | 72 (13.7) |
| 70–79% | 104 (19.8) |
| 80–89% | 91 (17.3) |
| 90–99% | 104 (19.7) |
| 100% | 16 (3.0) |
| Unknown | 353 |
| PS ECOG, n (%) | |
| 0 | 366 (42.0) |
| 1 | 435 (49.9) |
| 2 | 71 (8.1) |
| Unknown | 8 |
| Smoking history, n (%) | |
| Ex smoker | 251 (46.3) |
| Smoker | 186 (34.3) |
| Not smoker | 105 (19.4) |
| Unknown | 338 |
| Presence of brain metastases, n (%) | |
| Yes | 134 (15.7) |
| No | 717 (84.3) |
| Unknown | 29 |
| Variable | Number of Patients | Number of Events | Median Rw-PFS (95% CI) | p-Value (Log-Rank Test) |
|---|---|---|---|---|
| All cases | 880 | 666 | 8.6 (7.6–10.0) | - |
| Age | ||||
| <75 yrs | 651 | 488 | 8.5 (7.1–10.1) | 0.640 |
| ≥75 yrs | 229 | 178 | 9.1 (7.4–10.8) | |
| Sex | ||||
| Male | 618 | 480 | 8.3 (7.2–10.0) | 0.108 |
| Female | 262 | 186 | 9.2 (7.2–12.1) | |
| Histologic types | ||||
| Squamo | 158 | 133 | 8.4 (6.2–12.1) | 0.092 |
| No squamo | 721 | 532 | 8.6 (7.5–10.1) | |
| PDL1 | ||||
| 50–69% | 212 | 176 | 6.9 (5.4–8.9) | 0.032 |
| 70–89% | 195 | 155 | 7.0 (5.6–10.0) | |
| ≥90% | 120 | 79 | 13.9 (8.9–19.0) | |
| PS ECOG | ||||
| 0 | 366 | 256 | 11.1 (9.8–14.8) | <0.001 |
| 1 | 435 | 339 | 7.8 (6.4–9.5) | |
| 2 | 71 | 64 | 2.8 (1.7–5.1) | |
| Smoking history | ||||
| Never smoker | 105 | 86 | 5.9 (3.9–8.6) | 0.007 |
| Smoker or former smoker | 437 | 317 | 10.8 (8.9–13.1) | |
| Presence of brain metastases | ||||
| Yes | 134 | 108 | 6.3 (4.2–8.9) | 0.053 |
| No | 717 | 538 | 9.3 (7.7–10.4) |
| Variable | Number of Patients | Number of Events | Median Rw-OS (95% CI) | 12-Months Rw-OS (95% CI) | 24-Months Rw-OS (95% CI) | p-Value Log-Rank Test |
|---|---|---|---|---|---|---|
| All cases | 880 | 467 | 25.5 (21.8–31.6) | 64.6 (61.3–67.7) | 50.8 (47.4–54.1) | - |
| Age | ||||||
| <75 yrs | 651 | 326 | 30.7 (23.6–48.7) | 66.8 (63.0–70.3) | 53.7 (49.7–57.6) | 0.002 |
| ≥75 yrs | 229 | 141 | 15.4 (12.4–22.5) | 58.3 (51.6–64.4) | 42.8 (36.2–49.3) | |
| Sex | ||||||
| Male | 618 | 347 | 22.3 (17.8–28.9) | 62.9 (59.0–66.6) | 48.1 (44.1–52.1) | 0.014 |
| Female | 262 | 120 | 39.1 (25.5-NE) | 68.5 (62.5–73.8) | 57.2 (50.8–63.1) | |
| Histologic types | ||||||
| Squamo | 158 | 101 | 18.5 (15.5–22.8) | 64.6 (56.6–71.5) | 40.4 (32.5–48.2) | 0.010 |
| No squamo | 721 | 365 | 29.9 (23.5–39.1) | 64.7 (61.1–68.1) | 53.2 (49.4–56.8) | |
| PDL1 | ||||||
| <50–69% | 212 | 138 | 15.5 (10.7–19.8) | 54.7 (47.8–61.1) | 39.3 (32.6–45.9) | 0.117 |
| 70–89% | 195 | 131 | 15.3 (11.6–21.3) | 56.9 (49.7–63.5) | 38.4 (31.4–45.4) | |
| ≥90% | 120 | 66 | 22.5 (14.1–40.4) | 66.5 (57.2–74.2) | 49.4 (39.9–58.2) | |
| PS ECOG | ||||||
| 0 | 366 | 171 | 38.5 (29.5-NE) | 73.9 (69.1–78.1) | 59.1 (53.7–64.0) | <0.001 |
| 1 | 435 | 241 | 18.6 (14.1–28.6) | 60.4 (55.6–64.8) | 47.2 (42.4–51.9) | |
| 2 | 71 | 51 | 5.9 (2.8–15.5) | 42.3 (30.7–53.4) | 29.3 (18.8–40.5) | |
| Smoking history | ||||||
| Never smoker | 105 | 48 | NR | 69.5 (59.8–77.4) | 58.6 (48.5–67.4) | 0.044 |
| Smoker or former smoker | 437 | 246 | 22.4 (18.7–28.9) | 64.6 (59.9–68.9) | 48.1 (43.2–52.9) | |
| Presence of brain metastases | ||||||
| Yes | 134 | 78 | 23.5 (13.5–29.9) | 60.5 (51.6–68.2) | 48.8 (39.8–57.1) | 0.291 |
| No | 717 | 377 | 25.5 (20.0–35.7) | 65.2 (61.6–68.6) | 50.9 (47.2–54.6) |
| Toxicities | N° Patients n = 880 (%) | |
|---|---|---|
| At Least One Adverse Reaction | 351 (39.9%) | |
| Any Grade | Grade 3–4 | |
| Infections and infestations | 22 (2.5) | 1 (0.1) |
| Blood and lymphatic system disorders | 74 (8.4) | 6 (0.7) |
| Immune system disorders | 5 (0.6) | 0 (0.0) |
| Endocrine disorders | 60 (6.8) | 0 (0.0) |
| Metabolism and nutrition disorders | 92 (10.5) | 2 (0.2) |
| Psychiatric disorders | 13 (1.5) | 0 (0.0) |
| Nervous system disorders | 48 (5.5) | 0 (0.0) |
| Eye disorders | 13 (1.5) | 0 (0.0) |
| Cardiac disorders | 23 (2.6) | 1 (0.1) |
| Vascular disorders | 12 (1.0) | 0 (0.0) |
| Respiratory, thoracic, and mediastinal disorders | 140 (15.9) | 1 (0.1) |
| Gastrointestinal disorders | 209 (23.8) | 14 (1.6) |
| Hepatobiliary disorders | 14 (1.6) | 0 (0.0) |
| Skin and subcutaneous tissue disorders | 197 (22.4) | 13 (1.5) |
| Musculoskeletal and connective tissue disorders | 90 (10.2) | 2 (0.2) |
| Renal and urinary disorders | 15 (1.7) | 1 (0.1) |
| General disorders and administration site conditions | 203 (36.6) | 13 (1.5) |
| Diagnostic exams | 89 (10.1) | 13 (1.5) |
| Paper, Publication Year | Patients Number | Nations Involved | Median Follow Up | Median Overall Survival | Median Progression Free Survival | Others Surrogate Endpoints Evaluated | Note |
|---|---|---|---|---|---|---|---|
| [14] Velcheti V; February 2022 | 1044 | USA, multicentric | 34 months | / | / | rwTOT = 7.4 months (95%CI: 6.3–8.1) | |
| [15] Bérard G; March 2023 | 279 | Canada (Quebec), multicentric | 7.53 months | 17.3 months (95% CI: 12.9–NR) | 9.4 months (95% CI: 6.6–11.2) | / | 2 patients with PD-L1 < 50%; 1 patient with unknown expression of PD-L1; 35 patients with stage III NSCLC |
| [16] Jiménez Galán R; September 2021 | 88 | Spain, monocentric | 23.0 months | 7.9 months (95% CI: 1.2–14.6) | 3.9 months (95% CI, 2.3–5.6) | / | 2 patients with stage III B NSCLC; 7 patients with ECOG PS 3; 34.6% patients with ECOG PS ≥ 2 |
| [17] Pons-Tostivint E; June 2023 | 141 | France, multicentric | 11.5 months | 12-month survival rate: 66.1% (95% CI: 58–75.3) | 10.6 months (95% CI 7.2—NR | / | / |
| [18] Izano MA, June 2023 | 341 | USA: multicentric | 10 months | 18 months (95% CI: 14–22) | / | / | 28 patients with PD-L1 < 50%, and 78 patients with unknown expression; 7 patients EGFR-mutated; 2 patients ALK-positive |
| [19] Descourt R, January 2023 | 845 | France, multicentric | 25.8 months | 22.6 months (95% CI 18.5–27.4) | 8.2 months (95% CI: 6.9- 9.5) | / | / |
| [20] Amrane K; April 2020 | 108 | France, multicentric | 8.2 months | 15.2 months (95% CI, 13.9–NR) | 10.1 months (95% CI: 8.8 to 11.4) | / | 14 patients with stage III NSCLC |
| [21] Velcheti V; March 2022 | 566 (EHR cohort) | USA: multicentric | 35.1 months | 19.6 months (95% CI: 16.6–24.3) | / | / | All Patients had PS ECOG <2 |
| 288 (spotlight coohrt) | 38.4 months | 21.1 (95% CI 16.2–28.9) | 7.3 months (95% CI: 5.7–9.2) | / | |||
| [22] Tamiya M; December 2019 | 213 | Japan: Multicentric | 11 months | 17.8 months (95% CI: 17.8–NR) | 8.3 months (95% CI: 6.0–10.7) | / | 9 patients with ECOG PS 3 and 1 patients with ECOG PS 1; 6 patients EGFR mutated, 38 patients stage III. |
| [23] Goto Y, 5 August 2022 | 441 | Japan: multicentric | 13.5 months | 12 and 24 months OS rate 72.2% (95% CI: 67.5–76.3) and 57.9% (95% CI: 50.8–64.3) | 10 months (95% CI: 8.2–11.8) | rwTOT 5.6 (95% CI 4.4–6.7) | 19% of patients with stage III NSCLC |
| [24] Mountzios G, March 2021 | 265 | Italy, Spain, Greece, Switzerland: multicentric | / | 22.5 months | / | TTP: 10.4 months | 2 patients with ECOG PS 3 |
| [25] Dudnik E; January 2021 | 203 | Israel: multicentric | 22.3 months | 12.5 months (95% CI: 9.8–16.4) | / | TTD: 4.9 months (95% CI, 3.1–7.6) | 9 patients with stage III NSCLC |
| [26] Tambo Y; September 2020 | 95 | Japan: multicentric | 8.8 months | 12- and 24-month survival rate: 78.3% and 58.3% | 6.1 months (95% CI: 3.64–8.56) | / | 10 patients ECOG-PS 3–4; 29 patients with non metastatic NSCLC |
| [27] Cavaille F, February 2021 | 38 | France: monocentric | 7.6 months | 11.08 months (95% CI: 5.98–NR) | 6 months (95% CI 3–NR) | / | 5 patients with ECOG PS 3; 2 patients with stage III NSCLC |
| [28] Frost N.; September 2021 | 153 | Germany: multicentric | 26.9 months | 22.0 months (95% CI: 15.4–28.6) | 8.2 months (95% CI 5.1–11.4) | / | 6 patients with ECOG PS3; 29 patients with stage III NSCLC |
| [29] Cortellini A; November 2020 | 1026 | Multicentric: Italy | 14.6 months | 17.2 months (95% CI: 15.3–22.3) | 7.9 months (95% CI: 6.9–9.5) | / | / |
| [30] Velcheti V; November 2019 | 423 | Multicentric: USA | 18.4 | 18.9 months (95% CI 14.9–25.5) | 6.8 months (95% CI 5.3–8.1) | / | / |
| 188 | 15.5 | 19.1 months (95% CI:12.6–NR) | / | / | 15.4% of patients with non metastatic NSCLC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cafaro, A.; Foca, F.; Nanni, O.; Chiumente, M.; Coppola, M.; Russi, A.; Svegliati, E.; Baldo, P.; Orzetti, S.; Enrico, F.; et al. Real-World Safety and Outcome of First-Line Pembrolizumab Monotherapy for Metastatic NSCLC with PDL-1 Expression ≥ 50%: A National Italian Multicentric Cohort (“PEMBROREAL” Study). Cancers 2024, 16, 1802. https://doi.org/10.3390/cancers16101802
Cafaro A, Foca F, Nanni O, Chiumente M, Coppola M, Russi A, Svegliati E, Baldo P, Orzetti S, Enrico F, et al. Real-World Safety and Outcome of First-Line Pembrolizumab Monotherapy for Metastatic NSCLC with PDL-1 Expression ≥ 50%: A National Italian Multicentric Cohort (“PEMBROREAL” Study). Cancers. 2024; 16(10):1802. https://doi.org/10.3390/cancers16101802
Chicago/Turabian StyleCafaro, Alessandro, Flavia Foca, Oriana Nanni, Marco Chiumente, Marina Coppola, Alberto Russi, Elena Svegliati, Paolo Baldo, Sabrina Orzetti, Fiorenza Enrico, and et al. 2024. "Real-World Safety and Outcome of First-Line Pembrolizumab Monotherapy for Metastatic NSCLC with PDL-1 Expression ≥ 50%: A National Italian Multicentric Cohort (“PEMBROREAL” Study)" Cancers 16, no. 10: 1802. https://doi.org/10.3390/cancers16101802
APA StyleCafaro, A., Foca, F., Nanni, O., Chiumente, M., Coppola, M., Russi, A., Svegliati, E., Baldo, P., Orzetti, S., Enrico, F., Foglio, F., Pinnavaia, D., Ladisa, V., Lauria Pantano, C., Lerose, R., Nardulli, P., Ferraiuolo, S., Maiolino, P., De Stasio, I., ... Masini, C. (2024). Real-World Safety and Outcome of First-Line Pembrolizumab Monotherapy for Metastatic NSCLC with PDL-1 Expression ≥ 50%: A National Italian Multicentric Cohort (“PEMBROREAL” Study). Cancers, 16(10), 1802. https://doi.org/10.3390/cancers16101802







